Abstract
Changes in appetite in older adults may result in unhealthy weight change and negatively affect overall nutrition. Research examining gustatory processing in young adults has linked changes in patterns of the hemodynamic response of gustatory and motivation related brain regions to the physiological states of hunger and satiety. Whether the same brain regions are involved in taste processing in older adults is unknown. The current study used functional magnetic resonance imaging (fMRI) to examine age-related changes in gustatory processing during hedonic assessment. Caffeine, citric acid, sucrose, and NaCl were administered orally during two event-related fMRI sessions, one during hunger and one after a pre-load. Participants assessed the pleasantness of the solutions in each session. Increased activity of the insula was seen in both age groups during hunger. Activity of secondary and higher order taste processing and reward regions such as the orbitofrontal cortex, amygdala, hippocampus, thalamus, and caudate nucleus was also observed. Hunger and satiety differentially affected the hemodynamic response, resulting in positive global activation during hunger and negative during satiety in both age groups. While in a state of hunger, the frequency and consistency of positive activation in gustatory and reward processing regions was greater in older adults. Additional regions not commonly associated with taste processing were also activated in older adults. Investigating the neurological response of older adults to taste stimuli under conditions of hunger and satiety may aid in understanding appetite, health, and functional changes in this population.
Keywords: Taste, Gustatory, fMRI, Neuroimaging, reward value, aging
Introduction
Unhealthy weight change in older adults can be damaging to physical and psychological health. Obesity in middle age has been identified as a serious risk factor in the development of dementia later in life (Whitmer et a., 2005a). Other cardiovascular risk factors such as diabetes, high cholesterol, and hypertension also have an association with the development of dementia (Whitmer et al., 2005b). Late in life, reduced intake is common and can lead to weight loss and nutritional deficiencies in many elderly individuals (Hays & Roberts, 2006). Non-physiological and physiological mechanisms have been investigated as possible factors in the reduction of nutritional intake. Social factors such as a higher prevalence of solitary eating habits in older adults (Wright et al., 2006), lower socioeconomic status, and psychological factors such as depression (DiPietro et al., 1992) and dementia have been noted as possible non-physiological factors in weight change and nutritional deficiencies in older adults.
Chemosensory and other physiological impairments are also believed to play a role in the deregulation of intake in the elderly by disrupting naturally occurring processes that facilitate proper eating behaviors. An important phenomenon known as sensory specific satiety has been shown to play a major role in the motivation of an individual to consume a varied and balanced diet (B. J. Rolls et al., 1981); sensory specific satiety has been shown to decrease with age (B. J. Rolls & McDermott, 1991). Age-related chemosensory changes such as a decrease in gustatory and olfactory functioning are possible contributors to reduced sensory specific satiety and an overall decline in health and nutrition in aging populations.
Diminished chemosensory functioning is common in older individuals and can have an effect on appetite and the hedonic appreciation of eating (Cerf-Ducastel & Murphy, 2003; Ferdon & Murphy, 2003; Fukunaga et al., 2005; Hays & Roberts, 2006; Murphy, 2008; Murphy & Gilmore, 1989; Murphy et al., 2002; B. J. Rolls & McDermott, 1991; Schiffmann et al., 1979). Age-related chemosensory processing changes combined with non-physiological factors place this population at a greater risk for nutritional deficiencies.
Efforts to understand the cortical substrates of taste began with studies aimed at mapping the gustatory system of nonhuman primates. The primary gustatory cortex in the rostral part of the frontal operculum and insula, a secondary taste processing region in the caudolateral orbitofrontal cortex (OFC), and anatomical and functional connections with reward, motivation, and homeostatic regions such as the amygdala, hypothalamus, hippocampus, and the OFC have been demonstrated to be involved in taste and reward processing (Pritchard et al., 1986; E. T. Rolls & Scott, 2003; Verhagen et al., 2003; Yaxley et al., 1988).
The effects of hunger and satiety on neuronal responsiveness in gustatory processing regions of non-human primates have also been examined. Studies reported that while activity in the primary gustatory cortex was unaffected by a transition from hunger to satiety, activity in the caudolateral OFC, amygdala, and hypothalamus was greatly reduced once satiety was reached (Burton et al., 1976; E. T. Rolls et al., 1989; Scott et al., 1993; Yan & Scott, 1996; Yaxley et al., 1988). This shift in activation is thought to be a regulatory mechanism, which facilitates proper energy intake.
In more recent studies, noninvasive neuroimaging techniques such as functional magnetic resonance imaging (fMRI) have been useful tools for examining representations of gustatory functioning in the human brain. Unlike results of nonhuman primate studies, hunger has been shown to modulate brain activity of the insula in response to pure tastes during hedonic evaluation (Haase et al., 2009a), in response to a chocolate stimulus (Small et al., 2001), and in response to visual and gustatory stimuli (Uher et al., 2006). Activity in gustatory related regions such as the OFC, amygdala, and hippocampus has also been shown to be modulated by hunger in studies involving responsiveness to food-related stimuli (Arana et al., 2003; Haase et al., 2009a; LaBar et al., 2001; Morris & Dolan, 2001). Medial and lateral OFC activation correlates with the pleasantness ratings of odors and flavor (de Araujo et al., 2003; Kringelback et al., 2003; Rolls et al., 2003) and medial OFC activation is related to pleasantness ratings of oral fat texture (Grabenhorst et al., 2009). Examining the complex relationships between physiological state and neuronal activity in response to gustatory stimuli is important in understanding normal eating behavior and gaining insight into factors which might disrupt these processes.
While studies have reported a reduction of activation in olfactory and reward processing areas, as well as in regions receiving olfactory projections in older adults (Cerf-Ducastel & Murphy, 2003; Ferdon & Murphy, 2003; Suzuki et al., 2001; Wang et al., 2005), age-related effects on activation during gustatory processing are unknown. Differential brain activation to gustatory stimuli with different taste qualities (sweet, bitter, sour, salty) in normal young persons (Haase et al., 2009a), as well as previous psychophysical studies employing threshold, magnitude estimation and Weber Ratio techniques which report that age effects differ for stimuli with different tastes (Cowart, 1989; Gilmore & Murphy, 1989; Murphy, 2008; Murphy & Gilmore, 1989; Razani, et al., 2008; Schiffman et al., 1994) all suggest the importance of investigating gustatory stimuli of different taste qualities in order to extract commonalities and differences associated with quality. The purpose of the present study is to investigate age-related changes in gustatory, motivation, and reward regions of the brain in response to taste stimuli that represent the basic taste qualities of sweet, bitter, sour and salty, under the conditions of hunger and satiety.
Methods
Participants
Twenty young adults (M=23.9), and 20 older adults (M=72.2) participated in the current study. Due to technical difficulties during the collection of functional images, data from one young female were not included in analyses. The gender of participants was split equally in the older group, while the young sample included 9 females and 10 males. Participants received monetary compensation for participation. This study was approved by the Institutional Review Boards at San Diego State University and the University of California, San Diego.
