Abstract
The epidemic of human immunodeficiency virus in Zambia has led to a dramatic rise in the incidence of human herpesvirus-8 (HHV-8)–associated Kaposi's sarcoma in both adults and children. However, there is a paucity of knowledge about the routes of HHV-8 transmission to young children. The Zambia Children's KS-HHV8 Study, a large, prospective cohort study in Lusaka, Zambia, was launched in 2004 to investigate the role of household members as a source of HHV-8 infection in young children and social behaviors that may modify the risk of HHV-8 acquisition. This cohort is distinct from other epidemiologic studies designed to investigate HHV-8 incidence and transmission because it recruited and followed complete households in the urban central African context. Between July 2004 and March 2007, 1,600 households were screened; 368 households comprising 464 children and 1,335 caregivers and household members were enrolled. Follow-up of this population continued for 48 months postrecruitment, affording a unique opportunity to study horizontal transmission of HHV-8 and understand the routes and sources of transmission to young children in Zambia. The authors describe the study rationale, design, execution, and characteristics of this cohort, which provides critical data on the epidemiology and transmission of HHV-8 to young children in Zambia.
Keywords: cohort studies; herpesvirus 8, human; incidence; sarcoma, Kaposi; Zambia
Human herpesvirus-8 (HHV-8), also known as Kaposi's sarcoma (KS)–associated herpesvirus, is the most recently described human gamma-herpesvirus reported to be associated with all forms of KS (1, 2). KS is an illness defining acquired immunodeficiency syndrome (AIDS) and is the most common malignancy present in patients infected with human immunodeficiency virus (HIV) (3). Because of the ongoing HIV epidemic in sub-Saharan Africa, KS has become one of the most frequently diagnosed cancers in this region (4). Global seroprevalence of HHV-8 varies greatly and is generally high in areas where non-HIV-associated forms of KS (classic or endemic) have been common (5). Epidemiologic studies from sub-Saharan Africa report an HHV-8 seroprevalence of 20%–68% in adolescents (6–8). One such country is Zambia, a part of the “KS belt” where endemic KS was prevalent and where a significant increase in KS incidence in adults and children has coincided with the emergence of the HIV-1 epidemic (9–11). By 1992, KS accounted for approximately 25% of all childhood cancers diagnosed in Lusaka, the capital of Zambia (12).
Because a vaccine to prevent HHV-8 infection is not on the horizon, efforts to reduce HHV-8 transmission are needed. However, routes of HHV-8 transmission and factors associated with increased risk of HHV-8 acquisition have yet to be delineated. On the basis of results from our previous cohort study in Zambia conducted from 1998 to 2003, we were the first known to report that 1) early childhood infection with HHV-8 is common—on the basis of 1,532 child-years of follow-up, we reported that HHV-8 seroconversion occurs early in life and that the incidence rate of HHV-8 seroconversion is 13.8 infections per 100 child-years by 48 months of age; 2) HHV-8 may be transmitted perinatally, albeit rarely; and 3) HHV-8 is detected in saliva but not in breast milk (13–15). Taken together, these results suggest that the major mode of HHV-8 transmission to children is by person-to-person contact, likely via saliva. Several studies have reported salivary transmission, leading to the emergence of saliva as the major route of HHV-8 transmission (16–18).
We have also reported that HHV-8 infection in young children could occur independently of the HHV-8 serostatus of the mother, suggesting the possible role of other household members or others in the community in transmitting infection (15). Therefore, it is critical to evaluate the role of household contacts in transmitting infection to young children, especially in HHV-8-endemic areas such as Zambia. To date, we know of no cohort study designed to recruit and follow entire households to investigate HHV-8 transmission within households. Socioeconomic factors such as low parental educational levels, use of water from a communal source, and low income levels have been implicated as risk factors associated with HHV-8 seroprevalence; these studies were cross-sectional in design and thus were unable to associate specific risk factors with incident HHV-8 infection (19–21). Therefore, the Zambia Children's KS-HHV8 Study was designed to include a longitudinal 4-year follow-up of recruited households, with the specific objective of determining the rate and source of horizontal transmission of HHV-8 to young children. We aimed to investigate whether HHV-8 could be transmitted to young children through casual, person-to-person contact within a household.
