Abstract
Preliminary evidence suggests that daytime sleepiness may predate clinical diagnosis of Parkinson disease. The authors examined daytime napping and nighttime sleeping durations, reported in 1996–1997 by 220,934 US NIH-AARP Diet and Health Study participants, in relation to Parkinson disease diagnoses at 3 clinical stages: established (cases diagnosed before 1995, n = 267), recent (1995–1999, n = 396), and prediagnostic (2000 and after, n = 770). Odds ratios and 95% confidence intervals were derived from multivariate logistic regression models. Longer daytime napping was associated with higher odds of Parkinson disease at all 3 clinical stages: the odds ratios comparing long nappers (>1 hour/day) with nonnappers were 3.9 (95% confidence interval: 2.8, 5.6) for established cases, 2.2 (95% confidence interval: 1.7, 3.0) for recent cases, and 1.5 (95% confidence interval: 1.2, 1.9) for prediagnostic cases. Further control for health status or nighttime sleeping duration attenuated the association for established cases but made little difference for recent or prediagnostic cases. In the nighttime sleeping analysis, a clear U-shaped association with Parkinson disease was observed for established cases; however, this association was attenuated markedly for recent cases and disappeared for prediagnostic cases. This study supports the notion that daytime sleepiness, but not nighttime sleeping duration, is one of the early nonmotor symptoms of Parkinson disease.
Keywords: Parkinson disease, prospective studies, sleep
Parkinson disease is clinically diagnosed by the presence of motor dysfunctions including tremor at rest, rigidity, and bradykinesia. However, Parkinson disease patients also suffer from a variety of nonmotor symptoms, some of which may predate clinical onset of the disease and may be an integral part of the underlying pathogenesis (1–3). Recognizing and characterizing these nonmotor symptoms at different stages of the disease may eventually lead to earlier Parkinson disease diagnosis, better clinical management, and improvement of our understanding of the pathoetiology and progression of the disease (4). Atypical sleep habits such as excessive daytime sleepiness are common among Parkinson disease patients, which may be partially explained by usage of dopaminergic medications (5). It is unclear, however, whether Parkinson disease patients have atypical or altered sleep habits prior to their clinical diagnosis. Therefore, we examined the associations between durations of daytime napping or nighttime sleeping and Parkinson disease occurrence during 3 clinical periods (i.e., before, peri-, or after Parkinson disease diagnosis) in a large cohort of older men and women in the National Institutes of Health (NIH)-AARP Diet and Health Study.
MATERIALS AND METHODS
Study population and case identification
The cohort was established in the mid-1990s by the National Cancer Institute and recruited 566,402 members of AARP (formerly the American Association of Retired Persons), aged 50–71 years, from 6 states and 2 metropolitan areas of the United States (6). The baseline survey was conducted in 1995–1996, focusing on dietary habits (6). Between 1996 and 1997, 334,908 participants of the original cohort also answered a risk factor questionnaire to provide more details on their health behaviors, including numbers of hours of daytime napping and nighttime sleeping.
In 2004–2006, a follow-up questionnaire was mailed to all surviving participants of the original cohort to update their health status and to ascertain the occurrence of cancers and other major chronic diseases, including Parkinson disease. On the follow-up questionnaire, participants were asked whether they had ever been diagnosed by a physician as having Parkinson disease and the year of first diagnosis (before 1985, 1985–1994, 1995–1999, or 2000 and after). A total of 318,261 participants answered the follow-up questionnaire.
The base population of the current study therefore included 220,934 participants who answered both the risk factor survey in 1996–1997 and the follow-up survey in 2004–2006. Of these participants, 1,681 reported physician-diagnosed Parkinson disease on the 2004–2006 follow-up survey. We excluded 242 self-reports that were later found to be erroneous by the validation procedure described below and 6 additional cases for whom data on sleep habits were missing. Of the eligible participants who did not report a Parkinson disease diagnosis, we excluded 4,916 missing data on Parkinson disease status and 452 missing data on sleep habits. The final analyses included 1,433 Parkinson disease cases and 213,885 individuals without Parkinson disease. In reference to the exposure assessment in 1996–1997, these cases included 267 that were established (diagnosed before 1995), 396 that were recent (diagnosed between 1995 and 1999), and 770 that were prediagnostic (diagnosed in 2000 and after).
