Abstract
The airway defensive response to tussive agents, such as capsaicin, is frequently assessed by counting the number of cough sounds, or expulsive events. This method does not identify or differentiate important respiratory events that occur in the respiratory muscles and lungs, which are critical in assessing airway defensive responses. The purpose of this study was to characterize the airway defensive behaviours (cough and expiration reflex) to capsaicin exposure in humans. We observed complex motor behaviours in response to capsaicin exposure. These behaviours were defined as cough reacceleration (CRn) and expiration reflex (ERn), where n is the number of expulsive events with and without a preceding inspiratory phase, respectively. Airway defensive responses were defined in terms of frequency (number of expulsive events), strength (activation of abdominal muscles) and behaviour type (CRn vs. ERn). Thirty-six subjects (15 females, 24±4 yr) were instrumented with EMG electrodes placed over the rectus abdominis (RA), external abdominal oblique (EO) and the 8th intercostal space (IC8). A custom-designed mouth pneumotachograph was used to assess the airflow acceleration, plateau velocity and phase duration of the expulsive phase. Subjects inhaled seven concentrations of capsaicin (5–200 μM) in a randomized block order. The total number of expulsive events (frequency) and the sum of integrated EMG for the IC8, RA and EO (strength) increased in a curvilinear fashion. Differentiating the airway defense responses into type demonstrated predominately CR1 and CR2 (i.e. inspiration followed by one and two expulsive events, respectively) with very few ER’s at <50 μM capsaicin. At higher concentrations (>50 μM) ER’s with one or more expulsive events (≥ER1) appeared, and the number of CR’s with three or more expulsive events (≥CR3) increased. The decrease in EMG activation and airflow measurements with each successive expulsive event suggests a decline in power and shear force as the number of expulsive events increased. Therefore, the airway defensive response to capsaicin is a complex motor pattern that functions to coordinate ER’s and CR’s with differing numbers of expulsive events possibly to prevent aspirations and keep air moving to promote clearance.
Keywords: Cough reacceleration, Expiration reflex, Cough strength, Cough frequency, Tussives, Capsaicin
1. Introduction
Cough is the second most common reason patients seek medical attention [1] and in many cases it is the only symptom of respiratory diseases. Clinicians assess cough to determine the progress of the respiratory disease or disorder, and the effectiveness of drug treatments, such as antitussives. Currently, the most common method of assessing cough is to count the number of coughs based on “cough sounds” collected by automated cough monitors or manually by the clinician. However, there are other parameters that are equally important in assessing and defining cough. For instance, antitussives such as codeine reduce cough strength (as determined by rectus abdominis EMG signal amplitude) as well as cough number [2]. Hypoxic hypercapnia, a hallmark of several respiratory diseases, reduces cough strength (as determined by intrapleural pressure) but not cough number [3]. Cough in patients with motor neural disease, such as Parkinson’s disease, are reduced in strength but higher in frequency [4,5]. Cough strength can vary with or independently of cough frequency; thus it is difficult to infer cough strength from only cough frequency measurements. From a patient’s perspective, cough strength is a critical component of the cough response as it determines whether a cough is effective in moving material from the airway and lungs or whether it is too strong resulting in distress and even rib fractures (e.g. cough associated with some high-altitude sojourners) [6].
Another important aspect of assessing airway defensive responses is differentiating cough from expiration reflexes (ER) [7]. While both produce a similar “cough sound”, they have different functions. Cough begins with a priming inspiration that draws air into the lungs to enhance the force of the expiration thus promoting clearance of material from the airways and lungs. An ER consists of a forced expiration (i.e. expulsive event) that is not preceded with an inspiratory phase, and likely functions to prevent entry of material into the airways [8]. Furthermore, the number of expulsive events associated with each cough and ER also varies [9-12]. In this study we defined cough as cough reacceleration (CRn), where n is the number of expulsive events following a single priming inspiration (i.e. CR2 consists of an inspiratory phase followed by two expulsive events), and expiration reflexes as ERn. These expulsive events are the result of an interruption of expiration, which reaccelerates the expiratory airflow. Similarly, ERn are one or more expulsive events but with no priming inspiration. Since these different types of airway defensive responses serve different functions, it is particularly important to distinguish between them when assessing airway defensive response in patients. The motor pattern of airway defensive responses, defined in this paper as the frequency (number of expulsive events), strength (abdominal muscle EMG and airflow dynamics), and type of airway defense behaviour (CR vs. ER), is likely different for different diseases and disorders of the respiratory tract. To date this has not been fully investigated in the same group of subjects.
Studies that investigated variations of the airway defensive response, specifically the cough response, in different subject pools and patient populations, used the C2/C5 technique. With this technique, capsaicin concentrations (or other tussives such as citric acid and distilled water) are doubled until two and five “cough events” (typically defined by the number of expulsive events, or “cough sounds”) are achieved, representing the threshold and suprathreshold response, respectively. These studies have shown differences in the cough response between ages [13], genders [14-16] and diseases [4,5,13,17]. However, these data cannot infer changes in expiratory muscle activation, duration of expulsive phases and the airflow dynamics with increasing capsaicin concentration, all which are essential components in defining the cough response. Equally important, they do not distinguish between types of airway defensive behaviour; thus it is unknown if the expulsive events, or “cough sounds”, measured are CR’s or ER’s.
Characterization of the motor pattern of airway defensive responses is important because it provides information about the changes in motorneural pathways involved in generating CR and ER in disease vs. the changes in respiratory mechanics. For instance, bronchitis exhibit lower peak airflows during the expulsive phase, but the duration of the expulsive phase is the same as in healthy controls [9]. Combined study of EMG and airflow dynamics provides information on both motorneural activation and pulmonary mechanics thus allowing for a more comprehensive evaluation of airway defensive motor responses.