Preliminary Screening
Screening for exclusionary criteria such as anosmia and agueusia was conducted prior to participation in the study. Taste and odor detection thresholds were obtained and those with extremely low sensitivity were excluded from the study (Cain, et al., 1983; modified as in Murphy et al., 1990).
Neuroimaging Stimuli
The following stimuli were delivered orally during the neuroimaging procedures outlined in the following section: Caffeine, 0.04M; citric acid, 0.01M; citral/sucrose combination, 0.02% citral, 0.64M sucrose; citral, 0.02%; sucrose, 0.64M; NaCl, 0.16M (See Haase, Cerf-Ducastel, & Murphy, 2009a,b). Stimulus delivery methods are outlined completely in Haase et al. (2007).
Neuroimaging Procedure
Participants completed neuroimaging sessions on 2 different days, no more than 7 days apart. On both of the test days, participants fasted 12 hours prior to the scanning. A subjective rating of the participants' level of hunger was recorded before beginning the scan using a general labeled magnitude scale (gLMS). The only distinction between the two imaging sessions was the physiological state of the participant. In one of the sessions the participant received a nutritional preload containing 700 kcal of Ensure Plus prior to scanning. In the session investigating the effects of hunger on gustatory activation, the participant received no preload. The order in which participants participated in either the hunger or satiety conditions was randomized through the use of a random number set. Stimulus presentation was pseudo-random with a 10s inter-stimulus interval between each stimulus. Each stimulus was presented a total of 8 times per run. In order to rinse the mouth, distilled water was presented immediately following every taste. A second presentation of distilled water immediately followed the first as a neutral baseline for comparisons that will be discussed in further sections. The duration of each run was 24 minutes and 11 seconds. Ratings of stimulus intensity or pleasantness were recorded through the use of a joystick and a Matlab program in separate runs. The present analyses considered scans where pleasantness was evaluated. A real time image of the corresponding gLMS with a cursor controlled by the participant was projected onto a screen viewable while receiving the taste stimuli. After receiving a stimulus, participants moved the cursor to the appropriate location on the gLMS to reflect subjective experience. Further details of the neuroimaging paradigm can be found in Haase et al. (2007).
Data Acquisition
Imaging sessions took place at the University of California, San Diego Center for Functional Magnetic Resonance Imaging using a 3T General Electric Signa Excite short bore scanner. Structural imaging parameters are as follows: T1-weighted whole brain MP-RAGE sequence, field of view (FOV) = 25cm, slice thickness = 1mm, resolution 1×1×1 mm3, echo time (TE) = 30ms, Locs per slab = 136, flip angle = 15°. Functional imaging parameters are as follows: T2*-weighted images, 24 axial slices, FOV = 19cm, matrix size = 64×64, spatial resolution = 3×3×3 mm3, flip angle = 90°, TE = 30ms, repetition time (TR) = 2000ms.
Data Analysis
Independent samples t-tests were used to determine whether there were age-related differences in taste and olfactory thresholds, pleasantness ratings of each of the 4 stimuli, and hunger ratings before and after the preload.
Analysis of Functional NeuroImages (AFNI) software was used in all of the processing and analyses of the structural and functional data (Cox, 1996). Preprocessing of the data consisted of correction for head movement, temporal and spatial smoothing, concatenation of the runs, and automasking. Deconvolution was then applied to the concatenated runs. Deconvolution, a multiple regression process which matches specific time points in the data set with the impulse response function (IRF) of the hemodynamic response, enables precise discrimination of “contrasts of interests”. In the present study, a contrast is defined as the specific time points where a participant is in a state of hunger or satiety and rating a specific stimulus. For example, after running deconvolution on the data set, it is possible to view activation for each individual while receiving only the sucrose stimulus, in the hunger condition, while rating the pleasantness of the stimulus.
For the present study, whole brain group analyses were conducted. T-tests were run comparing young participants to older participants on the basis of the levels of activation and regions of activation in response to the gustatory stimuli. AlphaSim (Ward, 1997) was run in AFNI to correct for multiple comparisons at p < 0.05. Using 10,000 Monte Carlo simulations, the program estimates the probability of occurrence of clusters composed of voxels exceeding a specific p value that is maintained for a study performed with the expressed parameters on the whole brain volume. Results of the AlphaSim prompted the application of a threshold in which voxels exceeding p < 0.015 and located in a cluster of at least 13 were considered significantly activated. Activity significantly greater than baseline (water) will be referred to as positive activation, while activation significantly less than baseline will be referred to as negative activation. For direct statistical comparisons between the young and older adult groups, an analysis was conducted in AFNI in which significant differences in activity levels between the groups are displayed.
Results
Psychophysics
No significant differences were found between young and older participants in taste or odor thresholds. Older adults found citric acid to be significantly less pleasant than the young adults in their initial taste test (t(36) = .154, p = .029); no differences were found in response to sucrose, NaCl, or caffeine. In response to the initial hunger ratings after a 12 hour fast, older adults reported significantly lower levels of hunger than young adults (t(36) = 1.23, p = .005), while no significant age-related differences were found between post preload hunger ratings.
Functional Neuroimaging
To view activation in response to all taste stimuli, a contrast was created in which time points during the presentation of caffeine, sucrose, NaCl, and citric acid were processed as a single stimulus using 3dDeconvolution. Activation in the young group was subtracted from activation in the older group in order to illustrate areas of significant age-related differences in hedonic-evaluation-related gustatory activity. The results are shown in Figure 1.
Fig. 1.
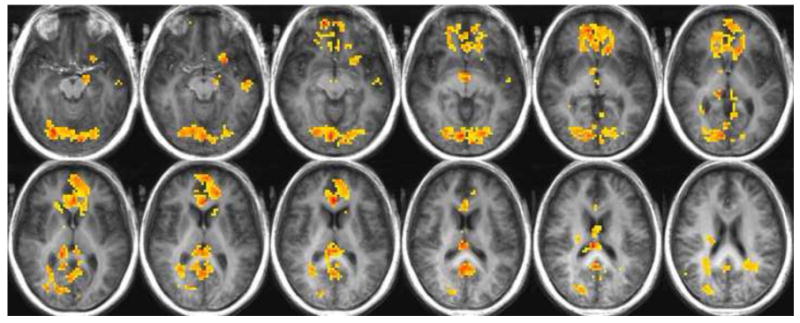
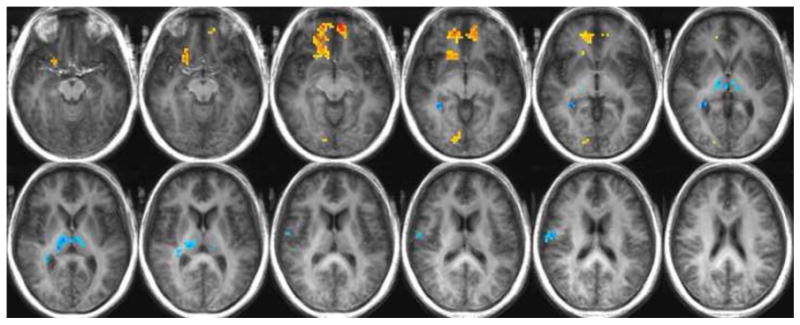
Fig. 1a. Regions of significantly increased or decreased activity in the older adults relative to the young adults while in a state of hunger in response to all stimuli: warm colors indicate greater activity in the older adults; cool colors indicate a greater response in the young adults.
