Summary
Cytoplasmic second messengers, Ca2+ and cAMP, regulate nerve growth cone turning responses induced by many guidance cues, but the causal relationship between these signaling pathways has been unclear. We here report that, for growth cone turning induced by a gradient of myelin-associated glycoprotein (MAG), cAMP acts by modulating MAG-induced Ca2+ signaling. Growth cone repulsion induced by MAG was accompanied by localized Ca2+ signals on the side of the growth cone facing the MAG source, due to Ca2+ release from intracellular stores. Elevating cAMP signaling activity or membrane depolarization enhanced MAG-induced Ca2+ signals and converts growth cone repulsion to attraction. Directly imposing high- or low-amplitude Ca2+ signals with an extracellular gradient of Ca2+ ionophore was sufficient to trigger either attractive or repulsive turning, respectively. Thus distinct Ca2+ signaling, which can be modulated by cAMP, mediates the bi-directional turning responses induced by MAG.
Keywords: growth cone guidance, myelin-associated glycoprotein, Ca2+ signaling, cAMP signaling
Myelin-associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994; Schafer et al., 1996), a key inhibitory component in the myelin of the central nervous system, is partly responsible for preventing axon regeneration after spinal cord injury. A soluble form of MAG, which is released endogenously from myelin (Sato et al., 1982; Tang et al., 1997), inhibits neurite outgrowth (Tang et al., 1997) and induces repulsion of neuronal growth cones (Song et al., 1998; Ming et al., 1999; Ming et al., 2001) in cell culture. Recent studies (Domeniconi et al., 2002; Liu et al., 2002; Wang et al., 2002; Wong et al., 2002) have shown that MAG binds with high affinity to the Nogo-66 receptor (NgR), which forms a complex with the low affinity pan-neurotrophin receptor p75NTR to mediate its cytoplasmic signaling. Activation of the NgR-p75NTR receptor complex by MAG leads to an increase in cytoplasmic Ca2+ ([Ca2+]i) (Wong et al., 2002) and activation of protein kinase C (PKC) (Sivasankaran et al., 2004), the small GTPase RhoA, and its effector Rho-associated kinase (Yamashita et al., 2002). Although the signal transduction pathway underlying growth cone responses to MAG remains to be fully elucidated, two second messengers – Ca2+ and cAMP – appear to play key regulatory roles (Song et al., 1998, Ming et al., 2001, Henley and Poo, 2004). In cultured Xenopus spinal neurons, repulsive turning of the growth cone induced by an extracellular gradient of MAG can be converted to attraction by elevating cAMP in the neuron (Song et al., 1998). Because the depletion of extracellular Ca2+ ([Ca2+]o) abolishes both repulsive and attractive turning induced by MAG (Song et al., 1998), and MAG (Wong et al., 2002) and Nogo (Bandtlow et al., 1993; Loschinger et al., 1997) both induce an elevation of [Ca2+]i, it appears that Ca2+ may mediate MAG signaling in the neuron, similar to that found for growth cone turning responses in a netrin-1 gradient (Hong et al., 2000; Ming et al., 2002; Nishiyama et al., 2003). However, two outstanding issues concerning Ca2+ and cAMP signaling at the growth cone remain unresolved. First, the precise spatiotemporal pattern of Ca2+ signals that leads to attractive versus repulsive turning is unclear. We do not know whether it is the absolute magnitude of Ca2+ elevation or the polarity of Ca2+ gradient that determines the turning response. Second, the causal relationship between Ca2+- and cAMP-dependent signaling remains to be determined.
In cultured Xenopus neurons, attractive and repulsive turning of growth cones can be induced by an extracellular gradient of a low or high concentration of ryanodine, respectively (Hong et al., 2000). Ryanodine is known to open Ca2+ release channels in the ER membrane at low concentrations but blocks these channels at high concentrations (Zucchi and Ronca-Testoni, 1997). Thus a gradient of high concentration might induce a reverse Ca2+ gradient across the growth cone (Hong et al., 2000). On the other hand, focal elevation of [Ca2+]i by photoactivated release of caged Ca2+ on one side of the growth cone can result in repulsive turning away from the irradiated side (Zheng, 2000). It is unclear whether these direct experimental manipulations of [Ca2+]i mimic Ca2+ signaling accompanying growth cone repulsion induced by endogenous factors such as MAG. Thus it is important to determine the magnitude and polarity of Ca2+ elevation across the growth cone in response to a gradient of MAG that induces repulsive turning.
In the present study, we first showed that MAG induces a transient and polarized increase in [Ca2+]i at the growth cone that is higher on the side facing the MAG source before the onset of repulsive turning. This Ca2+ signal, which is of lower amplitude than that induced by netrin-1, is caused by Ca2+ release from intracellular stores rather than Ca2+ influx through plasmalemmal channels. Furthermore, we found that increased cAMP signaling activity, which induces switching of the MAG-induced turning response from repulsion to attraction, elevates basal [Ca2+]i in the growth cone and leads to a higher amplitude Ca2+ signal in response to MAG. In addition, we found that elevating basal [Ca2+]i to a similar extent by depolarization with a high-K+ medium also elevates the MAG-induced Ca2+ signal and switches growth cone turning from repulsion to attraction. We then confirmed the notion that gradients of high and low amplitude Ca2+ signals of the same polarity are sufficient to trigger attractive and repulsive turning, respectively, using an extracellular gradient of Ca2+ ionophore in the presence of defined [Ca2+]o. To further address the causal relationship between Ca2+ and cAMP signaling, we showed that attractive turning induced by direct Ca2+ elevation with a gradient of ionophore is independent of cAMP signaling activity, whereas attractive turning induced by a gradient of cAMP requires Ca2+ elevation in the growth cone. Taken together, these findings support the notion that coincident, modulatory cAMP-signals act by elevating [Ca2+]i to convert MAG-induced repulsion to attraction, and that attractive and repulsive turning of the growth cone are mediated by [Ca2+]i elevations of the same polarity but differing magnitude.
