Abstract
The authors sought to investigate trends in the incidence of human immunodeficiency virus (HIV) infection, evaluate changes in risk behavior, and assess associations between syringe access programs and HIV seroconversion among injection drug users (IDUs) in Montreal, Canada, who were recruited and followed for a prospective cohort study between 1992 and 2008. Methods included Kaplan-Meier survival analysis and time-varying Cox regression models. Of 2,137 HIV-seronegative IDUs at enrollment, 148 became HIV-positive within 4 years (incidence: 3.3 cases/100 person-years; 95% confidence interval: 2.8, 3.9). An annual HIV incidence decline of 0.06 cases/100 person-years prior to 2000 was followed by a more rapid annual decline of 0.24 cases/100 person-years during and after 2000. Behavioral trends included increasing cocaine and heroin use and decreasing proportions of IDUs reporting any syringe-sharing or sharing a syringe with an HIV-positive person. In multivariate analyses, HIV seroconversion was associated with male gender, unstable housing, intravenous cocaine use, and sharing syringes or having sex with an HIV-positive partner. Always acquiring syringes from safe sources conferred a reduced risk of HIV acquisition among participants recruited after 2004, but this association was not statistically significant for participants recruited earlier. In conclusion, HIV incidence has declined in this cohort, with an acceleration of the reduction in HIV transmission after 2000.
Keywords: HIV; HIV seropositivity; needle-exchange programs; substance abuse, intravenous
For nearly 3 decades, injection drug use has been recognized for its ongoing impact on the transmission of human immunodeficiency virus (HIV) and perpetuation of the HIV epidemic in developed and developing countries (1–3). Longitudinal studies have enabled the tracking and analysis of changes in HIV risk behaviors and HIV transmission over time (4).
Decreases in or stabilization of HIV incidence rates have been documented in cohorts of injection drug users (IDUs) followed in many settings (5, 6). Such changes have been partly attributed to reduction of high-risk behaviors and the implementation of prevention programs providing access to sterile syringes (4, 7–9). Reduced HIV transmission in long-term prospective studies could also be due to the aging of cohort members (4). Participation in a longitudinal study involving serial HIV counseling and testing could itself reduce risky behavior and lower the risk of HIV transmission. Nevertheless, reviews worldwide have concluded that community outreach and individual interventions, along with access to sterile injection equipment through needle exchange programs (NEPs) and drug abuse treatment, are effective in reducing the risk of HIV transmission among IDUs (10–13). However, recent reviews have suggested that the effectiveness of provision of sterile injecting equipment in preventing HIV transmission may be lower than is widely thought, and investigators have called for more research in this area (14, 15).
Montreal, Quebec, Canada, was among the first of cities in North America to implement NEPs, beginning in the late 1980s. In 1995, NEPs in Montreal attracted international attention when we reported that the incidence of HIV infection was higher among NEP clients than among nonclients, even after adjustment for confounding variables (16). Since then, public health officials have taken steps to continuously improve harm reduction strategies in many ways. Montreal now has one of the most liberal syringe distribution policies in North America, including no limits on the number of syringes that can be obtained from NEPs, provision of IDU outreach workers and services at local health service centers, and the implementation of a network of pharmacies selling low-cost syringes to IDUs. In line with policy changes, the distribution of syringes per year by NEPs and local health service centers increased from 340,000 syringes in 1996 to 950,000 syringes in 1999; annual distribution stabilized after 1999, with an average distribution of 800,000 syringes annually (17). However, this estimate does not include syringes sold or distributed by pharmacies and outreach workers. Whether the changes implemented in recent years and the diversity of syringe sources now available in Montreal have had an impact on risk behaviors and HIV incidence has not been established.
In this study, we had 2 objectives. The first was to investigate secular trends in HIV incidence and to evaluate changes in risk behavior and HIV infection among IDUs followed between 1992 and 2008 in Montreal's St. Luc Cohort. The second objective was to assess associations between NEP participation, safe syringe acquisition, and HIV seroconversion.
MATERIALS AND METHODS
Recruitment criteria for the St. Luc Cohort included being 18 years of age or older and having injected drugs within the past 6 months. From the study's outset in 1988, the cohort was designed as an open cohort, with new recruitment occurring each year. From 2001 to November 2004, however, recruitment of new participants was interrupted by loss of funding. Follow-up of the remaining participants was maintained. Using the original eligibility criteria, recruitment of new participants was reinstated in November 2004. Similar questionnaires and HIV testing measures were used. The 4-year gap in recruitment yielded 2 distinct waves of follow-up, 1988–2001 and 2004–2008. The current analyses include both waves of follow-up; however, first-wave participants recruited prior to 1992 were excluded because of changes in the questionnaire used since 1992.
First- and second-wave cohort participants volunteered to participate in response to direct street-level recruitment or word-of-mouth referral (57%) or community programs (43%). All participants provided their informed consent as approved by the institutional review board of the Centre Hospitalier de l'Université de Montréal. Cohort visits were scheduled after 3 months for the first follow-up visit and at 6-month intervals thereafter. Behavioral questionnaires were administered by trained interviewers, with venous blood samples being drawn at each visit for HIV antibody testing. Participants were asked to return within 2 weeks for their serostatus test results, at which time post-test counseling and referrals were provided. At each interview, participants were given a small stipend, initially Can$10, later increased to Can$20.
To study HIV incidence, all cohort members were tested for HIV-1 antibodies at baseline and at each follow-up visit by enzyme immunoassay (Axsym, Abbott Laboratories), with results being confirmed by Western blot or radioimmunoprecipitation assay at the Quebec Provincial Public Health Laboratory. Sociodemographic characteristics—age, gender, education, drug-use patterns, injection behaviors, sexual behavior, and access to clean syringes through NEP or pharmacies—were examined as potential determinants of HIV seroconversion. Consistent with previous studies, an unstable housing arrangement was defined as living on the street, in a shelter, or in an apartment or hotel room rented on a monthly basis (indicating a rapid turnover compared with typical 12-month rent-lease accommodation standards in Montreal) (18).