Fig. 1b. Regions of significantly increased or decreased activity in the older adults relative to the young adults while in a state of satiety in response to all stimuli: warm colors indicate greater activity in the older adults; cool colors indicate a greater response in the young adults.
In response to all stimuli, and while in a state of hunger, older participants showed significantly greater activity than the young in the majority of significantly activated regions. Increased activation relative to the young was seen in the posterior cingulate, thalamus, parahippocampal gyrus, lingual gyrus, occipital gyrus, anterior cingulate, medial frontal gyrus, caudate nucleus, superior frontal gyrus, OFC BA 47, OFC BA 11, middle frontal gyrus, cuneus, middle and superior temporal gyri, putamen, lentiform nucleus, claustrum, hypothalamus, inferior frontal gyrus, precentral gyrus, amygdala, and uncus. Conversely, the young adults had significantly greater activation in parts of the thalamus, hippocampus, caudate tail, and postcentral gyrus (See Table 1). Figure 1a illustrates significant differences between the young and older adults in response to all stimuli during a state of hunger.
Table 1. Regions of significantly increased or decreased activity in the older adults relative to the young adults while in a state of hunger in response to all stimuli.
| Region | Hem. | Tlrc coord. | # Voxels Max. Int. | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ALL STIMULI | ||||||
|
| ||||||
| Lingual Gyrus | R | -10 | 75 | -1 | 852 | 4.85 |
| Posterior Cingulate | R | -3 | 53 | 21 | ||
| Posterior Cingulate | L | 1 | 51 | 21 | ||
| Thalamus | R | -7 | 25 | 12 | ||
| Thalamus | L | 7 | 28 | 9 | ||
| Parahippocampal Gyrus | R | -14 | 36 | 5 | ||
| Parahippocampal Gyrus | L | 8 | 45 | 5 | ||
| Middle Occipital Gyrus | R | -31 | 75 | 5 | ||
| Lingual Gyrus | L | 18 | 82 | 2 | ||
| Cerebellum | R | 5 | 56 | 2 | ||
| Inferior Occipital Gyrus | L | 35 | 76 | -6 | ||
| Fusiform Gyrus | L | 24 | 78 | -12 | ||
| Fusiform Gyrus | R | -24 | 81 | -12 | ||
| Cerebellum | L | 26 | 72 | -16 | ||
| Anterior Cingulate | L | 1 | -39 | 3 | 642 | 4.85 |
| Medial Frontal Gyrus | L | 11 | -40 | 14 | ||
| Anterior Cingulate | R | -2 | -29 | 14 | ||
| Medial Frontal Gyrus | R | -11 | -41 | 11 | ||
| Caudate Body | L | 13 | -21 | 6 | ||
| Caudate Body | R | -16 | -21 | 6 | ||
| Caudate Head | L | 13 | -19 | 3 | ||
| Caudate Head | R | -15 | -19 | 3 | ||
| Superior Frontal Gyrus | R | -21 | -43 | -3 | ||
| Medial Frontal Gyrus | L | 20 | -43 | -3 | ||
| Superior Frontal Gyrus | L | 10 | -55 | -7 | ||
| OFC BA 47 | L | 21 | -28 | -7 | ||
| Middle Frontal Gyrus | R | -24 | -37 | -7 | ||
| OFC BA 11 | R | -16 | -59 | -11 | ||
| Precuneus | L | 6 | 47 | 31 | 269 | 4.36 |
| Precuneus | R | -10 | 54 | 34 | ||
| Cuneus | L | 23 | 71 | 32 | ||
| Angular Gyrus | L | 44 | 60 | 32 | ||
| Middle Temporal Gyrus | L | 39 | 59 | 27 | ||
| Superior Temporal Gyrus | L | 35 | 57 | 27 | ||
| Cingulate Gyrus | L | 4 | 56 | 27 | ||
| Medial Frontal Gyrus | R | -2 | -46 | 30 | 76 | 3.47 |
| Superior Frontal Gyrus | L | 19 | -36 | 30 | ||
| Superior Frontal Gyrus | R | -20 | -45 | 30 | ||
| Medial Frontal Gyrus | R | -7 | -45 | 30 | ||
| Posterior Cingulate | R | -26 | 63 | 8 | 48 | 3.87 |
| Cuneus | R | -21 | 79 | 17 | 40 | 3.52 |
| Middle Occipital Gyrus | R | -27 | 78 | 21 | ||
| OFC BA 47 | L | 25 | -15 | -11 | 33 | 4.15 |
| Putamen | L | 20 | -13 | -3 | ||
| Lentiform Nucleus | L | 20 | -13 | -3 | ||
| Claustrum | L | 27 | -14 | -6 | ||
| Hypothalamus | R | -7 | 4 | -2 | 31 | 4.23 |
| Precuneus | R | -9 | 72 | 38 | 29 | 4.05 |
| Cuneus | R | -12 | 75 | 35 | ||
| Middle Temporal Gyrus | L | 52 | 17 | -9 | 26 | 3.75 |
| Superior Temporal Gyrus | R | -51 | 57 | 24 | 26 | 3.87 |
| Inferior Frontal Gyrus | R | -50 | -10 | 24 | 24 | -3.44 |
| Precentral Gyrus | R | -60 | 1 | 24 | ||
| Middle Temporal Gyrus | L | 35 | 56 | 20 | 22 | 3.23 |
| Parahippocampal Gyrus | L | 16 | 12 | -12 | 15 | 3.86 |
| Amygdala | L | 20 | 8 | -12 | ||
| Middle Frontal Gyrus | R | -38 | -4 | 39 | 13 | 3.01 |
| Anterior Cingulate | R | -16 | -33 | -6 | 141 | 4.44 |
| Superior Frontal Gyrus | R | -21 | -43 | -3 | ||
| Medial Frontal Gyrus | R | -14 | -53 | -3 | ||
| Middle Frontal Gyrus | R | -24 | -39 | -6 | ||
| Caudate Head | R | -7 | -16 | -6 | ||
| Inferior Frontal Gyrus | R | -25 | -15 | -11 | ||
| OFC BA 11 | R | -23 | -28 | -11 | ||
| OFC BA 47 | R | -24 | -14 | -14 | ||
| Thalamus | R | -10 | 16 | 5 | 56 | -3.72 |
| Thalamus | L | 11 | 18 | 6 | ||
| Cerebellum | L | 17 | 60 | -22 | 49 | -3.63 |
The comparison of activation in response to all stimuli during satiety yielded less unidirectional results. While the older participants exhibited significantly greater levels of activation in the anterior cingulate, superior frontal gyrus, medial frontal gyrus, caudate head, inferior frontal gyrus, OFC BA 47, OFC BA 11, inferior parietal lobule, precentral gyrus, lingual gyrus, parahippocampal gyrus, and uncus; the young showed increases relative to the old in the thalamus, hippocampus, caudate tail, parahippocampal gyrus, postcentral gyrus, precentral gyrus, and cerebellum (See Table 2). Figure 1b illustrates significant differences between the young and older adults in response to all stimuli during a state of satiety.