Results
A MAG gradient induces a gradient of Ca2+ signals in the growth cone
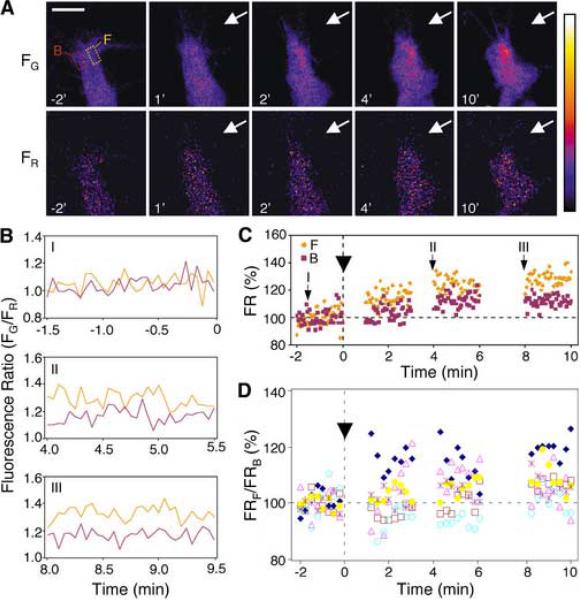
In cultures of Xenopus spinal neurons, an extracellular gradient of MAG applied to the growth cone induced an elevation of [Ca2+]i (Figure 1A) and caused a subsequent repulsive turning of the growth cone (Song et al., 1998; Ming et al., 1999; Ming et al., 2001; Wong et al., 2002) (see also Figure 3). The amplitude of [Ca2+]i elevation in the growth cone, as monitored by fluorescence ratio imaging with the Ca2+-sensitive probes Oregon Green BAPTA and Fura Red, corresponded to an average [Ca2+]i elevation throughout the growth cone of about 45 nM over the basal level of 65 nM (see Experimental Procedures). This increase (~4% in the fluorescence ratio, Figures 2A and 2B) was about half of that associated with the attractive turning induced by a netrin-1 gradient (Hong et al., 2000; Ming et al., 2002). When fluorescence imaging was taken at a time resolution of 3 s, we noted more frequent transient elevations of [Ca2+]i on the side of the growth cone facing (`F') the MAG gradient than the back side (`B') (Figures 1A–1C). This led to localized Ca2+ transients across the growth cone, each lasting for tens of seconds. These gradients, which were absent during the control period, appeared within minutes after the onset of the MAG gradient but before repulsive growth cone turning could be detected. Figures 1A–1C depict the time course of [Ca2+]i changes during the first 10 min after the onset of a MAG gradient, with fluorescence ratios assayed at two sides of the growth cone. Figure 1D summarizes the results of all six experiments in which Ca2+ gradients were mapped in the form of normalized ratios of [Ca2+]i on the two sides. Gradients of Ca2+, which were detected in 5/6 cases during this time frame, always exhibited the same polarity, with [Ca2+]i highest on the side of the growth cone facing the MAG source. The existence of such localized Ca2+ transients supports the notion that Ca2+ signals can convey directional cues provided by the MAG gradient.
Figure 1.
Localized Ca2+ signals in the growth cone induced by an extracellular MAG gradient.
(A) Fluorescence images of the growth cone of a cultured Xenopus spinal neuron, which was co-injected with the Ca2+-sensitive fluorescence indicators Oregon Green BAPTA-dextran (FG) and Fura Red (FR), are shown in pseudocolor, with blue and white representing lowest and highest fluorescence intensities, respectively. The time (in min) before and after the onset of the MAG gradient (arrows), and regions of interest (ROIs) used to quantify fluorescence intensities on either side of the growth cone (F and B), are indicated (boxes in top left panel). Scale bar, 10 μm. (B) Traces depict [Ca2+]i, determined from the mean fluorescence ratio (FR = FG/FR) within each ROI shown in (A), at various times before and after exposure to a MAG gradient. Yellow and purple traces indicate the fluorescence ratio (acquired every 3 s) localized to the `front' (F) side of the growth cone facing the MAG gradient or the opposite `back' (B) side, respectively. (C) Data points represent the fluorescence ratio (FR) within each ROI shown in (A), normalized to the mean fluorescence ratio during the 2 min period before application of the MAG gradient (at time `0', arrowhead). Time points representing the start of each trace in (B) are indicated (arrows). (D) Summary of all growth cones imaged as in (B) that showed a MAG-induced [Ca2+]i increase. Data points (averaged over 15-s bins) represent the fluorescence ratio of the front ROI (FRF) divided by the fluorescence ratio of the back ROI (FRB), normalized to the mean FRF/FRB ratio during the 2 min period before starting the MAG gradient (arrowhead) for each case (symbols represent 6 different growth cones).
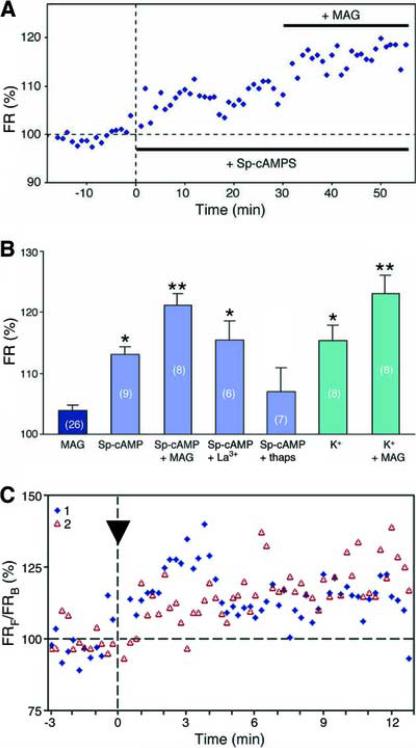
Figure 3.
MAG-induced Ca2+ signals in the growth cone are modulated by cAMP.
(A) An example showing changes in [Ca2+]i in the growth cone with time after treatment with Sp-cAMPS and subsequent exposure to a MAG gradient. The [Ca2+]i level over the entire growth cone was determined by fluorescence ratio imaging and data points show the fluorescence ratio (FR) normalized to the mean value for the 10-min period prior to adding Sp-cAMPS to the external solution. Images were collected every 30 s and the data are shown in 1-min bins. (B) Summary of percentage changes in [Ca2+]i. The [Ca2+]i level in the entire growth cone was determined by ratio imaging as in (A). The mean for data points acquired during the last 10 min of a 30-min treatment (with 20 μM Sp-cAMPS, 20 μM Sp-cAMPS and 100 μM La3+, 20 μM Sp-cAMPS and 250 nM thapsigargin, or 9.3 mM K+), or 5 – 15 min after superimposing the MAG gradient, as indicated, were normalized to the mean value for the 10-min period prior to any treatment. Data are the mean ± s.e.m. (n = number indicated; *P < 0.01, compared to the MAG alone group, Mann-Whitney U-test; **P < 0.01, compared to the same treatment without the MAG gradient). (C) Summary of all growth cones imaged as in Figure 1B to detect gradients of [Ca2+]i. Data points (averaged over 15-s bins) represent the fluorescence ratio of the front ROI (FRF) divided by the fluorescence ratio of the back ROI (FRB), normalized to the mean FRF/FRB value during the 2 min period before starting the MAG gradient (arrowhead) for each case (1 and 2).