Conducting prospective cohort studies is a challenging endeavor, particularly in resource-poor settings such as Zambia, which has a poor infrastructure, high poverty levels, and a limited number of skilled personnel. The aim of this paper is to describe the rationale, design, execution, logistics, and characteristics of a large, observational, household-based cohort study in Lusaka, Zambia.
MATERIALS AND METHODS
Study population and site
The Zambia Children's KS-HHV8 Study is a community-based cohort study executed by investigators at the University Teaching Hospital in Lusaka, Zambia, in collaboration with investigators at the University of Nebraska-Lincoln. The study coordinating office was based at the University Teaching Hospital, with an independent clinic for recruitment and follow-up of study participants and a fully equipped laboratory for biospecimen processing, testing, and storage. The study office also houses the data management team and the study coordinator; together, they manage the paper and electronic versions of questionnaires, study-related forms, and laboratory results. All study personnel at the clinic and laboratory are Zambians, and all study-related discussions and decisions are made by consensus by the study coordinator, Zambian- and US-based study directors, and principal investigators. This study was approved by the institutional review board at the University of Nebraska-Lincoln and the Ethics Board of the University of Zambia.
Screening for enrollment
Recruitment and long-term follow-up of participants in Zambia can be a difficult process. There is general disinterest in participating in research studies because the economic and educational status of an average household is low, with approximately 64% of Zambian households living below the poverty line (in 2006) (22). Zambia is one of the poorest countries in sub-Saharan Africa, with a large percentage of a highly mobile population living in unorganized and densely populated settlements called “compounds,” making it difficult to locate and track study participants.
To achieve a high follow-up rate, we tried a novel method to create interest in the study and encourage follow-up visits. Community health workers, who were trained and educated about the study goals, spread the information about the goals of the study in their communities. These workers were residents of Lusaka and were instructed to reach potential study participants not only from within their residential areas and clinics but also from areas and clinics in other compounds. Community health workers provided potential study participants with basic information about the study goals and expectations and gave them an opportunity to ask questions. If interested, participants were directed to visit the study office at the University Teaching Hospital, where trained study nurses provided comprehensive information about the study and answered all relevant questions. HIV and HHV-8 counseling was also given, and written informed consent was obtained to participate in the study. Upon providing consent, each household and each member of the household was given a unique identification number. Participation incentives included the availability of basic health care at the study clinic, counseling for all study participants, common medications at no cost, and reimbursement of travel costs to the study office and clinic.
Our objective was to enroll complete households that had at least one HHV-8–negative child (referred to as the index child). The 4 eligibility criteria were as follows:
Each household with at least one HHV-8–negative index child between 6 and 24 months of age (children younger than age 24 months were screened for HHV-8 serostatus). Children less than age 6 months were not recruited to avoid detecting residual maternal antibodies, which can be detected in children up to 6 months of age (23).
The household members had to reside in Lusaka District. Our early research experience suggested that participants living outside Lusaka were difficult to recruit or follow up because of their inability to travel or their disinterest in traveling to the study site at the University Teaching Hospital. According to the 2000 census, 14% of the Zambian population lived in Lusaka District; we therefore decided to limit our research to study participants residing in Lusaka District (24).
All members of the household, up to a total of 10, had to agree to participate and visit the study office for scheduled follow-up visits. The size of the household was limited to 10 to help control the number of possible contacts with the index child.
The child could not be seriously ill (e.g., with tuberculosis, cancer, or AIDS) on the day of screening to ensure timely follow-up visits. Seriously ill children were excluded only because of their inability to travel or because of health risks involved in traveling to the study clinic.
On meeting all eligibility criteria, all members of the household had to return to the study office for enrollment within 1 month of screening. In addition, a small number (n = 34) of HHV-8–positive children younger than 24 months of age were enrolled as a control group to investigate the risk factors associated with HHV-8 infection for a baseline cross-sectional analysis.
Enrollment and follow-up
The HHV-8–negative children, along with their primary caregiver, were requested to return for follow-up visits every 4 months until age 48 months; the other household members were followed annually. During each visit, all study participants received a physical examination and free medications for common ailments such as worm infestation, fever, and minor aches and pains as well as multivitamin supplements in case of malnourishment. During enrollment and at each follow-up visit, various biologic specimens were collected (as described below), health assessments were completed, and questionnaires to obtain demographic and behavioral information were administered (Table 1). Throughout the duration of the study, additions to the household that increased its size were enrolled either as index children if they were 2 years of age or younger or as household members if they were older than age 2 years. Community health workers tracked study participants who did not return for scheduled visits to remind them of their missed visits or, if they decided to withdraw, determine the reasons for study withdrawal.