Participants consented to the study by returning survey questionnaires. The study protocol was approved by the institutional review board of the National Institute of Environmental Health Sciences and the Special Studies Institutional Review Board of the National Cancer Institute.
We started to validate the diagnoses of surviving Parkinson disease cases in this cohort in 2007. With permission from cases who self-reported Parkinson disease, we asked their treating neurologists to complete a Parkinson disease diagnostic form and to send us a copy of the patients’ medical records. The medical records were subsequently reviewed by a movement disorder specialist, and a self-report of clinical Parkinson disease diagnosis was accepted if the treating neurologist confirmed the diagnosis or if medical record review by the movement disorder specialist showed at least 2 of the 4 cardinal Parkinson disease signs (with one being rest tremor or bradykinesia), a progressive course, and the absence of unresponsiveness to dopaminergic treatment or other features suggesting an alternative diagnosis. This protocol has been successfully implemented in other large cohorts (7, 8). Of the 1,069 responses from physicians and medical record review we received to date, 940 (87.9%) Parkinson disease diagnoses were confirmed. The confirmation rate was similar across all 3 groups of patients: 91.3% for established cases, 88.0% for recent cases, and 87.2% for prediagnostic cases.
Exposure assessment
At the risk factor survey in 1996–1997, participants were asked how many hours were spent napping during daytime or sleeping at nighttime in a typical 24-hour period over the past 12 months. Five choices were available for the daytime napping question (hours): none, <1, 1–2, 3–4, and ≥5. For nighttime sleeping, 4 categories were provided (hours): <5, 5–6, 7–8, and ≥9. The risk factor survey also asked participants to report how often they participated in moderate to vigorous physical activities in the past 10 years, providing 6 categories (never, rarely, <1, 1–3, 4–7, and >7 hours/week). At the dietary survey in 1995–1996, participants were asked whether they had ever smoked more than 100 cigarettes during their lifetime and, for ever smokers, the typical amount of smoking, current smoking status, and number of years since last smoking. The dietary survey also collected information on date of birth, sex, race, self-evaluated general health status, and consumption of coffee and other caffeinated drinks (6). Unlike other covariates, information on depression was not collected until the follow-up survey in 2004–2006, where physician-diagnosed depression was asked about in the same format as Parkinson disease (none; year of first diagnosis: before 1985, 1985–1994, 1995–1999, 2000 and after).
Statistical analysis
We used multivariate logistic regression models to calculate odds ratios and 95% confidence intervals. Because sleep habits were asked about in 1996–1997, the exposure information was collected after diagnosis for established cases, within 2 years before or after the diagnosis for recent cases, and at least 3–4 years prior to the diagnosis for prediagnostic cases.
Using participants reporting no napping as the reference, we examined the relation between daytime napping and Parkinson disease occurrence for all 3 case groups. In these analyses, we combined participants with ≥1 hour of napping because few participants reported more than 2 hours of napping. In the analysis for nighttime sleeping, we used 7–8 hours of sleeping as the reference. Covariates included age (in 5-year age groups), sex, race (non-Hispanic Caucasian vs. others), smoking status (never smoker, past smoker (years since last smoking: ≥35, 30–34, 20–29, 10–19, 5–9, and 1–4), and current smoker (cigarettes per day: 1–10, 11–20, >20)), daily caffeine intake (mg/day, quintiles), and moderate to vigorous physical activity (never, rarely, <1, 1–3, 4–7, and ≥7 hours/week). We additionally adjusted for self-reported health status (excellent, very good, good, fair, or poor) to examine the potential impact of general health status on the analyses. Depression is associated with both sleeping behaviors (9) and the occurrence of Parkinson disease (10, 11). However, we did not have information on depression at the time that sleeping and napping durations were asked about. We therefore conducted additional sensitivity analyses to further adjust for ever presence of depression until the follow-up survey.
