Abstract
Studies show that ambient temperature and air pollution are associated with cardiovascular disease and that they may interact to affect cardiovascular events. However, few epidemiologic studies have examined mechanisms through which ambient temperature may influence cardiovascular function. The authors examined whether temperature was associated with heart rate variability (HRV) in a Boston, Massachusetts, study population and whether such associations were modified by ambient air pollution concentrations. The population was a cohort of 694 older men examined between 2000 and 2008. The authors fitted a mixed model to examine associations between temperature and air pollution and their interactions with repeated HRV measurements, adjusting for covariates selected a priori on the basis of their previous studies. Results showed that higher ambient temperature was associated with decreases in HRV measures (standard deviation of normal-to-normal intervals, low-frequency power, and high-frequency power) during the warm season but not during the cold season. These warm-season associations were significantly greater when ambient ozone levels were higher (>22.3 ppb) but did not differ according to levels of ambient fine (≤2.5 μm) particulate matter. The authors conclude that temperature and ozone, exposures to both of which are expected to increase with climate change, might act together to worsen cardiovascular health and/or precipitate cardiovascular events via autonomic nervous system dysfunction.
Keywords: air pollution, heart rate, interaction, ozone, particulate matter, temperature
Changes in the frequency and intensity of extreme weather and climate events have had profound effects on both human society and the natural environment (1–3). Recent studies have indicated that variation in ambient temperature is correlated with human morbidity and mortality, especially for cardiovascular disease (4–7). Similarly, ambient particulate matter and ozone have been consistently associated with cardiovascular death (8–13). Because heart rate variability (HRV), a noninvasive and sensitive measure of cardiac autonomic function, is predictive of sudden death from cardiovascular disease, it is often used as a measure of cardiovascular function in physiologic and epidemiologic studies (14–18).
Suggested mechanisms linking air pollution, especially particulate matter, to cardiovascular diseases include changes in the response of the autonomic nervous system through direct pulmonary reflexes from airways; chemical effects on ion channel function in myocardial cells; ischemic response in the myocardium; and pulmonary and systemic oxidative stresses and inflammatory responses that trigger endothelial dysfunction, atherosclerosis, and thrombosis (19–21). Recently, many experimental and epidemiologic studies have indicated that autonomic nervous system response and oxidative stress are key mechanisms involved in the influence of air pollution exposure on cardiovascular disease occurrence (18, 22–25).
Compared with the robust literature exploring mechanisms by which air pollution may influence cardiovascular health, relatively few studies have examined these mechanisms with respect to ambient temperature, and most of them have been experimental studies (26–31). Since humans are often only intermittently exposed to the extreme ambient temperatures evaluated in experiments, and since experiments are limited to examination of short-term exposures only, the implications of these findings for general population exposure to ambient temperatures are unclear.
Recently, many epidemiologic studies have shown that ambient temperature and air pollution interact to affect morbidity and mortality (6, 8, 32–34). For example, Ren et al. (6, 8, 32) reported that ambient temperature, together with particulate matter and ozone, enhances the adverse associations with all natural-cause mortality as well as cardiorespiratory-cause mortality in Australia and multiple US cities. In studies from diverse locations, other investigators have made similar observations (33, 34). However, the biologic mechanisms for the interaction between ambient temperature and air pollution remain unclear.
Both ambient temperature and air pollution have been separately related to alterations in cardiovascular function and adverse cardiovascular health outcomes, and other studies have shown that they might act jointly (32, 33). Therefore, the exploration of potential biologic mechanisms by which ambient temperature could influence cardiovascular disease is an important priority in environmental epidemiology, especially since relatively little is known about such mechanisms based on research in community-dwelling human populations. Thus, we examined whether ambient temperature changes were associated with cardiac autonomic function as measured by HRV in a longitudinal analysis (2000–2008) of the Normative Aging Study population. We also assessed whether such associations were modified by ambient particulate matter and ozone.
MATERIALS AND METHODS
Study population
The Normative Aging Study is a longitudinal study of aging established in 1963, when men aged 21–80 years from Greater Boston, Massachusetts, who were free of known chronic medical conditions were recruited (35). Written informed consent was provided by all participants. The present investigation was approved by all relevant institutional review boards. Since 1963, every 3–5 years, participants have undergone routine physical examinations, laboratory tests, collection of medical history information, and completion of questionnaires on smoking history, educational level, food intake, and other factors that may influence health.
The present study included the participants who underwent examinations between November 2000 and December 2008, because both HRV and air pollution data were available for that period. A total of 694 participants were evaluated for HRV during this time period. Each of these 694 men had either 1 (n = 233), 2 (n = 265), 3 (n = 194), or 4 (n = 2) HRV measurements, for a total of 1,353 visits.