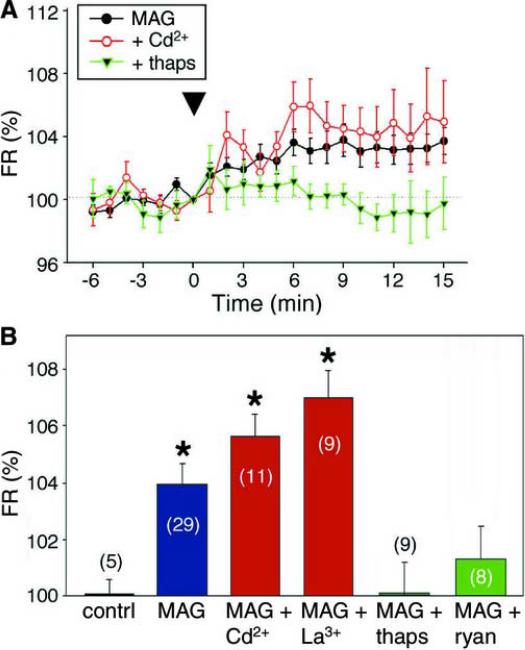
Figure 2.
MAG induces Ca2+ release from internal stores.
(A) Summary of [Ca2+]i changes observed at the entire growth cone induced by a MAG gradient (onset at time `0') either alone (MAG) or after adding cadmium (50 μM; Cd2+) or thapsigargin (250 nM; thaps) to the external solution. Images were acquired every 30 s and the fluorescence ratio (FR) measured at each time point was normalized to the mean value obtained during the 5-min period before the onset of the MAG gradient for each growth cone. Data points represent the mean ± s.e.m. for 1-min bins (n = 29, 11, and 9, for MAG, Cd2+, and thaps, respectively). (B) Summary of percentage changes in [Ca2+]i. The [Ca2+]i level in the entire growth cone was determined by the fluorescence ratio (FR) as in (Figure 1D), and the mean for data points acquired 5 – 15 min after starting the gradient was normalized to the mean for the 5-min period prior to the onset of the gradient. The [Ca2+]i change in response to gradients of the saline solution (contrl) and MAG either alone or after the treatments with 50 μM Cd2+ (MAG + Cd2+), 100 μM La3+ (MAG + La3+), 250 nM thapsigargin (MAG + thaps) or 100 μM ryanodine (MAG + ryan) are indicated. The number associated with each bar is the total number of growth cones examined for each group. Data are the mean ± s.e.m. Values that are significantly different from that of the external solution control are marked (*P < 0.01, Mann-Whitney U-test).
It has been shown that growth cone turning responses induced by netrin-1 require both Ca2+ influx through plasmalemmal Ca2+ channels and Ca2+-induced Ca2+ release from internal stores (Hong et al., 2000). We therefore examined the source of MAG-induced Ca2+ signals. As shown in Figures 2A and 2B, we found that treatment of the culture with thapsigargin (Thastrup et al., 1990), which depletes internal Ca2+ stores, abolished MAG-induced Ca2+ signals. Treatment with a high concentration of ryanodine, which inhibits Ca2+ release from ryanodine-sensitive channels in the ER, also reduced MAG-induced Ca2+ signals. In contrast, treatment with Cd2+ (50 μM) or La3+ (100 μM), which are known to block plasmalemmal Ca2+ channels, had no effect on MAG-induced Ca2+ signals. Thus, unlike netrin-1-induced attraction, MAG-induced repulsive Ca2+ signals do not require Ca2+ influx, but Ca2+ release from internal stores is required in both cases.
Cyclic AMP modulates MAG-induced Ca2+ signals
Previous studies (Song et al., 1998) have shown that uniform bath application of membrane-permeable cAMP analogues results in a conversion of MAG-induced growth cone turning from repulsion to attraction. To understand the interaction between cAMP and Ca2+ signals, we first examined whether cAMP-induced switching of growth cone turning is due to a cAMP-dependent modulation of Ca2+ signals. We found that incubation with the cAMP analogue Sp-cAMPS (20 μM), which leads to activation of protein kinase A (PKA), indeed increased [Ca2+]i (Figure 3). Importantly, in the presence of elevated cAMP, a MAG gradient induced a further [Ca2+]i elevation, which superimposed on top of the higher basal [Ca2+]i. This resulted in a larger Ca2+ signal, as compared to that induced by the MAG gradient alone (Figure 3B). The MAG-induced Ca2+ signals were not suppressed by incubation with Rp-cAMPS (data not shown), a competitive antagonist of cAMP for PKA, suggesting that the effects of MAG are not mediated by cAMP signaling (see also Ming et al., 2001). Furthermore, in the presence of elevated cAMP, a gradient of MAG continues to induce asymmetric [Ca2+]i elevations in the growth cone, with [Ca2+]i highest on the side facing the source of MAG (Figure 3C). Taken together, these results support the notion that although cAMP is not directly in the MAG signaling pathway, it can regulate MAG-induced growth cone responses by modulating the absolute [Ca2+]i of the growth cone, with a lower level (~100 nM) mediating repulsive turning and a higher level (~200 nM) mediating attraction.
