Abstract
Background
Prevalence of moderate to severe cognitive impairment among hemodialysis patients is more than double the prevalence in the general population. This study describes cognitive impairment occurrence in a peritoneal dialysis cohort compared with a cohort without chronic kidney disease (CKD).
Study Design
Cross-sectional study.
Setting and Participants
51 English-speaking peritoneal dialysis patients from two urban dialysis units, compared with 338 hemodialysis patients from 16 urban dialysis units and 101voluntary controls without CKD from urban general medicine clinics.
Predictor
A 45-minute battery of nine validated neuropsychological tests (cognitive domains memory, executive function, language).
Outcomes
Mild, moderate, or severe cognitive impairment, classified according to a previously designed algorithm.
Results
Of the peritoneal dialysis cohort, 33.3% had no or mild, 35.3% moderate, and 31.4% severe cognitive impairment; corresponding values were 60.4%, 26.7%, and 12.9% of the non-CKD cohort, and 26.6%, 36.4%, and 37.0% of the hemodialysis cohort. A logistic regression model including age, sex, race, education, hemoglobin, diabetes, and stroke showed that only non-white race (P = 0.002) and education (P = 0.002) were associated with moderate to severe cognitive impairment in the peritoneal dialysis cohort. Compared with hemodialysis patients, more peritoneal dialysis patients had moderate to severe memory impairment (60% vs. 52%), but fewer had impaired executive function (one-third vs. one-half). Peritoneal dialysis was associated with a more than 2.5-fold increased risk of moderate to severe cognitive impairment compared with no CKD (OR, 2.58; 95% confidence interval 1.02-6.53), as was hemodialysis (OR, 3.16; 95% CI, 1.91-5.24), in an adjusted logistic regression model.
Limitations
Small sample size, participation rate somewhat low.
Conclusions
Similar to hemodialysis patients, two-thirds of peritoneal dialysis patients had moderate to severe cognitive impairment, enough to interfere with safely self-administering dialysis and adhering to complex medication regimens. These patients could benefit from cognitive assessment before and periodically after dialysis initiation.
Dialysis patients are at increased risk of cognitive impairment due to their older age, high prevalence of cardiovascular risk factors,1-3 stroke,4 uremia, inflammation,5 and multiple metabolic disturbances.6 The burden of cognitive impairment will likely grow as the dialysis population ages and the prevalence of diabetes and vascular disease increases.
We previously reported that the prevalence of moderate to severe cognitive impairment is more than double in hemodialysis patients compared with the general population; up to 70% of hemodialysis patients aged 55 years and older are moderately to severely cognitively impaired.1 Whether cognitive impairment is as common in peritoneal dialysis patients is unclear.
We sought to fill this information gap by measuring the frequency of patients with cognitive impairment in an urban cohort of peritoneal dialysis patients. We compared the risk of moderate to severe cognitive impairment in peritoneal dialysis patients to risk in a cohort of hemodialysis patients and a control group of patients without chronic kidney disease (CKD). We conducted preliminary analyses to measure factors associated with severe cognitive impairment in the peritoneal dialysis cohort. Common occurrence of cognitive impairment would raise questions regarding the ability of these patients to safely administer peritoneal dialysis, comply with complex medication regimens, or make informed decisions regarding initiating and continuing hemodialysis.
METHODS
Study Population
Participants were recruited from two peritoneal dialysis units in downtown Minneapolis and St. Paul, Minnesota, during the first year of a 3-year longitudinal study. Cognitive testing was conducted using a previously designed neuropsychological battery from our previous study of cognitive impairment in hemodialysis patients.1 Eligible participants spoke English as their primary language, were aged 18 years or older, and had no documented history of recent chemical dependency or acute psychoses. Participants used continuous ambulatory or overnight peritoneal dialysis. All cognitive testing was conducted during off-dialysis time, with an interval of at least 2 hours from the time of last dialysis.
We compared the results of the current study to previously published results of our study in a cohort of 338 hemodialysis patients and a voluntary non-CKD control group of 101 independently living participants using the identical cognitive battery.1 The control group was recruited from outpatient internal medicine and geriatrics clinics, and from the metropolitan community. Inclusion criteria for the hemodialysis cohort and control group were the same as for the peritoneal dialysis cohort, except that participants were aged 55 years and older, and the control group had no documented history of CKD. The control group was recruited before the current study to approximately match the mean age and education of the hemodialysis cohort, because age and education are the strongest risk factors for cognitive impairment in previous population studies of non-CKD patients. Given the relative scarcity of peritoneal dialysis patients, and because many risk factors for cognitive impairment are the same for younger and older dialysis patients, age eligibility was expanded to 18 years and older to facilitate recruitment. Thus, we were unable to age match the control group to the peritoneal dialysis cohort. The Minneapolis Medical Research Foundation institutional review board approved the study protocol.