HRV measurements
After a 5-minute rest, HRV was measured for 7 minutes in a sitting position with a 2-channel (5-lead) electrocardiograph (Trillium 3000 model with a sampling rate of 256 Hz per channel; Forest Medical LLC, East Syracuse, New York). The electrocardiogram was digitally recorded, and the best 4-consecutive-minute interval was used for the HRV calculations. The standard deviation of normal-to-normal beat intervals (SDNN), high-frequency power (0.15–0.4 Hz), and low-frequency power (0.04–0.15 Hz) were calculated with a fast Fourier transform using Trillium 3000 PC Companion Software (Forest Medical LLC); the procedure conforms to established guidelines (the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996)). Detailed information has been published elsewhere (36).
Air pollution and weather data
Hourly weather measurements were recorded at Boston's Logan Airport monitoring site, about 5 miles (8 km) from the physical examination site, through the whole study period. Weather conditions recorded included hourly temperature, humidity, and wind speed. Hourly maximum and daily moving averages of temperature and apparent temperature were used as temperature indices in this study, with various averages of these indices, up to 20 days prior to the examination day. Apparent temperature (AT) was calculated using the following formula:
where Ta is air temperature and Td is dew-point temperature (37, 38). Ambient particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5) and ozone were measured at a stationary Environmental Protection Agency monitoring site using a tapered-element oscillating microbalance (model 1400A; Rupprecht & Pataschnick Company, East Greenbush, New York) (18) and an ultraviolet absorption ozone analyzer (model 400E; Teledyne Advanced Pollution Instrumentation, Inc., San Diego, California), respectively. Daily averages of PM2.5 and ozone were evaluated as potential effect modifiers.
Statistical analysis
Because our previous analyses found skewed distributions of HRV, we conducted log10 transformations of HRV measures to improve normality and stabilize the variance. The following covariates were chosen a priori for the analysis on the basis of previous studies: age, body mass index (weight (kg)/height (m)2), mean arterial pressure, fasting blood glucose concentration, cigarette smoking (never, former, or current smoker), alcohol consumption (≥2 drinks per day; yes/no), use of β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, examination room temperature, and season (18, 22, 39, 40). Because high temperatures in the warm season and low temperatures in the cold season are associated with increased cardiovascular mortality (4, 41, 42), we divided the data into 2 portions, the warm season (May–September) and the cold season (November–March), excluding April and October. During each season, we assumed that relations between temperatures and HRV were linear (4). Because of the repeated measurements of HRV in our data, we fitted a linear mixed-effects model in R software (version 2.9.1; R Foundation for Statistical Computing, Vienna, Austria) that applied a random intercept for each subject in order to explore associations between ambient temperature and HRV. We separately estimated associations between HRV (SDNN, high-frequency power, and low-frequency power) and different-day moving averages of temperature measures up to 20 days (1-hour maximal and daily temperature and apparent temperature) for both the cold and warm seasons. We adjusted for daily relative humidity while assessing the effect of air temperature but not while assessing apparent temperature, because humidity is part of the calculation of apparent temperature (37, 38).
We first estimated the main effects of temperature on HRV and then examined potential effect modification by ozone and PM2.5 by categorizing the pollutant data into 2 levels using their median values as cutpoints. We selected these pollutants because a large body of literature shows that they are associated with health outcomes and can interact with temperature to influence cardiovascular outcomes, as described previously. To evaluate effect modification by these pollutants, we included a term for the main effect of the categorically defined pollutant and added a term for interaction between temperature and the pollutant. We then linearly combined the coefficients of the reference group and the interaction term to calculate the associations of temperature (using the averaging time that showed the strongest association) across pollution levels and estimated the variance via the sum of corresponding variances and twice the covariance of the 2 terms (22, 40). Similarly to this method, we can obtain the combined coefficients and variance by fitting the model excluding the main term of the temperature (43).
As a sensitivity analysis, we separately examined associations between ambient temperature and HRV using 2 data subsets—the first- and last-visit data subsets—by fitting linear regression models adjusting for the same covariates included in the above main mixed-effects model because the small number of observations for each subject limited any analysis of intrasubject variability. To examine whether the relation between temperature and HRV was linear, we fitted penalized spline models using a generalized additive mixed model (GAMM in R, version 2.9.1) in the warm season. We conducted sensitivity analyses to examine effect modification by the pollutants using 2- and 5-day moving averages of particles and the 2-day moving average of ozone, in addition to the daily average. In previous research in this cohort, the longer-term average metric of particles was more strongly associated with HRV than the shorter-term metric, whereas ozone had a shorter-term effect (44). Finally, to explore whether the confidence intervals we calculated for the high-PM2.5 and high-ozone results, which accounted for covariance between the main effect and interaction terms, differed from results obtained using a stratified approach, we also divided the whole data set in the warm season by levels of PM2.5 and ozone to fit the models.