Table 1.
Questionnaires, Biologic Specimens Collected, and Laboratory Measures for the Zambia Children's KS-HHV8 Study, Lusaka, Zambia, 2004–2009
| Visit and Questionnaire | Samples Collected | Tests Performed |
| Screening | ||
| Screening questionnaire for eligibility | Blood | HHV-8 |
| Enrollment | ||
| Enrollment questionnaire Basic medical examination Demographic characteristics Baseline data Household behavioral data Developmental milestones |
Blood, buccal swab | HIV, CD4 count, HHV-8 serology, DNA detection in buccal swabs, others if indicated (malaria, syphilis) |
| Follow-up | ||
| Follow-up questionnaire Basic medical examination Changes in medical conditions Household behavioral data Developmental milestones |
Blood, buccal swab | HIV, CD4 count, HHV-8 serology, DNA detection in buccal swabs, others if indicated (malaria, syphilis) |
Abbreviations: HHV-8, human herpesvirus 8; HIV, human immunodeficiency virus; KS, Kaposi's sarcoma.
The major outcome variable of interest was HHV-8 serostatus at each visit. Outcome assessment was conducted by testing plasma collected from each infant during each follow-up visit for evidence of HHV-8 infection, as described previously (25). In addition, HHV-8 was detected in buccal swabs at each visit.
Data collection instruments and exposure assessment
Structured interview questionnaires were developed to collect information on factors that may be associated with increased risk of horizontal transmission of HHV-8 to the index child. The content of these questionnaires was based on discussions with focus groups conducted in March 2004 (26). Discussions with men and women from diverse ethnic and socioeconomic backgrounds were conducted to determine various behavioral and sociocultural practices that could impact the risk of the index child acquiring HHV-8. The questionnaires were administered to the caregiver during enrollment and at each follow-up visit in English or by using the local language (translated into commonly used languages, Bemba or Nyanja, by the interviewer). Questions pertained to household living conditions (electricity, water source, toilet facilities, number of rooms/sleeping areas, household density), behaviors involving food and drinks (premastication, sharing sweets and/or drinks), health and personal care practices (e.g., bathing habits, oral hygiene, use of traditional medicine and use of saliva to soothe injuries), demographic variables (sex, age, education of the primary caregiver, household size, playmates), medical history (ailments and hospitalizations), and health assessments of the primary caregiver and the child. Developmental milestones of the child were also recorded.
Data management
Answers from the printed copies of the questionnaires and laboratory test results were recorded in the database and managed locally. Databases were generated by entering each form twice and comparing both entries to identify and correct entry errors. At study initiation, we used optical character recognition software to transfer information from each form to the database. However, character recognition and record posting problems were frequently encountered, leading to extensive manual data cleaning. Subsequently, manual entry using Microsoft Access software (Microsoft Corporation, Redmond, California) forms was used, which significantly improved data quality. Copies of the database were regularly provided to the study staff at the University of Nebraska-Lincoln, where analytic data sets were generated and are currently being used for analysis, review, and publication by study investigators.
Biospecimen collection and processing
All samples collected at the clinic were processed by the study laboratory within 2 hours of collection. Venous blood was collected from all study participants at every visit, and plasma was separated and stored. The peripheral blood mononuclear cell pellet was divided into 2 vials; one was stored at −80°C, and the other was lysed for DNA extraction. During the screening visit, both the child and the caregiver were tested for the presence of HHV-8 and HIV-1 antibodies, as described previously (25). Screening for the presence of HIV-1 antibodies was performed by using Abbott Determine (Abbott Laboratories, Chicago, Illinois) and was confirmed by using Unigold (Trinity Biotech, Bray, Ireland) test kits, according to the HIV-1 testing guidelines of the Zambia Ministry of Health. For children younger than 18 months of age, polymerase chain reaction was performed in the study laboratory using dried blood spots. Buccal cells were also collected at each visit by scraping the buccal cavity with a cotton swab; the cells were then stored in a collection tube containing Cell Lysis Solution (Qiagen, Valencia, California) to stabilize the DNA until DNA extraction was conducted. DNA extracted from buccal cell lysates was used for investigating HHV-8 detection in the oral cavity, frequency of detection, and HHV-8 viral load.