The primary analyses were first conducted by including all participants and then by sex, age (years: ≤62 vs. >62), smoking status (never vs. ever smoker), and caffeine intake (above or below the median). In the analysis of napping, we tested the statistical significance for a linear trend by assigning a value to each category of the variable napping (0 for no napping, 0.5 for <1 hour, and 1.5 for ≥1 hour) and including it as a continuous variable in the regression model. To further examine whether the relation between napping and Parkinson disease was influenced by atypical nighttime sleeping, we conducted analysis by further controlling for nighttime sleeping duration or limited the analysis to participants who reported the typical 7–8 hours of sleeping. All statistical analysis was conducted by using SAS software (version 9.2; SAS Institute, Inc., Cary, North Carolina), and the significance tests were 2-tailed with α = 0.05.
RESULTS
Table 1 shows population characteristics of Parkinson disease patients diagnosed at different time periods and of those without the disease. As expected, Parkinson disease patients were older than those without Parkinson disease; they also were more likely to be men and Caucasians but were less likely to be current smokers, consumed less caffeine, and were less physically active. Parkinson disease patients were more likely to report daytime napping and less optimal (excellent or very good) health. They were also more likely to report an ever diagnosis of depression at the follow-up survey. Refer to Web Table 1 (posted on the Journal’s Web site (http://aje.oupjournals.org/)) for population characteristics by hours of daytime napping and nighttime sleeping.
Table 1.
Population Characteristicsa of Parkinson Disease Cases and Individuals Without Parkinson Disease, NIH-AARP Diet and Health Study
| Parkinson Disease Caseb |
No Parkinson Disease (n = 213,885) |
|||||||
| Established (n = 267) |
Recent (n = 396) |
Prediagnostic (n = 770) |
||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Age, years | 64.4 (4.8) | 64.9 (5.0) | 65.1 (4.7) | 62.6 (5.3) | ||||
| Male sex | 77.5 | 74.5 | 73.9 | 57.7 | ||||
| Race | ||||||||
| Caucasian | 95.5 | 96.5 | 94.8 | 93.2 | ||||
| Others | 3.4 | 2.8 | 4.3 | 5.8 | ||||
| Missing | 1.1 | 0.8 | 0.9 | 1.0 | ||||
| Cigarette smoking | ||||||||
| Never smoker | 44.6 | 46.0 | 40.1 | 38.6 | ||||
| Past smoker | 49.1 | 48.5 | 53.8 | 51.0 | ||||
| Current smoker | 3.8 | 4.8 | 4.7 | 9.3 | ||||
| Missing | 2.6 | 0.8 | 1.4 | 1.1 | ||||
| Daily caffeine intake, mg | 234.1 (309.7) | 311.9 (345.9) | 315.5 (336.2) | 358.9 (367.6) | ||||
| Physical activity, hours/week | ||||||||
| Never or rare | 15.0 | 11.4 | 14.8 | 12.9 | ||||
| <1 | 12.0 | 8.6 | 11.7 | 10.1 | ||||
| 1–3 | 30.3 | 31.1 | 25.3 | 25.2 | ||||
| 4–7 | 25.8 | 31.6 | 26.9 | 26.4 | ||||
| >7 | 16.1 | 16.4 | 20.4 | 24.4 | ||||
| Missing | 0.8 | 1.0 | 0.9 | 1.1 | ||||
| General health status | ||||||||