Drug use patterns and injection behaviors were assessed by asking about the type of drug used and the frequency of injections in the past month and whether, in the past 6 months, the participant had shared syringes, shared syringes with a person known to be HIV-seropositive, or had practiced “booting” (a practice likely to contaminate the injection equipment with blood) (19). Sexual risk indicators in the past 6 months included having sexual relations with person(s) known to be seropositive, prostitution activities for females, and sexual relations with a male partner for males. Accessibility of clean syringes was measured by 2 dichotomous indicators: 1) having visited a NEP to get clean syringes in the past 6 months and 2) having obtained 100% of syringes used through safe sources (i.e., NEPs, pharmacies or related venues, including community health centers, or outreach workers).
Statistical analyses
Descriptive analyses were used to characterize the study population during the 2 recruitment waves. Cochran-Armitage trend tests were used to compare the characteristics of participants recruited during 4 time intervals, 3 for wave 1 (1992–1994, 1995–1997, and 1998–2001) and 1 for wave 2 (2004–2008).
Primary analyses employed time-to-event methods to assess trends in HIV incidence between 1992 and 2008. Generalized additive models with loess smoothers (20) were used to model nonlinear changes in annual HIV incidence rates between 1992 and 2008.
To investigate HIV secular trends and changes in risk behavior, we truncated the follow-up of all participants at 48 months, taking into account only those seroconversions that occurred within 4 years following the participant's entry into the cohort. This strategy ensured the comparability of results for participants enrolled in different waves, all of whom had the opportunity to be followed for up to 4 years.
Analyses of factors associated with HIV incidence involved the use of univariate and multivariable Cox proportional hazards regression models (21) to estimate crude and adjusted hazard ratios, respectively, with corresponding 95% confidence intervals. The outcome was defined as the time from entry into the cohort until seroconversion, assumed to have occurred at the midpoint between the dates of the participant's visits corresponding to the last negative and the first positive HIV test. Except for baseline age, gender, education, and time of recruitment, all variables were modeled as time-dependent covariates. Thus, at any time during follow-up, we used the values from the most recent visit. To examine and account for secular trends in annual HIV incidence, the effect of calendar time was represented by a binary indicator of recruitment period (2004–2008 vs. 1992–2001).
Three separate multivariable Cox models were fitted to examine relations between the risk of HIV seroconversion and, respectively, 1) sociodemographic variables and risk behaviors; 2) sociodemographic variables, risk behaviors, and NEP participation; and 3) sociodemographic variables, risk behaviors, and whether 100% of syringes used had been obtained from safe sources. Baseline age and dichotomized recruitment period were forced into each final model. Other variables were entered into the final models using a forward selection procedure with P < 0.05 as the criterion for entry. Because of correlation between cocaine use and frequency of injection (Pearson correlation coefficient: r = 0.44), only the former was included as a covariate in the final models.
We calculated adjusted population attributable risk percentages for selected behaviors that showed 1) a positive association between a given behavior and HIV and 2) decreased prevalence over time in that same behavior, to investigate the contribution of such behaviors to the observed reductions in incidence. Adjusted population attributable risk percentages were calculated using standard methods (22) employed in IDU studies (23).
To investigate whether the effects of particular risk factors on the hazard of HIV seroconversion varied between the 2 cohort waves, multivariable Cox regression analyses tested 2-way interactions between a dichotomized recruitment period (1992–2001 vs. 2004–2008) and each potential risk factor. All statistically nonsignificant interactions (P > 0.05 for a 2-tailed Wald test) were then excluded from the final multivariable models. In the case of a significant interaction, we estimated separate hazard ratios for the associations between a corresponding factor and HIV incidence in each of the 2 waves. All analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
A total of 3,120 persons who had injected drugs in the previous 6 months were screened during the entire study period. Among these, 329 (10.5%) tested positive for HIV at baseline, with the remaining 2,791 (89.5%) participants being eligible for inclusion in the cohort. A total of 2,137 HIV-negative participants (77% of those eligible) had at least 1 follow-up visit and were included in the present analyses. Compared with IDUs included in the analyses, participants with no follow-up assessments were slightly younger (mean age = 32.2 years vs. 33.6 years; P < 0.0001), more likely to have used heroin (37% vs. 32%; P = 0.02), more likely to have injected drugs more than 30 times in the last month (56% vs. 51%; P = 0.01), and less likely to have visited a NEP in the previous 6 months (51% vs. 58%; P = 0.001). Distributions of gender, education, cocaine use, and unstable housing conditions were similar between the 2 groups (data not shown).
Prior to seroconversion or censoring at 48 months of follow-up, participants contributed a total of 4,501 person-years of observation. The median time between consecutive visits was 6.1 months in wave 1 and 5.8 months in wave 2. The majority of participants were male (80.5%). At enrollment, the mean age was 33.6 years (standard deviation, 8.7), and 34.9% of the participants were under 30 years of age. The mean duration of injecting drugs was 9.9 years (standard deviation, 8.4). As many as 39.3% of participating IDUs did not finish high school, and 82.2% reported French as their mother tongue.
Table 1 compares baseline characteristics of participants recruited in different periods. The distributions of gender, mother tongue, and educational level at enrollment did not change throughout the study period, whereas the proportions of participants younger than age 30 years and living in unstable housing conditions increased over time. Notable trends in drug use patterns and injection behaviors included increases in intravenous cocaine, crack, and heroin use in the previous month, increases in the proportion of IDUs reporting frequent injections in the past month, and increases in “booting.” Conversely, the proportions of IDUs who reported any syringe-sharing in the past 6 months or sharing with a known seropositive person declined significantly in the second wave of recruitment.
Table 1.