RESULTS
The study population comprised 694 older men who were predominantly non-Hispanic white (97%). Table 1 presents basic statistics for the HRV outcomes, exposures of interest, and covariates. At their first study visit, the average age of participants was 73.3 years (standard deviation, 6.7). Median values for daily apparent temperature, daily mean temperature, daily average PM2.5, and daily average ozone were 10.55°C, 12.25°C, 13.2 μg/m3, and 17.94 ppb at all visits and 19.25°C, 19.37°C, 12.9 μg/m3, and 22.4 ppb at the warm-season visits, respectively. Although outdoor temperatures varied considerably (mean = 11.1°C; standard deviation, 9.8), the temperature of the examination room was relatively constant (mean = 23.9°C; standard deviation, 1.6).
Table 1.
Distribution of Demographic, Health, and Environmental Factors Among Participants With Heart Rate Variability Measured Between November 2000 and December 2008, Normative Aging Study, Boston, Massachusetts
| Variable | First Study Visit (n = 694) |
All Study Visits (n = 1,353) |
||||
| Mean (SD) | No. of Participants | % | Mean (SD) | No. of Participants | % | |
| Age, years | 73.26 (6.68) | 74.71 (6.67) | ||||
| Body mass indexa | 28.20 (4.11) | 28.11 (4.24) | ||||
| Mean arterial pressure, mm Hg | 92.73 (10.67) | 89.88 (10.9) | ||||
| Fasting blood glucose concentration, mg/dL | 107.16 (26.49) | 106.56 (23.41) | ||||
| Season | ||||||
| Spring | 172 | 24.78 | 331 | 24.46 | ||
| Summer | 166 | 23.92 | 335 | 24.76 | ||
| Fall | 212 | 30.55 | 417 | 30.82 | ||
| Winter | 144 | 20.75 | 270 | 19.96 | ||
| Smoking status | ||||||
| Never smoker | 200 | 28.82 | 407 | 30.08 | ||
| Current smoker | 31 | 4.47 | 46 | 3.40 | ||
| Former smoker | 463 | 66.71 | 900 | 66.52 | ||
| Alcohol consumption of ≥2 drinks/day | 130 | 18.98 | 243 | 18.08 | ||
| Use of β-blockers | 283 | 40.73 | 556 | 41.09 | ||
| Use of calcium channel blockers | 149 | 21.47 | 271 | 20.03 | ||
| Use of angiotensin-converting enzyme inhibitors | 202 | 29.11 | 419 | 30.97 | ||
| Log10 SDNN, m/second2 | 1.59 (0.32) | 1.59 (0.34) | ||||
| Log10 high-frequency power, m/second2 | 2.08 (0.83) | 2.12 (0.85) | ||||
| Log10 low-frequency power, m/second2 | 2.10 (0.65) | 2.10 (0.69) | ||||
| Relative humidity, % | 67.9 (15.5) | 66.9 (15.3) | ||||
| Examination room temperature, °C | 24.23 (1.79) | 23.87 (1.62) | ||||
| 48-hour moving average of PM2.5, μg/m3 | 16.89 (10.54) | 13.32 (7.21) | ||||
| 4-hour moving average of ozone, ppb | 18.26 (11.54) | 15.39 (9.73) | ||||
| 1-hour maximal temperature, °C | 15.46 (10.56) | 15.27 (10.56) | ||||
| Daily mean temperature, °C | 11.18 (9.74) | 11.13 (9.77) | ||||
| 1-hour maximal apparent temperature, °C | 16.03 (9.62) | 15.90 (9.65) | ||||
| Daily apparent temperature, °C | 11.98 (8.83) | 11.94 (8.88) | ||||
Abbreviations: PM2.5, particulate matter ≤2.5 μm in aerodynamic diameter; SD, standard deviation; SDNN, standard deviation of normal-to-normal beat intervals.
Weight (kg)/height (m)2.