Ca2+ signals are essential for MAG-induced bi-directional turning
If higher Ca2+ signals mediate the attractive turning induced by a MAG gradient, direct elevation of [Ca2+]i in the growth cone should convert MAG-induced repulsion to attraction. We tested this idea by depolarizing the neuron with an elevated K+ concentration in the external solution (from 1.3 to 9.3 mM). This treatment caused a significant elevation of [Ca2+]i (Figure 3B) that was sustained for a period of at least 90 min (data not shown). As shown in Figure 3B, a MAG gradient induced a further increase of [Ca2+]i on top of the higher basal [Ca2+]i, similar to that found with elevated cAMP signal (Figures 3A and 3B). When the turning response to a MAG gradient was examined in the normal K+ solution (1.3 mM), marked repulsion was observed as expected (Figure 4). However, in the presence of the high K+ external solution (9.3 mM), we found that MAG-induced repulsion of growth cones was converted to attraction. Thus the level of [Ca2+]i appears to determine the polarity of MAG-induced turning responses.
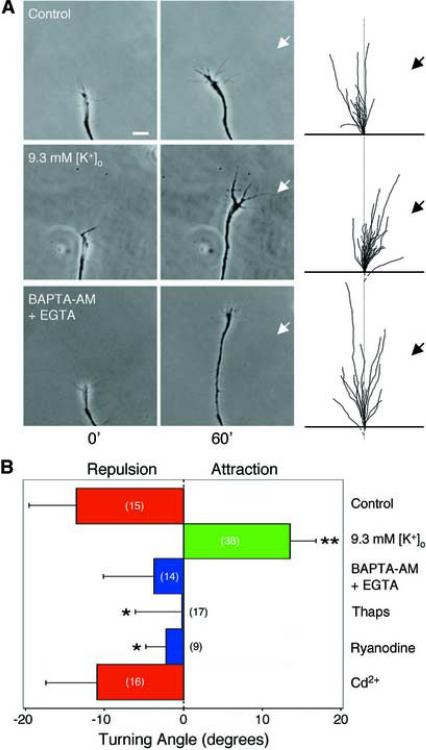
Figure 4.
Ca2+ signals mediate growth cone turning responses induced by MAG.
(A) Images of the growth cone of a cultured Xenopus spinal neuron at the onset (left) and the end (right) of a 1-hr exposure to a MAG gradient (arrow). Superimposed traces show the trajectory of neurite extension during the 1-hr period. The origin was the center of the growth cone at the onset of the assay and the original direction of extension was aligned vertical. Assays were done in normal saline, depolarizing saline (with 9.3 mM [K+]o), or low Ca2+ saline (with EGTA) for neurons preloaded with BAPTA-AM. Scale bar, 10 μm. (B) Average turning angles for the data illustrated in (A), in addition to that for growth cones exposed to a MAG gradient after treatment with 250 nM thapsigargin (Thaps) and 100 μM ryanodine (indicated) to inhibit Ca2+ release from internal stores or 50 μM Cd2+ to inhibit Ca2+ influx. For each experimental condition, the average angular position of all growth cones at the end of the 1-hr exposure to the MAG gradient is plotted. Data significantly different from that of the MAG alone control are marked (**P < 0.001 and *P < 0.02, Kolmogorov-Smirnov test).
If Ca2+ signals are necessary to mediate both attractive and repulsive growth cone turning induced by a MAG gradient, then growth cones should lose their responsiveness to MAG when Ca2+ signals are suppressed. To test this, neurons were preincubated with BAPTAAM (1 μM), which prevents elevation of [Ca2+] by its effective Ca2+ buffering capacity. Growth cone turning assays were then performed in a 35-nM Ca2+ external solution (see Experimental Procedures). As shown in Figure 4, under this essentially Ca2+-free condition, the trend was no preferential growth cone turning response to the MAG gradient. In support of this finding, inhibiting Ca2+ release from internal stores by treatment with thapsigargin or ryanodine also suppressed MAG-induced repulsive turning (Figure 4B). However, repulsive turning induced by MAG was not inhibited by treatment with Cd2+, indicating Ca2+ influx is not essential. These results are consistent with the previous finding (Song et al., 1998) that growth cones show no preferential turning to a gradient of MAG when Ca2+ is removed from the external bathing solution, which presumably lowers [Ca2+]i. Taken together, we conclude that Ca2+ signaling is essential for MAG-induced bi-directional turning responses: High-level Ca2+ signals correlate with attractive turning, whereas low-level Ca2+ signals correlate with repulsion.
Ca2+ signals are sufficient for triggering bi-directional turning
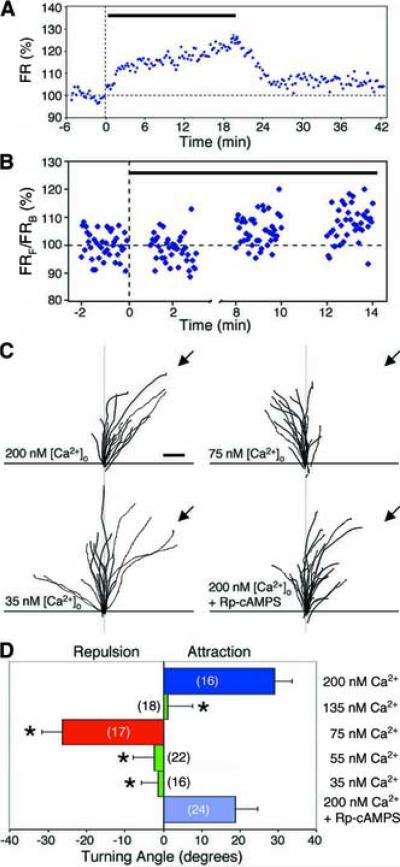
If high- and low-level Ca2+ signals mediate MAG-induced attractive and repulsive turning of the growth cone, respectively, a gradient of high- and low-Ca2+ signals created in the absence of any MAG gradient should cause similar growth cone turning responses. This idea was tested by the following experiments. Cultured spinal neurons were incubated in a bathing solution containing distinct preset Ca2+ levels, using defined concentrations of EGTA and Ca2+ (see Experimental Procedures). An extracellular gradient of ionomycin (Liu and Hermann, 1978), a Ca2+-selective ionophore that renders the plasma membrane permeable to Ca2+, was used to establish a gradient of Ca2+ across the growth cone. As shown in Figures 5A, the average growth cone [Ca2+]i was elevated soon after exposure to the ionomycin gradient. Measurements across the growth cone showed that a gradient of [Ca2+]i can be established by the ionomycin gradient (Figure 5B). In the presence of ionomycin, the maximal [Ca2+]i is set by[Ca2+]o. For the assay ofgrowth cone turning, we first set [Ca2+]o to a level measured in the growth cone during attraction (200 nM, see Figure 3B). We found that a gradient of ionomycin indeed caused growth cone attraction (Figures 5C and 5D). We next set [Ca2+]o at 75 nM, which corresponds to a level measured in the growth cone during repulsion (Figures 2 and 3B). To ensure precise control of [Ca2+]i by [Ca2+]o at this low level, thapsigargin (100 nM) was added 30 min before the onset of the experiment to deplete Ca2+ from the intracellular stores. Under this condition, we found that growth cones indeed exhibited repulsive turning in the ionomycin gradient (Figures 5C and 5D). To confirm that Ca2+ signals are necessary for an ionomycin gradient to trigger growth cone turning, we further reduced [Ca2+]o to a low level of 35 nM (see Experimental Procedures) and found no preferential turning responses under this condition. This latter experiment also served as a control to show that turning responses were not due to factors unrelated to Ca2+. Finally, at intermediate levels of 55 and 135 nM, we observed no preferential turning (Figure 5D). Taken togther, these results showed that distinct Ca2+ signals are sufficient to trigger both attractive and repulsive growth cone turning.