Neuropsychological Testing
A 45-minute battery of nine validated neuropsychological tests was administered to study participants (Table S1, provided as on-line supplementary material). Cognitive assessment was rescheduled for participants who were ill for any reason until after they recovered. We did not record causes of these acute illness episodes; specifically, episodes of peritonitis were not recorded. The full battery consisted of the Modified Mini-Mental State Examination (3MS)7 to test overall (global) cognitive function, the Hopkins Verbal Learning Test-Revised (HVLRT-R)8 to test verbal memory (12-word list), Color Trails 1 and 2 (a test similar to Halsted-Reitan Trails A and B, but using alternative colors instead of numbers for Trail B)9 to test executive function including decision-making and processing speed, the Stroop Interference test10 to test executive function and shifting paradigms/abstraction, the Brief Visuospatial Memory Test-Revised (BVMT-R)11 to test visual memory, the Controlled Oral Word Association Test (COWAT)12 to test word-finding (generation) ability, the Clock-Drawing Test13;14 to briefly test executive function and visual spatial skills, the Wechsler Digit Span15 to test attention, and the Geriatric Depression Scale (Short Form).16 We used published norms for each test from the corresponding test manuals to classify raw score results by number of standard deviations (SD) below age- and education-adjusted means.
Based on number of SDs below the normative mean scored on each test, participants were classified as having no, mild, moderate, or severe cognitive impairment using a previously designed algorithm from our study in hemodialysis patients. The algorithm parallels the Mayo Clinic Petersen criteria for mild cognitive impairment17 and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for dementia, as outlined in Box 1.18 Moderate cognitive impairment is approximately equivalent to the clinical diagnosis of mild to moderate dementia, and severe cognitive impairment to moderate to severe dementia, based on the algorithm we used. The algorithm requires test results for at least one test in each of at least two of three cognitive domains (memory, executive function, or language) for a classification of severe cognitive impairment. Participants were classified using test results in the domains of memory (HVLT-R, verbal; BVMT-R, visual), executive function (Stroop Interference, Color Trails), and language/verbal (COWAT). The 3MS, Clock-Drawing Test, and Digit Span were administered to characterize the cohort, but not to classify level of cognitive impairment.
Box 1. Cognitive Impairment Algorithm.
|
Normal cognitive function:Scored ≤ 1.49 SD below the age-adjusted mean on all tests in all domains.A |
| Mild cognitive impairment: Scored 1.50-1.99 SD below the age-adjusted mean in 1 domain. |
| Moderate cognitive impairment: Scored 1.50-1.99 SD below the age-adjusted mean in ≥ 2 domains, or ≥ 2.00 SD below the mean in 1 domain. |
| Severe cognitive impairment b: Scored ≥ 2.00 SD below the below the age-adjusted mean in ≥ 2 domains. |
SD, standard deviation.
The cognitive domains of memory, language, and executive function.
Classification requires results of at least 1 test in each of at least 2 of the 3 domains.
Data Analyses
Only participants who were able to complete testing in at least two cognitive domains were included in the analyses. First, the percentage of participants scoring at each level of cognitive impairment (none, mild, moderate, severe) was calculated. Next, analysis of variance (ANOVA) and chi-squared analyses measured the relation between the following independent variables and moderate to severe cognitive impairment in the peritoneal dialysis patients: age, sex, race, education, continuous ambulatory vs. overnight peritoneal dialysis, diabetes, hypertension, dialysis duration, hemoglobin, depression, and stroke. Then, variables associated at the P ≤ 0.10 level with moderate to severe cognitive impairment were entered into a logistic regression analysis with severe cognitive impairment in the peritoneal patients as the dependent variable, adjusted for age. Lastly, the odds of moderate to severe cognitive impairment in peritoneal dialysis patients compared with hemodialysis patients and with the non-CKD control group were calculated using logistic regression, adjusting for age, sex, race, education, stroke, and variables associated at the P ≤ 0.10 level with moderate to severe cognitive impairment in the peritoneal dialysis cohort.
RESULTS
Of the 94 eligible peritoneal dialysis patients from the two dialysis clinics, five transitioned to hemodialysis or underwent kidney transplant and three died before testing could be conducted. Of the remaining 86 patients, 34 were feeling too ill or declined to participate for other reasons. Thus, 52 of 86 (60.5%) peritoneal dialysis patients were enrolled. Because of Health Insurance Portability and Accountability Act (HIPAA) consent issues, information regarding demographic characteristics of the non-participants was unavailable.
Of the 52 enrolled peritoneal dialysis patients, 51 (97.2%) completed testing in at least two cognitive domains and were included in analyses. Most peritoneal dialysis patients were aged younger than 65 years, white, and relatively well educated (Table 1). Mean peritoneal dialysis duration was 23 ± 15.6 months. Of the 51 included patients, 8 used continuous ambulatory and the remainder overnight peritoneal dialysis. Age, sex, and race distribution differed significantly among the three groups, but education did not. Peritoneal dialysis patients were younger, a lower percentage were women, and the race distribution was broader.
Table 1.