The purpose of this study was to characterize the airway defensive motor pattern to increasing capsaicin concentrations in healthy adults. While EMG activity has been recorded in response to citric acid [18], ultrasonically nebulized distilled water [19,20] and tartaric acid [10], and airflow has been assessed in bronchitis [9], the EMG and airflow response has not been evaluated simultaneously in the same subject pool and to the same tussive agent. Thus, direct comparisons of respiratory muscle activation and airway mechanics have not been made. We hypothesized that strength (determined by expiratory muscle activation, airflow dynamics and phase duration) and frequency (number of expulsive events), will increase with capsaicin concentration. We focussed on the compressive and expiratory phases since most disease-related changes in airway defensive responses occur in these phases. In addition, we focussed on expiratory muscle activity since the role of inspiratory muscles during the expulsive phase is virtually quiescent [21]. We also hypothesized that the type of airway defensive behaviour (CR vs. ER) will change with increasing capsaicin concentration suggesting a complex pattern in motorneural activity.
2. Methods
2.1. Subjects
Thirty-six healthy adult non-smoking subjects (15 females; 24±4 yr) with no history of chronic respiratory or neurological disease participated in this study. All subjects consented to participating as per the regulations set forth by the Institutional Review Board at the University of Florida.
2.2. Apparatus
2.2.1. Capsaicin administration
After the subject was instrumented, the subject was seated comfortably in a chair in front of an airflow fume hood. The airflow fume hood prevented exposure of the subject to the nebulized solution except through inspiration via the mouthpiece. Subjects leaned into the airflow fume hood and inspired deeply through a mouthpiece connected to the nebulizer. The outflow neublizer gas was passed through an isopropyl alcohol solution to remove capsaicin from the vented gas.
2.2.2. Frequency of expulsive events
A microphone was fixed to the neck in the region of the larynx to record tracheal–pharyngeal sound. This provided an auditory record of expulsive events that would later be compared with the number of expulsive events counted by the experimenter during the experiment.
2.2.3. Airflow dynamics
Airway mechanical events were measured using a custom-designed mouthpiece with an integral pneumotachograph. A dental impression was made of the subject’s upper teeth and a plastic retainer was constructed and a plastic pneumotachograph was cemented to the palatal surface of the retainer. The pneumotachograph was connected to a differential pressure transducer by flexible tubing. This device allowed the subjects to close their mouths around the nebulizer mouthpiece and cough while measuring mouth airflow velocity, but it did not allow the measurement of airflow volume because not all of the mouth airflow passed through the pneumotachograph. This device did permit unobstructed breathing, aerosol inhalation, cough and measurement of airflow pattern. The pressure transducer was connected to a computer processing system (ADInstruments, Inc.).
2.2.4. Respiratory muscle activity
Respiratory motor patterns were recorded with abdominal and thoracic surface EMGs. Active EMG electrodes (Delsys, Inc.) with integral amplifiers and band pass filters were placed over the RA, external abdominal oblique (EO), and the 8th intercostals space (IC8). The signals were amplified, band-pass filtered (100–1000 Hz) and recorded on a computer. Intra-subject variability in EMG was controlled by leaving the electrodes positioned on the subject in place while presenting all the trials on the same day. Placing electrodes in slightly different locations on different subjects may add to inter-subject variability by affecting the amplitude: signal amplitude decreases as the distance between the muscle fibres and the recording site increases, and the homogeneity of the signal which may inadvertently include activity from underlying muscle layers.
Airflow pattern, tracheal–pharyngeal sound (from the neck) and respiratory muscle EMGs were digitized (Power-Lab, ADInstruments, Inc.) and recorded on a computer polygraphing system (Chart, ADInstruments, Inc).
2.3. Protocol
During the experimental trials, subjects were given a verbal cue to lean into the airflow fume hood, place the mouthpiece into their mouth and take a single deep inspiration of the test-nebulized air. The experimenter and subjects were blinded to the capsaicin concentration. Each test inspiration was separated by an interval of 2 min during which the subject was breathing outside the airflow fume hood. The experimental trial consisted of eight test solutions: 0, 5, 10, 25, 50, 100, 150 and 200 μM capsaicin in 80% physiological saline, 10% Tween 20, and 10% ethanol. Each test solution was presented three times in a randomized block order for a total of 24 test solution presentations.
2.4. Data analysis
2.4.1. Frequency of expulsive events
The number of expulsive events was counted for 30 s following the presentation of each capsaicin test solution during the experiment subsequent to the experiment from auditory recordings from the tracheal–pharyngeal region of the neck. The lowest capsaicin concentration by both methods of measurement associated with at least two and five expulsive events were referred to as C2 and C5, respectively. The capsaicin concentration associated with C2 and C5 for each of the three randomized trials was determined for both methods of measurement and averaged.
EMG and airflow recordings were also used to count the number of expulsive events, and to differentiate CRs (inspiration followed by one or more expulsive event) from ER (one or more expulsive event without a preceding inspiratory phase). The number of CR’s and ER’s were counted from the computer data record following each capsaicin test solution presentation.
2.4.2. Respiratory muscle activity
Raw EMG activity was processed to obtain the moving time average (time constant: 50 ms). Activities of the RA, EO and IC8 muscles were assessed by calculating the area of the moving time averaged EMG signals for each CR and ER.
2.4.3. Airflow dynamics
Signal recordings from the pneumotachograph were used to calculate the mean airflow during the expulsive event of each CR and ER. In addition, the acceleration of air (i.e. slope of the airflow signal) at the onset of each expulsive event was determined.
2.4.4. Duration of expulsive events
The computer signals from airflow recordings were used to determine the duration of compression and expiration (i.e. expulsive) phases. Expiration duration was further divided into two segments: time for airflow rate to reach plateau levels, and duration of airflow at plateau levels.
2.4.5. Statistics
The results were compared using a paired t-test, repeated measures ANOVA and post-hoc between group analyses performed with Student–Newman–Keuls method for paired multiple comparisons where appropriate. The significance criterion was set at p<0.05.