Figure 5.
Ca2+ signals can directly mediate bi-directional growth cone turning responses.
(A) An example showing changes in [Ca2+]i, measured by fluorescence ratio imaging over the entire growth cone, in response to an ionomycin gradient (marked by the bar) in an external solution containing 200 nM Ca2+. The fluorescence ratio (FR) measured at each time point was normalized to the mean value obtained during the 5-min period before the onset of the ionomycin gradient, as in Figure 2A. (B) An example showing Ca2+ gradients measured across the growth cone in response to an ionomycin gradient similar to that in (A). The mean [Ca2+]i level (FR) within ROIs at the `front' (facing the ionomycin gradient) and the `back' of the growth cone were measured as in Figures 1B–1D. Data are expressed as the fluorescence ratio in the `front'/`back' ROIs (FRF/FRB), normalized to the mean value measured during the 2 min period before starting the ionomycin gradient (black bar). Data points represent 15-s bins. (C) Superimposed traces depict the trajectories of neurite extension in response to an ionomycin gradient, similar to Figure 4. The origin was the center of the growth cone at the onset of the assay and the original direction of extension was aligned vertical. Extracellular Ca2+ was adjusted to 200 nM, 75 nM or 35 nM, as indicated. Thapsigargin (100 nM) was present in the external solutions containing 35 nM and 75 nM Ca2+. Rp-cAMPS (20 μM) was added as indicated. Scale bar, 10 μm. (D) Average turning angles for the data in (C) in addition to that obtained with [Ca2+]o adjusted to 135 nM and 55 nM, as indicated. Significant differences from that found in 200-nM [Ca2+]o are indicated (*P < 0.001, Kolmogorov-Smirnov test).
To further elucidate the causal relationship between Ca2+ and cAMP signaling in triggering growth cone turning, we examined whether Ca2+ signals can cause turning responses independent of cAMP signaling activity in the growth cone. Addition of Rp-cAMPS (20 μM) to the culture did not abolish the attractive turning response in the ionomycin gradient in the presence of 200 nM [Ca2+]o, indicating that the attractive turning response does not require cAMP signaling.
Ca2+ signals are necessary for turning induced by a cAMP gradient
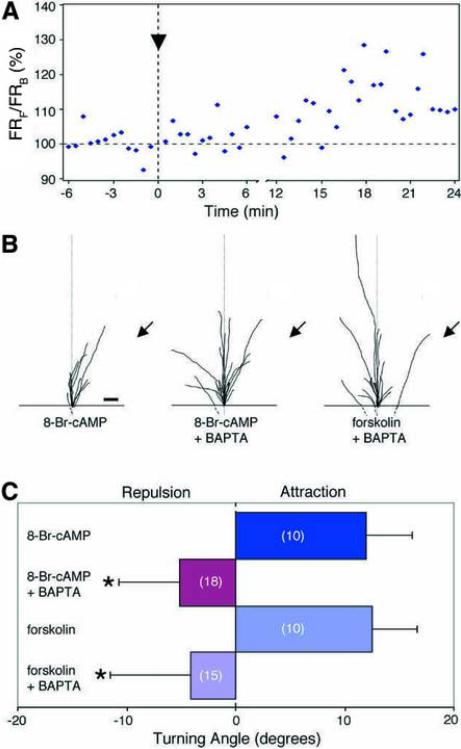
Extracellular gradients of forskolin or the membrane permeable cAMP analogue 8-Br-cAMP are known to induce attractive turning responses of these neuronal growth cones (Lohof et al., 1992; Song et al., 1997). The above results showed that a Ca2+ gradient can mediate growth cone turning in the absence of cAMP signaling. It is of interest to know whether a cAMP gradient can induce turning in the absence of Ca2+ signaling. We first determined whether a gradient of cAMP signaling induces any Ca2+ signals across the growth cone. As shown in Figure 6, an extracellular gradient of 8-Br-cAMP was indeed capable of inducing a substantial elevation of [Ca2+]i, with higher elevation on the side of the growth cone facing the source of cAMP (Figure 6A). We next examined whether Ca2+ signals are required for attractive turning induced by the cAMP gradient. Turning assays were performed in a 35-nM [Ca2+]o solution with or without loading the neurons with BAPTA-AM (1 μM). Consistent with a previous report (Lohof et al., 1992), growth cones exhibited attractive turning to a gradient of either forskolin or 8-Br-cAMP in the absence of BAPTA-AM loading (Figures 6B and 6C). In contrast, no preferential turning responses to the 8-Br-cAMP or forskolin gradient were observed in neurons preloaded with BAPTA-AM. Thus, Ca2+ signals are necessary and most likely mediate the attractive turning response to a gradient of cAMP signaling activity, which may be induced by a number of axon guidance cues.
Figure 6.
Focal Ca2+ signals in the growth cone mediate the attractive turning response induced by a cAMP gradient.