RESULTS
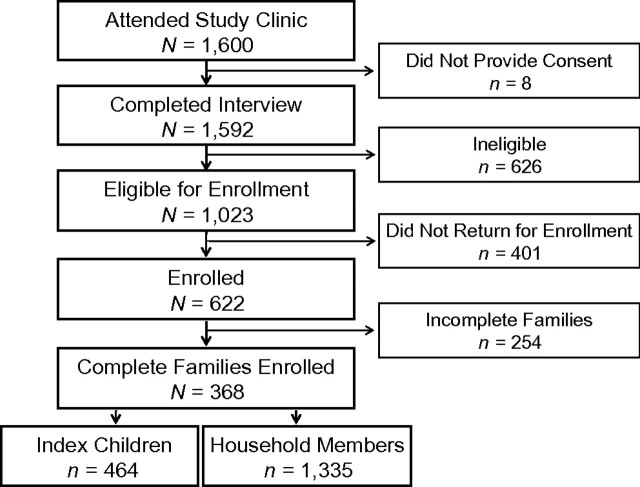
From July 2004 to March 2007, caregivers from 1,600 Zambian households, including 1,657 index children younger than age 24 months, visited the study clinic to participate in the study. All were residents of 1 of 40 townships within Lusaka District (refer to Web Figure 1, which is posted on the Journal's Web site (http://aje.oupjournals.org/)). Although our attempt was to sample from all areas of the city, we were unable to sample equally from all residential areas, especially those containing residents of higher socioeconomic status. One of the major objectives of this study was to determine increased risk of HHV-8 acquisition by the index child when an HHV-8–positive member was present in the household. To achieve this objective, our goal was to recruit and retain at least 370 index children to ensure 85% power to detect change in the HHV-8 seroconversion difference from 12% in HHV-8–negative households to 22% in HHV-8–positive ones over a 4-year period, at a significance level of 0.05. By the end of the study period, 464 index children and 1,335 household members were enrolled (Figure 1).
Figure 1.
Flowchart describing recruitment of the study cohort for the Zambia Children's KS-HHV8 Study, Lusaka, Zambia, 2004–2009. HHV-8, human herpesvirus 8; KS, Kaposi's sarcoma.
During the first visit to the clinic, eligibility was determined after the caregiver provided informed consent to participate in the study. Refusal to participate at this visit was rare (0.5%) because caregivers had a basic understanding of the study from community health workers before they attended the study clinic. The most common reason for refusal was fear of blood collection. Eligibility criteria were met by participants of 1,023 households, who were invited to return with the entire household within 1 month (Figure 1). Of these, all members of 368 households (1,799 total study participants) were enrolled and followed. In 254 households, one or more household member(s) could not attend the study clinic within the specified enrollment period; thus, these households were considered incomplete and were not followed at later time points. The enrollment rate of complete households compared with households initially screened was 23.0%. Variation was noted in enrollment rates between townships (P < 0.0001), most likely as a result of distance and accessibility to the study site (data not shown).
The annual enrollment and characteristics of 464 index children and 1,335 household members during the enrollment period are summarized in Table 2. Of the 464 index children initially enrolled, 395 (85%) returned for one or more visits. The baseline characteristics of the 368 enrolled, complete households are summarized in Table 3. Homes were generally small in size (mean, 2 rooms), with a mean of 4.7 household members per household. A majority of the households were of lower socioeconomic status, having no electricity, running water, or in-home toilet facilities.
Table 2.