3. Results
3.1. Frequency of expulsive events
The total number of expulsive events for each subject determined by the auditory signal, airflow tracing and the EMG signal were averaged to generate a group mean (Table 1). There was no difference in the frequencies of expulsive events (i.e. “cough sounds”) based on the method. However, when the expulsive events were grouped into CR’s and ER’s the total number of airway defensive behaviours was 50% less (p<0.05). The reason for this was that some of the CR’s included multiple expulsive events (i.e. CR2 is one airway defensive behaviour but two expulsive events).
Table 1.
Counting of airway defensive behaviours using different sources of measurement
| CR | ER | Total | |
|---|---|---|---|
| Auditory | — | — | 49.2±6.2 |
| Airflow tracing | 44.7±6.2 | 4.1±1.2 | 48.9±6.6 |
| EMG signal (# expiratory events) | 44.1±6.1 | 4.1±1.2 | 48.2±6.6 |
| EMG signal (# of each type) | 21.3±3.5 | 3.2±0.8 | 23.9±3.8* |
Significantly different from auditory, airflow and EMG (# expiratory events) totals (p<0:05). The last row shows reduced numbers for CR because it is based on counting the number of CR and ER behaviours rather than individual expulsive events. As such, a CR2 would be counted as a single behaviour rather than two expulsive events.
The total number of expulsive events counted at each capsaicin concentration based on airflow tracing is illustrated in Fig. 1. Total number of expulsive events, CR’s and ER’s, increased in a curvilinear fashion with capsaicin concentration. The average capsaicin concentration at C2 and C5 based on auditory sounds occurred at 50 and 200 μM, which is in agreement with the calculations using the total number of expulsive events based on airflow tracing (i.e. approximately two and five expulsive events were recorded at 50 and 200 μM of capsaicin). Thus randomized presentations of capsaicin produced similar capsaicin concentrations at C2 and C5 compared with the more commonly used “doubling dose” method. The mode for C2 and C5 was 50 and 150 μM, and the median was 50 and 100 μM. While the “threshold” or C2 measurement is consistent with the statistical method used to describe the group response, the “suprathreshold” or C5 measurement varied.
Fig. 1.
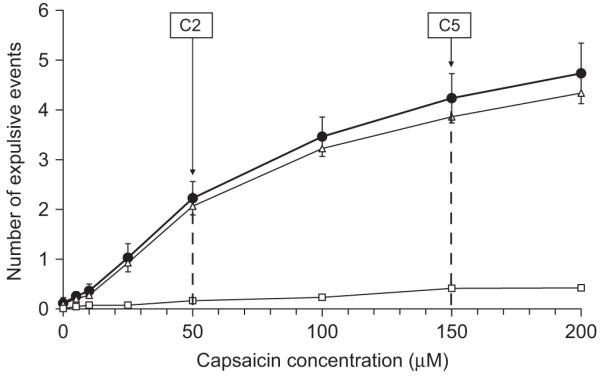
The average increase in the number of expulsive events at each capsaicin concentration as determined by EMG tracings (closed circles). Data are shown for expulsive events associated with CR’s (open triangles) and ER’s (open squares). C2 and C5, indicating threshold and suprathreshold responses, occurred at 50 and 150 μM of capsaicin, respectively.
3.2. Type of airway defensive behaviour
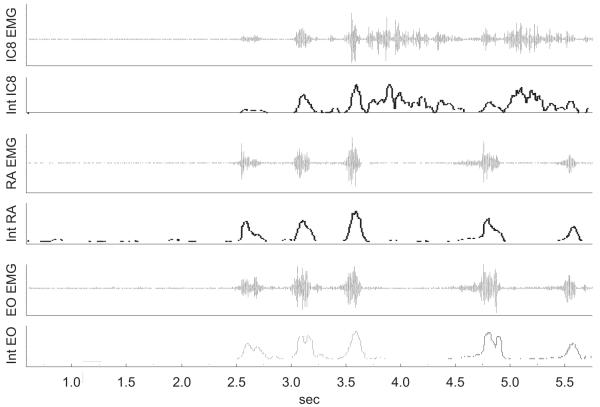
Airway defensive behaviours differentiated into CR and ER was determined using the airflow tracing. Fig. 2 illustrates an example of a CR3 (i.e. an inspiration followed by three successive expulsive events) and 1 CR2.
Fig. 2.
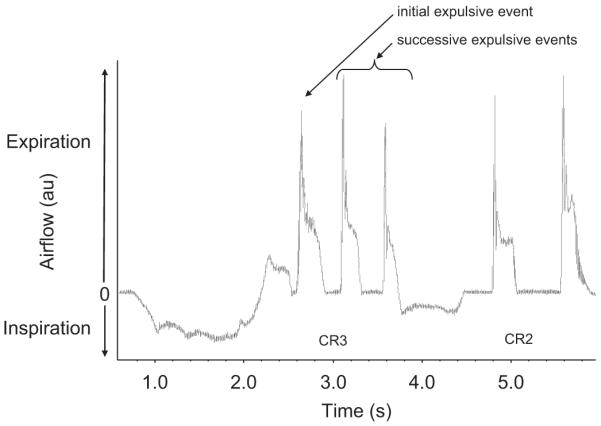
Representative airflow tracing from one subject illustrating a CR3 and a CR3 following inhalation of 150 μM of capsaicin. A CR is defined as an inspiration followed by one or more expulsive events.
The total numbers of each type of CR (i.e. CR1–CR7) and ER (i.e. ER1–ER4) at every capsaicin concentration were added and expressed as a percentage of total CR’s and ER’s (Fig. 3). There are three points to note. First, as capsaicin concentration was raised, the total number of CR’s and ER’s increased. Second, CR’s and ER’s with a greater number of expulsive events were recruited at higher capsaicin concentrations (>50 μM). Third, the most common airway defensive behaviour at all concentrations was CR2. The proportion of subjects responding at each capsaicin concentration followed a similar pattern. For instance, the percentage of subjects producing a CR1, CR2, CR3, CR4, ER1 and ER2 at 200 μM were 61%, 78%, 69%, 31%, 25% and 3%, respectively, suggesting that the average airway defensive response illustrated in Fig. 3 is a representation of the response across all subjects.