Table 2.
Regions of significantly increased or decreased activity in the older adults relative to the young adults while in a state of satiety in response to all stimuli.
| Region | Hem. | Tlrc coord. | # Voxels Max. Int. | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| ALL STIMULI | |||||||
|
| |||||||
| Anterior Cingulate | R | -16 | -33 | -6 | 141 | 4.44 | |
| Superior Frontal Gyrus | R | -21 | -43 | -3 | |||
| Medial Frontal Gyrus | R | -14 | -53 | -3 | |||
| Middle Frontal Gyrus | R | -24 | -39 | -6 | |||
| Caudate Head | R | -7 | -16 | -6 | |||
| Inferior Frontal Gyrus | R | -25 | -15 | -11 | |||
| OFC BA 11 | R | -23 | -28 | -11 | |||
| OFC BA 47 | R | -24 | -14 | -14 | |||
| Thalamus | R | -10 | 16 | 5 | 56 | -3.72 | |
| Thalamus | L | 11 | 18 | 6 | |||
| Cerebellum | L | 17 | 60 | -22 | 49 | -3.63 | |
| Inferior Parietal Lobule | L | 52 | 36 | 29 | 46 | 3.26 | |
| Medial Frontal Gyrus | L | 10 | -50 | -5 | 38 | 4.71 | |
| Anterior Cingulate | L | 14 | -46 | -2 | |||
| Precentral Gyrus | R | -34 | -21 | 35 | 23 | 2.81 | |
| Hippocampus | L | -29 | 40 | 2 | 21 | -4.21 | |
| Caudate Tail | R | -31 | 36 | 6 | |||
| Parahippocampal Gyrus | R | -32 | 42 | -3 | |||
| Lingual Gyrus | R | -12 | 82 | -3 | 15 | 3.08 | |
| Postcentral Gyrus | R | -57 | 10 | 16 | 15 | -3.54 | |
| Precentral Gyrus | R | -58 | 7 | 12 | |||
| Parahippocampal Gyrus | R | -17 | 9 | -19 | 13 | 3.24 | |
| Uncus | R | -21 | 7 | -22 | |||
Functional Neuroimaging: Individual Taste Qualities
To further investigate the robust effect of age in the hunger condition, we compared activation to individual gustatory stimuli in older and younger adults. Results of a direct comparison between gustatory-related activation in the hungry adults indicated a significantly greater response in the older adults during the hedonic evaluation of caffeine, citric acid, and sucrose, relative to the young (See Table 3). Figure 2 illustrates greater activation in the older group relative to the young adults in the hunger condition, in response to sucrose, citric acid and caffeine.
Table 3.
Regions of significantly increased or decreased activity in the older adults relative to the young adults while in a state of hunger.
| Region | Hem. | Tlrc coord. | # Voxels Max. Int. | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| SUCROSE | ||||||
|
| ||||||
| Cingulate Gyrus | R | -1 | 55 | 26 | 29 | 1.58 |
| Precuneus | L | 11 | 46 | 33 | ||
| Cingulate Gyrus | L | 4 | 59 | 27 | ||
| Precuneus | R | -8 | 59 | 27 | ||
| Posterior Cingulate | L | 4 | 57 | 24 | ||
| Posterior Cingulate | R | -5 | 59 | 24 | ||
| Middle Temporal Gyrus | L | 58 | 41 | -7 | 13 | 1.78 |
|
| ||||||
| CITRIC ACID | ||||||
|
| ||||||
| Anterior Cingulate | L | 17 | -40 | 3 | 56 | 1.69 |
| BA 10 | L | 3 | -59 | 8 | ||
| Medial Frontal Gyrus | R | -3 | -59 | 8 | ||
| BA 10 | R | -3 | -59 | 8 | ||
| Medial Frontal Gyrus | L | 2 | -59 | 8 | ||
| Lentiform Nucleus | L | 21 | -16 | 0 | ||
| Putamen | L | 21 | -16 | 0 | ||
| Caudate Head | L | 15 | -21 | 0 | ||
| OFC BA 47 | L | 27 | -11 | -14 | ||
| Inferior Frontal Gyrus | L | 31 | -15 | -12 | ||
| Posterior Cingulate | R | -2 | 55 | 13 | 38 | 2.94 |
| Precuneus | L | 3 | 64 | 18 | ||
| Posterior Cingulate | L | 1 | 58 | 15 | ||
| Cuneus | R | -4 | 62 | 6 | ||
| Putamen | L | 20 | -11 | -8 | 29 | 2.72 |
| Lentiform Nucleus | L | 20 | -15 | 0 | ||
| Claustrum | L | 28 | -11 | -6 | ||
| OFC BA 47 | L | 27 | -20 | -9 | ||
| Inferior Frontal Gyrus | L | 31 | -14 | -12 | ||
| Posterior Cingulate | L | 27 | 23 | 60 | 9 | 0.99 |
| Lingual Gyrus | L | 26 | 57 | 4 | ||
| Middle Occipital Gyrus | L | 28 | 58 | 4 | ||
| Caudate Head | R | -12 | -23 | 4 | 22 | 1.31 |
| Anterior Cingulate | R | -8 | -32 | 9 | ||
| Thalamus | L | 9 | 31 | 6 | 14 | 1.75 |
|
| ||||||
| CAFFEINE | ||||||
|
| ||||||
| Lingual Gyrus | R | -6 | 81 | -3 | 482 | 4.16 |
| Cuneus | R | -17 | 79 | 18 | ||
| Middle Occipital Gyrus | R | -20 | 85 | 15 | ||
| Middle Occipital Gyrus | L | 32 | 78 | 6 | ||
| Cuneus | L | 1 | 81 | 6 | ||
| Lingual Gyrus | L | 23 | 76 | 3 | ||
| Inferior Occipital Gyrus | L | 35 | 76 | -3 | ||
| Inferior Occipital Gyrus | R | -30 | 84 | -3 | ||
| Cerebellum | R | -3 | 79 | -11 | ||
| Cerebellum | L | 6 | 78 | -11 | ||
| Medial Frontal Gyrus | L | 13 | -49 | 10 | 83 | 2.55 |
| Caudate Head | L | 11 | -22 | 1 | ||
| Anterior Cingulate | L | 11 | -36 | 4 | ||
| BA 10 | L | 11 | -51 | 10 | ||
| Medial Frontal Gyrus | R | -2 | -55 | 10 | ||
| BA 10 | R | -2 | -55 | 10 | ||
| Anterior Cingulate | R | -2 | -32 | 8 | 59 | 3.71 |
| Anterior Cingulate | L | 1 | -30 | 15 | ||
| Medial Frontal Gyrus | R | -11 | -55 | 0 | ||
| Superior Frontal Gyrus | R | -17 | -52 | 0 | ||
| Posterior Cingulate | R | -26 | 66 | 9 | 47 | 1.2 |
| Cingulate Gyrus | R | -19 | 19 | 27 | ||
| Parahippocampal Gyrus | R | -27 | 50 | 5 | ||
| Fusiform Gyrus | R | -37 | 47 | -9 | ||
| Precuneus | R | -18 | 66 | 30 | 37 | 1.29 |
| Cuneus | R | -4 | 68 | 30 | ||
| Cuneus | L | 9 | 70 | 30 | ||
| Precuneus | L | 8 | 60 | 30 | ||
| Precuneus | L | 18 | 70 | 38 | 34 | 2.38 |
| Middle Occipital Gyrus | L | 33 | 70 | 17 | 32 | 1.71 |
| Middle Temporal Gyrus | L | 35 | 75 | 24 | ||
| Superior Temporal Gyrus | L | 48 | 60 | 21 | ||
| Superior Frontal Gyrus | R | -18 | -53 | -2 | 26 | 2.62 |
| BA 10 | R | -12 | -53 | -1 | ||
Fig. 2.