Characteristics of the Cohorts and Comparison Group
| PD Cohort | Control Group |
Primary HD Cohort |
P | |
|---|---|---|---|---|
| No. | 51 | 101 | 338 | |
| Age category | < 0.001 a | |||
| < 55 y | 37.3 | 0.0 | 0.0 | |
| 55-64 y | 33.3 | 40.6 | 29.6 | |
| ≥ 65 y | 29.4 | 59.4 | 70.4 | |
| age, yr | 57.5 +/−14.8 | 68.5 +/−9.6 | 71.2 +/−9.5 | < 0.001 a |
| Women | 33.3 | 56.4 | 45.9 | 0.007 a |
| Education category | 0.3 a | |||
| 0-8 y | 4.0 | 4.0 | 11.2 | |
| 9-12 y | 42.0 | 28.7 | 43.5 | |
| > 12 y | 54.0 | 67.3 | 45.3 | |
| Education duration, yr | 13.2 +/−2.4 | 14.3 +/−3.0 | 12.8 +/−3.0 | 0.03 a |
| Race | < 0.001 a | |||
| African American | 27.5 | 7.9 | 11.2 | |
| White | 54.9 | 89.1 | 82.5 | |
| Other | 17.7 | 3.0 | 6.2 | |
| Dialysis duration category | 0.7 b | |||
| 0-12 mo | 27.6 | - | 28.5 | |
| 13-24 mo | 31.0 | - | 24.3 | |
| > 24 mo | 41.4 | - | 47.2 | |
| dialysis duration, mo | 23.0 +/−15.6 | - | 32.8 +/−32.8 | 0.005 b |
| Diabetes | 41.2 | 22.8 | 46.8 | 0.01 a |
| Stroke | 11.8 | 7.9 | 23.4 | 0.4 a |
Note: Categorical variables are presented as column percentages; continuous variables are presented as mean +/− standard deviation. Control group consisted of individuals without CKD.
The chi-square test for categorical variables was used to compare the PD cohort and the comparison group.
The chi-square test for categorical variables was used to compare the PD cohort and HD cohorts. Abbreviations: CKD, chronic kidney disease; HD, hemodialysis; PD, peritoneal dialysis.
Table 2 shows the mean cognitive test scores for the three cohorts. A higher score is better for all tests, including the 3MS, except for Color Trails and the Stroop interference test, which are timed; a longer time in seconds is worse. The percentages of peritoneal dialysis, hemodialysis, and control patients who scored ≤ 1.49 SD, 1.50-1.99 SD (moderate impairment), and ≥ 2.00 SD (severe impairment) below the age-adjusted norms are shown for each test. Between 21% and 46% of peritoneal dialysis patients scored ≥ 2 SD (severe impairment) below the norms on each test in the memory and executive function domains, and 25% in the verbal domain. Slightly less than a third (29.4%) scored ≥ 5 points on the Geriatric Depression Scale, suggesting depression. Compared with hemodialysis patients, more peritoneal patients had moderate to severe verbal memory impairment (58.3% vs. 51.2%), but fewer had moderate to severe impairment in executive function (decision-making) on the Color Trails (28.2% vs. 43%) and Stroop tests (33.3 % vs. 50%).
Table 2.
Percentage of Participants With Mean Scores Below Adjusted Means on Cognitive Tests
| Cognitive Test | Primary Cognitive Domain Measured |
Raw Score, Mean/Max a (SD) |
No. of SDs Below Adjusted Population Norms b |
||
|---|---|---|---|---|---|
| < 1.50 | 1.50-1.99 | ≥ 2.0 | |||
| Peritoneal Dialysis | |||||
| 3MS (total score) | Global cognitive function |
93.0/100 (6.7) | 86.3 | 7.8 | 5.9 |
| Hopkins Verbal Learning (delayed, words) |
Verbal memory | 6.1/12.0 (3.6) | 41.7 | 12.5 | 45.8 |
| Color Trails 2 (time, s) | Executive function | 134.5 (66.8) | 71.7 | 6.5 | 21.7 |
| BVMT-R (delayed, figures) | Visual-spatial memory | 6.4/12.0 (3.6) | 56 | 10 | 34 |
| Stroop Interference Test(s) | Executive function | 84.4 (27.7) | 66.7 | 8.3 | 25 |
| COWAT (total words) | Language | 28.8 (11.7) | 77.6 | 4.1 | 18.4 |
| Digit Span | Attention | 16.7/30.0 (3.5) | 100 | 0.0 c | |
| Clock-drawing | Executive function, visual-spatial |
3.6/4.0 (0.6) | 4.1 d | ||
| Geriatric Depression Scale | Depression | 3.1 (2.8) | 29.4 e | ||
| Hemodialysis | |||||
| 3MS (total score) | Global cognitive function |
88.3/100 (8.6) | 59.5 | 27.5 | 13.0 |
| Hopkins Verbal Learning (delayed, words) |
Verbal memory | 5.1/12.0 (3.2) | 48.5 | 13.3 | 38.2 |
| Color Trails 2 (time, s) | Executive function | 156.5 (53.9) | 57.0 | 7.2 | 35.8 |
| BVMT-R (delayed, figures) | Visual-spatial memory | 4.7/12.0 (3.0) | 45.7 | 18.5 | 35.8 |
| Stroop Interference Test(s) | Executive function | 110.4 (43.2) | 49.8 | 9.0 | 41.1 |
| COWAT (total words) | Language | 26.4 (11.1) | 71.3 | 17.8 | 10.9 |
| Digit Span | Attention | 14.8/30.0 (3.7) | 97.3 | 2.7 f | |
| Clock-drawing | Executive function, visual-spatial |
3.3/4.0 (0.8) | 14.2 g | ||
| Geriatric Depression Scale | Depression | 3.1 (2.6) | 25.0 h | ||
| Non-CKD Control Group | |||||
| 3MS (total score) | Global cognitive function |
94.3/100 (5.7) | 87.1 | 9.9 | 3.0 |
| Hopkins Verbal Learning (delayed, words) | Verbal memory | 6.8/12.0 (3.1) | 69.3 | 10.9 | 19.8 |
| Color Trails 2 (time, s) | Executive function | 117.1 (46.0) | 78.2 | 8.9 | 12.9 |
| BVMT-R (delayed, figures) | Visual-spatial memory | 6.8/12.0 (2.8) | 76.2 | 7.9 | 15.8 |
| Stroop Interference Test(s) | Executive function | 71.2 (23.8) | 80.2 | 7.9 | 11.9 |
| COWAT (total words) | Language | 43.9 (15.8) | 92.1 | 3.0 | 5.0 |
| Digit Span | Attention | 18.2/30.0 (4.2) | 99 | 1.0 i | |
| Clock-drawing | Executive function, visual-spatial |
3.6/4.0 (0.6) | 5.9 j | ||
| Geriatric Depression Scale | Depression | 1.6 (1.8) | 3.0 k | ||
Note: Values shown are percentages, unless otherwise indicated. For all tests, higher score is better, except for the two timed tests, Color Trails and Stroop interference test, for which a longer time in seconds is worse.