Fig. 3.
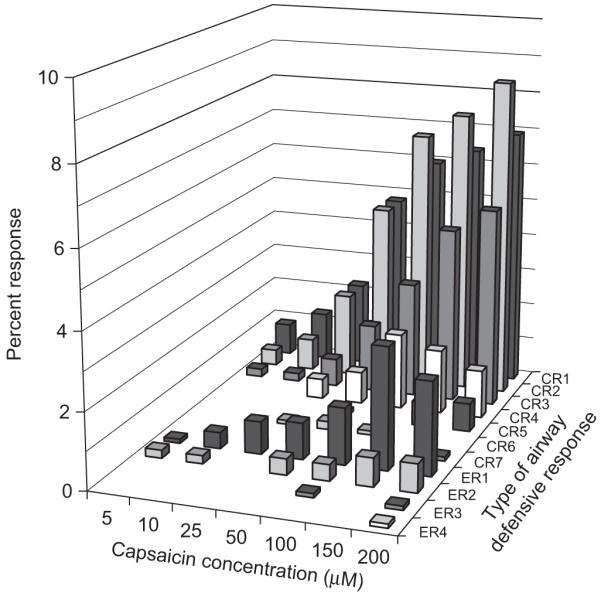
Number of airway defensive behaviours (expressed as a percentage of total CR and ER) for each type (CRn and ERn) increased with increasing capsaicin concentration. CR’s and ER’s with a greater number of expulsive events appeared at higher concentrations. CR2 was the most common response at all concentrations.
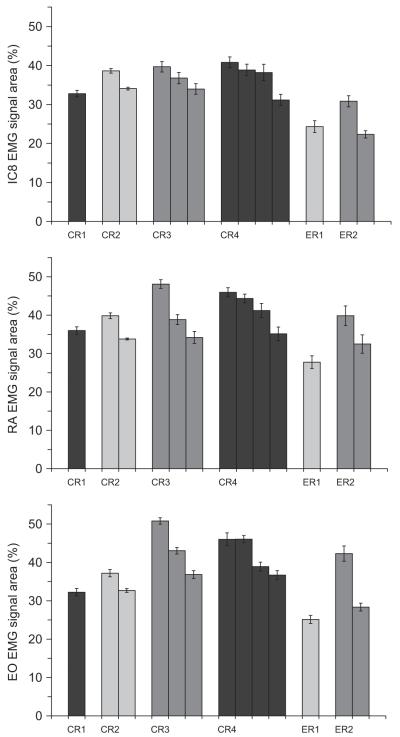
3.3. Strength of airway defensive behaviours
Fig. 4 illustrates an example of the raw and averaged EMG signals used to calculate areas for IC8, RA and EO. The total sum of the integrated EMG area for a given CR and ER was averaged at each capsaicin concentration (Fig. 5). All three muscle groups demonstrated the same response; thus Fig. 5 shows the results from IC8 EMG recordings which are representative for the other muscle groups. CR’s with a greater number of expulsive events had a greater overall expiratory muscle activation. The same pattern was noted with the ER’s; however, they exhibited lower overall expiratory muscle activation compared with their CR counterparts (i.e. CR2>ER2). Strength of a given CR and ER was the same at all capsaicin concentrations. There was a general increasing trend in strength for CR3 and CR4; however, the differences were between low (5–50 μM) and high (100–200 μM) concentration ranges rather than individual concentrations. Total strength at a single capsaicin concentration (calculated by adding the total sum of integrated EMG areas for all CR’s and ER’s) increased significantly with increasing capsaicin concentration (p<0.05) and followed a curvilinear trend (r2 = 0.98) similar to the frequency response.
Fig. 4.
Representative tracing of raw and integrated EMG signals for the IC8, RA and EO from the same subject illustrated in Fig. 2. Tracings show EMG activation for a CR3 and a CR2.
Fig. 5.
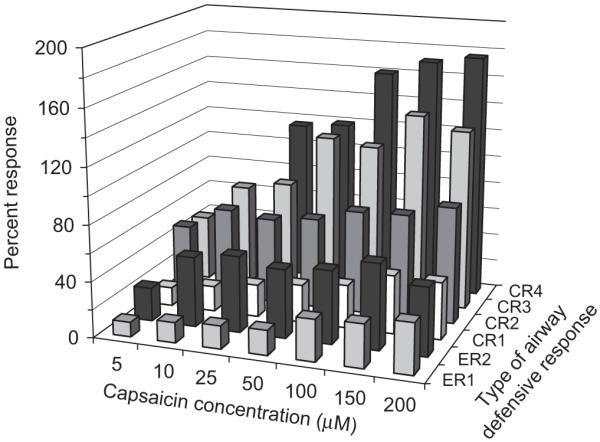
Total sum of the integrated IC8 EMG signal. CR’s greater than 4 and ER’s greater than 2 were not included since not all subjects exhibited these responses. While total muscle activation was greater for airway defense behaviours with more expulsive events, there was no effect of capsaicin concentration on the muscle activation.
Since the integrated EMG area for individual CR and ER did not change with capsaicin concentration, the integrated EMG areas for every expulsive event associated with each CR and ER were averaged across all capsaicin concentrations. Fig. 6 shows the average integrated EMG areas for CR1–4 and ER1–2 expiratory events in the IC8, RA and EO. The integrated EMG area of the initial expulsive event was the greatest and decreased with subsequent expulsive events, indicating a decline in strength. The strength for ER1 and ER2 were less than CR1 and CR2.
Fig. 6.
Percent increase in integrated EMG for IC8, RA and EO for expulsive events from each type of airway defensive behaviour. Muscle activation was greatest with the initial expulsive event, and subsequently declined with successive expulsive events. Bars indicate SEMs.