| Excellent | 4.5 | 15.7 | 14.8 | 19.6 | ||||
| Very good | 14.6 | 31.8 | 37.4 | 37.8 | ||||
| Good | 38.6 | 38.6 | 33.3 | 32.8 | ||||
| Fair | 33.0 | 9.9 | 11.6 | 7.9 | ||||
| Poor | 6.0 | 2.3 | 1.2 | 0.8 | ||||
| Missing | 3.4 | 1.8 | 1.8 | 1.2 | ||||
| Depression | ||||||||
| No | 71.2 | 71.5 | 78.2 | 82.0 | ||||
| Yes | 12.7 | 14.7 | 11.6 | 9.5 | ||||
| Missing | 16.1 | 13.9 | 10.3 | 8.6 | ||||
| Hours of daytime napping/day | ||||||||
| 0 | 28.8 | 41.7 | 41.8 | 52.0 | ||||
| <1 | 50.6 | 41.4 | 45.7 | 39.7 | ||||
| ≥1 | 20.2 | 16.4 | 12.2 | 8.0 | ||||
| Missing | 0.4 | 0.5 | 0.3 | 0.3 | ||||
| Hours of nighttime sleeping/day | ||||||||
| <5 | 7.5 | 2.0 | 1.6 | 2.5 | ||||
| 5–6 | 41.6 | 22.7 | 30.0 | 30.9 | ||||
| 7–8 | 46.8 | 69.7 | 64.2 | 63.2 | ||||
| ≥9 | 4.1 | 5.6 | 4.2 | 3.3 | ||||
| Missing | 0.0 | 0.0 | 0.1 | 0.1 | ||||
Abbreviations: NIH, National Institutes of Health; SD, standard deviation.
Sex, race, smoking status, daily caffeine intake, and general health status were derived from the dietary survey (1995–1996); age, physical activity, and napping/sleeping duration were obtained from the risk factor survey (1996–1997); and depression was ascertained from the follow-up survey (2004–2006).
Established cases: diagnosed before 1995; recent cases: diagnosed in 1995–1999; prediagnostic cases: diagnosed in 2000 and after.
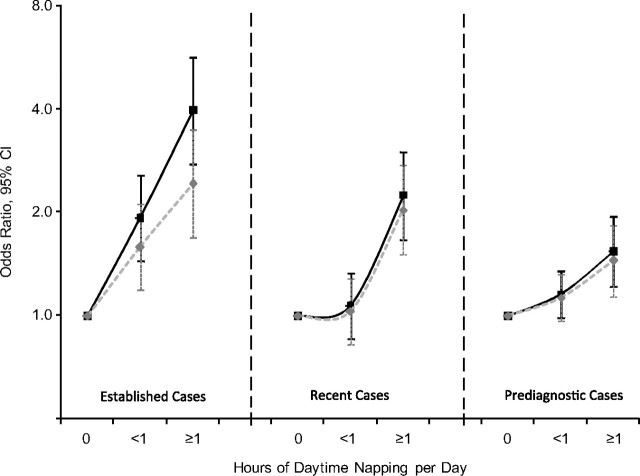
Longer daytime napping was associated with higher odds of having a Parkinson disease diagnosis at all 3 time periods (Figure 1). The association was strongest for established cases diagnosed before 1995 (multivariate odds ratio comparing ≥1 hour with no napping = 3.9, 95% confidence interval: 2.8, 5.6; P for trend <0.0001), followed by recent cases diagnosed in 1995–1999 (corresponding odds ratio = 2.2, 95% confidence interval: 1.7, 3.0; P for trend <0.0001) and prediagnostic cases diagnosed in 2000 and after (corresponding odds ratio = 1.5, 95% confidence interval: 1.2, 1.9; P for trend = 0.0003). Additionally adjusting for self-reported health status (Figure 1) or hours of nighttime sleeping (Table 2), or limiting the analysis to participants who reported 7–8 hours of sleep (Table 2), modestly attenuated the association for established cases but made little difference for recent or prediagnostic cases. Further adjusting for depression up to the follow-up survey barely changed the results. For example, on the basis of the fully adjusted model shown in Figure 1, the odds ratios comparing ≥1 hour with no napping with additional adjustment for depression were 2.4 (95% confidence interval: 1.6, 3.4; P for trend <0.0001) for established cases, 1.9 (95% confidence interval: 1.4, 2.6; P for trend <0.0001) for recent cases, and 1.4 (95% confidence interval: 1.1, 1.8; P for trend = 0.0043) for prediagnostic cases. Stratified analyses according to subgroups of age, sex, smoking, and caffeine intake generally produced similar results despite lower statistical power (data not shown).