3MS, Modified Mini-Mental State Examination; BVMT-R, Brief Visuospatial Memory Test Revised; COWAT, Controlled Oral Word Association Test; SD, standard deviation; CKD, chronic kidney disease
Maximum score provided only if maximum exists.
Published normative scores were adjusted for age for the Hopkins, BVMT-R, and Stroop; for age and education for Color Trials; and for age, education, and race for the COWAT.
0.0% scored > 1.50 SD below normal mean.
4.1% scored ≤ 2 out of 4.
29.4 % scored ≥ 5 points, suggesting depression.
2.7% scored > 1.50 SD below normal mean.
14.2% scored ≤ 2 out of 4.
25.0 % scored ≥ 5 points, suggesting diagnosis of depression.
1.0% scored > 1.50 SD below normal mean.
5.9% scored ≤ 2 out of 4.
3.0% scored ≥ 5 points, suggesting diagnosis of depression.
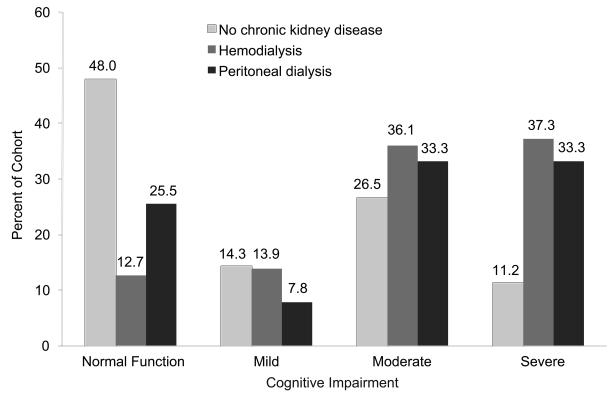
Table 3 describes the percentage of peritoneal dialysis, hemodialysis, and non-CKD control patients with cognitive impairment by age, sex, education, and race using the cognitive impairment algorithm. Of the peritoneal dialysis patients, 25.5% had no cognitive impairment, 7.8% mild cognitive impairment, 35.3% moderate cognitive impairment, and 31.4% severe cognitive impairment. Of the non-CKD cohort, 60.4% had no or mild, 26.7% moderate, and 12.9% severe cognitive impairment; corresponding values were 26.6%, 36.4%, and 37.0% of the hemodialysis cohort. None of the peritoneal patients had a documented medical history of memory impairment or dementia. Of note, only three of the patients with moderate cognitive impairment were living alone, and none of the patients with mild or severe cognitive impairment were living alone.
Table 3.