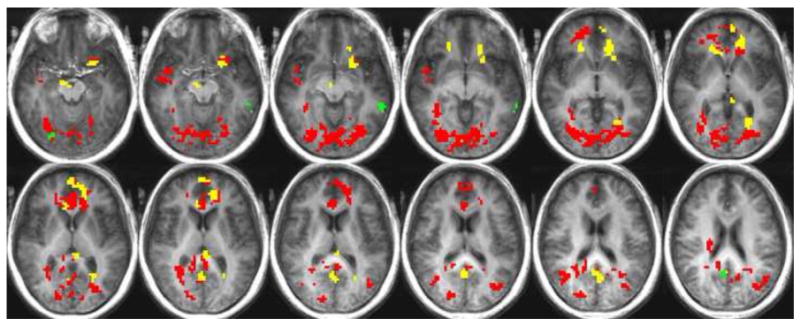
Significantly greater activation in the older group relative to the young in the hunger state during hedonic evaluation is shown in response to sucrose (green), citric acid (yellow), and caffeine (red).
In the hunger condition, during the evaluation of pleasantness of caffeine, the older adults had a significantly greater hemodynamic response in the insula, OFC BA 47, caudate nucleus, anterior cingulate, medial frontal gyrus, superior frontal gyrus, posterior cingulate, parahippocampal gyrus, middle temporal gyrus, superior temporal gyrus, claustrum, lingual gyrus, fusiform gyrus, and cuneus.
Hedonic rating during the presentation of citric acid in the hunger condition evoked a significantly greater neuronal response in the older adults relative to the young in the anterior cingulate, medial frontal gyrus, lentiform nucleus, putamen, caudate nucleus, OFC BA 47, inferior frontal gyrus, posterior cingulate, cuneus, lingual gyrus, and thalamus.
During the presentation and rating of sucrose while in a state of hunger, the older adults showed activation significantly greater than the young adults in the cingulate gyrus, precuneus, middle temporal gyrus, and posterior cingulate.
No significant differences were found between the young and older adult groups with respect to their activation levels during the presentation and hedonic assessment of NaCl, while in the hunger condition.
When participants were satiated, differences in activation varied as a function of stimuli (See Table 4). Interestingly, during the hedonic evaluation of sucrose, older adults showed increased activation relative to young in the anterior cingulate, medial frontal gyrus, and superior frontal gyrus, while decreased activation relative to the young adults was seen in the insula, superior temporal gyrus, postcentral gyrus, and precentral gyrus.
Table 4.
Regions of significantly increased or decreased activity in the older adults relative to the young adults while in a state of satiety.
| Region | Hem. | Tlrc coord. | # Voxels Max. Int. | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| SUCROSE | ||||||
|
| ||||||
| Anterior Cingulate | R | -3 | -51 | -1 | 166 | 4.55 |
| Medial Frontal Gyrus | L | 14 | -52 | 6 | ||
| Medial Frontal Gyrus | R | -2 | -52 | 6 | ||
| Superior Frontal Gyrus | R | -22 | -59 | 3 | ||
| Postcentral Gyrus | R | -56 | 14 | 16 | 45 | -2.5 |
| Insula | R | -46 | 10 | 17 | ||
| Superior Temporal Gyrus | R | -59 | 28 | 17 | ||
| Precentral Gyrus | R | -54 | 13 | 12 | ||
| Postcentral Gyrus | L | 50 | 17 | 20 | 16 | -1.55 |
| Insula | L | 48 | -21 | 20 | ||
|
| ||||||
| CITRIC ACID | ||||||
|
| ||||||
| Cerebellum | R | -30 | 50 | -25 | 45 | -2.32 |
| Cerebellum | L | 3 | 57 | -24 | 38 | -2.07 |
| Inferior Parietal Lobule | L | 45 | 34 | 27 | 21 | 2.17 |
| Superior Temporal Gyrus | R | -60 | 20 | 12 | 20 | -2.22 |
| Postcentral Gyrus | R | -61 | 19 | 18 | ||
| Transverse Temporal Gyrus | R | -54 | 20 | 12 | ||
| Thalamus | L | 11 | 4 | 6 | 19 | -0.99 |
| Postcentral Gyrus | R | -55 | 9 | 16 | 18 | -1.9 |
| Insula | R | -45 | 8 | 16 | ||
| Precentral Gyrus | R | -57 | 7 | 12 | ||
|
| ||||||
| CAFFEINE | ||||||
|
| ||||||
| Medial Frontal Gyrus | L | 15 | -51 | 9 | 1156 | 4.69 |
| Insula | L | 32 | -2 | 17 | ||
| Inferior Frontal Gyrus | L | 45 | -29 | 15 | ||
| Caudate Body | L | 15 | -14 | 15 | ||
| Anterior Cingulate | L | 16 | -32 | 15 | ||
| Middle Frontal Gyrus | R | -25 | -50 | 12 | ||
| Claustrum | R | -27 | -12 | 12 | ||
| Anterior Cingulate | R | -7 | -30 | 12 | ||
| Precentral Gyrus | L | 47 | -3 | 12 | ||
| Middle Frontal Gyrus | L | 30 | -52 | 9 | ||
| Claustrum | L | 34 | 3 | 9 | ||
| Caudate Body | R | -13 | -17 | 9 | ||
| Medial Frontal Gyrus | R | -10 | -57 | 9 | ||
| Putamen | L | 22 | -9 | 6 | ||
| Putamen | R | -22 | -11 | 6 | ||
| Caudate Head | L | 10 | -14 | 3 | ||
| Caudate Head | R | -11 | -16 | 3 | ||
| Superior Frontal Gyrus | L | 19 | -49 | 3 | ||
| Superior Frontal Gyrus | R | -20 | -52 | 3 | ||
| OFC BA 47 | L | 34 | -16 | -6 | ||
| OFC BA 47 | R | -33 | 17 | -6 | ||
| Parahippocampal Gyrus | R | -30 | 4 | 11 | ||
| Superior Temporal Gyrus | R | 47 | 4 | -10 | 118 | 3.06 |
| Putamen | L | 29 | 5 | -3 | ||
| Lentiform Nucleus | L | 28 | 3 | -3 | ||
| Claustrum | L | 34 | 10 | -6 | ||
| Middle Temporal Gyrus | L | 52 | 4 | -9 | ||
| Superior Temporal Gyrus | L | 49 | 2 | -9 | ||
| Parahippocampal Gyrus | L | 30 | 4 | -12 | ||
| Amygdala | L | 31 | 4 | -14 | ||
| Putamen | L | 26 | 4 | 14 | 37 | 0.89 |
| Insula | L | 30 | 22 | 18 | ||
| Middle Frontal Gyrus | R | -44 | -30 | 15 | 33 | 1.23 |
| Anterior Cingulate | R | -16 | -33 | 15 | ||
| Precuneus | L | 5 | 63 | 39 | 32 | 2.05 |
| Precuneus | R | -14 | 57 | 39 | ||
| Middle Frontal Gyrus | L | 34 | -34 | -3 | 22 | 1.68 |
| Inferior Frontal Gyrus | L | 35 | -35 | 0 | ||
| OFC BA 47 | L | 31 | -28 | -4 | ||
| Parahippocampal Gyrus | R | -22 | 14 | -17 | 15 | 1.58 |
| Amygdala | R | -23 | 9 | -18 | ||
|
| ||||||
| NACL | ||||||
|
| ||||||
| Postcentral Gyrus | L | 59 | 16 | 20 | 15 | -3.24 |
In response to citric acid, young adults elicited greater activation when compared to the older sample in the insula, precentral gyrus, thalamus, superior temporal gyrus, and postcentral gyrus. Similarly, greater activity of the young adults was recorded during the assessment of NaCl in the postcentral gyrus.
Caffeine showed greater activation in the older participants in the insula, medial frontal gyrus, inferior frontal gyrus, caudate body, anterior cingulate, middle frontal gyrus, claustrum, precentral gyrus, putamen, caudate head, superior frontal gyrus, OFC BA 47, parahippocampal gyrus, amygdala, and precuneus.
Discussion
Over all stimuli (Figure 1a,b), older adults showed significantly greater activation when evaluating the pleasantness of gustatory stimuli than young adults did. When hungry, greater activation in the older adults was observed in areas of the brain previously associated, in both non-human primate electrophysiological studies and human neuroimaging studies, with chemosensory function and with reward value. Younger adults showed greater negative activation than older adults when sated.