Figure 1.
Numbers of hours of daytime napping in relation to Parkinson disease diagnosis at different clinical stages for NIH-AARP Diet and Health Study participants, 1996–2006. Black lines: adjusted for age, sex, race, physical activity, smoking status, and caffeine intake; grey lines: also adjusted for self-reported health status. All P for trend < 0.0001 except for prediagnostic cases in the basic model (P = 0.0003) and with additional adjustment for self-reported health status (P = 0.0022). Established cases: diagnosed before 1995; recent cases, diagnosed in 1995–1999; prediagnostic cases: diagnosed in 2000 and after. Actual values for odds ratios and 95% confidence intervals are provided in Web Table 2 (which is posted on the Journal’s Web site (http://aje.oupjournals.org/)). CI, confidence interval; NIH, National Institutes of Health.
Table 2.
Daytime Napping in Relation to Parkinson Disease at Different Stages,a With Further Adjustment for Nighttime Sleeping or Limited to Participants Reporting 7–8 Hours of Nighttime Sleeping, NIH-AARP Diet and Health Study
| Clinical Stage in Reference to Exposure Assessment in 1996–1997b |
||||||||||||
| Established |
Recent |
Prediagnostic |
||||||||||
| PD | No PD | ORc | 95% CI | PD | No PD | ORc | 95% CI | PD | No PD | ORc | 95% CI | |
| Further adjustment for hours of nighttime sleeping | ||||||||||||
| None (ref) | 77 | 111,237 | 1.0 | 165 | 111,237 | 1.0 | 322 | 111,237 | 1.0 | |||
| <1 hour | 135 | 84,971 | 1.9 | 1.4, 2.5 | 164 | 84,971 | 1.1 | 0.9, 1.4 | 352 | 84,971 | 1.2 | 1.0, 1.3 |
| ≥1 hours | 54 | 16,997 | 3.4 | 2.4, 4.9 | 65 | 16,997 | 2.3 | 1.7, 3.1 | 94 | 16,997 | 1.5 | 1.2, 2.0 |
| P for trend | <0.0001 | <0.0001 | 0.0004 | |||||||||
| Limited to participants reporting 7–8 hours of nighttime sleeping | ||||||||||||
| None (ref) | 39 | 73,409 | 1.0 | 126 | 73,409 | 1.0 | 214 | 73,409 | 1.0 | |||
| <1 hour | 72 | 52,918 | 2.1 | 1.4, 3.1 | 108 | 52,918 | 1.0 | 0.8, 1.3 | 227 | 52,918 | 1.2 | 1.0, 1.4 |
| ≥1 hour | 13 | 8,485 | 2.4 | 1.3, 4.5 | 40 | 8,485 | 2.4 | 1.7, 3.5 | 51 | 8,485 | 1.6 | 1.2, 2.2 |
| P for trend | 0.0009 | <0.0001 | 0.0019 | |||||||||
Abbreviations: CI, confidence interval; NIH, National Institutes of Health; OR, odds ratio; PD, Parkinson disease; ref, reference.
Established cases: diagnosed before 1995; recent cases, diagnosed in 1995–1999; prediagnostic cases: diagnosed in 2000 and after.
PD: number of Parkinson disease cases; no PD: number of participants without Parkinson disease.
Covariates included age, sex, race, smoking status, daily caffeine intake, physical activity, daytime napping, and nighttime sleeping.