Baseline Characteristics of Injection Drug Users According to Period of Recruitment Into the St. Luc Cohort, 1992–2008, Montreal, Quebec, Canada
| Variable | Cohort Wave 1 |
Cohort Wave 2 (2004–2008) (n = 380) |
P Value From Trend Testa | ||||||||
| Total (1992–2001) (n = 1,757) |
1992–1994 (n = 642) |
1995–1997 (n = 619) |
1998–2001 (n = 496) |
||||||||
| % | SD | % | SD | % | SD | % | SD | % | SD | ||
| Age <30 years at time of recruitment | 34.3 | 1.1 | 32.7 | 1.9 | 31.5 | 1.9 | 39.9 | 2.2 | 37.4 | 2.5 | 0.015 |
| Female gender | 19.4 | 0.9 | 20.1 | 1.6 | 18.6 | 1.6 | 19.6 | 1.8 | 20.1 | 2.1 | 0.979 |
| No high school completion | 39.4 | 1.2 | 41.0 | 1.9 | 37.0 | 1.9 | 40.5 | 2.2 | 38.7 | 2.5 | 0.701 |
| French as mother tongue (vs. another language) | 82.5 | 0.9 | 82.2 | 1.5 | 80.6 | 1.6 | 85.1 | 1.6 | 80.8 | 2.0c | 0.856 |
| Living in unstable housing conditions | 36.6 | 1.1 | 33.8 | 1.9 | 39.3 | 2.0 | 36.9 | 2.2 | 49.9 | 2.6 | <0.001 |
| Cocaine use in the past month | 64.8 | 1.1 | 61.5 | 1.9 | 64.0 | 1.9 | 70.0 | 2.1 | 80.5 | 2.0 | <0.001 |
| Heroin use in the past month | 30.6 | 1.1 | 29.6 | 1.8 | 25.7 | 1.8 | 37.9 | 2.2 | 40.3 | 2.5 | <0.001 |
| “Crack” use in the past month | 26.9 | 1.1 | 25.1 | 1.7 | 25.2 | 1.7 | 31.3 | 2.1 | 60.5 | 2.5 | <0.001 |
| >30 injections in the past month | 47.1 | 1.2 | 46.3 | 2.0 | 41.7 | 2.0 | 54.8 | 2.2 | 66.8 | 2.4 | <0.001 |
| Sharing syringes in the past 6 months | 78.7 | 1.0 | 80.8 | 1.6 | 79.2 | 1.6 | 75.4 | 1.9 | 53.7 | 2.6 | <0.001 |
| Sharing a syringe with an HIV-positive person in the past 6 months | 12.5 | 0.8 | 11.5 | 1.3 | 13.6 | 1.4 | 12.3 | 1.5 | 5.0 | 1.1 | 0.006 |
| “Booting” | 13.0 | 0.8 | 16.2 | 1.5 | 8.7 | 1.1 | 14.3 | 1.6 | 21.6 | 2.1 | 0.018 |
| Having sex with a person known to be HIV-positive | 2.0 | 0.3 | 1.1 | 0.4 | 2.3 | 0.6 | 2.8 | 0.7 | 2.6 | 0.8 | 0.049 |
| Prostitution in the past 6 months (females) | 45.5 | 2.7 | 42.6 | 4.4 | 53.0 | 4.7 | 40.2 | 5.0 | 36.8 | 5.5 | 0.252 |
| Sexual relations with a same-sex partner (males) | 14.6 | 0.9 | 9.2 | 1.3 | 13.9 | 1.5 | 22.6 | 2.1 | 14.5 | 2.0 | <0.001 |
| Participation in a needle exchange program in the past 6 months | 55.2 | 1.2 | 52.2 | 2.0 | 51.9 | 2.0 | 63.0 | 2.2 | 73.2 | 2.3 | <0.001 |
| Obtaining 100% of syringes from safe sources | 61.6 | 1.2 | 61.8 | 1.9 | 63.2 | 1.9 | 59.3 | 2.2 | 59.0 | 2.5 | 0.213 |
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
P value from a Cochran-Armitage trend test assessing the trend across the 4 time periods.
There was an increase in the proportion of IDUs reporting visiting a NEP for their syringe supply, while the proportion of those obtaining 100% of their syringes from safe sources remained stable over time.
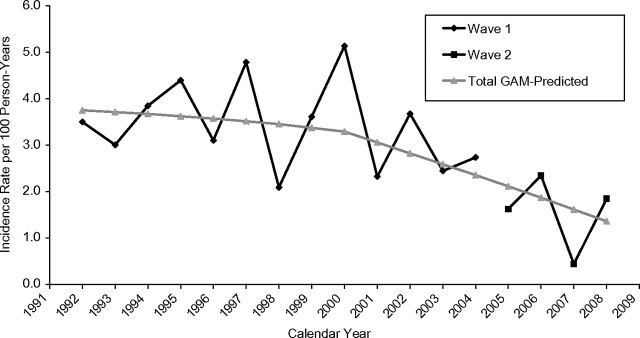
A total of 148 IDUs seroconverted within the first 48 months of follow-up, representing a crude incidence rate of 3.3 per 100 person-years (95% confidence interval (CI): 2.8, 3.9). Table 2 and Figure 1 present annual HIV incidence rates and the corresponding smoothed trends over time, respectively, estimated with generalized additive models. The estimate from the generalized additive model suggested a bilinear model with 2 distinct phases. Specifically, a relatively slow annual decline in HIV incidence of 0.06 cases/100 person-years prior to 2000 was followed by a 4 times' faster decline of 0.24 cases/100 person-years per year during and after 2000.
Table 2.