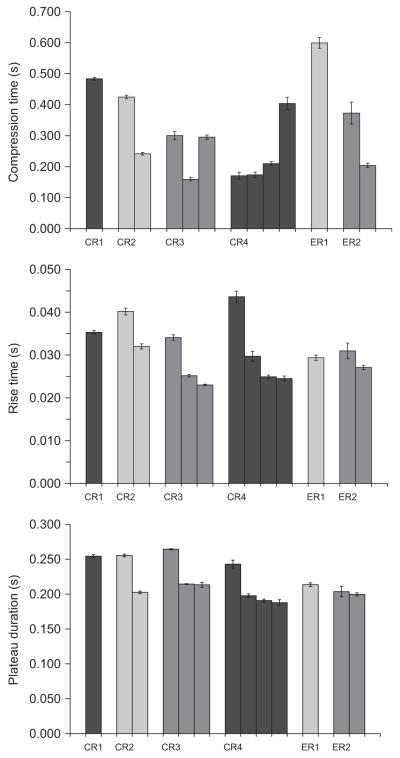
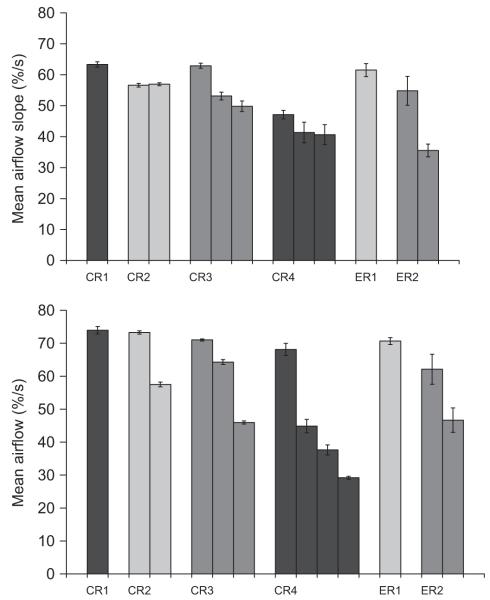
Average airflow dynamics and expulsive phase durations for each CR and ER (illustrated in Fig. 7) did not show significant differences with increasing capsaicin concentration; thus these data were averaged for each expulsive event (Figs. 8 and 9). In conjunction with EMG area, mean airflow slope (i.e. airflow acceleration) and mean airflow (i.e. airflow velocity) were greatest with the initial expulsive event, declining with subsequent expulsive events. Rise time and plateau duration were longest for the initial expulsive event. Subsequent expulsive events were shorter but not significantly different from one another. There were significant differences in the group means for compression time, but the there was no clear pattern between the initial and subsequent expulsive events.
Fig. 7.
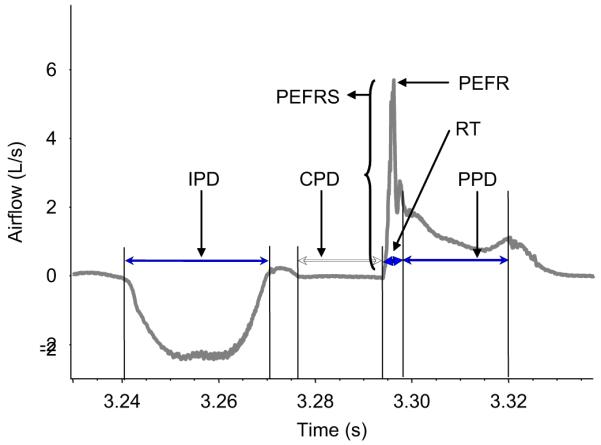
Illustration of the parameters measured from the airflow tracing. IPD: inspiratory phase duration; CPD: compressive phase duration; PEFRS: peak expiratory flow rate slope (i.e. airflow acceleration); PEFR: peak expiratory flow rate; RT: rise time; PPD: plateau phase duration.
Fig. 8.
Duration of the compressive phase (CPD), rise time (RT) and plateau phase (PPD) for expulsive events from each type of airway defensive behaviour. Rise time and plateau duration were greatest with the initial expulsive event, and subsequently declined with successive expulsive events. Bars indicate SEM.
Fig. 9.
Percent increase in mean airflow slope (i.e. airflow acceleration) and mean airflow (i.e. plateau velocity) for expulsive events from each type of airway defensive behaviour. Similar to muscle EMG, airflow acceleration and velocity were significantly greater in the initial expulsive event and subsequently decline with successive expulsive events.
4. Discussion
The main results from this study show that increasing capsaicin concentration in a randomized fashion increased frequency and strength of airway defensive responses. The total number of expulsive events and the sum of integrated EMG areas for the IC8, RA and EO increased in a curvilinear fashion. There were no differences between the methods of counting expulsive events (EMG, airflow or sound tracings). Total integrated EMG and airflow velocity, both measures of strength, rose simultaneously with increasing capsaicin concentration, illustrating for the first time a coordinated response of the respiratory muscles to airflow mechanics in the same group of subjects. Differentiating the airway defensive response into type (CRn and ERn) illustrated complex motor patterns. At lower capsaicin concentrations (<50 μM) there were very few ER’s and most of the CR’s were associated with one or two expulsive events (CR1 and CR2). At higher concentrations (>50 μM) ER’s with one or more expulsive events (≥ER1) appeared, and the number of CR’s with three or more expulsive events (≥CR3) increased. Examining the motor pattern for each type of CR and ER demonstrated a decrease in the force of contraction, airflow acceleration and plateau velocity and an increase in compression and expiration phase durations with each successive expulsive event.
4.1. Motor neural activation
Electrical activity measured with EMG is proportional to force of contraction; hence it serves as a good indicator of cough strength [22]. While EMG is frequently used to assess cough strength, the method of analysing EMG data is diverse, making it difficult to compare cough strength from different studies. In the present study, normalizing integrated EMG area for EO demonstrated a 32.2±1.0% increase in activity during a single cough reacceleration (CR1) in response to capsaicin. Lasserson et al. [10] showed a much higher activation of 79.7±41.6% following tartaric acid inhalation. Normalizing peak EMG activity for EO produced a 50% increase in cats with mechanical stimulation of the larynx [23] and 78–83% in humans using fog [5,17,19]. In addition to method of analysis, the type of tussive agent used and the species of the test subject will also add variability to the data. If EMG data are to be used as a tool to diagnose and define airway diseases, and as a method of comparing airway defensive responses including cough from different patient populations or assessing treatment regiments, then a more standardized method of analysis is required.