Enrollment Characteristics of Study Participants During Each Year of the Zambia Children's KS-HHV8 Study, Lusaka, Zambia, 2004–2009
| 2004 |
2005 |
2006 |
2007 |
2008 |
Total |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Index children | 11 | 171 | 151 | 92 | 39 | 464 | ||||||
| Sex | ||||||||||||
| Female | 6 | 54.5 | 81 | 47.4 | 62 | 41.1 | 46 | 50.0 | 24 | 61.5 | 219 | 47.2 |
| Male | 5 | 45.5 | 90 | 52.6 | 89 | 58.9 | 46 | 50.0 | 15 | 38.5 | 245 | 52.8 |
| Age, months | ||||||||||||
| 0–5 | 0 | 0 | 9 | 15 | 2 | 26 | ||||||
| 6–12 | 4 | 61 | 64 | 45 | 33 | 207 | ||||||
| 13–18 | 6 | 42 | 36 | 19 | 4 | 107 | ||||||
| 19–24 | 1 | 51 | 41 | 10 | 0 | 103 | ||||||
| 25–29 | 0 | 17 | 1 | 3 | 0 | 21 | ||||||
| Household members | 38 | 567 | 500 | 226 | 4 | 1,335 | ||||||
| Sex | ||||||||||||
| Female | 19 | 50.0 | 321 | 56.6 | 288 | 57.6 | 136 | 60.2 | 2 | 50.0 | 766 | 57.4 |
| Male | 19 | 50.0 | 246 | 43.4 | 212 | 42.4 | 90 | 39.8 | 2 | 50.0 | 569 | 42.6 |
| Age, years | ||||||||||||
| ≤5, child | 9 | 104 | 89 | 39 | 0 | 241 | ||||||
| 6–15, youth | 9 | 197 | 171 | 70 | 2 | 449 | ||||||
| >15, adult | 20 | 266 | 240 | 117 | 2 | 645 | ||||||
| Total enrolled | 49 | 738 | 651 | 318 | 43 | 1,799 | ||||||
Abbreviations: HHV-8, human herpesvirus 8; KS, Kaposi's sarcoma.
Table 3.
Characteristics of 368 Enrolled Households in the Zambia Children's KS-HHV8 Study, Lusaka, Zambia, 2004–2009a
| Characteristic | No. | % |
| Household members | ||
| ≤3 | 87 | 23.6 |
| 4–5 | 175 | 47.6 |
| >5 | 106 | 28.8 |
| Rooms in the home | ||
| 1 | 89 | 24.2 |
| 2–3 | 223 | 60.6 |
| ≥4 | 23 | 6.3 |
| Electricity in the home | ||
| Yes | 64 | 17.4 |
| No | 270 | 73.4 |
| Toilet in the home | ||
| Shared outhouse (latrine) | 329 | 89.4 |
| Flush toilet in own house | 6 | 1.6 |
| Water source | ||
| Community tap or shared with neighbors | 291 | 79.1 |
| Tap outside on property or in own house | 44 | 12.0 |
Abbreviations: HHV-8, human herpesvirus 8; KS, Kaposi's sarcoma.
All numbers do not equal total household numbers because of missing values.
Table 4 summarizes the characteristics of the primary caregiver and his or her relationship with the enrolled index child. A number of different household structures were represented, including multiple index children in a single household. The second index child could be a younger sibling, a niece, a nephew, or another extended family member living in the household. Of the 464 enrolled index children from 368 households, 97% of caregivers were the mothers of these children, while others included grandmothers, aunts, fathers, and even cousins. A majority of primary caregivers were between 20 and 29 years of age (54.3%; age range, 14–78 years). Households were classified as “2 parent” if both a mother and a father of at least 1 index child in the household were present; “single parent” if only 1 parent was available in the household (all the single parents in our enrolled cohort were mothers); and, if there were no parents or if the household included another adult such as an aunt or uncle, these households were classified as “extended family.” Nearly half (49.7%) of the households were classified as either “single parent” or “extended family,” which reflects a community continuing to suffer a high adult mortality rate from HIV/AIDS and related conditions. Most of the primary caregivers in the cohort (54%) had completed primary education; no primary caregiver reported education beyond high school.
Table 4.
Characteristics of the Primary Caregivera in the Zambia Children's KS-HHV8 Study, Lusaka, Zambia, 2004–2009b
| Characteristic | No. | % |
| Primary caregiver relationship to the index child (N = 464) | ||
| Mother | 449 | 96.8 |
| Grandmother | 6 | 1.3 |
| Aunt | 7 | 1.5 |
| Father | 1 | 0.2 |
| Cousin | 1 | 0.2 |
| Primary caregiver's age, years (N = 368) | ||
| <20 | 25 | 6.8 |
| 20–29 | 200 | 54.3 |
| 30–39 | 120 | 32.6 |
| ≥40 | 23 | 6.3 |
| Parental caregiver status within households (N = 368) | ||
| Households with 2 parents | 185 | 50.3 |
| Households with a single parent | 130 | 35.3 |
| Households with extended family | 53 | 14.4 |
| Education of the primary caregiver (N = 368)b | ||
| None | 33 | 9.0 |
| Primary | 200 | 54.3 |
| Secondary | 102 | 27.7 |
Abbreviations: HHV-8, human herpesvirus 8; KS, Kaposi's sarcoma.