Annual Incidence of Human Immunodeficiency Virus Infection Per 100 Person-Years Among Injection Drug Users Followed for Up To 48 Months Between 1992 and 2008 in Montreal, Quebec, Canada
| Calendar Year | Incidence Rate Per 100 Person-Years | 95% Confidence Interval |
| 1992 | 3.5 | 0.9, 9.5 |
| 1993 | 3.0 | 1.3, 5.9 |
| 1994 | 3.8 | 2.2, 6.3 |
| 1995 | 4.4 | 2.8, 6.7 |
| 1996 | 3.1 | 1.8, 5.0 |
| 1997 | 4.8 | 3.1, 7.1 |
| 1998 | 2.1 | 1.1, 3.7 |
| 1999 | 3.6 | 2.1, 5.7 |
| 2000 | 5.1 | 3.0, 8.2 |
| 2001 | 2.3 | 0.9, 4.8 |
| 2002 | 3.7 | 1.5, 7.7 |
| 2003 | 2.4 | 0.4, 8.1 |
| 2004 | 2.7 | 0.1, 13.5 |
| 2005 | 1.6 | 0.1, 8.0 |
| 2006 | 2.3 | 0.7, 5.7 |
| 2007 | 0.4 | 0.0, 2.2 |
| 2008 | 1.8 | 0.5, 5.0 |
Figure 1.
Annual trends in human immunodeficiency virus incidence per 100 person-years among injection drug users followed for 48 months between 1992 and 2008 in Montreal, Quebec, Canada. GAM, generalized additive model.
Table 3 shows crude associations between sociodemographic and behavioral characteristics and the risk of HIV seroconversion, based on separate univariate Cox models. Female and younger IDUs were less likely to become infected. Among male participants, having had sexual relations with a same-sex partner in the past 6 months was associated with HIV seroconversion. IDUs reporting unstable housing conditions were more likely to seroconvert to HIV. Several injection-related practices were associated with an increased risk of HIV infection: syringe-sharing, sharing a syringe with a known seropositive partner, and booting. Higher injection frequency and cocaine injection were both associated with an increased risk of HIV seroconversion, whereas heroin injection was associated with a lower risk. Participants who reported visiting a NEP to obtain clean syringes had a higher risk of seroconversion, while in univariate analyses, obtaining 100% of one's syringes from safe sources was not associated with HIV risk. The adjusted population attributable risks of sharing syringes and of sharing a syringe with a person known to be HIV-positive were estimated at 18% (95% CI: 1, 29) and 5% (95% CI: 4, 6), respectively.
Table 3.
Crude Incidence of Human Immunodeficiency Virus Infection According to Sociodemographic Characteristics, Behavioral Risk Factors, and Recruitment Period for Injection Drug Users in Montreal, Quebec, Canada, 1992–2008
| Variable | No. of Seroconversions | Person-Years of Follow-up | Incidence Rate Per 100 Person-Years | 95% CI | Hazard Ratioa | 95% CI |
| Age ≥30 years | ||||||
| No | 34 | 1,459.20 | 2.33 | 1.7, 3.2 | 1 | |
| Yes | 114 | 2,990.83 | 3.81 | 3.2, 4.6 | 1.66 | 1.13, 2.44 |
| Gender | ||||||
| Male | 135 | 3,554.59 | 3.80 | 3.2, 4.5 | 1 | |
| Female | 13 | 891.91 | 1.46 | 0.8, 2.4 | 0.39 | 0.22, 0.68 |
| Education | ||||||
| High school or more | 90 | 2,696.37 | 3.34 | 2.7, 4.1 | 1 | |
| Less than high school | 58 | 1,753.67 | 3.31 | 2.5, 4.2 | 0.99 | 0.71, 1.38 |
| Mother tongue | ||||||
| Language other than French | 15 | 780.46 | 1.92 | 1.1, 3.1 | 1 | |
| French | 133 | 3,669.58 | 3.62 | 3.1, 4.3 | 1.91 | 1.12, 3.26 |
| Living in unstable housing conditions | ||||||
| No | 61 | 3,090.50 | 1.97 | 1.5, 2.5 | 1 | |
| Yes | 87 | 1,359.27 | 6.40 | 5.2, 7.9 | 3.08 | 2.22, 4.28 |
| Cocaine use in the past month | ||||||
| No | 20 | 2,111.56 | 0.95 | 0.6, 1.4 | 1 | |
| Yes | 128 | 2,338.48 | 5.47 | 4.6, 6.5 | 5.45 | 3.40, 8.75 |
| Heroin use in the past month | ||||||
| No | 127 | 3,376.33 | 3.76 | 3.2, 4.5 | 1 | |
| Yes | 21 | 1,073.71 | 1.96 | 1.3, 2.9 | 0.48 | 0.30, 0.76 |
| “Crack” use in the past month | ||||||
| No | 99 | 3,265.94 | 3.03 | 2.5, 3.7 | 1 | |
| Yes | 49 | 1,184.10 | 4.14 | 3.1, 5.4 | 1.30 | 0.92, 1.84 |
| >30 injections in the past month | ||||||
| No | 62 | 2,914.17 | 2.13 | 1.7, 2.7 | 1 | |
| Yes | 86 | 1,535.86 | 5.60 | 4.5, 6.9 | 2.42 | 1.74, 3.37 |
| Sharing syringes in the past 6 months | ||||||
| No | 35 | 2,215.02 | 1.58 | 1.1, 2.2 | 1 | |
| Yes | 113 | 2,235.02 | 5.06 | 4.2, 6.1 | 2.94 | 2.00, 4.32 |
| Sharing a syringe with a person known to be HIV-positive | ||||||
| No | 100 | 4,125.77 | 2.42 | 2.0, 2.9 | 1 | |
| Yes | 48 | 324.27 | 14.80 | 11.1, 19.5 | 5.90 | 4.17, 8.34 |
| “Booting” | ||||||
| No | 113 | 4,110.52 | 2.75 | 2.3, 3.3 | 1 | |
| Yes | 35 | 339.52 | 10.31 | 7.3, 14.2 | 3.40 | 2.32, 5.00 |
| Having sex with a person known to be HIV-positive | ||||||
| No | 135 | 4,354.81 | 3.10 | 2.6, 3.7 | 1 | |
| Yes | 13 | 95.23 | 13.65 | 8.0, 22.8 | 4.44 | 2.51, 7.85 |
| Prostitution in the past 6 months (females) | ||||||
| No | 5 | 559.52 | 0.89 | 0.3, 2.0 | 1 | |
| Yes | 8 | 332.39 | 2.41 | 1.1, 4.6 | 2.58 | 0.84, 7.91 |
| Sexual relations with a same-sex partner (males) | ||||||
| No | 110 | 3,181.80 | 3.46 | 2.9, 4.2 | 1 | |
| Yes | 25 | 372.79 | 6.71 | 4.5, 9.7 | 1.85 | 1.20, 2.86 |
| Period of recruitment | ||||||
| 1992–1994 | 55 | 1,428.13 | 3.85 | 2.9, 5.0 | 1 | |