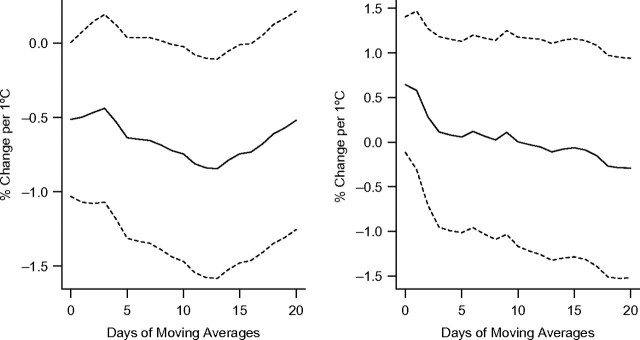
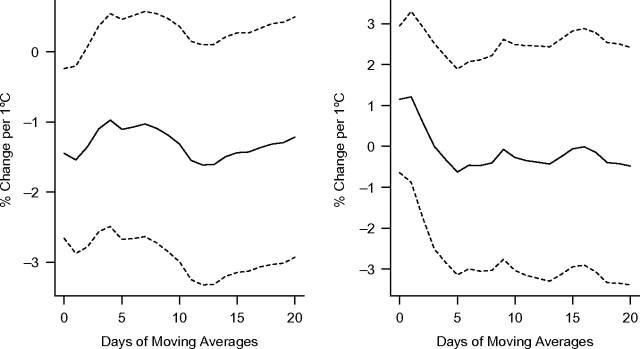
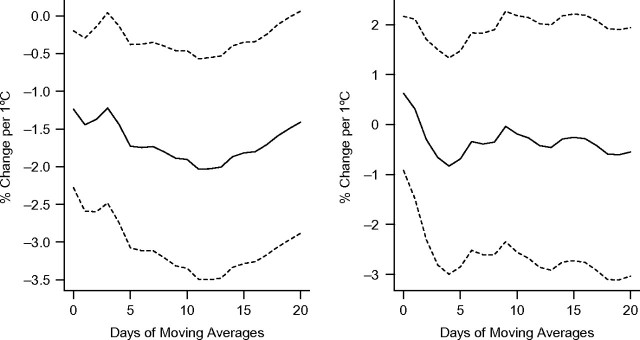
Figure 1, Figure 2, and Figure 3 show associations between apparent temperature and HRV using different-day moving averages up to 20 days in the warm and cold seasons. We found no evidence that temperature measures were associated with HRV during the cold season. Therefore, we did not further examine effect modification by pollution in that season. In the warm season, all HRV measures were significantly associated with the different temperature measures (1-hour minimal, maximal, and daily temperature and apparent temperature) (P < 0.05). Two-week moving averages of daily temperature were more strongly associated with decreased SDNN and low-frequency power. Averages of the current and previous days' temperatures were more strongly associated with decreased high-frequency power. Overall, temperature was more strongly associated with low-frequency power than SDNN and high-frequency power (see Figures 1–3 and Web Figure 1 (http://aje.oxfordjournals.org/)). Effect estimates for the association of 1-hour maximum temperature and apparent temperature, respectively, with HRV were very similar (results not presented here).
Figure 1.
Percent change in the standard deviation of normal-to-normal beat intervals per 1°C increase in apparent temperature at different daily moving averages in the warm (left) and cold (right) seasons, Normative Aging Study, Boston, Massachusetts, 2000–2008. Results were adjusted for age, body mass index, mean arterial pressure, fasting blood glucose, cigarette smoking (never, former, or current smoker), alcohol consumption (≥2 drinks per day; yes/no), use of β-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors, examination room temperature, and season. Dashed lines, 95% confidence interval.
Figure 2.
Percent change in high-frequency power per 1°C increase in apparent temperature at different daily moving averages in the warm (left) and cold (right) seasons, Normative Aging Study, Boston, Massachusetts, 2000–2008. Results were adjusted for age, body mass index, mean arterial pressure, fasting blood glucose, cigarette smoking (never, former, or current smoker), alcohol consumption (≥2 drinks per day; yes/no), use of β-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors, examination room temperature, and season. Dashed lines, 95% confidence interval.
Figure 3.
Percent change in low-frequency power per 1°C increase in apparent temperature at different daily moving averages in the warm (left) and cold (right) seasons, Normative Aging Study, Boston, Massachusetts, 2000–2008. Results were adjusted for age, body mass index, mean arterial pressure, fasting blood glucose, cigarette smoking (never, former, or current smoker), alcohol consumption (≥2 drinks per day; yes/no), use of β-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors, examination room temperature, and season. Dashed lines, 95% confidence interval.
We then examined potential effect modification of the association of temperature with HRV by the 2-level categories of air pollution (daily ozone and PM2.5), divided at their median values (>22.3 ppb vs. ≤22.3 ppb and >12.9 μg/m3 vs. ≤12.9 μg/m3, respectively). Analyses were limited to the warm season. We used 13-day moving averages of 1-hour and daily temperatures to examine effect modification by pollution for SDNN and low-frequency power and 1-day moving averages of temperature for high-frequency power because those averaging periods showed the strongest effects on HRV in the main-effect analysis. Ozone significantly modified associations of temperature (1-hour and daily temperature and apparent temperature) with SDNN and low-frequency power at the 0.05 significance level, but it did not modify associations for high-frequency power. We found no evidence that PM2.5 modified associations between temperature and HRV (Figure 4).
Figure 4.