(A) An example showing Ca2+ gradients measured across the growth cone in response to an extracellular gradient of 8-Br-cAMP. The mean fluorescence ratio was measured within ROIs at the `front' (FRF; facing the 8-Br-cAMP gradient) and `back' (FRB) of the growth cone before and after the onset of the 8-Br-cAMP gradient (arrowhead). Data are presented in a similar manner as in Figure 1D. (B) Summary of growth cone turning in response to a gradient of 8-Br-cAMP or forskolin. Turning assays were done in 35-nM Ca2+ saline and neurons were preloaded with BAPTA-AM where indicated. Scale bar, 10 μm. (C) Average turning angles of the data shown in (B). For each experimental condition, the average angular position of all growth cones at the end of the 1-hr exposure to the 8-Br-cAMP or forskolin gradient is plotted. Data represent the mean ± s.e.m. Average turning angles that were significantly different from that of the 8-Br-cAMP alone group are indicated (*P < 0.02, Kolmogorov-Smirnov test).
Discussion
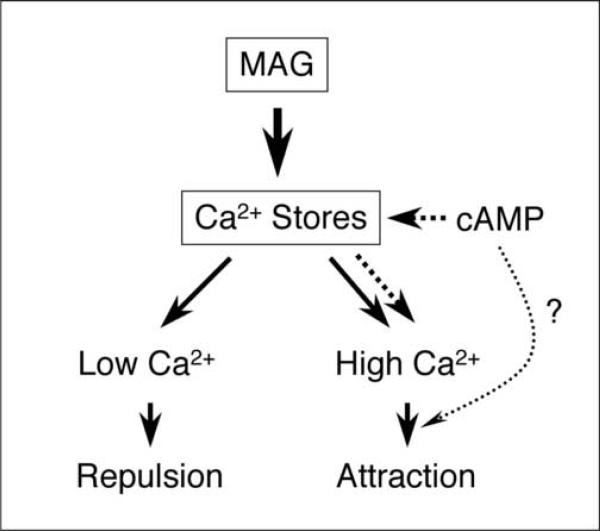
Appropriate responses of the growing axon to extracellular factors are critical for axon pathfinding during early development and for axon regeneration after nerve injury. Previous studies have shown that MAG-induced inhibition of axon growth can be overcome by treatments that elevate cytoplasmic cAMP activity (Cai et al., 1999; Neumann et al., 2002; Qiu et al., 2002). The inhibitory action of MAG is also reflected by the repulsive growth cone turning induced by a MAG gradient applied across the growth cone at an oblique angle. Previous results (Song et al., 1998) have shown that such MAG-induced repulsion of growth cones can be converted to attraction by elevating cAMP signaling activity by incubation with membrane permeable cAMP analogues. The same conversion was also found when the neuron was exposed to a brief period of electrical stimulation that in turn elevates the endogenous production of cAMP through a Ca2+-dependent adenylate cyclase (Ming et al., 2001). Furthermore, depleting extracellular Ca2+ abolished both attractive and repulsive turning of the growth cone in response to a MAG gradient (Song et al., 1998). In the present study, we showed that a MAG gradient induces both attractive and repulsive turning by releasing Ca2+ from internal stores, which establishes a cytoplasmic Ca2+ gradient at the growth cone on top of a high or low basal [Ca2+], respectively. The effect caused by the depletion of [Ca2+]o found previously (Song et al., 1998) is most likely to due to the indirect depletion of internal Ca2+ stores and lowering of basal [Ca2+]i. Importantly, cAMP-dependent switching of the growth cone turning response can be attributed to modulatory actions of events upstream from Ca2+ signaling (see Figure 7).
Figure 7.
Model for cAMP-dependent modulation of Ca2+ signals that mediate bidirectional growth cone turning induced by MAG.
An extracellular gradient of MAG and MAG receptor activation induces Ca2+ release from internal stores. This creates a gradient of low-level [Ca2+]i that is highest on the side of the growth cone facing the MAG source and leads to repulsive turning of the growth cone. Coincident elevation of cAMP, through a parallel signaling pathway, can also lead to Ca2+ release from internal stores, creating a high-level Ca2+ signal that mediates attractive turning of the growth cone. Thus, cAMP elevations can impinge on MAG-induced Ca2+ signals to effectively switch the growth cone turning response from repulsion to attraction.
Cyclic AMP and Ca2+ are prominent second messengers with numerous cytoplasmic effectors that could participate in the growth cone response to MAG. Furthermore, cAMP and Ca2+ signals can interact with each other. For example, Ca2+-induced activation of Ca2+-dependent adenylate cyclase elevates the endogenous cAMP level (Tang et al., 1991; Choi et al., 1992; Xia and Storm, 1997), and the consequent activation of PKA can modulate membrane Ca2+ channels, leading to an increase in Ca2+ influx through the plasma membrane (Sculptoreanu et al., 1993) or Ca2+ release from internal stores (Furuichi et al., 1994; Hain et al., 1995; Bruce et al., 2002). In the present study, we demonstrated directly that elevation of cAMP can induce immediate [Ca2+]i elevation in the growth cone (see Figures 3 and 6). Conversely, a recent study using a fluorescent indicator to directly measure cytoplasmic cAMP showed Ca2+ elevations in these cultured spinal neurons can also lead to a rise of cAMP levels (Gorbunova and Spitzer, 2002). Furthermore, Ca2+ influx triggered by firing a train of action potentials leads to the conversion of MAG-induced repulsion to attraction, an effect that was abolished by PKA inhibitors, again suggesting Ca2+-dependent activation of cAMP synthesis by endogenous adenylate cyclases (Ming et al., 2001). Given this reciprocal relationship between Ca2+ and cAMP and the finding that a gradient of membrane permeable cAMP analogues or forskolin can trigger attractive turning of the growth cone of these Xenopus neurons (Lohof et al., 1992; Song et al., 1997) (see also Figure 6), one might expect that MAG-induced Ca2+ signals could trigger a cAMP gradient, which in turn mediates the turning response. However, our results showed that this is not the case. We found that the attractive growth cone turning induced directly by a Ca2+ gradient is independent of cAMP activity (see Figure 5), whereas the attractive turning induced by a cAMP gradient requires [Ca2+]i elevation (see Figure 6). Thus, although a cAMP gradient can trigger growth cone turning even in a low [Ca2+]o medium (Lohof et al., 1992); see also Figure 6), our results suggest that this cAMP effect may be mediated by creating a Ca2+ gradient through internal release of Ca2+. It has been reported recently (Guirland et al., 2003) that growth cone attraction to a gradient of pituitary adenylate cyclase-activating polypeptide, which activates a G protein-coupled receptor (GPCR) and presumably creates a cAMP gradient, is independent of [Ca2+]i elevation. It is possible that turning of these Xenopus growth cones induced by a gradient of GPCR activation is mediated by another cAMP-independent pathway, since our results showed that turning induced by a cAMP gradient requires elevation of [Ca2+]i. Finally, uniformly elevating cAMP activity in the growth cone by treatment with Sp-cAMPS, which should saturate and thereby prevent any gradient of cAMP activity, does not abolish the attractive turning response induce by a MAG gradient (Song et al., 1998), arguing against the notion that a cAMP gradient mediates MAG-induced turning.