Because neuroimaging has demonstrated differential brain response to gustatory stimuli of different qualities (Haase et al., 2009a) and psychophysical studies have demonstrated differences in the effects of age on gustatory stimuli of different qualities (Murphy, 2008), we investigated age-related differences with respect to sucrose, caffeine, NaCl and citric acid which represent the basic qualities of sweet, bitter, salty and sour. Interestingly, in the current study, the most profound difference between young and older adults' cortical activation to gustatory stimuli was in response to the bitter stimulus (caffeine), while a smaller age-related difference was seen in response to the sweet stimulus (sucrose). Psychophysical studies investigating potential age-related changes in perceived intensity for taste show age effects that are ordered bitter>sour>salty>sweet (Murphy & Gilmore, 1989). Weber ratios also show greater age-related deficits in bitter than in sweet taste (Gilmore & Murphy, 1989) and in sour than in salty taste (Razani, Markison, Nordin & Murphy, 2008). While some inconsistencies exist in the literature, the vast majority of the psychophysical research on taste has reported that age has its greatest effect in depressing bitter perception and the least on sweet perception (Gilmore & Murphy, 1989; Cowart, 1989; Schiffman et al., 1994). The current study suggests a cortical substrate for the differential effects of age on different taste stimuli, and supports multiple mechanisms for age-related decline in taste function. From a larger chemosensory perspective, it is interesting to note that psychophysical studies show significantly greater effects of age on olfaction than on taste (Murphy, 2008); and the results of the current study taken with those of fMRI studies of olfactory function in older adults (Cerf-Ducastel & Murphy, 2003: Ferdon & Murphy, 2003 Suzuki et al., 2001; Wang et al., 2005) suggest that older adults generate greater activation in response to sensory loss in taste than in olfaction.
Age-Related Functional Changes
In the present study, the physiological state of the participants altered global activation in both the young and older adults. The majority of activation in the hunger condition was significantly greater than baseline, while negative activation was dominant after participants were satiated (see Supplementary Tables 5 & 6). A similar shift in response has been reported in neurophysiological taste studies in non-human primates (Burton et al., 1976; E.T. Rolls et al., 1989; Scott et al., 1993; Yan & Scott, 1996; Yaxley et al., 1988), and in neuroimaging studies in young adults (Haase et al., 2009a; Small et al., 2001; Uher et al., 2006).
Within the primary gustatory system, the insula showed consistent positive activation when the participants of both age groups were hungry. Higher-order taste and reward regions were active with a much greater consistency in the older adults. Significant activation of these regions in the young adult sample was much less robust and varied among stimuli (See Supplementary Table 5).
Additionally, brain regions not typically associated with neuroimaging of taste or reward processing were also activated in the older adults (See Supplementary Tables 5 and 6). This finding raises the possibility of functional reorganization of gustatory processing in older adults, though the cross-sectional design of the study does not address this directly and longitudinal study would be required to confirm this. In comparison to the young adults, older participants generated higher levels of activity in regions that process gustatory stimuli. The compensation hypothesis postulates that reduced efficiency of older brains may result in such effects (Cabeza et al., 2002).
Brain Activation in Older Adults
To our knowledge, this is the first investigation of the effects of brain aging on gustatory processing utilizing functional neuroimaging. The results reveal significant differences in processing hedonic information about taste in older and younger brains under the conditions of hunger and satiety. We report evidence for greater activation and activation in additional areas in older than younger adults in order for the brain to process the hedonic aspects of gustatory information.
Exactly how the aged brain functions differently from that of a younger brain has been studied in a number of paradigms and modalities. Studies focusing on brain functioning during motor tasks have consistently reported greater activation in older individuals relative to young (Calautti et al., 2001; Mattay et al., 2002; Ward & Frackowaik, 2003). Heuninckx et al. (2008) reported activity in older adults in motor coordination regions, as well as additional regions not activated in young adults such as frontal and higher level sensory motor regions. In studies examining activation during memory tasks, higher levels of prefrontal activity in older adults than younger adults have been reported (Grady et al., 2002; Rosen et al., 2002). Decreased activity in frontal and medial temporal regions has also been reported in older adults relative to younger adults during memory tasks (Grady et al., 1995; Stebbins et al., 2002). During cued recall older adults sometimes show reduced prefrontal activity or activity in different regions than younger adults (Anderson et al., 2000; Schacter et al., 1996) and during retrieval, increased activation has been observed in left prefrontal cortex. Investigating working memory for verbal and spatial information, Reuter-Lorenz et al. (2000) observed left lateralized activity to verbal material and right lateralized activity to spatial material in young adults, while older adults showed bilateral activity in both tasks.