| 1995–1997 | 50 | 1,368.70 | 3.65 | 2.7, 4.8 | 0.94 | 0.64, 1.38 |
| 1998–2001 | 34 | 1,031.92 | 3.29 | 2.3, 4.6 | 0.84 | 0.55, 1.28 |
| 2004–2008 | 9 | 621.29 | 1.45 | 0.7, 2.7 | 0.34 | 0.17, 0.68 |
| Period of recruitment (dichotomized) | ||||||
| 1992–2001 | 139 | 3,828.75 | 3.63 | 3.1, 4.3 | 1 | |
| 2004–2008 | 9 | 612.29 | 1.45 | 0.7, 2.7 | 0.36 | 0.18, 0.71 |
| Participation in a needle exchange program | ||||||
| No | 41 | 1,661.03 | 2.47 | 1.8, 3.3 | 1 | |
| Yes | 102 | 1,993.80 | 5.12 | 4.2, 6.2 | 2.02 | 1.41, 2.90 |
| Obtaining 100% of syringes from safe sources | ||||||
| No | 53 | 1,237.17 | 4.28 | 3.2, 5.6 | 1 | |
| Yes | 90 | 2,417.66 | 3.72 | 3.0, 4.6 | 0.88 | 0.63, 1.24 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Crude (nonadjusted) hazard ratio from separate univariate Cox models.
Results from 3 separate multivariable Cox models, adjusted for period of recruitment, are presented in Table 4. Variables that were independently statistically significantly associated with an increased risk of HIV acquisition in all 3 models included male gender, unstable housing conditions, intravenous cocaine use, booting, and syringe-sharing or having sexual relations with a known seropositive partner. In multivariable models, the effect of having sexual relations with a same-sex partner became nonsignificant (for model 1, hazard ratio = 1.34, 95% CI: 0.85, 2.12).
Table 4.
Hazard Ratiosa for Associations Between Human Immunodeficiency Virus Seroconversion, Sociodemographic Characteristics, and Behavioral Risk Factors Among Injection Drug Users in Montreal, Quebec, Canada, 1992–2008
| Variable | Model 1 |
Model 2 (Including NEP Participation) |
Model 3 (Including Obtaining 100% of Syringes From Safe Sources) |
|||
| Adjusted HR | 95% CI | Adjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Age ≥30 years | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.14 | 0.77, 1.70 | 1.23 | 0.82, 1.83 | 1.15 | 0.77, 1.71 |
| Gender | ||||||
| Male | 1 | 1 | 1 | |||
| Female | 0.53 | 0.29, 0.98 | 0.54 | 0.30, 0.99 | 0.52 | 0.29, 0.95 |
| Living in unstable housing conditions | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 2.05 | 1.46, 2.88 | 1.96 | 1.39, 2.75 | 2.07 | 1.47, 2.90 |
| Cocaine use in the past month | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 3.77 | 2.32, 6.12 | 2.87 | 1.66, 4.97 | 3.21 | 1.86, 5.55 |
| Heroin use in the past month | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 0.69 | 0.43, 1.12 | 0.63 | 0.39, 1.03 | 0.67 | 0.41, 1.09 |
| Sharing a syringe with a person known to be HIV-positive | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 3.03 | 2.08, 4.41 | 2.87 | 1.97, 4.18 | 2.93 | 2.01, 4.27 |
| “Booting” | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 2.38 | 1.59, 3.55 | 2.43 | 1.63, 3.62 | 2.40 | 1.61, 3.60 |
| Having sex with a person known to be HIV-positive | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 2.36 | 1.28, 4.33 | 2.25 | 1.23, 4.12 | 2.46 | 1.34, 4.54 |
| Period of recruitment | ||||||
| 1992–2001 | 1 | 1 | ||||
| 2004–2008 | 0.30 | 0.15, 0.59 | 0.27 | 0.14, 0.55 | ||
| NEP participationb | ||||||
| No | 1 | |||||
| Yes | 1.78 | 1.22, 2.58 | ||||
| Obtaining 100% of syringes from a safe sourceb | ||||||
| Recruited during 1992–2001 | 1.05 | 0.73, 1.51 | ||||
| Recruited during 2004–2008 | 0.18 | 0.04, 0.89 | ||||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; NEP, needle exchange program.
Hazard ratios were obtained from multivariable Cox regression analyses.
Includes only visits at which participants reported having injected drugs.
No statistically significant interactions were found between recruitment period and any of the other risk factors. As shown in Table 4 (model 3), always acquiring syringes from safe sources conferred a reduced risk of HIV acquisition among participants recruited in 2004–2008 (hazard ratio = 0.18, 95% CI: 0.04, 0.89); however, this association was not statistically significant for participants recruited earlier, up to 2001.
DISCUSSION
Our results indicate declining HIV incidence in a large cohort of IDUs recruited between 1992 and 2008 in Montreal, Quebec, Canada, with an acceleration of the reduction in HIV transmission after 2000. A number of possible explanations may underlie this decline, including reduction of risk behaviors over time, older age of the participants at enrollment, and aging of the cohort over time. Unlike other studies (4, 24), the age of participants in this cohort was not strongly related to HIV incidence, after accounting for other risk factors. In addition to an older age at recruitment, the lack of an age effect may be partially explained by the 48-month maximum follow-up period applied in our study; this restriction was invoked to account for the potential effect of exposure to multiple testing and counseling on long-term cohort members.