Frequency of Cognitive Impairment
| PD Cohort (n = 51) | Non-CKD Control Group (n =101) | HD Cohort (n = 338) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No, | Cognitive Impairment | No. | Cognitive Impairment | No. | Cognitive Impairment | |||||||
| None/Mild | Moderate | Severe | None/Mild | Moderate | Severe | None/Mild | Moderate | Severe | ||||
| No. | 17 | 18 | 16 | 61 | 27 | 13 | 90 | 122 | 126 | |||
| Age | ||||||||||||
| < 55 y | 19 | 42.1 | 26.3 | 31.6 | 0 | NA | NA | NA | NA | NA | NA | NA |
| 55-64 y | 17 | 29.4 | 29.4 | 41.2 | 41 | 65.9 | 19.5 | 14.6 | 100 | 22.0 | 37.0 | 41.0 |
| ≥ 65 y | 15 | 26.7 | 53.3 | 20.0 | 60 | 56.7 | 31.7 | 11.7 | 238 | 28.6 | 35.7 | 35.7 |
| Sex | ||||||||||||
| Women | 17 | 58.8 | 23.5 | 17.6 | 57 | 70.2 | 19.3 | 10.5 | 155 | 22.0 | 40.6 | 37.4 |
| Men | 34 | 20.6 | 41.2 | 38.2 | 44 | 47.7 | 36.4 | 15.9 | 183 | 30.6 | 32.2 | 37.2 |
| Education | ||||||||||||
| 0-8 y | 2 | 0.0 | 50.0 | 50.0 | 4 | 0.0 | 25.0 | 75.0 | 38 | 13.2 | 31.5 | 55.3 |
| 9-12 y | 21 | 42.9 | 28.6 | 28.6 | 29 | 58.6 | 24.1 | 17.2 | 147 | 19.7 | 39.5 | 40.8 |
| > 12 y | 27 | 29.6 | 40.7 | 29.6 | 68 | 64.7 | 27.9 | 7.4 | 153 | 36.6 | 34.0 | 29.4 |
| Race | ||||||||||||
| African American | 14 | 28.6 | 42.9 | 28.6 | 8 | 37.5 | 25.0 | 37.5 | 38 | 5.3 | 42.1 | 52.6 |
| White | 28 | 39.3 | 35.7 | 25.0 | 90 | 63.3 | 26.7 | 10.0 | 279 | 30.8 | 35.5 | 33.7 |
| Other | 9 | 22.2 | 22.2 | 55.6 | 3 | 33.3 | 33.3 | 33.3 | 21 | 9.6 | 33.3 | 57.1 |
| Total | 51 | 33.3 | 35.3 | 31.4 | 101 | 60.4 | 26.7 | 12.9 | 338 | 26.6 | 36.1 | 37.3 |
Note: Values are percents unless otherwise indicated.
Abbreviations: CKD, chronic kidney disease; HD, hemodialysis; PD, peritoneal dialysis; NA, not applicable.
Figure 1 describes the percentage of peritoneal, hemodialysis, and control group patients with each level of cognitive impairment, using the cognitive impairment algorithm in Box 1, data from Table 3, and data from our previous study of hemodialysis patients.1 On ANOVA and chi-squared analyses to measure factors associated at the P < 0.10 level with moderate to severe cognitive impairment in the peritoneal dialysis cohort (age; sex; race; education; continuous ambulatory vs. overnight peritoneal dialysis; duration of dialysis; hypertension; systolic blood pressure; previously diagnosed diabetes, depression, and stroke; and living alone vs. with friends or family, spouse, or other), hemoglobin was significantly associated (P = 0.02), as were stroke (P = 0.07) and sex (P = 0.01). A logistic regression model including age, sex, race, education, hemoglobin, diabetes, and stroke showed that only race (P = 0.002) and education (P = 0.002) were associated with moderate to severe cognitive impairment.
Figure 1.
Percentages of the peritoneal dialysis, hemodialysis, and non-chronic-kidney-disease comparison cohorts with cognitive impairment.
In a logistic regression model with covariates for each of the three cohorts and moderate to severe cognitive impairment as the outcome, adjusted for age, sex, race, education, diabetes, and stroke (Table 4), peritoneal dialysis was associated with a more than 2.5-fold increased risk of severe cognitive impairment compared with no CKD (odds ratio 2.58, 95% confidence interval [CI] 1.02-6.53), as was hemodialysis (OR, 3.16; 95% CI, 1.9-5.24). Non-white race and low education were also strongly associated with moderate to severe cognitive impairment. In a sensitivity analysis using the same model and excluding peritoneal dialysis patients aged < 55 years, results did not change appreciably except that the effect of race was no longer significant. In addition, a sensitivity analysis using the same covariates with ordinal logistic regression (using each of the four categories of cognitive impairment as outcomes) was carried out, with very similar results.
Table 4.
Odds of Moderate to Severe Cognitive Impairment in PD and HD Patients Compared With Patients Without CKD
| Variables | OR (95% CI) |
|---|---|
| Age | |
| < 55 y | 1.00 (reference) |
| 55-64 y | 2.76 (0.74-10.35) |
| ≥ 65 y | 2.54 (0.66-9.68) |
| Diabetes | |
| No | 1.00 (reference) |
| Yes | 1.37 (0.89-2.12) |
| Stroke | |
| No | 1.00 (reference) |
| Yes | 1.47 (0.84-2.59) |
| Race | |
| White | 1.00 (reference) |
| African American | 3.47 (1.55-7.77) |
| Other | 3.36 (1.16-9.72) |
| Education | |
| > 12 y | 1.00 (reference) |
| < 8 y | 4.85 (1.78-13.21) |
| 8-12 y | 1.71 (1.11-2.64) |
| Sex | |
| Men | 1.00 (reference) |
| Women | 0.85 (0.56-1.28) |
| Cohort | |
| Non-CKD | 1.00 (reference) |
| HD | 3.16 (1.91-5.24) |
| PD | 2.58 (1.02-6.53) |
CI, confidence interval; CKD, chronic kidney disease; OR, odds ratio, PD, peritoneal dialysis; HD, hemodialysis.
