Abstract
Lymphatic filariasis is a mosquito borne parasitic infection that cause severe economic burden in several parts of the world. Currently there is no vaccine available to prevent this infection in human. Multidrug therapy is effective, however, requires annual treatment and there is significant concern of drug resistance. In this manuscript we describe development of a multivalent DNA based vaccine comprising BmALT-2 and BmHSP antigens of lymphatic filariasis. Challenge experiments using third stage infective larvae of Brugia malayi in a mouse model suggested that nearly 90% protection can be achieved using the multivalent formulation in a DNA prime protein boost approach. The vaccination regimen induced significant IgG antibody responses and ELISPOT analysis for secreted cytokines from the spleen cells of vaccinated animals showed that these cells produce significant amount of IL-4. Results from this study thus show that a multivalent vaccine formulation of BmALT-2 and BmHSP is an excellent vaccine for lymphatic filariasis and significant protection can be achieved against a challenge infection with B. malayi in a mouse model.
Keywords: Brugia malayi, multivalent vaccine, BmALT-2, BmHSP, mouse
1. Introduction
Lymphatic filariasis caused by the filarial nematodes Wuchereria bancrofti, Brugia malayi, and Brugia timori, affects more than 120 million people worldwide [1]. Mass drug administration program by the World Health Organization, is significantly reducing the incidence rate of lymphatic filariasis in many parts of the world [2]. Nevertheless, lack of effectiveness to the mass drug administration has been reported from several endemic regions mainly due to noncompliance [3, 4]. In addition, drug resistance has been reported to at least one of the drugs in the mass drug combination [5, 6]. Since yearly administration of the mass drugs is required for effective control, there is an alarming concern for selecting drug resistant parasites. Therefore, there is an immediate need for a multipronged approach in controlling this mosquito borne infection. Combining vaccination with chemotherapy is an excellent strategy for controlling this infection, especially to achieve the targeted elimination date of 2020 by the Global Programme for Elimination of Lymphatic Filariasis [7]. Unfortunately, there are no effective vaccines currently available to control this infection. Several subunit candidate vaccine antigens have been tested in laboratory animals with variable results [8–14]. Lymphatic filariasis is a multicellular organism with complex life cycle and produce large array of host modulatory molecules. Thus, fighting against this infection with a single antigen vaccine can be difficult. By screening a phage display cDNA expression library of the B. malayi parasite with sera from immune individuals, we identified several potential vaccine candidates [15]. Varying degree of protection was achieved with each of the candidate vaccine antigens when given as a DNA, protein or prime boost vaccine [14]. Therefore, in this study, we selected the two most promising candidate antigens, Brugia malayi Abundant larval transcript-2 (BmALT-2) and B. malayi small heat shock protein (BmHSP) for further development as a multivalent vaccine. We also compared the efficacy of the vaccine when given as a monovalent formulation or as a multivalent formulation. Previous studies showed that a DNA prime protein boost gave maximum protection. Therefore, in this study we used a prime boost approach to evaluate the multivalent vaccine formulation.
2. Materials and methods
Parasite
Brugia malayi L3s were obtained from the NIAID/NIH Filariasis Research Reagent Resource Center (FR3) at the University of Georgia, Athens, GA.
Construction of monovalent and multivalent DNA vaccines
Monovalent DNA vaccine consisted of Bmhsp or Bmalt-2 in pVAX1 vector. To prepare the monovalent vaccine, codon optimized Bmhsp or Bmalt-2 genes were cloned into the eukaryotic expression vector pVAX1 (Invitrogen, Carlsbad, CA) using insert specific primers [15]. The multivalent vaccine consisted of Bmhsp and Bmalt-2 genes in the same pVAX1 vector. Codon optimized Bmhsp gene was first cloned into pVAX1 vector with no stop codon in the reverse primer (5’-CCGGAATTCTCACTTGTCGTTGGTG-3’) but contained a pst I site. Codon optimized Bmalt-2 gene was then inserted into this clone using gene specific primers [15]. PCR parameters for all the three constructs were: 94°C denaturation for 30 s, 50°C primer annealing for 30 s, 72°C primer extension for 30 s for 30 cycles; a final extension of 5 min was performed at 72° C. Insert DNA was finally sequenced to ensure authenticity of the cloned nucleotide sequence on both strands. Plasmids were maintained and propagated in E.coli Top10F’ cells. Plasmids were purified using endotoxin free plasmid extraction kit (Qiagen, Valencia, CA). DNA was analyzed by agarose gel electrophoresis and quantified in a spectrophotometer (OD 260/280, ratio>1.8).