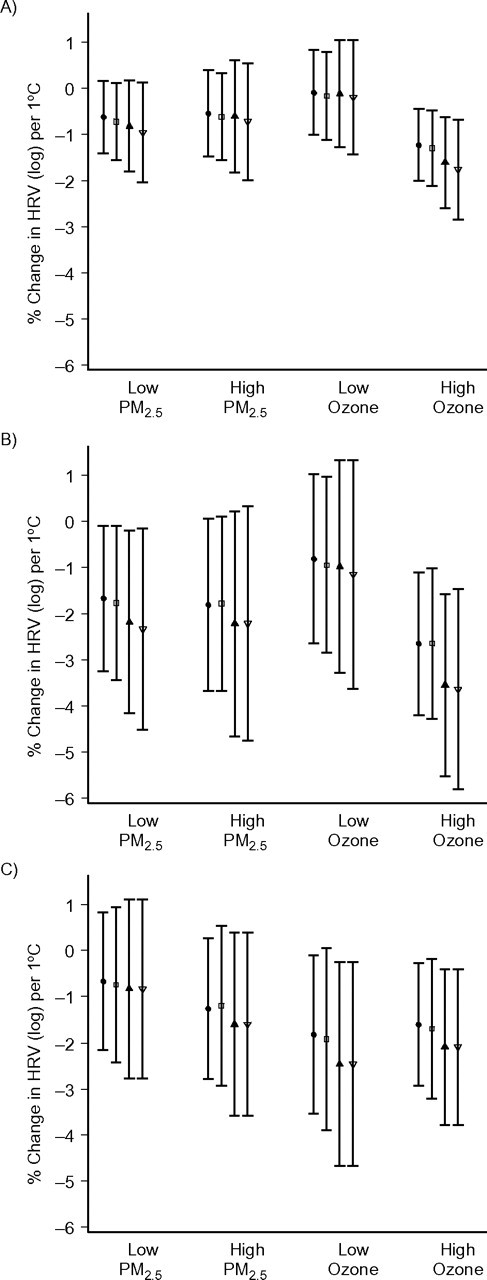
Percent change in heart rate variability (HRV) per 1°C increase in temperature according to levels of air pollutants (particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5) and ozone) during the warm season, Normative Aging Study, Boston, Massachusetts, 2000–2008. Results were adjusted for age, body mass index, mean arterial pressure, fasting blood glucose, cigarette smoking (never, former, or current smoker), alcohol consumption (≥2 drinks per day; yes/no), use of β-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors, examination room temperature, and season. Thirteen-day moving averages of 1-hour maximal and daily temperature and apparent temperature were used to examine effect modification by pollutants for the standard deviation of normal-to-normal beat intervals (panel A) and low-frequency power (panel B), and 1-day moving averages of 1-hour maximal and daily temperature and apparent temperature were used to examine effect modification by pollutants for high-frequency power (panel C). The circles, boxes, filled triangles, and open triangles indicate the point estimates for daily apparent temperature, 1-hour maximal apparent temperature, daily mean temperature, and 1-hour maximal temperature, respectively. Low PM2.5, ≤12.9 μg/m3; high PM2.5, >12.9 μg/m3; low ozone, ≤22.3 ppb; high ozone, >22.3 ppb. Bars, 95% confidence interval.
In the sensitivity analyses, we found that HRV (SDNN, high-frequency power, and low-frequency power) was significantly associated with temperature or apparent temperature at different-day moving averages in the warm season but not in the cold season, when we used the first-visit data subset. We also found the similar links between HRV and temperature using the last-visit data subset, but such estimated associations were not significant.
We did not find any evidence that the slopes of associations changed with elevation of temperature when we fitted penalized spline models, and a linear relation minimized the cross-validation score (Web Figure 2). We found no evidence of effect modification by air pollution using 2- and 5-day moving averages of PM2.5. The 2-day moving average of ozone had the same general pattern of effect modification as the daily average. Finally, for the model fitted to data sets stratified by levels of ozone and PM2.5, the confidence intervals were similar to those of the interactive or joint models.
DISCUSSION
Ambient temperature was associated with a decrease in HRV in this population of elderly males during the warm season, but not during the cold season. The association of temperature with high-frequency power was immediate, whereas reduced low-frequency power was associated more with the 2-week average temperature. In addition, associations of ambient temperature with all HRV parameters except high-frequency power were higher when ambient ozone was higher than when ambient ozone was lower.
To our knowledge, this is the first epidemiologic study of the relation between ambient temperatures and HRV, as well as modification of this relation by air pollution. Our findings of an association between high temperatures and HRV suggest that mechanistic pathways through which high temperatures affect cardiovascular function may include the autonomic nervous system, and these effects may be amplified in the presence of high ozone levels. Given that global warming is likely to increase both heat waves and ozone formation, such an interaction may be important for public health.