DISCUSSION
Our study shows that approximately 75% of this peritoneal dialysis patient cohort was cognitively impaired; about a third were moderately and a third severely impaired. Peritoneal dialysis patients were more than 2.5 times more likely than non-CKD participants to develop moderate to severe cognitive impairment. These rates are also significantly higher than the estimated prevalence of dementia in people aged 65 years and older without ESRD in community-based population studies, which ranges from about 10% for all people aged older than 65 years to 35% to 40% for those aged older than 85 years.19;20 In this study, moderate to severe cognitive impairment was almost as common in peritoneal dialysis patients as in hemodialysis patients in our previous study (35.3% moderate and 31.4% severe cognitive impairment in peritoneal dialysis patients; 36.1% moderate and 37.3% severe cognitive impairment in hemodialysis patients).1 This is despite the peritoneal dialysis cohort being on average 11 years younger than the hemodialysis cohort (57.5 vs. 68.5 years).
In contrast, in the much larger US Renal Data System (USRDS) database, dementia rates for hemodialysis patients are about 1.5- to 2-fold higher than for peritoneal dialysis patients, with a strong age effect.21 For example, among African Americans aged 65-74 years in the USRDS, dementia prevalence was about 8% for 2003 prevalent hemodialysis patients and 4% for peritoneal dialysis patients; among African Americans aged 75-84 years, prevalence was 17% for hemodialysis patients and 9% for peritoneal dialysis patients. However, because peritoneal dialysis patients may come to medical attention less often than hemodialysis patients, lower prevalence of dementia among peritoneal dialysis patients may be due to ascertainment bias. In addition, because dialysis patients are rarely assessed for dementia, these Medicare claims-based rates are underestimated by two- to three-fold. We did not see a strong age effect of severe cognitive impairment in our cohort, likely due to our use of age- and education-adjusted norms on the individual cognitive tests used to classify cognitive impairment.
Our study was not designed to determine how impairment in specific cognitive domains or severity of impairment affects ability to administer peritoneal dialysis safely and effectively; this would require a clinical outcomes study. However, even mild cognitive impairment is associated with changes in ability to perform instrumental activities of daily living22-24; activities most affected are taking medication, keeping appointments, managing belongings, preparing meals, and talking about recent events.23 About one-third of our peritoneal dialysis cohort was moderately to severely impaired in executive function, reflected by low scores on the Color Trails and Stroop tests. These tests measure judgment and ability to make decisions, apply abstract concepts, and change paradigms. Self-administering peritoneal dialysis is a complex activity that involves multiple aspects of executive function, including assessing the timing and safety of the procedure.
Verbal memory was also moderately to severely impaired in almost 60% of our cohort, as measured by the HVLRT-R. Impaired memory and executive function greatly affect ability to set up a pill box and to remember which medication is for what indication and when to take it.25 Taken together, these findings raise serious concerns regarding the ability of peritoneal dialysis patients to consistently and safely perform the complex tasks of self-administering dialysis and medications and avoid costly iatrogenic hospitalizations.
Cognitive impairment also increases costs associated with dialysis care. In one study, a low Mini-Mental Status Exam score in hemodialysis patients is significantly associated with increased dialysis technician time and increased hospitalizations.26 In USRDS studies, dementia in hemodialysis patients increases the risk of hospitalization, annual Medicare costs, and mortality.26
There has been little previous work on cognitive impairment in peritoneal dialysis patients. One study found that the Mini-Mental State exam score was slightly lower in 25 continuous ambulatory peritoneal dialysis patients (mean age 44.2 ± 3.9 years) compared with 25 controls (29.0 ± 0.3 vs. 30 ± 0.2, P < 0.001), but higher than in 17 hemodialysis patients (mean age 37.3 ± 2.7 years) predialysis (score 26.0 ± 1.5, P < 0.001) but not postdialysis. P300 event-related potential latencies were significantly shorter for peritoneal dialysis (337 ± 14.1 msec) than for hemodialysis patients (378 ± 8.9 msec, P < 0.05), but were not significantly different from controls, possibly reflecting slowed cognitive processing for the hemodialysis patients. However, diabetic patients were excluded from the study.27
The pathophysiology of cognitive impairment in peritoneal dialysis patients is undefined, but likely has a large vascular ischemic component combined with neurodegenerative pathology, exacerbated by chronic inflammation.6;28-30 On brain magnetic resonance imaging (MRI), Kim et al found that white matter disease (leukoaraiosis) was present in 68% of 57 peritoneal dialysis patients.31 White matter disease found on MRI corresponds to vascular degenerative morphology consistent with chronic hypoxia and vascular hypoperfusion on autopsy. These pathologic changes include hyalinosis of blood vessels, demyelination of neurons, endothelial and microglial activation, and increased levels of molecular markers of hypoxia such as hypoxiainducible factors and matrix metalloproteinase-7.32 White matter disease is associated with increased risk of stroke, disability, cognitive impairment, and decline.33;34
In hemodialysis patients, the dialysis process results in large acute intravascular volume loss and fluid shifts leading to cerebral edema, decreased cerebral perfusion, and cerebral ischemia, all of which may contribute to cognitive impairment.35;36 However, peritoneal dialysis does not involve the same extent of acute fluid and electrolyte shifts; thus, the high prevalence of stroke and elevated levels of inflammation, uremia, and cardiovascular risk factors among peritoneal dialysis patients may be the strongest contributors to cognitive impairment.