Understanding the differences in degree of activation and the instances of brain activation in additional areas in older than younger adults is a complex task and its pursuit has led to a number of hypotheses. One potential explanation that has been proposed for cognitive age-related changes is the compensation hypothesis, which states that age-related functional changes in the brain may be the result of decreased processing resources and inefficiency in some regions in older brains, resulting in greater levels of activity and the recruitment of brain regions not required in young adults in order to achieve comparable performance on a task (Cabeza et al., 2002). It has also been argued that older adults may engage in different strategies than younger adults do in order to perform a task. Addressing these hypotheses directly would require longitudinal data.
The aim of the current study was to investigate differences in young and older adults in processing taste information during hedonic assessment with functional neuroimaging. Below we discuss the observed differences in activation between older and younger adults as the brain processes gustatory information and the individual produces an hedonic assessment.
Insula
The insular-opercular cortex is considered the primary gustatory cortex in nonhuman primates (Pritchard et al., 1986; Yaxley et al., 1988). Human neuroimaging studies of young adults have consistently reported insular activity in response to taste stimuli (de Araujo et al., 2003; Haase et al., 2009; Uher et al., 2006). In the current study, the insula was a common site of localized activity regardless of age, with physiological state affecting insula activation similarly in the two age groups.
Orbitofrontal Cortex
The caudolateral OFC is known as secondary gustatory cortex. This area has been shown to receive input from the insular-opercular cortex (primary taste area), the amygdala, rhinal sulcus, substantia innominata, and surrounding OFC. The OFC has been implicated in reward processing, incentive motivation, and goal selection (Arana et al., 2003; Gottfried et al., 2003; Gottfried & Dolan, 2004; Morris & Dolan, 2001). Activation in medial and lateral OFC correlates with the pleasantness ratings of odors and flavor (de Araujo et al., 2003; Kringelback et al., 2003; Rolls et al., 2003) and activation in medial OFC is also correlated with pleasantness ratings of oral fat texture (Grabenhorst et al., 2009).
In the present study, group analysis of the young and older adults indicated that secondary gustatory activity (OFC BA 47) was differentially activated by age. Older adults had more robust and consistent activity during the task of hedonic assessment in this region (See Table 3), suggesting that the older adults required greater activation in order to assess hedonic aspects of the stimuli presented. The differences between the age groups in activity levels of OFC BA 47 further supports the hypothesis that functional compensation is occurring in the older sample.
Amygdala
The amygdala is known to be associated with hedonic tone, motivation, and emotion and is an intermediary region between the primary and secondary gustatory cortices. Activity in this region is affected by gustatory stimuli in nonhuman primates (Yan & Scott, 1996). Human subjects show reduced activity within the amygdala while viewing food related stimuli in a state of satiety relative to a hungry state (La Bar et al., 2003; Haase et al., 2009a).
In the current study, physiological state affected activity in the amygdala in both age groups, with positive activation to some stimuli during hunger, and negative activation or baseline activation during satiety. The shift in activity from positive to negative as a result of the physiological state of the participants may influence motivational factors associated with the reward value of the stimuli presented. Similar to the age-related effects seen in OFC BA 47, activation of the amygdala was more robust and consistent across stimuli in the older adults, relative to the young (see Table 3).
Caudate Nucleus
The caudate nucleus located in the striatum is involved in reward related processing in a variety of tasks. The caudate is sensitive to intrinsically (Tricomi et al., 2006) and extrinsically motivating rewards. Monetary rewards are commonly used to evoke reward anticipatory activation within these regions. Higher incentive to perform a task has been shown to elicit higher levels of caudate activity (Delgado et al., 2003; Delgado et al., 2004).
Interestingly, factors such as drug craving and anticipation of drug-related reward are believed to affect caudate and striatum activity during monetary reward anticipation tasks (Wilson et al., 2008). Age has also been shown to affect striatal activity. Older adults show decreased activation in anticipation of monetary losses but do not differ from the young in anticipation of rewards (Samenez Larkin et al., 2007). The caudate is seldom discussed in imaging studies of taste, yet it is commonly activated (Haase et al., 2009; Small et al., 2001; Uher et al., 2006). In the current study older adults had more robust signals with greater consistency across stimuli than younger adults (Supplementary Table 5). Caudate activity was robust during a state of hunger but not when sated. In addition to a greater consistency of activation in the caudate relative to baseline, the direct comparison of activation between the age groups also reports significantly higher levels of caudate activity in the older adults (See Table 3). This integral function of reward processing may be critical to motivational factors involved in eating behavior.
It is important to appreciate that the nature of the task will impact brain activation to gustatory stimuli and that we have focused here on brain activation while participants are judging stimulus pleasantness. Experiments attending to or evaluating stimulus intensity have been shown to engage different cortical regions and produce different levels of activation than studies investigating affective processing of taste or olfactory stimuli (Small et al., 2003; Cerf-Ducastel, Haase, Kemmotsu, Jacobson, Green & Murphy, 2006; Grabenhorst & Rolls, 2008; Rolls, Grabenhorst, Margot, de Silva & Velazco, 2008). For example, greater levels of OFC activity have been observed when attention is placed on the affective value of taste or olfactory stimuli in comparison to attending to intensity (Grabenhorst & Rolls, 2008; Rolls, et al., 2008). Interestingly, primary gustatory regions show activation when attending to intensity and during taste detection and selective attention to taste (Veldhuizen, Bender, Constable & Small, 2007; Grabenhorst & Rolls, 2008). Thus, while the results of the current study delineate age-related effects on affective processing of taste stimuli, we note that age-related differences in stimulus quality and intensity processing may potentially differ from age-related differences in affective processing of taste.
Conclusion
This is the first investigation, to our knowledge, of the effects of brain aging on gustatory processing utilizing functional neuroimaging. The results reveal significant differences in processing hedonic information about taste stimuli in older and younger brains under the conditions of hunger and satiety. These results support greater activation and activation in additional areas in older than younger adults when evaluating gustatory information.