Annual HIV incidence rates declined in this study 4 times faster after 2000 than before 2000, raising the possibility that changes in and enhancement of prevention strategies reported after 1998 may have largely contributed to a reduction of HIV transmission in Montreal.
These results are consistent with recent findings from SurvUDI, a provincial IDU epidemiologic surveillance network for HIV infection that targets active injectors recruited mainly through NEPs (25). Our results are potentially more generalizable than those of the SurvUDI network, as we analyzed data from a broader population base of IDUs, some of whom did not visit NEPs and thus were not captured in SurvUDI.
Consistent with data previously reported from our group and other investigators (26–30), the present results confirm the importance of intravenous cocaine use, unsafe injection practices (such as syringe-sharing and bingeing), and unstable housing conditions as the main drivers of the HIV epidemic among IDUs. Our present results also confirm the contribution of sexual transmission in this population, independent of injection practices (4, 29, 31). Contrary to findings from other North American cities (31, 32), women in our cohort were 2 times less likely to acquire HIV, even after adjustment for known confounders, and this difference was constant across all recruitment periods. We previously reported a lower HIV prevalence at baseline among women who were enrolled in the cohort between 1988 and 1998 (33). Our findings suggest that a gender discrepancy in risk of HIV acquisition continues still. Likewise, unlike previous reports from Baltimore, Maryland (34) and San Francisco, California (35), men who had sex with men were at higher risk of HIV seroconversion, but this association did not remain statistically significant when other covariates were accounted for.
An increase in the proportions of IDUs reporting characteristics and drug-use behaviors associated with HIV incidence in other settings, such as living in unstable housing conditions (26, 36, 37), cocaine use (28, 29), and frequent injection behavior (27, 30), was observed across the follow-up period. These temporal trends did not support the hypothesis that reductions in adverse circumstances or risk behavior could have driven a reduction in incidence. On the other hand, significant reductions in the proportion of IDUs reporting any syringe-sharing or sharing with a person known to be HIV-seropositive were observed. Estimation of the adjusted population attributable risks for these 2 related risk behaviors suggests that reductions may have modestly contributed to reduced HIV incidence. Underreporting of HIV status by syringe-sharing partners is likely to have yielded underestimation of the true impact of a reduced prevalence of sharing with HIV-positive partners (the reported prevalence of such behavior seems much lower than the expected true prevalence). Other investigators have similarly reported decreased HIV incidence associated with reduced injection risk behavior for IDU communities (5, 38, 39).
Period of recruitment interacted in relation to HIV incidence only with access to syringes from safe sources. IDUs recruited after 2001 and those who always obtained syringes from safe sources were 5 times less likely to become HIV-infected than IDUs reporting other sources as well as safe sources for the same recruitment period. In contrast to other North American cities and to another Canadian city, Vancouver (40), the IDU population in Montreal is widely distributed rather than concentrated, with only half living in the downtown area (41). We recently reported that Montreal NEPs and designated pharmacies selling syringes have been instituted where they are most needed relative to the dwelling places of IDUs (18). Moreover, we have demonstrated that IDUs obtaining some of their sterile syringes from unreliable sources have a higher proportion of high-risk injection behavior, consistent with findings reported in other North American cities (18, 32, 42, 43). IDUs who obtained their clean syringes exclusively from either a pharmacy or a NEP had a lower prevalence of high-risk injection behavior, relative to those obtaining syringes from mixed sources (18).
Though the incidence of HIV seroconversion and the frequency of some risk behaviors changed across the study period, other potential risk factors did not vary in their associations with HIV across the recruitment period. This suggests that the impact of such risk factors on the hazard of HIV seroconversion is stable over time.
Several limitations apply to this study. Participants were not randomly selected and thus cannot be considered representative of all IDUs in Montreal. Males and chronic cocaine-using IDUs are overrepresented in the sample in comparison with Quebec provincial data on IDUs (44). This study was conducted in the specific setting of a planned syringe supply strategy system. Unfortunately, data were not available on the size of the IDU population and the level of syringe coverage relative to the true needs of this community, limiting interpretation of our findings. Regardless, the context of this study might still serve to increase understanding of the potential impact of syringe supply services relevant to reduction of HIV transmission for IDUs in other settings. Even if our follow-up rates were acceptable for a drug-using population, our data could have been influenced by losses to follow-up. Although our study suggests possible positive effects of changes in syringe-sharing behaviors and syringe access strategies on HIV transmission, the small number of seroconversions occurring during wave 2 and the related lack of statistical power warrant caution in interpreting results. The recruitment gap from 2001 to 2004 did not allow for longitudinal analysis of a potential secular trend from 2001 onwards. Additionally, as for other cohort studies, a lead-time bias exists wherein potentially important risk-behavior events that may have occurred before participants joined the cohort could not be measured or accounted for. Residual confounding of our results by such influences is possible.
The reduction in HIV incidence documented by this study among IDUs in Montreal is a welcome result for public health authorities. Likewise, the protective effect on HIV transmission observed for always acquiring syringes from safe sources suggests a positive impact of syringe access policies that evolved in Montreal during the past decade. Nevertheless, the stability of the continued high prevalence of high-risk drug and injection behaviors suggests a need for primary prevention in order to reduce initiation of injection drug use. As Wood et al. (6) have noted previously, an association between NEP participation, expressed dichotomously, and HIV seroconversion is not in itself a strong marker of safe injection behavior. Given distribution policies, syringes obtained by IDUs not visiting NEPs might come from safe sources, directly or through secondary exchange. Most importantly, our study adds to knowledge on which components within the range of IDU syringe access services work to reduce HIV transmission.