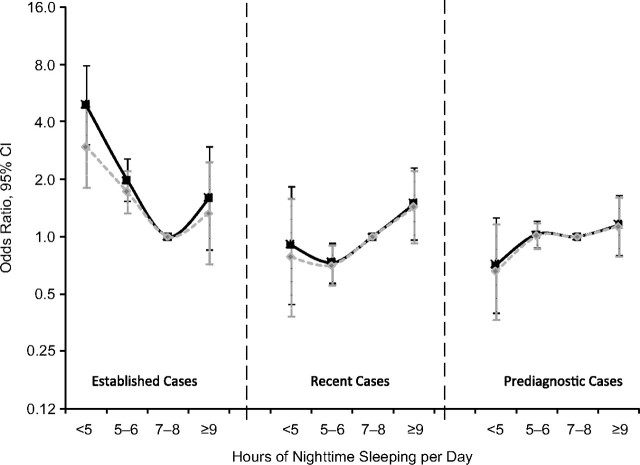
In the analysis for nighttime sleeping, both shorter and longer sleeping seemed to be associated with higher odds of reporting established Parkinson disease diagnosed prior to 1995 (Figure 2). Compared with that for participants who slept 7–8 hours per night, the multivariate odds ratios for having established Parkinson disease were 4.9 (95% confidence interval: 3.0, 7.9) for participants reporting <5 hours of nighttime sleeping, 2.0 (95% confidence interval: 1.5, 2.6) for 5–6 hours, and 1.6 (95% confidence interval: 0.9, 3.0) for ≥9 hours. However, this association was much attenuated after further adjustment for self-reported health status (Figure 2). In contrast to prevalent cases, this U-shaped relation was less apparent for recent cases and disappeared for prediagnostic cases (Figure 2). Similar to the napping analysis, further adjustment for depression or stratifying analyses by age, sex, smoking, and caffeine intake generally showed similar results (data not shown).
Figure 2.
Numbers of hours of nighttime sleeping in relation to Parkinson disease diagnosis at different clinical stages for NIH-AARP Diet and Health Study participants, 1996–2006. Black lines: adjusted for age, sex, race, physical activity, smoking status, and caffeine intake; grey lines: also adjusted for self-reported health status. Established cases: diagnosed before 1995; recent cases, diagnosed in 1995–1999; prediagnostic cases: diagnosed in 2000 and after. Actual values for odds ratios and 95% confidence intervals are provided in Web Table 3 (which is posted on the Journal’s Web site (http://aje.oupjournals.org/)). CI, confidence interval; NIH, National Institutes of Health.
DISCUSSION
In this large population of older adults in the United States, we demonstrated that, in addition to strong associations for established and recent cases, longer daytime napping was also associated with future Parkinson disease occurrence. Compared with nonnappers, participants who napped ≥1 hour per day in 1996–1997 had an approximately 50% higher chance of reporting a Parkinson disease diagnosis in 2000 and after. This association with prediagnostic cases could not be explained by Parkinson disease medications or by inadequate nighttime sleeping and deteriorating health status, as shown in our sensitivity analyses. In contrast to napping, although atypical sleeping durations were more common among established Parkinson disease cases, they showed no relation with future Parkinson disease diagnosis.
It is well known that most Parkinson disease patients experience sleep abnormalities, ranging from difficulty in sleep maintenance, to sleep fragmentation, to conditions such as excessive daytime sleepiness and rapid eye movement sleep behavior disorder (5, 12). This disorder is rare in the general population but affects up to 27% of Parkinson disease patients over time (13) and has been suggested to precede the clinical onset of Parkinson disease (13–17). Not surprisingly, the current study supports the association of atypical sleep habits with Parkinson disease. As expected, the strongest associations were found for established Parkinson disease cases, which could be partially explained by their poor health status.