In cats, the RA plays a major role in the expulsive phase [7,24], whereas in humans the EO is thought to be the major abdominal muscle [18,19,25]. We did not compare the degree of activation of the IC8, EO or RA, but we do show that the relative increase in activation of these muscle groups was the same with increasing capsaicin concentration. Thus, measuring EMG activity in any of these muscle groups is adequate in assessing strength of airway defensive responses such as CR’s and ER’s in humans.
In addition, we showed an increase in total EMG activation with increasing capsaicin concentration. This has also been reported with citric acid [18] and fog [19] in humans. The shape of the dose/response curve is equivocal. Fontana et al. [19] report a linear relationship between EO EMG and fog, while Cox et al. [18] show an exponential rise in EO EMG with citric acid. Redrawn and modelled with a line of best fit, the data sets, including ours, demonstrate a curvilinear relationship (data not shown). The EMG response is likely a function of frequency since the number of expulsive events plotted over capsaicin concentration exhibited the same curvilinear trend. The shape of the EMG response curve is particularly relevant if EMG recordings are used to assess cough strength in people with reduced muscle fibre content such as older adults [26,27], or patients with neuromuscular diseases such as Parkinson’s and muscular dystrophy [4,5,28].
4.2. Airway mechanics
Airway flow dynamics during the expulsive phase has also been used as a measure of cough strength [9,12]. Greater airway narrowing (due to greater abdominal muscle contraction during the compressive phase) results in higher velocities thus displacing and accelerating sputum more effectively. Patients with obstructive diseases [12] and chronic bronchitis [9] exhibit lower peak and plateau flows during an expulsive event and hence they have less sputum clearance [12]. In support of this view, we report a comparable increase in airflow and abdominal muscle activity to increasing capsaicin concentration. Similar observations were made in previous studies [25,29]. Furthermore, the coordinated relationship between airflow and abdominal muscle activity is preserved in some disease states and with age. For instance, airflow and EMG decrease in parallel during cough in laryngectomized patients [17], and inspiratory muscle training in older adults (whose respiratory muscles are weakened due to age-related changes) improves peak and plateau airflow (unpublished observation). Therefore airflow relates to abdominal muscle activity in health and disease, and both can be used to measure strength of airway defensive behaviours such as cough.
However, the correlation between peak airflow and integrated or peak EMG is weak in response to citric acid [18], and negative in response to tartaric acid [10]. Fontana et al. [17] show a stronger correlation between rate of rise of EMG to peak airflow compared to peak EMG, suggesting the parameter used to assess EMG activity (i.e. peak, integrated area, rate of rise) may be important in determining the relationship between muscle activity and airflow during cough. Lung volume at the onset of an expulsive event may also explain the contradictory reports [9]. Voluntary cough starting from TLC produced more consistent and stronger correlations between airflow and EMG [10,19] than coughs beginning at lung volumes less than TLC [30,31].
4.3. Motor pattern of airway defensive behaviours
In this study, we separated airway defensive responses into CR’s and ER’s. A CR is defined by an inspiration followed by one or more expulsive events whereas an ER consists of expulsive event(s) without a preceding inspiratory phase. They are affected differently by the same stimuli suggesting they may follow different motor path-ways and/or be regulated differently. For example, hypercapnia [3,27] and anaesthesia [27] depress ER’s more than CR’s, whereas slow-wave sleep inhibits CR’s but not ER’s [32,33]. Furthermore, they likely serve different functions; CR clears the airway while ER prevents entry of material into the airway [11]. Difficulties with ER generation may cause aspirations leading to pneumonia [34], a problem for patients with stroke, Parkinson’s disease and motoneurone disease [5]. Also, an inadequate CR will prevent sputum movement, leading to airway infections [12,34]. Therefore, distinction between types of airway defensive behaviours is important when comparing the expulsive motor responses between diseases, assessing the effectiveness of treatments, or determining the effect of secondary symptoms on the airway defensive responses such as cough.
This is the first study to show a distinction in strength between ER and CR in humans. For the same number of expulsive events (i.e. CR2 vs. ER2), integrated EMG activity during ER was less than that for CR. This observation is supported by findings in the cat transversus abdominis muscle [35]. In addition, Poliacek et al. [35] report an increase in peak muscle activity and duration during an ER to the same stimulus in cats. While we did not measure peak EMG activity, we observed shorter expulsive phase duration with ER. These data support the hypothesis that ER and CR are controlled by different motor neural pathways and suggest that they differ between species. ER strength is less probably because high linear velocities are not required to prevent entry of foreign material into the airway whereas removal of foreign materials requires greater accelerations. Indeed, we found airflow acceleration and plateau velocity were less with ER compared to CR.
Differences between CR and ER frequency were also observed. There was a greater number of CR’s than ER’s at every capsaicin concentration; 92% of total coughs were CR and the remaining 8% were ER. This is in agreement with the airway defensive response to mechanical stimulation of the laryngeal folds in cats, where 94% were CR and 6% were ER [36]. Considering the different functions of the CR and ER, these data show that CR’s were recruited more frequently with inhaled capsaicin probably because the need to remove foreign material was greater than the need to block its entry into the airway.
The frequency of CR’s with three or more expulsive events (≥CR3) increased at higher capsaicin concentrations (≥50 μM). The relevance of recruiting CR’s with multiple expulsive events is that it keeps material moving through the airway since expiratory flow is not being interrupted by inspirations. It follows that five CR1’s will not be as effective in moving material from the airway as one CR5. Indeed, Young et al. [12] report that sputum expectoration usually occurs after groups of expulsive events following single inspirations rather than a single expulsive event. In addition, the pattern of CR and ER during a “coughing episode” is important because recruitment of CR and ER may be a coordinated effort to prevent further inhalation of foreign substances and to promote their removal. While we did not assess the pattern of CR’s and ER’s in the present study, three patterns of “coughing episodes” have been described in patients [8]. Thus counting the number of expulsive events without differentiating them into airway defensive behaviour types or patterns limits assessment of the patients’ cough response and inferences made about the cough motor pattern.