Acknowledgments
Author affiliations: Centre de Recherche du CHUM (CRCHUM), Centre Hospitalier de l'Université de Montréal, Montréal, Québec, Canada (Julie Bruneau, Mark Daniel, Geng Zang, François Lamothe, Jean Vincelette); Department of Family Medicine, Faculty of Medicine, Université de Montréal, Montréal, Québec, Canada (Julie Bruneau); Department of Social and Preventive Medicine, Faculty of Medicine, Université de Montréal, Montréal, Québec, Canada (Mark Daniel); Division of Health Sciences, School of Health Sciences, University of South Australia, Adelaide, Australia (Mark Daniel); Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montréal, Québec, Canada (Michal Abrahamowicz); and Department of Microbiology and Immunology, Faculty of Medicine, Université de Montréal, Montréal, Québec, Canada (François Lamothe, Jean Vincelette).
This work was supported by the Canadian Institutes of Health Research (grant MOP 135260), the US National Institute on Drug Abuse (grant R01 DA11591), and the Réseau SIDA et Maladies Infectieuses du Fonds de la Recherche en Santé du Québec (grant FRSQ 5227). Dr. Julie Bruneau holds a senior clinical research career award from the Fonds de la Recherche en Santé du Québec. Dr. Michal Abrahamowicz is a James McGill Professor at McGill University. During the conduct of this study, Dr. Mark Daniel held a Canada Research Chair (Population Health) awarded by the Canadian Institutes of Health Research.
The authors thank Dr. Jean-François Boivin, Professor of Epidemiology and Biostatistics at McGill University, for his contribution to an earlier version of this analysis. They also thank the St. Luc Cohort study staff.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- HIV
human immunodeficiency virus
- IDU
injection drug user
- NEP
needle exchange program
References
- 1.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86(5):642–654. doi: 10.2105/ajph.86.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV and AIDS. Report on the Global AIDS Epidemic. Geneva, Switzerland: United Nations; 2008. [Google Scholar]
- 4.Nelson KE, Galai N, Safaeian M, et al. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156(7):641–653. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- 5.Des Jarlais DC, Marmor M, Friedmann P, et al. HIV incidence among injection drug users in New York City, 1992–1997: evidence for a declining epidemic. Am J Public Health. 2000;90(3):352–359. doi: 10.2105/ajph.90.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood E, Lloyd-Smith E, Li K, et al. Frequent needle exchange use and HIV incidence in Vancouver, Canada. Am J Med. 2007;120(2):172–179. doi: 10.1016/j.amjmed.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 7.van Ameijden EJ, Coutinho RA. Maximum impact of HIV prevention measures targeted at injecting drug users. AIDS. 1998;12(6):625–633. doi: 10.1097/00002030-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Des Jarlais DC, Perlis T, Friedman SR, et al. Declining seroprevalence in a very large HIV epidemic: injecting drug users in New York City, 1991 to 1996. Am J Public Health. 1998;88(12):1801–1806. doi: 10.2105/ajph.88.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson DR, Flynn NM, Perales D. Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 2001;15(11):1329–1341. doi: 10.1097/00002030-200107270-00002. [DOI] [PubMed] [Google Scholar]
- 10.Monterroso ER, Hamburger ME, Vlahov D, et al. Prevention of HIV infection in street-recruited injection drug users. The Collaborative Injection Drug User Study (CIDUS) J Acquir Immune Defic Syndr. 2000;25(1):63–70. doi: 10.1097/00042560-200009010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Metzger DS, Navaline H. Human immunodeficiency virus prevention and the potential of drug abuse treatment. Clin Infect Dis. 2003;37(suppl 5):S451–S456. doi: 10.1086/377548. [DOI] [PubMed] [Google Scholar]
- 12.Ball AL, Rana S, Dehne KL. HIV prevention among injecting drug users: responses in developing and transitional countries. Public Health Rep. 1998;113(suppl 1):S170–S1181. [PMC free article] [PubMed] [Google Scholar]
- 13.Copenhaver MM, Johnson BT, Lee IC, et al. Behavioral HIV risk reduction among people who inject drugs: meta-analytic evidence of efficacy. J Subst Abuse Treat. 2006;31(2):163–171. doi: 10.1016/j.jsat.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmateer N, Kimber J, Hickman M, et al. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105(5):844–859. doi: 10.1111/j.1360-0443.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 15.Tilson H, Aramrattana A, Bozzette SA, et al. Preventing HIV Infection Among Injecting Drug Users in High-Risk Countries: An Assessment of the Evidence. Washington, DC: Institute of Medicine, National Academy of Sciences; 2007. [Google Scholar]
- 16.Bruneau J, Lamothe F, Franco E, et al. High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am J Epidemiol. 1997;146(12):994–1002. doi: 10.1093/oxfordjournals.aje.a009240. [DOI] [PubMed] [Google Scholar]
- 17.Leclerc P, Tremblay C, Morissette C. Monitorage des Centres d'Accès au Mtériel stérile d'injection. Rapport régional: Avril 2007 à Mars 2008. Montreal, Quebec, Canada: Agence de la Santé et des Services sociaux de Montréal, Direction de Santé publique de Montréal; 2009. [Google Scholar]
- 18.Bruneau J, Daniel M, Kestens Y, et al. Associations between HIV-related injection behaviour and distance to and patterns of utilisation of syringe-supply programmes. J Epidemiol Community Health. 2008;62(9):804–810. doi: 10.1136/jech.2007.064154. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield L, Bigelow GE, Brooner RK. HIV risk behavior in drug users: increased blood “booting” during cocaine injection. AIDS Educ Prev. 1992;4(2):95–107. [PubMed] [Google Scholar]
- 20.Hastie T, Tibshirani R. Generalized Additive Models. New York, NY: Chapman & Hall, Inc; 1990. [Google Scholar]