Excessive daytime sleepiness is the well-documented sleep disturbance that affects 16%–50% of Parkinson disease patients (18). Longitudinal data among Parkinson disease patients show that the prevalence of excessive daytime sleepiness increases as the disease progresses, and Parkinson disease medications such as dopamine agonists may, at least partially, contribute to this problem (18). However, most studies did not clarify whether the presence of excessive daytime sleepiness is secondary to inadequate sleep at night and/or side effects of Parkinson disease treatments, or whether it actually develops prior to Parkinson disease diagnosis. To our knowledge, only one prospective study has investigated whether daytime sleepiness appears before Parkinson disease diagnosis (2). The analysis was conducted in the Honolulu Asia Aging Study with 43 incident cases and 7 years of follow-up of 3,078 Japanese-American men. The study showed that men who felt sleepy most of the day were about 3 times more likely to develop Parkinson disease than men who did not. Another study found an association for nurses who slept longer hours in a 24-hour period, compared with shorter sleepers, with higher future Parkinson disease occurrence (19). However, this study did not differentiate between daytime and nighttime sleeping and might have been affected by the fact that nurses had altered sleep habits due to shift-work schedules.
The current study is substantially larger than the previous ones, included both men and women, and simultaneously examined daytime napping and nighttime sleeping. The results of our study show that longer daytime napping, but not atypical nighttime sleeping durations, is associated with future Parkinson disease occurrence. Furthermore, our sensitivity analyses of participants with a typical night sleeping duration (7–8 hours) suggest that the association of longer day napping and future Parkinson disease occurrence could not be explained simply by short night sleeping.
The lack of an association between nighttime sleeping and future Parkinson disease occurrence may need to be approached cautiously. Although these data may suggest that indeed nighttime sleeping is not disturbed during the premotor stage of Parkinson disease, this finding may be due to measurement errors regarding nighttime sleeping duration. Self-reported night sleeping duration is both approximate and subjective, and it may not reflect the quality of nighttime sleeping and the various sleep cycles. Objective, yet relatively expensive polysonographic assessment provides much better data on the quality and quantity of nighttime sleeping but is not suitable for use in large populations. Because daytime napping generally involves less than one sleep cycle, people tend to arise if they awake from a daytime nap; thus, reports of daytime napping time may be more accurate.
Although this study has several strengths, as mentioned earlier, it also has some limitations. Daytime napping is only a surrogate for excessive daytime sleepiness, because whether or not one can actually nap depends on several factors in addition to his or her tendency to sleep. Therefore, our result may not be directly comparable to those from Abbott et al. (2), who aimed to directly ask about daytime sleepiness. In the current study, we adjusted for and stratified by some known Parkinson disease risk factors and conducted several sensitivity analyses to examine the robustness of our findings; however, we did not have data on some other potential confounders such as head injury that may be associated with both sleep duration and Parkinson disease (20, 21). Therefore, we were unable to exclude the possibility of unmeasured confounding.
We relied on self-reports for case identification because it was necessary in such a large population-based cohort. It is likely that some cases were not identified and some noncases were misclassified as cases. On the other hand, the ongoing validation study suggests that most self-reports can be confirmed by the treating neurologists or by expert medical record review. Furthermore, we previously reported the well-known association of smoking with Parkinson disease in this cohort, which indirectly supports our case identification strategy (22). We did not have information on the cohort's use of dopaminergic medicines, which may alter sleeping/napping habits; therefore, we were unable to examine how the use of dopaminergic medicines might have affected our analyses for prevalent and recent cases.
As in many other epidemiologic studies (23, 24), hours of napping was self-reported; therefore, misclassification of exposure is also possible. For incident cases diagnosed in 2000 and after, however, these misclassifications were likely nondifferential and therefore might have underestimated the strength of the association between napping and future Parkinson disease. The analyses were limited to participants of the follow-up survey in 2004–2006, which included approximately only 66.0% of the risk factor survey participants. A selection bias could have been introduced if deaths or losses to follow-up had been differentially related to napping habits by Parkinson disease status. Finally, previous studies show rapid eye movement sleep behavior disorder may predate Parkinson disease by a decade (14), whereas our study did not collect data on this disorder and had a shorter follow-up interval. Nonetheless, our study suggests that daytime napping, not atypical nighttime sleep duration, is associated with a higher future risk of Parkinson disease.