A primary caregiver is defined as the household member with the most contact with the child.
All numbers do not equal total household numbers because of missing values.
DISCUSSION
In this paper, we describe the study rationale, design, and methods of the Zambia Children's KS-HHV8 Study in Lusaka, Zambia. This study is the only one known to recruit and follow complete households in an HIV/AIDS- and KS-endemic area such as Zambia. Our objective was to design and implement a study to examine HHV-8 incidence and factors associated with HHV-8 acquisition by young children. Collection of a wide range of demographic, medical, and behavioral data and of biologic samples during each visit enabled us to meet our objectives.
This is the second cohort study being undertaken by our group. Several steps implemented in this study ensured its success in terms of recruitment and follow-up. It enabled us to establish a fully equipped, modern diagnostic laboratory on the premises of the University Teaching Hospital, which provided no-cost service to University Teaching Hospital patients, helping build positive relationships with local health officials. Additionally, establishing the study clinic helped to provide basic medical care to study participants who seldom have access to quality medical care, encouraging participation and high follow-up rates. The study emphasized follow-up of children for childhood health and wellness, prevention of common childhood illnesses with regular household checkups, and a focus on the well-being of the child rather than only an emphasis on testing for HIV-1 infection. This process was chosen to increase participation rates because some households are not eager to take part in HIV-1 studies compared with studies emphasizing the general well-being of the child; participation in HIV studies may still be associated with a stigma. In addition, some participants still do not want to know their HIV status. Therefore, child health was the most emphasized point during each visit.
Close collaboration with local community health workers was of immense help during screening and recruitment of households. Educational level is low in most communities in Lusaka, which made it difficult to spread the correct message regarding the study objectives. Community health workers helped to educate the clients about the objectives, and they addressed participant fears and questions in the local language and a familiar setting, achieving high consent rates among the participants who attended the study clinic. Community health workers also reduced the need for lengthy study descriptions at the clinic because they had previously been provided, allowing more time to be dedicated to medical or counseling needs. In addition, all study personnel in Zambia were local people who helped establish contacts and gain the trust of study participants, which was critical to achieving enrollment goals and high follow-up rates. In our experience, research studies in Zambia will greatly benefit by clearly defining and explaining the benefits to study participants. A high poverty level makes it difficult to garner interest about research studies among these participants; their individual problems are so severe that they have little time, energy, or resources to participate in research studies.
Cohort studies are a challenge to plan and execute, even more so in a low-resource setting such as Zambia. In our experience, high-tech equipment does not work well without constant technical support. We think that one reason for the malfunctioning scanning software was the lack of firewall protection for the University Teaching Hospital network of computers. Affordable Internet service in Zambia is often slow and unreliable, making many tasks tedious. Another common problem faced by the study personnel was ensuring that the enrolled household was indeed one unit. To establish this fact, all clients were asked to clearly define the relationships. For example, a client describing her sister's son as “like a son” was further queried to determine whether the client was taking care of the child briefly or whether the child was indeed living with the household. The household structure in Zambia is markedly different from that in Western countries, with a common joint family system, leading to a large household size. Addition of new household members is also common in Zambia because of relatives staying for extended periods of time and because young children for whom one or both parents have died are cared for. Careful planning and explanation of the definition of “household” is essential for such studies.
This study design had several strengths. Several elegant studies that have examined HHV-8 seroprevalence in children have been reported (17, 21, 27, 28). However, most are cross-sectional in design and cannot provide incidence estimates during early childhood in relation to the factors that may be associated with increased incidence. In addition, very few studies have focused on assessing infection within households, especially in endemic areas such as Zambia. Unlike in Western countries, where primary infection occurs mostly after adolescence, our previous studies have shown that children are infected in Zambia at a young age (15). We had a unique opportunity to study whether the source of infection is within or outside the household.
Nevertheless, despite careful planning, this cohort may still have certain limitations. We were unable to recruit households from more affluent backgrounds, thus limiting the ability to generalize our findings to different living situations. However, with 64% of Zambian households living in poverty, our results should be generalizable to the majority of the Zambian population. In addition, since we had to limit the household size to 10, we may have missed the effect of larger household size on HHV-8 acquisition, but we could still analyze the effect of increasing family size on HHV-8 acquisition for as many as 10 household members. Because we did not collect saliva samples, we could have potentially missed detecting cell-free virus shed in the oral cavity. Another potential limitation could be self-selection bias; because medical benefits were provided, the study participants may have been of poorer overall health because of HIV infection, malaria, parasitic infections, or tuberculosis and had more of an incentive to enroll as well as to continue follow-up. If a selection bias was present, we will still be able to assess HHV-8 acquisition using information about the overall health of individuals as predictor variables.