- 21.Cox D. Regression models and life tables (with discussion) J R Stat Soc. 1972;34(2):187–220. [Google Scholar]
- 22.Cole P, MacMahon B. Attributable risk percent in case-control studies. Br J Prev Soc Med. 1971;25(4):242–244. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagan H, Thiede H, Weiss NS, et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91(1):42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fennema JS, Van Ameijden EJ, Van Den Hoek A, et al. Young and recent-onset injecting drug users are at higher risk for HIV. Addiction. 1997;92(11):1457–1465. [PubMed] [Google Scholar]
- 25.Leclerc P, Morissette C, Roy E. Le Volet Montréalais du Réseau SurvUDI. Vol 2. Données au 30 Juin 2008, Direction de Santé publique. Montreal, Quebec, Canada: Agence de la Santé et des Services sociaux de Montréal, Direction de Santé publique de Montréal; 2010. ( http://www.santepub-mtl.qc.ca/Publication/pdfudi/reseausurvudi_2.pdf). (Accessed September 10, 2010) [Google Scholar]
- 26.Boileau C, Bruneau J, Al-Nachawati H, et al. A prognostic model for HIV seroconversion among injection drug users as a tool for stratification in clinical trials. J Acquir Immune Defic Syndr. 2005;39(4):489–495. doi: 10.1097/01.qai.0000153424.56379.61. [DOI] [PubMed] [Google Scholar]
- 27.Craib KJ, Spittal PM, Wood E, et al. Risk factors for elevated HIV incidence among aboriginal injection drug users in Vancouver. CMAJ. 2003;168(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17(6):887–893. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 29.Hankins C, Alary M, Parent R, et al. Continuing HIV transmission among injection drug users in Eastern Central Canada: the SurvUDI Study, 1995 to 2000. J Acquir Immune Defic Syndr. 2002;30(5):514–521. doi: 10.1097/00126334-200208150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Miller CL, Kerr T, Frankish JC, et al. Binge drug use independently predicts HIV seroconversion among injection drug users: implications for public health strategies. Subst Use Misuse. 2006;41(2):199–210. doi: 10.1080/10826080500391795. [DOI] [PubMed] [Google Scholar]
- 31.Strathdee SA, Galai N, Safaiean M, et al. Sex differences in risk factors for HIV seroconversion among injection drug users: a 10-year perspective. Arch Intern Med. 2001;161(10):1281–1288. doi: 10.1001/archinte.161.10.1281. [DOI] [PubMed] [Google Scholar]
- 32.Tyndall MW, Bruneau J, Brogly S, et al. Satellite needle distribution among injection drug users: policy and practice in two Canadian cities. J Acquir Immune Defic Syndr. 2002;31(1):98–105. doi: 10.1097/00126334-200209010-00013. [DOI] [PubMed] [Google Scholar]
- 33.Bruneau J, Lamothe F, Soto J, et al. Sex-specific determinants of HIV infection among injection drug users in Montreal. CMAJ. 2001;164(6):767–773. [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta SH, Galai N, Astemborski J, et al. HIV incidence among injection drug users in Baltimore, Maryland (1988–2004) J Acquir Immune Defic Syndr. 2006;43(3):368–372. doi: 10.1097/01.qai.0000243050.27580.1a. [DOI] [PubMed] [Google Scholar]
- 35.Kral AH, Lorvick J, Gee L, et al. Trends in human immunodeficiency virus seroincidence among street-recruited injection drug users in San Francisco, 1987–1998. Am J Epidemiol. 2003;157(10):915–922. doi: 10.1093/aje/kwg070. [DOI] [PubMed] [Google Scholar]
- 36.Des Jarlais DC, Braine N, Friedmann P. Unstable housing as a factor for increased injection risk behavior at US syringe exchange programs. AIDS Behav. 2007;11(suppl 6):S78–S84. doi: 10.1007/s10461-007-9227-6. [DOI] [PubMed] [Google Scholar]
- 37.Corneil TA, Kuyper LM, Shoveller J, et al. Unstable housing, associated risk behaviour, and increased risk for HIV infection among injection drug users. Health Place. 2006;12(1):79–85. doi: 10.1016/j.healthplace.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Sabbatini A, Carulli B, Villa M, et al. Recent trends in the HIV epidemic among injecting drug users in Northern Italy, 1993–1999. AIDS. 2001;15(16):2181–2185. doi: 10.1097/00002030-200111090-00014. [DOI] [PubMed] [Google Scholar]
- 39.van Ameijden EJ, Coutinho RA. Large decline in injecting drug use in Amsterdam, 1986–1998: explanatory mechanisms and determinants of injecting transitions. J Epidemiol Community Health. 2001;55(5):356–363. doi: 10.1136/jech.55.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maas B, Fairbairn N, Kerr T, et al. Neighborhood and HIV infection among IDU: place of residence independently predicts HIV infection among a cohort of injection drug users. Health Place. 2007;13(2):432–439. doi: 10.1016/j.healthplace.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Généreux M, Bruneau J, Daniel M. Association between neighbourhood socioeconomic characteristics and high-risk injection behaviour amongst injection drug users living in inner and other city areas in Montréal, Canada. Int J Drug Policy. 2010;21(1):49–55. doi: 10.1016/j.drugpo.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Lorvick J, Bluthenthal RN, Scott A, et al. Secondary syringe exchange among users of 23 California syringe exchange programs. Subst Use Misuse. 2006;41(6-7):865–882. doi: 10.1080/10826080600669041. [DOI] [PubMed] [Google Scholar]
- 43.Huo D, Bailey SL, Hershow RC, et al. Drug use and HIV risk practices of secondary and primary needle exchange users. AIDS Educ Prev. 2005;17(2):170–184. doi: 10.1521/aeap.17.3.170.62900. [DOI] [PubMed] [Google Scholar]
- 44.Comité permanent de Lutte à la Toxicomanie, Ministère de la Santé et des Services sociaux, Gouvernement du Québec. Le Point sur la Situation de la Toxicomanie au Québec en l'An 2000. Montreal, Quebec, Canada: Ministère de la Santé et des Services sociaux, Gouvernement du Québec; 2000. ( http://publications.msss.gouv.qc.ca/biblio/CPLT/publications/0900pt.pdf). (Accessed January 25, 2009) [Google Scholar]