Supplementary Material
Acknowledgments
Author affiliations: Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Jianjun Gao, Honglei Chen); Department of Neurology, Pennsylvania State University-Milton S. Hershey Medical Center, Hershey, Pennsylvania (Xuemei Huang); Nutritional Epidemiology Branch, National Cancer Institute, Rockville, Maryland (Yikyung Park, Arthur Schatzkin); Occupational and Environmental Epidemiology Branch, National Cancer Institute, Rockville, Maryland (Aaron Blair); and AARP, Washington, DC (Albert Hollenbeck).
Drs. Gao and Huang contributed equally to this manuscript.
This study was supported by the intramural research program of the National Institutes of Health (NIH), the National Institute of Environmental Health Sciences (Z01-ES-101986), the National Cancer Institute (Z01 CP010196-02), and an NIH extramural grant (R01 NS060722) to Dr. Huang.
Conflict of interest: none declared.
References
- 1.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology. 2001;57(3):456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65(9):1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 3.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol. 2008;63(2):167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 4.Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. 2008;63(1):7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 5.Comella CL. Sleep disorders in Parkinson's disease: an overview. Mov Disord. 2007;22(suppl 17):S367–S373. doi: 10.1002/mds.21682. [DOI] [PubMed] [Google Scholar]
- 6.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58(6):963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Zhang SM, Hernán MA, et al. Diet and Parkinson's disease: a potential role of dairy products in men. Ann Neurol. 2002;52(6):793–801. doi: 10.1002/ana.10381. [DOI] [PubMed] [Google Scholar]
- 9.Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 10.Fang F, Xu Q, Park Y, et al. Depression and the subsequent risk of Parkinson's disease in the NIH-AARP Diet and Health Study. Mov Disord. 2010;25(9):1157–1162. doi: 10.1002/mds.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob EL, Gatto NM, Thompson A, et al. Occurrence of depression and anxiety prior to Parkinson's disease. Parkinsonism Relat Disord. 2010;16(9):576–581. doi: 10.1016/j.parkreldis.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhawan V, Healy DG, Pal S, et al. Sleep-related problems of Parkinson's disease. Age Ageing. 2006;35(3):220–228. doi: 10.1093/ageing/afj087. [DOI] [PubMed] [Google Scholar]
- 13.Gjerstad MD, Boeve B, Wentzel-Larsen T, et al. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008;79(4):387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 14.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46(2):388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 15.Boeve BF, Saper CB. REM sleep behavior disorder: a possible early marker for synucleinopathies. Neurology. 2006;66(6):796–797. doi: 10.1212/01.wnl.0000209264.61035.bb. [DOI] [PubMed] [Google Scholar]
- 16.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(pt 11):2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 17.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(pt 2):331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 18.Gjerstad MD, Alves G, Wentzel-Larsen T, et al. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology. 2006;67(5):853–858. doi: 10.1212/01.wnl.0000233980.25978.9d. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Schernhammer E, Schwarzschild MA, et al. A prospective study of night shift work, sleep duration, and risk of Parkinson's disease. Am J Epidemiol. 2006;163(8):726–730. doi: 10.1093/aje/kwj096. [DOI] [PubMed] [Google Scholar]
- 20.Orff HJ, Ayalon L, Drummond SP. Traumatic brain injury and sleep disturbance: a review of current research. J Head Trauma Rehabil. 2009;24(3):155–165. doi: 10.1097/HTR.0b013e3181a0b281. [DOI] [PubMed] [Google Scholar]
- 21.Goldman SM, Tanner CM, Oakes D, et al. Head injury and Parkinson's disease risk in twins. Ann Neurol. 2006;60(1):65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picarsic JL, Glynn NW, Taylor CA, et al. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2008;56(9):1674–1680. doi: 10.1111/j.1532-5415.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-García E, Faubel R, León-Muñoz L, et al. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87(2):310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.