Supplementary Material
Acknowledgments
Supported by NIH grant # AG04085 to CM. We would like to thank Lori Haase, M.A. for assistance with data acquisition, image analysis, and comments on the manuscript, Barbara Cerf-Ducastel, Ph.D., for technical assistance, Nobuko Kemmotsu, M.A. for technical support, and Delaney Downer and Lindsay Ramos for research assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FIM. The effects of divided attention on encoding-and retrieval related brain activity: A PET study of younger and older adults. Journal of Cognitive Neuroscience. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Arana SF, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Odoherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. J Neurophysiol. 2007;97:3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Rolls ET, Mora F. Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Experimental Neurology. 1976;51:668–677. doi: 10.1016/0014-4886(76)90189-8. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic memory. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cain WS, Gent J, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. American Journal of Otolaryngology. 1983;4:252–256. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during an auditory-cued thumb-to-index opposition: A positron emission tomography study. Stroke. 2001;32:139–146. doi: 10.1161/01.str.32.1.139. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Research. 2003;986:39–53. doi: 10.1016/s0006-8993(03)03168-8. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Haase L, Kemmotsu N, Jacobson A, Green E, Murphy C. Correlation between brain activity and online psychophysical measurement: how the evaluative task affects brain activation. Chem Senses. 2006;31:493. [Google Scholar]
- Cowart BJ. Relationships between taste and smell across the adult life span. Ann NY Acad Sciences. 1989;561:39–55. doi: 10.1111/j.1749-6632.1989.tb20968.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Araujo IET, Kringelback ML, Rolls ET, Hobden P. Representation of umami taste in the human brain. J Neurophysiol. 2003;90:313–3119. doi: 10.1152/jn.00669.2002. [DOI] [PubMed] [Google Scholar]
- de Araujo IET, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulation. Cognitive, Affective, Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- DePietro L, Anda RF, Williamson DF, Stunkard AJ. Depressive symptoms and weight change in a national cohort of adults. International Journal of Obesity and Related Metabolic Disorders. 1992;16:745–753. [PubMed] [Google Scholar]
- Ferdon S, Murphy C. The cerebellum and olfaction in the aging brain: An fMRI study. NeuroImage. 2003;20:12–21. doi: 10.1016/s1053-8119(03)00276-3. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Uematsu H, Sugimoto K. Influences of aging on taste perception and oral somatic sensation. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:109–113. doi: 10.1093/gerona/60.1.109. [DOI] [PubMed] [Google Scholar]
- Gilmore MM, Murphy C. Aging is associated with increased Weber ratios for caffeine, but not for sucrose. Perception & Psychophysics. 1989;46:555–559. doi: 10.3758/bf03208152. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neuroscience. 2004;7:1145–1153. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Selective attention to affective value alters how the brain processes taste stimuli. European Journal of Neuroscience. 2008;27:723–729. doi: 10.1111/j.1460-9568.2008.06033.x. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein L, Siegenthaler A, Beig S. The effects of encoding task on age-related differences in functional neuroanatomy of face memory. Psychology and Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Ungerleider LG, Schapiro MB, Carson PE, et al. Dissociation of object and spatial vision in human extrastriate cortex: Age related changes in activation of regional cerebral blood flow measured with [15O] water and positron emission tomography. Journal of Cognitive Neuroscience. 1992;4:23–34. doi: 10.1162/jocn.1992.4.1.23. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. Journal of Neuroscience Methods. 2007;159:98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage. 2009a;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. The effect of stimulus delivery technique on perceived intensity functions for taste stimuli: implications for fMRI studies. Atten Percept Psychophys. 2009b;71:1167–1173. doi: 10.3758/APP.71.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. The anorexia of aging in humans. Physiology Behavior. 2006;88:257–266. doi: 10.1016/j.physbeh.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. Journal of Neuroscience. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim Y, Nobre AC, Mesulam M. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. Journal of Neuroscience. 2001;21:5304–5310. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM. Quality-specific effects of aging on the human taste system. Perception Psychophysics. 1989;45:121–128. doi: 10.3758/bf03208046. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiology of Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Knutson B, Carstensen L. Affective dynamics, affective forecasting, and aging. Emotion. 2008;8:318–330. doi: 10.1037/1528-3542.8.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgen R. Projections of thalamic gustatory and lingual areas in the monkey, macaca fascicularis. Journal of Comparative Neurology. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Jonides J, Smith ES, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, McDermott TM. Effects of age on sensory-specific satiety. American Journal of Clinical Nutrition. 1991;54:988–996. doi: 10.1093/ajcn/54.6.988. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Behavior. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Scott TR. Central taste anatomy and neurophysiology. In: Doty RL, editor. Handbook of olfaction and gustation. 2nd. New York: Marcel Dekker; 2003. pp. 679–705. [Google Scholar]
- Rolls ET, Grabenhorst F, Margot C, de Silva MA, Velazco MI. Selective attention to affective value alters how the brain processes olfactory stimuli. Journal of Cognitive Neuroscience. 2008;20:1815–1826. doi: 10.1162/jocn.2008.20128. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IET. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Samanez Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory change: A PET study. NeuroReport. 1996;7:1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Hornack K, Reilly D. Increased taste thresholds of amino acids with age. American Journal of Clinical Nutrition. 1979;32:1622–1627. doi: 10.1093/ajcn/32.8.1622. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Gatlin FA, Frey AE, Heiman SA, Stagner WC, Cooper EC. Taste perception of bitter compounds in young and elderly persons: relation to lipophilicity of bitter compounds. Neurobiology of Aging. 1994;15:1622–1627. doi: 10.1016/0197-4580(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Scott TR, Karadi Z, Oomura Y, Nishino H. Gustatory neural coding in the amygdala of the alert macaque monkey. Journal of Neurophysiology. 1993;69:1810–1820. doi: 10.1152/jn.1993.69.6.1810. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans A, Jones-Gotman M. Changes in brain activity related to eating chocolate; from pleasure to aversion. NeuroReport. 2001;10:7–14. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, et al. Aging effects on memory encoding in the frontal lobes. Psychology and Aging. 2002;17:44–45. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, et al. Functional magnetic resonance imaging of odor identification: The effect of aging. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:756–760. doi: 10.1093/gerona/56.12.m756. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in phonological learning task. Journal of Cognitive Neuroscience. 2006;18:1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food related stimuli: Effects of fasting and gender. Brain. 2006;124:1720–1733. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Giza BJ, Scott TR. Responses to taste stimulation in the ventroposteriomedial nucleus in rats. Journal of Neurophysiology. 2003;89:265–275. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. Journal of Gerentology Series A: Biological Sciences and Medical Sciences. 2005;60:510–514. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowaik RS. Age-related changes in neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. British Medical Journal. 2005a doi: 10.1136/bmj.238446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston C, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005b;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. Journal of Abnormal Psychology. 2008;2:428–434. doi: 10.1037/0021-843X.117.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L, Hickson M, Frost G. Eating together is important: Using a dining room in an acute elderly medical ward increases energy intake. Journal of Human Nutrition and Dietetics. 2006;19:23–26. doi: 10.1111/j.1365-277X.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Scott TR. The effect of satiety on responses of gustatory neurons in the amygdala of alert cynomolgus macaques. Brain Research. 1996;740:193–200. doi: 10.1016/s0006-8993(96)00864-5. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ. The responsiveness of neurons in the insular gustatory cortex of the macaque monkey is independent of hunger. Physiology Behavior. 1988;42:223–229. doi: 10.1016/0031-9384(88)90074-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