Global elevation of [Ca2+]i throughout the growth cone can regulate growth cone motility and slow neurite extension (Kater and Mills, 1991; Gu and Spitzer, 1995). However, attractive turning induced by a gradient of attractive cues has been correlated with focal elevation of [Ca2+]i on the side of the growth cone facing the source of attractant (Zheng et al., 1994; Hong et al., 2000). For Ca2+ to serve as a mediator of MAG-induced attractive and repulsive turning responses, it should satisfy the following three criteria. First, [Ca2+]i elevation in the growth cone should be required for MAG-induced turning responses. We showed that when a [Ca2+]i rise was prevented by loading the cell with BAPTA-AM, the trend was no preferential growth cone turning in response to a MAG gradient. This supports a previous study showing extracellular Ca2+ is required for MAG-induced growth cone turning responses (Song et al., 1998). Second, MAG should stimulate an elevation of [Ca2+]i and a gradient of MAG should trigger a Ca2+ gradient across the growth cone. Our Ca2+ imaging results indeed revealed MAG-induced elevations of [Ca2+]i and transient asymmetric Ca2+ signals across the growth cone. These Ca2+ signals occurred within minutes after the onset of the MAG gradient and growth cone turning followed within tens of minutes. Finally, direct [Ca2+]i elevations of appropriate patterns should be sufficient for triggering either attractive or repulsive turning of the growth cone. We demonstrated this using a gradient of ionomycin in media containing different levels of [Ca2+]o. These latter experiments have further confirmed the notion suggested by previous studies (Hong et al., 2000; Zheng, 2000) that a Ca2+ gradient created upon different basal levels of [Ca2+]i can lead to opposite turning responses, with high [Ca2+]i favoring attraction and low [Ca2+]i favoring repulsion. Bidirectional turning responses of these growth cones induced by a gradient of netrin-1 are also known to be mediated by Ca2+ signaling (Hong et al., 2000; Nishiyama et al., 2003). Consistent with our present result that cAMP acts by impinging on Ca2+ signals, the netrin-1 induced bi-directional turning responses under different ratios of cAMP and cGMP levels in the growth cone are due to modulatory effects of the cAMP/cGMP ratio on the activity of Ca2+ channels in the growth cone membrane (Nishiyama et al., 2003).
By monitoring Ca2+ signals directly with fluorescence ratio imaging, we showed that the repulsive turning response induced by MAG is associated with a low-level [Ca2+]i elevation in the range of 100 nM (see Figures 1 and 2). Direct elevation of cAMP signaling by treatment with Sp-cAMPS can further elevate MAG-induced Ca2+ signals to > 200 nM (see Figure 3). These low- and high-level Ca2+ signals are associated directly with repulsive and attractive MAG-induced turning, respectively (see Figures 4 and 5). Different sets of signaling and effector proteins may be activated in response to these distinct Ca2+ signals. Thus, in response to low-amplitude Ca2+ signals localized to the side of the growth cone facing the gradient, molecular machinery may actively destabilize the cytoskeleton, thereby causing relatively more polymerization on the opposite side of the growth cone and a repulsive turning response. However, high-amplitude Ca2+ signals localized to the side of the growth cone facing the gradient may activate other effectors, which ultimately lead to more polymerization of the cytoskeleton to mediate an attractive turning response. Recent reports have shown that PKC, Rho and its effector Rho-associated kinase are activated in response to MAG (Yamashita et al., 2002; Sivasankaran et al., 2004). The Ca2+ -dependent role of these and other key effector proteins during repulsive and attractive turning responses remains to be determined.
In summary, the present study has established that cAMP-dependent modulation of MAG-induced growth cone turning responses acts by modulating Ca2+ signaling in the growth cone and that the magnitude of focal Ca2+ signals determines the polarity of growth cone turning (see Figure 7). Elevating cAMP signaling pathways by compounds that increase the cytoplasmic cAMP level has been shown to promote neurite growth in vitro and axon regeneration in several experimental paradigms of spinal cord injury (Cai et al., 1999; Neumann et al., 2002; Qiu et al., 2002), presumably through a mechanism similar to that shown by cAMP-dependent switching of growth cone repulsion to attraction (Song et al., 1998). Our findings further suggest that elevating Ca2+ signals within the range that permits neurite outgrowth (Kater and Mills, 1991; Henley and Poo, 2004), or activating their downstream effectors, may facilitate axon regeneration and functional recovery after nerve injury.
Experimental Procedures
Xenopus Primary Spinal Neuron Cultures
In vitro fertilization and culturing of Xenopus spinal neurons was performed as described previously (Spitzer and Lamborghini, 1976; Tabti et al., 1998). The cultures were used between 14–20 hrs after plating at room temperature (20–22° C). The culture medium consisted of 86% (v/v) Leibovitz medium (GIBCO, Gaithersburg, MD) containing 4 mM Hepes, 0.5% (v/v) fetal bovine serum (HyClone, Logan, UT), and 13.5% (v/v) saline solution (10 mM D-glucose, 5 mM Sodium pyruvate, 1.26 mM CaCl2, 1.34 mM Na2HPO4, 0.44 mM NaH2PO4 and 4 mM Hepes; pH 7.5). Experiments were performed in an external bathing solution (in mM: 130 NaCl, 5 Sodium pyruvate, 2 D-glucose, 1.26 CaCl2, 1.25 KCl, 0.8 MgCl2, 10 Hepes; pH 7.5). MAG was either purchased from R&D Systems (MAG-Fc; Minneapolis, Minnesota) or was purified from bovine brain according to McKerracher et al. (1994). All other compounds were purchased from Calbiochem (San Diego, California). Pharmacological agents were applied in the external bathing solution 30 min before experiments.