Most ESRD patients have diffuse vasculopathy with secondary elevated rates of cardiovascular events.37;38 Although stroke doubled the risk of severe cognitive impairment in our hemodialysis cohort,1 it was not a risk factor in the peritoneal dialysis cohort. This may be due to low occurrence and underreporting of stroke in this cohort, and to low statistical power. In the USRDS data, stroke increased the risk of dementia in peritoneal patients by more than 2.5-fold in adjusted models, and more than doubled the risk in the hemodialysis population.21
Diabetes was present in 41% of our peritoneal dialysis cohort. Diabetes as primary cause of ESRD in the USRDS data was associated with an odds ratio of 1.8 (95% CI, 1.14-2.79) for dementia in peritoneal dialysis patients.21 Daily prolonged exposure to the high glucose load in the peritoneal dialysis dialysate is associated with weight gain (fat deposition), hyperleptinemia, and dyslipidemia, which may lead to increased risk of cardiovascular disease including micro-and macrocerebrovascular disease.39 Mid-abdominal obesity and metabolic syndrome, prevalent in peritoneal dialysis patients, have also been identified as risk factors for dementia in nondialysis patients.40-42
We found probable depression in 29.4% of our peritoneal dialysis cohort, according to Geriatric Depression Scale scores, compared with 25% of our hemodialysis cohort. Depression can increase the risk of cognitive impairment for non-CKD patients, but was not found to be associated with severe cognitive impairment in either the peritoneal dialysis or the hemodialysis cohort. Depression also affects quality of life however, and warrants treatment with antidepressants.
None of the peritoneal dialysis patients with cognitive impairment had a previously documented history of it, implying that cognitive impairment in these patients is both unsuspected and undetected. Even if it were detected, currently dementia is not recordable as a comorbid condition on the Medical Evidence Report (Centers for Medicare & Medicaid Services form 2728, required for Medicare certification of payments for dialysis treatment). Most patients who choose peritoneal dialysis instead of hemodialysis are regarded as independent and capable of self-administering dialysis and medications, unless they have caregivers; a smaller number choose peritoneal dialysis after failed transplants or hemodialysis. The high level of cognitive impairment among the presumably healthier patients who chose to participate in our study sample suggests a need to assess patients' ability to chose and maintain peritoneal dialysis before and regularly after dialysis initiation.
Patients can easily be screened for cognitive impairment in clinic using the Montreal Cognitive Assessment tool (MOCA),43 an excellent instrument that is freely available online (www.mocatest.org), and easily administered by nonprofessional trained assistants in about 10 minutes, similar to the MMSE. The MOCA measures performance in the cognitive domains most important for daily function: executive function (attention and decision-making skills), verbal memory, language, and visual-spatial skills. A score < 26 on the MOCA indicates at least mild cognitive impairment and that medication supervision and help with decision-making including dialysis initiation are likely needed; mean score for dementia is 16, but the addition of functional impairment in activities of daily living to a score < 26 is also indicative of dementia. Patients who score < 26 on the MOCA should be referred to a geriatrician, neurologist, or psychiatrist for a detailed neurologic evaluation and brain MRI and, if appropriate, further neuropsychological testing.
The strengths of our study include use of a structured uniform cognitive battery and an algorithm to classify cognitive impairment. We used validated neuropsychological tests that measure cognitive function in the three cognitive domains most critical for daily functioning and ability to adhere to a complex medication and dialysis regimen. The generalizability of our results is limited by the small sample size, but the proportion of peritoneal dialysis patients (n = 51) compared with our previous study of hemodialysis patients (n = 338) is similar to the national percentage of approximately 12% of all dialysis patients.1;44 In addition, the mean age in our cohort was 56.0 years vs. 55.2 in the USRDS; our cohort included 27.5% African Americans vs. 26.2% in the USRDS, but a lower percentage of white peritoneal dialysis patients, 54.9% vs. 65.1% in the USRDS.44
Participation in this study was somewhat low and a healthy volunteer selection bias was unavoidable; however, since sicker patients and patients with severe cognitive impairment were less likely to participate, the measured frequency of cognitive impairment likely underestimates the true burden. We used age- and education-based norms for the neuropsychological tests, but because ethnicity-based norms were not available we could have overestimated cognitive impairment in nonwhite groups. Multiple studies in the general community population, however, have documented the increased risk of dementia and white matter disease in African Americans.45 The peritoneal dialysis cohort was significantly younger than the hemodialysis and control cohorts. Although this may have limited the robustness of our analyses to compare risk of cognitive impairment across the three cohorts, the strength of the increased risk of moderate to severe cognitive impairment in the peritoneal dialysis cohort (odds ratio 2.6) compared with the control cohort gives credence to our findings. In addition, the sensitivity analysis excluding patients aged < 55 years showed no substantial difference in the odds ratios for cognitive impairment.
Unfortunately, the burden of cognitive impairment does not appear to be substantially less in peritoneal dialysis patients than in hemodialysis patients, and cannot be ignored. Screening for cognitive impairment in peritoneal dialysis patients is urgently needed. Future studies in larger cohorts are needed to further evaluate the risk factors, prevalence, and natural history of cognitive impairment in the peritoneal dialysis population.
Supplementary Material
ACKNOWLEDGMENTS
Some results of this study were presented at the Alzheimer's Association International Conference on Alzheimer's Disease, Chicago, IL, July 28, 2008: Buot V, Pederson S, Kolste A, Zaun D, Murray AM. Cognitive impairment in peritoneal dialysis compared to hemodialysis and general medical patients. Alzheimers Dement. 2008;4(4 suppl 2):T403.