Several experimental studies have examined the relations between HRV and cold and heat exposures (27–31), but so far few epidemiologic studies have been conducted in community-dwelling populations. Low-frequency power peak is related to sympathetic and parasympathetic activity, whereas the high-frequency power peak is related to vagal activity (27, 45, 46). Yamamoto et al. (30) reported that after 6 healthy males were exposed to temperatures of 35°C for half an hour, their high-frequency power percentage (normalized high-frequency power) significantly decreased and the ratio of low-frequency power to high-frequency power significantly increased. However, Brenner et al. (47) conducted a similar experiment and found that heat exposure alone did not significantly change the autonomic balance as analyzed by HRV. Consistent with the findings of Yamamoto et al. (30), we found that high temperature was inversely associated with all HRV measures (SDNN, low-frequency power, and high-frequency power) in the present elderly population, suggesting an activation of sympathetic activity and a withdrawal of vagal activity. Moreover, we found stronger effects of longer-term (e.g., 2-week) exposure on low-frequency power. This suggests that temperature may have a different time course in its effects on the parasympathetic nervous system versus the sympathetic nervous system.
The nature and magnitude of associations between temperature and mortality/morbidity have been increasingly recognized (1, 4, 42, 48). Both hypothermia and hyperthermia are generally linked to human cardiovascular morbidity and mortality (4, 42). Seasonally stratified models have often found linear dose-response relations in each season (4). Our study found that ambient temperature was associated with HRV during the warm season but not during the cold season.
Our findings of no association between cold temperature and HRV differ from those of previous experimental studies of cold temperature and subclinical indicators of cardiovascular function. For example, Schneider et al. (26) found that decreased air temperature was associated with an increase in both C-reactive protein and interleukin-6. In their experimental study, Okamoto-Mizuno et al. (29) reported that HRV was related to low ambient temperature during sleep in humans. In the Greater Boston area, the assumption is that most families use heating and residential outdoor activities are very rare during the winter. Therefore, ambient temperature would be a poor surrogate for real temperature exposure of the local residents in the winter, and thus estimates based on ambient temperature indices might be severely biased because ambient temperature and indoor temperature are not correlated during the cold season.
In recent years, several groups of investigators have reported that temperature and air pollution interact to affect the occurrence of cardiovascular disease (6, 8, 32–34). This study found some evidence that temperature and ozone interact synergistically to affect HRV, but not obviously so for particulate matter. We found only slight sensitivity in our effect modification results to the choice of pollutant averaging times.
Biologic mechanisms for the observed effect modification of temperature associations by pollution levels are very complex, however, because causal pathways for both risks are unclear and the biologic and toxicologic reactions are complicated. Many studies have shown that exposure to ozone has significant biologic effects on the respiratory system, including acute and chronic effects, and further influence cardiovascular system functions (49, 50). Significant ambient temperature alterations can produce physiologic and psychological stress and thus change personal physiologic responses to toxic agents (51). The body may be able to maintain a stable core temperature in the face of temperature changes, but this may occur at the expense of activation of thermoeffectors and physiologic stress, which can alter the physiologic response to toxicant agents in many ways (51). For instance, activation of the thermoeffector system can have direct or indirect effects on the entry of toxicants into the body.
Additionally, higher ambient temperatures can alter sensitivities of toxicants in the body via complex biologic mechanisms. For example, the in vivo toxicity of most chemicals is exacerbated with rising body temperature, which decreases the 50% lethal dose of many toxicants observed in experimental studies (51–53). In addition, high ambient temperature can alter the generation, dispersion, and degeneration of toxicants, including ozone (54). The role of environmental stress in the physiologic response to chemical toxicants has been discussed elsewhere (51, 53). Our results provide new evidence that simultaneous exposures to high temperature and high ozone levels may lead to synergistic effects, especially on cardiovascular disease.
In this study, we examined associations between temperature and HRV, a measure of autonomic nervous system function, in an elderly population. Ambient temperature was associated with HRV, and such associations were significantly modified by ambient ozone. Limitations of this work include limited generalizability to other populations, since the Normative Aging Study cohort is an older, exclusively male, and predominantly non-Hispanic white population. In addition, weather conditions and air pollutant composition and concentration in Greater Boston differ from those in many other places. Therefore, further research on these issues needs to be conducted in other locations.
In conclusion, ambient temperature was associated with HRV during the warm season (May–September) but not during the cold season (November–March) in an elderly male study population in Greater Boston during the period 2000–2008. We also found that such associations were significantly modified by ambient ozone exposure but not by ambient particulate matter. The findings were consistent with those of other epidemiologic and experimental studies, suggesting that ambient temperature and ozone might interact to affect cardiovascular events through the autonomic nervous system.
Supplementary Material
Acknowledgments
Author affiliations: Exposure, Epidemiology, and Risk Program, Harvard School of Public Health, Boston, Massachusetts (Cizao Ren, Joel Schwartz); Departments of Environmental Health Sciences and Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Sung Kyun Park, Marie S. O'Neill); Veterans Affairs Boston Healthcare System, Boston, Massachusetts (David Sparrow, Pantel Vokonas); and Department of Medicine, School of Medicine, Boston University, Boston, Massachusetts (David Sparrow, Pantel Vokonas).