Abdominal muscle force (via EMG) and airflow (i.e. acceleration and plateau velocity) progressively decreased with each successive expulsive event. This has been previously shown in human EO EMG for CR’s with two expulsive events (CR2) only; CR’s with more expulsive events were not observed [10]. The cause for decline in abdominal muscle force is likely the result of decreasing lung volume with each successive expulsive event. Expiratory muscle length is less optimal as lung volume decreases; thus the force of contraction will be less resulting in lower airway velocity. On the other hand, bronchitis have relatively the same airflow plateau velocity with each successive expulsive event, possibly because these patients have a greater volume of air left in their lungs at the end of an expulsive event due to air trapping [8].
A decrease in EMG and airflow in CR’s and ER’s with successive expulsive events could indicate a progressive decrease in efficiency [26,37]. However, airflow measured at the mouth is not necessarily indicative of airflow in the smaller airways. Young et al. [12] proposed that a shift in the equal pressure point to more distal and smaller airways during coughs with multiple expulsive events will cause these airways to compress more resulting in an increase in linear velocity [28]. Thus, with each successive expulsive event, linear velocity will increase in smaller and smaller airways. Assuming that high linear velocities are required to move sputum [9,12], recruitment of CR’s and ER’s with multiple expulsive events may be a mechanism to remove sputum from very small airways that would not normally be removed following several single expulsive events (i.e. CR1, ER1). While deposition of capsaicin in our subjects likely did not reach smaller airways, the appearance of CR’s and ER’s with multiple expulsive events at higher capsaicin concentrations may serve as a protective reflex.
Muscle activity and airway dynamics for an expulsive event in the same position in a series of expulsive events from different CR’s were similar. For instance, EMG and airflow for the second expulsive event of a CR2 was the same as that for a CR4. While the significance of this finding is speculative, it may suggest that the motor drive for different CR’s is essentially the same, only differing by the duration or frequency of neural activity in the descending cough motor pathways allowing for greater or fewer expulsive events.
These results show that the airway defensive motor pattern changes with greater tussive stimulation. Frequency and strength of expulsive events increases with increasing capsaicin concentration suggesting an increase in overall motor drive. What is more interesting and novel is how the motor drive might be fine tuned with increasing tussive stimulation. CR’s were preferentially recruited over ER’s. Assuming both serve different functions these data suggest that CR’s were selectively chosen since the need to remove the irritant from the airway was greater than the need to prevent its entry. Moreover, the number of expulsive events associated with each CR and ER increased with increasing capsaicin concentration possibly because the ability to remove greater concentrations of airway irritants requires uninterrupted outward airflow and higher liner velocities in smaller airways. Thus assessing airway defensive responses such as cough based on sound alone omits several important parameters that can be extremely useful and essential in assessing changes in motorneural pathways in patients.
Acknowledgements
The authors would like to thank Erin Robertson and Patrick Shahan for their technical assistance. Support for this study was provided by Schering-Plough Research Institute.
References
- [1].National Ambulatory Care Survey: 1989 Results Series 13 # 36 (PHS) 92–1771 DHHS. 1992. Medical4. [Google Scholar]
- [2].Bolser DC, DeGennaro FC. Effect of codeine on the inspiratory and expiratory burst pattern during fictive cough in cats. Brain Res. 1994;662:30. doi: 10.1016/0006-8993(94)90792-7. [DOI] [PubMed] [Google Scholar]
- [3].Tatar M, Korpas J, Poliacek H, Zahradny V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetized cats. Respiration. 1986;49:114–21. doi: 10.1159/000194868. [DOI] [PubMed] [Google Scholar]
- [4].Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, et al. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124:1009–11. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- [5].Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson’s disease. Am J Respir Crit Care Med. 1998;158:458–64. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- [6].Litch JA, Tuggy M. Cough induced stress fracture and arthropathy of the ribs at extreme altitude. Int J Sports Med. 1998;19:220–2. doi: 10.1055/s-2007-971908. [DOI] [PubMed] [Google Scholar]
- [7].Korpas J, Tomori Z. Cough and other respiratory reflexes. S. Karger; Basel, New York: 1979. p. 356. [Google Scholar]
- [8].Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152:266–81. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- [9].Langlands J. The dynamics of cough in health and in chronic bronchitis. Thorax. 1967;22:88–96. doi: 10.1136/thx.22.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lasserson D, Mills K, Arunachalam R, Polkey M, Moxham J, Kalra L. Differences in motor activation of voluntary and reflex cough in humans. Thorax. 2006;61:699–705. doi: 10.1136/thx.2005.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Widdicombe J, Fontana G. Cough: what’s in a name? Eur Respir J. 2006;28:10–5. doi: 10.1183/09031936.06.00096905. [DOI] [PubMed] [Google Scholar]