Ca2+ Imaging
Cultured Xenopus spinal neurons were microinjected with Oregon Green 488 BAPTA-1 conjugated to dextran and Fura Red (Molecular Probes, Eugene, Oregon). Ca2+ imaging was done using a Leica confocal imaging system (TCS SP) equipped with a 63× objective (NA 1.32) as described previously (Ming et al., 2002). Excitation was at 488 nm and the Oregon Green BAPTA-dextran (FG) and Fura Red (FR) emission signals were collected at 500–560 nm and 620–720 nm, respectively. Fluorescence and transmission images (128 × 128 pixels; voxel size, 207 nm) were collected simultaneously at 3 — 30 s time intervals as indicated. Gradients of MAG, ionomycin or 8-Br-cAMP were produced as described previously (Lohof et al., 1992). Images were analyzed using Image J software (National Institutes of Health; http://rsb.info.nih.gov/ij/). The mean fluorescence intensity above the background threshold was measured over a fixed rectangular region of interest that covered the entire growth cone or, for detecting Ca2+ gradients, regions of interest 1/3 the width of the growth cone were positioned on opposite sides of the growth cone. Fluorescence ratios (FG/FR) were used to control for potential fluctuations in growth cone thickness or changes in focal plane. The fluorescence ratio at each time point was normalized to the average fluorescence ratio that was measured during a 2 to 10-min baseline period (prior to any treatments), as indicated. For quantification of Ca2+ signals, growth cones were imaged first in normal external bathing solution. Then the external solution was exchanged with one containing EGTA (0.5 mM) and various Ca2+ levels (0.22 – 0.41 mM) corresponding to distinct levels of free Ca2+ (43, 82, 106, 180, and 260 nM), as determined using MaxChelator software (Winmaxc v2.4; http://www.stanford.edu/~cpatton/winmaxc2.html). All other ion concentrations were kept constant. The Ca2+ -selective ionophore ionomycin (Liu and Hermann, 1978) (2 μM) was added to the bathing solutions to render the plasma membrane permeable to Ca2+ and thereby set [Ca2+]i to [Ca2+]o. Changes in the fluorescence ratio were then normalized to the average fluorescence ratio measured during the initial imaging period (in the normal external bathing solution) to obtain an in vivo calibration curve. Normalized fluorescence ratio values of 100, 105 and 115% corresponded to Ca2+ levels of 65, 110 and 220 nM, respectively.
Growth Cone Turning Assay
Gradients of MAG, ionomycin, 8-Br-cAMP and forskolin were produced by methods described previously (Lohof et al., 1992). Microscopic images of growing neurites were recorded with a CCD camera (Hitachi KP-M2U) attached to a phase contrast microscope (Olympus CK-40) and were analyzed using NIH Image 1.62 (National Institutes of Health). The final turning angle, defined by the angle between the original direction of neurite extension and a straight line connecting the positions of the center of the growth cone at the onset and the end of the 1-hr period, was measured from traces of each trajectory, using a digitizing pad and Sigma-Scan v3.10 software (Summagraphics, Scottsdale, Arizona). Only those growth cones with net extension > 7 μm over the one-hour period were included for analysis. For BAPTA loading, the external bathing solution was exchanged for one containing 0.63 mM Ca2+ and 1.6 mM EGTA to make [Ca2+]o 35 nM, as determined by MaxChelator software. Neurons were then incubated 30 min in 35-nM Ca2+ solution containing BAPTA-AM (1 μM, Calbiochem). Subsequent turning assays were performed in 35-nM Ca2+ solution. For the assays done using an ionomycin gradient, EGTA (0.8 – 1.6 mM) was added to external solutions containing 0.63 mM Ca2+ to set the [Ca2+]o at 200 nM, 135 nM, 75 nM, 55 nM or 35 nM, as determined using MaxChelator software. For these experiments it was necessary to use less than 1% DMSO in the pipette (equivalent to 0.001% at the growth cone (Lohof et al., 1992) to prevent potential turning responses induced by DMSO.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH NINDS. J.H. was supported by an NRSA award. We thank Scott Wong for helpful discussions and for purification of MAG, and Liwen Wu for technical assistance.
Footnotes
Competing interests statement The authors declare that they have no competing financial interests.
References
- Bandtlow CE, Schmidt MF, Hassinger TD, Schwab ME, Kater SB. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science. 1993;259:80–83. doi: 10.1126/science.8418499. [DOI] [PubMed] [Google Scholar]
- Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem. 2002;277:1340–1348. doi: 10.1074/jbc.M106609200. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Wong ST, Hinds TR, Storm DR. Calcium and muscarinic agonist stimulation of type I adenylylcyclase in whole cells. J Biol Chem. 1992;267:12440–12442. [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Kohda K, Miyawaki A, Mikoshiba K. Intracellular channels. Curr Opin Neurobiol. 1994;4:294–303. doi: 10.1016/0959-4388(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca(2+) spikes. Nature. 2002;418:93–96. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci. 2003;23:2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo M.-m. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschinger J, Bandtlow CE, Jung J, Klostermann S, Schwab ME, Bonhoeffer F, Kater SB. Retinal axon growth cone responses to different environmental cues are mediated by different second-messenger systems. J Neurobiol. 1997;33:825–834. doi: 10.1002/(sici)1097-4695(19971120)33:6<825::aid-neu9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;424:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Sato S, Quarles RH, Brady RO. Susceptibility of the myelin-associated glycoprotein and basic protein to a neutral protease in highly purified myelin from human and rat brain. J Neurochem. 1982;39:97–105. doi: 10.1111/j.1471-4159.1982.tb04706.x. [DOI] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron. 1996;16:1107–1113. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, et al. Protein kinase C mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Lamborghini JE. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabti N, Alder J, Poo M.-m. Culturing Nerve Cells. MIT Press; Cambridge, Massachusetts: 1998. [Google Scholar]
- Tang S, Woodhall RW, Shen YJ, deBellard ME, Saffell JL, Doherty P, Walsh FS, Filbin MT. Soluble myelin-associated glycoprotein (MAG) found in vivo inhibits axonal regeneration. Mol Cell Neurosci. 1997;9:333–346. doi: 10.1006/mcne.1997.0633. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Krupinski J, Gilman AG. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem. 1991;266:8595–8603. [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. Calmodulin-regulated adenylyl cyclases and neuromodulation. Curr Opin Neurobiol. 1997;7:391–396. doi: 10.1016/s0959-4388(97)80068-2. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.