An additional limitation could be information bias; some of the questions regarding eating habits or personal care habits may elicit a defensive response by the caregiver, resulting in answers that err toward more socially acceptable habits. To decrease this potential bias, the questionnaire was carefully worded to avoid possible negative connotations, and the interviewers were instructed to ask questions in a nonjudgmental manner. In addition, since participants were informed of their own and their child's HHV-8 status at follow-up visits, this factor could potentially alter their interactions with the child. The caregiver could assume that certain behaviors that were being asked about on a regular basis were important and may have changed their behavior. To avoid this potential problem, participants were not told that any questioned behaviors were known to be associated with HHV-8 transmission.
The results of this study have the potential to contribute to the design of effective health behavior interventions to prevent HHV-8 infection and to overall community health promotion. Designing effective behavioral interventions to prevent HHV-8 transmission to young children requires us to investigate the degree to which that behavior is influenced by attitudes, perceived norms, and self-efficacy. The questionnaire data collected from study participants will be critical in understanding these potential determinants of transmission. The laboratory and virologic data will help in understanding the biology of HHV-8 in the Zambian setting.
To summarize, this study was designed to yield critical clinical, demographic, laboratory, and behavioral data useful for answering a variety of questions about HHV-8 transmission: Does a child acquire HHV-8 infection from within a household? Does HIV-1 infection of the child or household member increase risk of HHV-8 acquisition in a child? What behavioral or lifestyle risk factors increase the risk that a child will acquire HHV-8?
Supplementary Material
Acknowledgments
Author affiliations: Nebraska Center for Virology, School of Biological Sciences, University of Nebraska-Lincoln, Lincoln, Nebraska (Veenu Minhas, Kay L. Crabtree, Charles Wood); CTS Global Inc., Los Angeles, California (Ann Chao); Department of Pediatrics, University of California San Francisco, San Francisco, California (Janet M. Wojcicki); Department of Paediatrics and Child Health, University Teaching Hospital, Lusaka, Zambia (Adrian M. Sifuniso, Catherine Nkonde, Chipepo Kankasa); and Department of Pediatrics, University of Miami School of Medicine, Miami, Florida (Charles D. Mitchell).
V. M. and K. L. C. contributed equally to this work.
This work was supported by the National Institutes of Health (PHS grant RO1 CA75903; Fogarty International Training Grant D43 TW01492 and T32 AI060547) and a National Center for Research Resources Centers of Biomedical Research Excellence grant (grant P20 RR15635) to C. W. K. L. C. was supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Allergy and Infectious Diseases and by the INBRE (IDeA Networks of Biomedical Research Excellence) program of the National Center for Research Resources (grant P20 RR016469). A. M. S. and C. N. were Fogarty Fellows.
The authors thank Chafye Siuluta, Ntazana Mandandi, all community health workers, and laboratory staff at University Teaching Hospital in Lusaka for their contributions to client recruitment, data collection and management, and sample processing. They also acknowledge Danielle Shea for providing technical assistance.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- HHV-8
human herpesvirus-8
- HIV
human immunodeficiency virus
- KS
Kaposi's sarcoma
References
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Huang YQ, Li JJ, Kaplan MH, et al. Human herpesvirus-like nucleic acid in various forms of Kaposi's sarcoma. Lancet. 1995;345(8952):759–761. doi: 10.1016/s0140-6736(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 3.Sunil M, Reid E, Lechowicz MJ. Update on HHV-8-associated malignancies. Curr Infect Dis Rep. 2010;12(2):147–154. doi: 10.1007/s11908-010-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feller L, Khammissa RA, Gugushe TS, et al. HIV-associated Kaposi sarcoma in African children. SADJ. 2010;65(1):20–22. [PubMed] [Google Scholar]