This study was supported by Environmental Protection Agency grants R827353 and R832416 and National Institute of Environmental Health Sciences grants RO1-ES015172, ES00002, RO1-ES016932, and PO1-ES008925. Dr. Sung Kyun Park was supported by grant KO1-ES016587 from the National Institute of Environmental Health Sciences. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the US Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Conflict of interest: none declared.
Glossary
Abbreviations
- HRV
heart rate variability
- PM2.5
particulate matter ≤2.5 μm in aerodynamic diameter
- SDNN
standard deviation of normal-to-normal beat intervals
References
- 1.Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002;24(2):190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- 2.Martens WJ. Climate change, thermal stress and mortality changes. Soc Sci Med. 1998;46(3):331–344. doi: 10.1016/s0277-9536(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 3.Patz JA, Engelberg D, Last J. The effects of changing weather on public health. Annu Rev Public Health. 2000;21:271–307. doi: 10.1146/annurev.publhealth.21.1.271. [DOI] [PubMed] [Google Scholar]
- 4.Zanobetti A, Schwartz J. Temperature and mortality in nine US cities. Epidemiology. 2008;19(4):563–570. doi: 10.1097/EDE.0b013e31816d652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina-Ramón M, Zanobetti A, Cavanagh DP, et al. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006;114(9):1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren C, Williams GM, Mengersen K, et al. Temperature enhanced effects of ozone on cardiovascular mortality in 95 large US communities, 1987–2000—assessment using the NMMAPS data. Arch Environ Occup Health. 2009;64(3):177–184. doi: 10.1080/19338240903240749. [DOI] [PubMed] [Google Scholar]
- 7.Basu R, Feng WY, Ostro BD. Characterizing temperature and mortality in nine California counties. Epidemiology. 2008;19(1):138–145. doi: 10.1097/EDE.0b013e31815c1da7. [DOI] [PubMed] [Google Scholar]
- 8.Ren C, Williams GM, Tong S. Does particulate matter modify the association between temperature and cardiorespiratory diseases? Environ Health Perspect. 2006;114(11):1690–1696. doi: 10.1289/ehp.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell ML, McDermott A, Zeger SL, et al. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292(19):2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gryparis A, Forsberg B, Katsouyanni K, et al. Acute effects of ozone on mortality from the “Air Pollution and Health: a European Approach” project. Am J Respir Crit Care Med. 2004;170(10):1080–1087. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz J. How sensitive is the association between ozone and daily deaths to control for temperature? Am J Respir Crit Care Med. 2005;171(6):627–631. doi: 10.1164/rccm.200407-933OC. [DOI] [PubMed] [Google Scholar]
- 14.Bigger JT, Jr, Fleiss JL, Steinman RC, et al. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Bigger JT, Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 16.Zuanetti G, Neilson JM, Latini R, et al. Prognostic significance of heart rate variability in post–myocardial infarction patients in the fibrinolytic era. The GISSI-2 results. Circulation. 1996;94(3):432–436. doi: 10.1161/01.cir.94.3.432. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz J, Park SK, O'Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172(12):1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson K, Stone V, Seaton A, et al. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utell MJ, Frampton MW, Zareba W, et al. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002;14(12):1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- 22.Ren C, Park SK, Vokonas PS, et al. Air pollution and homocysteine: more evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology. 2010;21(2):198–206. doi: 10.1097/EDE.0b013e3181cc8bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110(8):749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam T, McConnell R, Gauderman WJ, et al. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 2008;177(4):388–395. doi: 10.1164/rccm.200706-863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SK, O'Neill MS, Wright RO, et al. HFE genotype, particulate air pollution, and heart rate variability: a gene-environment interaction. Circulation. 2006;114(25):2798–2805. doi: 10.1161/CIRCULATIONAHA.106.643197. [DOI] [PubMed] [Google Scholar]
- 26.Schneider A, Panagiotakos D, Picciotto S, et al. Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology. 2008;19(3):391–400. doi: 10.1097/EDE.0b013e31816a4325. [DOI] [PubMed] [Google Scholar]