Support: Support for this work was provided by the Minneapolis Medical Research Foundation, Minneapolis, Minnesota, and National Institute on Aging Grant K021174A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org.
The authors thank the dialysis patients for their participation. They also thank DaVita, Inc, for access to its patients and for staff guidance, and Chronic Disease Research Group colleagues Anne Shaw, BA, and Shane Nygaard, BA, for manuscript preparation and Nan Booth, MSW, MPH, ELS, for manuscript editing.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Reference List
- 1.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 2.Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- 3.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 5.Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12:1549–1557. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
- 6.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. 2008;15:123–132. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 8.Brandt J, Benedict RHB. Hopkins Verbal Learning Test Revised. 2001 doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 9.D'Elia LF, Satz P, Lyons Uchiyama CL, White T. In: Color Trails Test. Professional Manual. Psychological Assessment Resources I, editor. Odessa, FL: 1996. [Google Scholar]
- 10.Delis D, Kaplan E, Kramer JH. In: Delis-Kaplan Executive Function System. The Psychological Corporation, editor. The Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- 11.Benedict RHB. Psychological Assessment Resources I, editor. Brief Visuospatial Memory Test - Revised. 1997 [Google Scholar]
- 12.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 13.Tuokko H, Hadjistavropoulos T, Miller JA, Beattie BL. The Clock Test: a sensitive measure to differentiate normal elderly from those with Alzheimer disease. J Am Geriatr Soc. 1992;40:579–584. doi: 10.1111/j.1532-5415.1992.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendez MF, Ala T, Underwood KL. Development of scoring criteria for the clock drawing task in Alzheimer's disease. J Am Geriatr Soc. 1992;40:1095–1099. doi: 10.1111/j.1532-5415.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. In: Wechsler Memory Scale. Administration and Scoring Manual. The Psychological Corporation, editor. The Psychological Corporation; San Antonio, Texas: 1997. [Google Scholar]
- 16.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-IV) 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 19.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population of older persons; higher than previously reported. JAMA. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- 21.U.S. Renal Data System . USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2005. pp. 140–143. [Google Scholar]
- 22.Perneczky R, Pohl C, Sorg C, et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing. 2006;35:240–245. doi: 10.1093/ageing/afj054. [DOI] [PubMed] [Google Scholar]
- 23.Ahn IS, Kim JH, Kim S, et al. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. 2009;6:180–184. doi: 10.4306/pi.2009.6.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55:570–581. doi: 10.1159/000228918. [DOI] [PubMed] [Google Scholar]
- 25.Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: three studies. Health Psychol. 2010;29:50–55. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 27.Tilki HE, Akpolat T, Tunali G, Kara A, Onar MK. Effects of haemodialysis and continuous ambulatory peritoneal dialysis on P300 cognitive potentials in uraemic patients. Ups J Med Sci. 2004;109:43–48. doi: 10.3109/2000-1967-109. [DOI] [PubMed] [Google Scholar]
- 28.Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12:1549–1557. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
- 29.Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Iturbe B, Garcia GG. The Role of Tubulointerstitial Inflammation in the Progression of Chronic Renal Failure. Nephron Clin Pract. 2010;116:c81–c88. doi: 10.1159/000314656. [DOI] [PubMed] [Google Scholar]
- 31.Kim CD, Lee HJ, Kim DJ, et al. High prevalence of leukoaraiosis in cerebral magnetic resonance images of patients on peritoneal dialysis. Am J Kidney Dis. 2007;50:98–107. doi: 10.1053/j.ajkd.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–2625. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- 34.Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postiglione A, Faccenda F, Gallotta G, Rubba P, Federico S. Changes in middle cerebral artery blood velocity in uremic patients after hemodialysis. Stroke. 1991;22:1508–1511. doi: 10.1161/01.str.22.12.1508. [DOI] [PubMed] [Google Scholar]
- 36.Hata R, Matsumoto M, Handa N, Terakawa H, Sugitani Y, Kamada T. Effects of hemodialysis on cerebral circulation evaluated by transcranial Doppler ultrasonography. Stroke. 1994;25:408–412. doi: 10.1161/01.str.25.2.408. [DOI] [PubMed] [Google Scholar]
- 37.Collins AJ, Li S, Ma JZ, Herzog C. Cardiovascular disease in end-stage renal disease patients. Am J Kidney Dis. 2001;38:S26–S29. doi: 10.1053/ajkd.2001.27392. [DOI] [PubMed] [Google Scholar]
- 38.Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16:101–105. doi: 10.1046/j.1525-139x.2003.16025.x. [DOI] [PubMed] [Google Scholar]
- 39.Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol. 2007;2:1317–1328. doi: 10.2215/CJN.02550607. [DOI] [PubMed] [Google Scholar]
- 40.Cereda E, Sacchi MC, Malavazos AE. Central obesity and increased risk of dementia more than three decades later. Neurology. 2009;72:1030–1031. doi: 10.1212/01.wnl.0000343499.72241.ea. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–328. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanaya AM, Lindquist K, Harris TB, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66:329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Renal Data System . USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2007. [Google Scholar]
- 45.Alzheimer's Association 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.