- 5.Cook-Mozaffari P, Newton R, Beral V, et al. The geographical distribution of Kaposi's sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78(11):1521–1528. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gessain A, Mauclère P, van Beveren M, et al. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int J Cancer. 1999;81(2):189–192. doi: 10.1002/(sici)1097-0215(19990412)81:2<189::aid-ijc4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77(6):817–820. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Mbulaiteye SM, Pfeiffer RM, Whitby D, et al. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187(11):1780–1785. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 9.Bayley AC. Occurrence, clinical behaviour and management of Kaposi's sarcoma in Zambia. Cancer Surv. 1991;10:53–71. [PubMed] [Google Scholar]
- 10.Patil P, Elem B, Zumla A. Pattern of adult malignancies in Zambia (1980–1989) in light of the human immunodeficiency virus type 1 epidemic. J Trop Med Hyg. 1995;98(4):281–284. [PubMed] [Google Scholar]
- 11.Patil PS, Elem B, Gwavava NJ. The pattern of paediatric malignancy in Zambia (1980–1989): a hospital-based histopathological study. J Trop Med Hyg. 1992;95(2):124–127. [PubMed] [Google Scholar]
- 12.Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Child. 1995;73(2):100–104. doi: 10.1136/adc.73.2.100. discussion 104–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brayfield BP, Kankasa C, West JT, et al. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J Infect Dis. 2004;189(12):2260–2270. doi: 10.1086/421119. [DOI] [PubMed] [Google Scholar]
- 14.Mantina H, Kankasa C, Klaskala W, et al. Vertical transmission of Kaposi's sarcoma-associated herpesvirus. Int J Cancer. 2001;94(5):749–752. doi: 10.1002/ijc.1529. [DOI] [PubMed] [Google Scholar]
- 15.Minhas V, Crabtree KL, Chao A, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am J Epidemiol. 2008;168(3):311–320. doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagni R, Whitby D. Kaposi's sarcoma-associated herpesvirus transmission and primary infection. Curr Opin HIV AIDS. 2009;4(1):22–26. doi: 10.1097/COH.0b013e32831add5a. [DOI] [PubMed] [Google Scholar]
- 17.de Souza VA, Sumita LM, Nascimento MC, et al. Human herpesvirus-8 infection and oral shedding in Amerindian and non-Amerindian populations in the Brazilian Amazon region. J Infect Dis. 2007;196(6):844–852. doi: 10.1086/520549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 19.Engels EA, Atkinson JO, Graubard BI, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196(2):199–207. doi: 10.1086/518791. [DOI] [PubMed] [Google Scholar]
- 20.Goedert JJ, Charurat M, Blattner WA, et al. Risk factors for Kaposi's sarcoma-associated herpesvirus infection among HIV-1-infected pregnant women in the USA. AIDS. 2003;17(3):425–433. doi: 10.1097/00002030-200302140-00017. [DOI] [PubMed] [Google Scholar]
- 21.Mbulaiteye SM, Biggar RJ, Pfeiffer RM, et al. Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr. 2005;38(4):474–479. doi: 10.1097/01.qai.0000132495.89162.c0. [DOI] [PubMed] [Google Scholar]
- 22.Living Conditions. Lusaka, Zambia: Central Statistical Office; 2006. ( http://www.zamstats.gov.zm/lcm.php). (Accessed June 1, 2010) [Google Scholar]
- 23.Lyall EG, Patton GS, Sheldon J, et al. Evidence for horizontal and not vertical transmission of human herpesvirus 8 in children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J. 1999;18(9):795–799. doi: 10.1097/00006454-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Summary Report for the 2000 Census of Population and Housing. Lusaka, Zambia: Central Statistical Office. 2000 ( http://www.zamstats.gov.zm/media/sum_rpt.pdf). (Accessed June 1, 2010) [Google Scholar]
- 25.Minhas V, Crosby LN, Crabtree KL, et al. Development of an immunofluorescence assay using recombinant proteins expressed in insect cells to screen and confirm presence of human herpesvirus 8-specific antibodies. Clin Vaccine Immunol. 2008;15(8):1259–1264. doi: 10.1128/CVI.00487-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojcicki JM. Traditional behavioural practices, the exchange of saliva and HHV-8 transmission in sub-Saharan African populations. Br J Cancer. 2003;89(10):2016–2017. doi: 10.1038/sj.bjc.6601390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler LM, Dorsey G, Hladik W, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis. 2009;200(3):430–438. doi: 10.1086/600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbulaiteye S, Marshall V, Bagni RK, et al. Molecular evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus in Uganda and K1 gene evolution within the host. J Infect Dis. 2006;193(9):1250–1257. doi: 10.1086/503052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.