- [12].Young S, Abdul-Sattar N, Caric D. Glottic closure and high flows are not essential for productive cough. Bull Eur Physiopathol Respir. 1987;23(suppl 10):11–7s. [PubMed] [Google Scholar]
- [13].Chang AB, Phelan PD, Sawyer SM, Del Brocco S, Robertson CF. Cough sensitivity in children with asthma, recurrent cough, and cystic fibrosis. Arch Dis Child. 1997;77:331–4. doi: 10.1136/adc.77.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dicpinigaitis PV, Allusson VR, Baldanti A, Nalamati JR. Ethnic and gender differences in cough reflex sensitivity. Respiration. 2001;68:480–2. doi: 10.1159/000050554. [DOI] [PubMed] [Google Scholar]
- [15].Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest. 1988;113:1319–21. doi: 10.1378/chest.113.5.1319. [DOI] [PubMed] [Google Scholar]
- [16].Fujimura M, Kasahara K, Kamio Y, Naruse M, Hashimoto T, Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996;9:1624–6. doi: 10.1183/09031936.96.09081624. [DOI] [PubMed] [Google Scholar]
- [17].Fontana GA, Pantaleo T, Lavorini F, Mutolo D, Polli G, Pistolesi M. Coughing in laryngectomized patients. Am J Respir Crit Care Med. 1999;160:1578–84. doi: 10.1164/ajrccm.160.5.9901093. [DOI] [PubMed] [Google Scholar]
- [18].Cox ID, Wallis PJ, Apps MC, Hughes DT, Empey DW, Osman RC, et al. An electromyographic method of objectively assessing cough intensity and use of the method to assess effects of codeine on the dose-response curve to citric acid. Br J Clin Pharmacol. 1984;18:377–82. doi: 10.1111/j.1365-2125.1984.tb02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fontana GA, Pantaleo T, Lavorini F, Boddi V, Panuccio P. A noninvasive electromyographic study on threshold and intensity of cough in humans. Eur Respir J. 1997;10:983–9. doi: 10.1183/09031936.97.10050983. [DOI] [PubMed] [Google Scholar]
- [20].Fontana GA, Pantaleo T, Lavorini F, Maluccio NM, Mutolo D, Pistolesi M. Repeatability of cough-related variables during fog challenges at threshold and suprathreshold stimulus intensity in humans. Eur Respir J. 1999;13:1447–50. [PubMed] [Google Scholar]
- [21].Kobayashi I, Kondo T, Suzuki H, Ohta Y, Yamabayashi H. Expiratory activity of the inspiratory muscles during cough. Jpn J Physiol. 1992;42:905–16. doi: 10.2170/jjphysiol.42.905. [DOI] [PubMed] [Google Scholar]
- [22].Bigland-Ritchie B. EMG/force relations and fatigue of human voluntary contractions. Exerc Sport Sci Rev. 1981;9:75–117. [PubMed] [Google Scholar]
- [23].Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J Appl Physiol. 2000;88:1207–14. doi: 10.1152/jappl.2000.88.4.1207. [DOI] [PubMed] [Google Scholar]
- [24].Belvisi MG, Bolser DC. Summary: animal models for cough. Pulm Pharmacol Ther. 2002;15:249–50. doi: 10.1006/pupt.2002.0349. [DOI] [PubMed] [Google Scholar]
- [25].Strohl KP, Mead J, Banzett RB, Loring SH, Kosch PC. Regional differences in abdominal muscle activity during various maneuvers in humans. J Appl Physiol. 1981;51:1471–6. doi: 10.1152/jappl.1981.51.6.1471. [DOI] [PubMed] [Google Scholar]
- [26].Lawson TV, Harris RS. Assessment of the mechanical efficiency of coughing in healthy young adults. Clin Sci. 1967;33:209–24. [PubMed] [Google Scholar]
- [27].Nishino T, Hiraga K, Honda Y. Inhibitory effects of CO2 on airway defensive reflexes in enflurane-anesthetized humans. J Appl Physiol. 1989;66:2–2646. doi: 10.1152/jappl.1989.66.6.2642. [DOI] [PubMed] [Google Scholar]
- [28].Macklem PT, Murphy B. The forces applied to the lung in health and disease. Am J Med. 1974;57:371–7. doi: 10.1016/0002-9343(74)90132-6. [DOI] [PubMed] [Google Scholar]
- [29].Goldman JM, Lehr RP, Millar AB, Silver JR. An electromyographic study of the abdominal muscles during postural and respiratory manoeuvres. J Neurol Neurosurg Psychiatry. 1987;50:866–9. doi: 10.1136/jnnp.50.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Knudson RJ, Mead J, Knudson DE. Contribution of airway collapse to supramaximal expiratory flows. J Appl Physiol. 1974;36:653–67. doi: 10.1152/jappl.1974.36.6.653. [DOI] [PubMed] [Google Scholar]
- [31].Yanagihara N, Von Leden H, Werner-Kukuk E. The physical parameters of cough: the larynx in a normal single cough. Acta Otolaryngol. 1966;61:495–510. doi: 10.3109/00016486609127088. [DOI] [PubMed] [Google Scholar]
- [32].Sullivan CE, Kozar LF, Murphy E, Phillipson EA. Arousal, ventilatory, and airway responses to bronchopulmonary stimulation in sleeping dogs. J Appl Physiol. 1979;47:17–25. doi: 10.1152/jappl.1979.47.1.17. [DOI] [PubMed] [Google Scholar]
- [33].Sullivan CE, Murphy E, Kozar LF, Phillipson EA. Waking and ventilatory responses to laryngeal stimulation in sleeping dogs. J Appl Physiol. 1978;45:681–9. doi: 10.1152/jappl.1978.45.5.681. [DOI] [PubMed] [Google Scholar]
- [34].Nakashima K, Maeda M, Tabata M, Adachi Y, Kusumi M, Ohshiro H, Tottori University Parkinson’s Disease Epidemiology (TUPDE) Study Group Prognosis of Parkinson’s disease in Japan. Eur Neurol. 1997;38(suppl 2):60–3. doi: 10.1159/000113485. [DOI] [PubMed] [Google Scholar]
- [35].Poliacek I, Stransky A, Jakus J, Barani H, Tomori Z, Halasova E. Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiol Res. 2003;52:749–62. [PubMed] [Google Scholar]
- [36].Bolser DC, Reier PJ. Inspiratory and expiratory patterns of the pectoralis major muscle during pulmonary defensive reflexes. J Appl Physiol. 1998;85:1786–92. doi: 10.1152/jappl.1998.85.5.1786. [DOI] [PubMed] [Google Scholar]
- [37].Harris RS, Lawson TV. The relative mechanical effectiveness and efficiency of successive voluntary coughs in healthy young adults. Clin Sci. 1968;34:569–77. [PubMed] [Google Scholar]