- 27.Yao Y, Lian Z, Liu W, et al. Heart rate variation and electroencephalograph—the potential physiological factors for thermal comfort study. Indoor Air. 2009;19(2):93–101. doi: 10.1111/j.1600-0668.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Lian Z, Liu Y. Heart rate variability at different thermal comfort levels. Eur J Appl Physiol. 2008;103(3):361–366. doi: 10.1007/s00421-008-0718-6. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto-Mizuno K, Tsuzuki K, Mizuno K, et al. Effects of low ambient temperature on heart rate variability during sleep in humans. Eur J Appl Physiol. 2009;105(2):191–197. doi: 10.1007/s00421-008-0889-1. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S, Iwamoto M, Inoue M, et al. Evaluation of the effect of heat exposure on the autonomic nervous system by heart rate variability and urinary catecholamines. J Occup Health. 2007;49(3):199–204. doi: 10.1539/joh.49.199. [DOI] [PubMed] [Google Scholar]
- 31.Bruce-Low SS, Cotterrell D, Jones GE. Heart rate variability during high ambient heat exposure. Aviat Space Environ Med. 2006;77(9):915–920. [PubMed] [Google Scholar]
- 32.Ren C, Williams GM, Morawska L, et al. Ozone modifies associations between temperature and cardiovascular mortality: analysis of the NMMAPS data. Occup Environ Med. 2008;65(4):255–260. doi: 10.1136/oem.2007.033878. [DOI] [PubMed] [Google Scholar]
- 33.Stafoggia M, Schwartz J, Forastiere F, et al. Does temperature modify the association between air pollution and mortality? A multicity case-crossover analysis in Italy. Am J Epidemiol. 2008;167(12):1476–1485. doi: 10.1093/aje/kwn074. [DOI] [PubMed] [Google Scholar]
- 34.Yi O, Hong YC, Kim H. Seasonal effect of PM10 concentrations on mortality and morbidity in Seoul, Korea: a temperature-matched case-crossover analysis. Environ Res. 2010;110(1):89–95. doi: 10.1016/j.envres.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Bell B, Rose C, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Aging Human Develop. 1972;3(1):4–17. [Google Scholar]
- 36.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 37.Steadman RG. The assessment of sultriness. Part II: effects of wind, extra radiation and barometric pressure on apparent temperature. J Appl Meteorol. 1979;18(5):874–885. [Google Scholar]
- 38.Kalkstein LS, Valimont KM, Valimont, et al. An evaluation of summer discomfort in the United States using a relative climatological index. Bull Am Meteorol Soc. 1986;67(7):842–848. [Google Scholar]
- 39.Baccarelli A, Cassano PA, Litonjua A, et al. Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008;117(14):1802–1809. doi: 10.1161/CIRCULATIONAHA.107.726067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren C, Baccarelli A, Wilker E, et al. Lipid and endothelium-related genes, ambient particulate matter, and heart rate variability—the VA Normative Aging Study. J Epidemiol Community Health. 2010;64(1):49–56. doi: 10.1136/jech.2008.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 2002;110(9):859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curriero FC, Heiner KS, Samet JM, et al. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155(1):80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 43.Roberts S. Interactions between particulate air pollution and temperature in air pollution mortality time series studies. Environ Res. 2004;96(3):328–337. doi: 10.1016/j.envres.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Park SK, O'Neill MS, Vokonas PS, et al. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113(3):304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayers BM. Analysis of heart rate variability. Ergonomics. 1973;16(1):17–32. doi: 10.1080/00140137308924479. [DOI] [PubMed] [Google Scholar]
- 46.Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 47.Brenner IK, Thomas S, Shephard RJ. Spectral analysis of heart rate variability during heat exposure and repeated exercise. Eur J Appl Physiol Occup Physiol. 1997;76(2):145–156. doi: 10.1007/s004210050227. [DOI] [PubMed] [Google Scholar]
- 48.Baccini M, Biggeri A, Accetta G, et al. Heat effects on mortality in 15 European cities. Epidemiology. 2008;19(5):711–719. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- 49.Thurston G, Ito K. Epidemiological studies of ozone exposure effects. In: Holsgate ST, Samet JM, Koren HS, editors. Air Pollution and Health. New York, NY: Academic Press, Inc; 1999. pp. 485–510. [Google Scholar]
- 50.Jörres RA, Holz O, Zachgo W, et al. The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am J Respir Crit Care Med. 2000;161(6):1855–1861. doi: 10.1164/ajrccm.161.6.9908102. [DOI] [PubMed] [Google Scholar]
- 51.Gordon CJ. Role of environmental stress in the physiological response to chemical toxicants. Environ Res. 2003;92(1):1–7. doi: 10.1016/s0013-9351(02)00008-7. [DOI] [PubMed] [Google Scholar]
- 52.Gordon CJ, Mohler FS, Watkinson WP, et al. Temperature regulation in laboratory mammals following acute toxic insult. Toxicology. 1988;53(2-3):161–178. doi: 10.1016/0300-483x(88)90211-9. [DOI] [PubMed] [Google Scholar]
- 53.Doull J. The effect of physical environmental factors on drug response. Essays Toxicol. 1972;3(1):37–63. [Google Scholar]
- 54.Viswanatham PN, Krishna Murti CR. Effect of temperature and humidity on ecotoxicology of chemicals. In: Bourdeau P, Haines JA, Kkein W, et al., editors. Ecotoxicology and Climate. New York, NY: John Wiley & Sons, Inc; 1989. pp. 139–154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.