Expression and purification of recombinant proteins
All the genes were cloned in pRSET-A vector (with an N-terminal hexahistidine tag) to produce recombinant proteins. Bmhsp and Bmalt-2 constructs were transformed into BL21(DE3) containing pLysS E.coli host (Invitrogen) to minimize toxicity due to the protein. When absorbance of the cultures reached 0.6 OD value, 1mM of IPTG (isopropyl thio-d-galacto pyranoside) was added to the cultures and incubated for an additional 3 hours to induce the gene expression. After lysing the cells, total proteins were separated in 15% and 12% SDS-PAGE to confirm the expression of his-tag recombinant BmHSP (rBmHSP) and rBmALT-2 proteins. The recombinant proteins were then purified using an immobilized cobalt metal affinity column chromatography (Clontech, Mountain View, CA) as per the manufacturer’s recommendations. Recombinant proteins were then separated in SDS-PAGE and stained with coomassie brilliant blue R250 and silver stain. These studies showed that a single band was obtained after column purification (data not shown). Endotoxins if any in the recombinant preparations were removed by passing the recombinant proteins through polymyxin B affinity columns (Thermo Fisher Scientific, Rockford, IL) and the levels of endotoxin in the final preparations were determined using an E-TOXATE kit (Sigma, St Louis, MO) as per manufacturer’s instructions. Endotoxin levels were below detection limits in these recombinant protein preparations (data not shown).
Immunization of mice
Six-weeks old male Balb/c mice purchased from Charles River Laboratories were used in these experiments. Humane use of animals in this study and the protocol was approved by the IACUC committee at the College of Medicine, University of Illinois Rockford. Mice were divided into four (4) groups of five (5) animals each. All mice were immunized subcutaneously using a DNA prime - protein boost vaccine regimen. All experimental groups of mice were primed with two injections of endotoxin free codon optimized DNA given in 50 μl volume and boosted with two doses of recombinant proteins suspended in alum (50 μl each) given at two weeks interval.
Group A mice were primed with 100 g of pVAXBmhsp and boosted with 15 g of rBmHSP; Group B mice were primed with 100 g of pVAX Bmalt-2 and boosted with 15 μg of rBmALT-2; Group C mice were primed with 100 g of pVAXBmhsp/Bmalt-2 DNA and boosted with 15 μg of rBmHSP and 15 μg of rBmALT-2. Group D mice received 100 μg of pVAX1 vector plus 50 l of alum and served as controls. Blood samples were collected from each mouse before immunization and one month after the last booster dose. Sera were separated and stored at −80°C.
Evaluation of antibody responses in mice
Levels of anti-BmHSP and anti-BmALT-2 antibodies were measured in the sera of immunized and control groups of mice using an indirect ELISA as described previously [14, 15]. Briefly, wells of 96 well microtitre plates were coated with rBmHSP, rBmALT-2 or rBmHSP (1 g/ml) in carbonate buffer (pH 9.6) overnight at 4°C. After washing the wells unbound sites were blocked with 3% BSA for 1 h at 37°C. Diluted sera samples were then added to the wells and incubated further overnight at 4°C. After washing the wells, HRP labelled rabbit anti-mouse IgG was added (1:5000) and incubated further for 1 hr at 37°C. Color was developed using ABTS [2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)] substrate. Absorbance was measured at 405 nm in a microplate reader (Bio-Rad, Hercules, CA).
Protection studies in mice
Vaccine potential of the monovalent and multivalent vaccine formulations were then evaluated in a mice model. Mice were immunized as described above using the prime boost approach. Vector plus alum group served as negative controls. Immunized and control animals were challenged using a micropore chamber method as described by [16]. Briefly, micropore chambers were assembled using 14 × 2 mm plexi rings (Millipore Corporations, Bedford, MA) and 5.0 μm nucleopore polycarbonate membranes (Millipore Corporations) that were attached to the plexi glass rings with cynoacrylic adhesive and dental cement. The chambers were immersed overnight at 37°C in sterile RPMI medium containing gentamycin and antimycotic solution. Before challenge experiments, 20 live infective L3s suspended in RPMI1640 medium supplemented with 15% heat inactivated fetal calf serum (FCS), were introduced into the micropore chambers and the opening was sealed with dental cement. Micropore chamber containing the L3s were then surgically implanted into the peritoneal cavity of each mice under anaesthesia. Aseptic conditions were followed for the surgical procedures. After 48 h of implantation, animals were sacrificed and the chambers were recovered from peritoneal cavity. Contents of each chamber were emptied and larvae were examined microscopically for adherence of cells and for larval death. Larval viability was determined microscopically at 100 x. The percentage of protection was expressed as the number of dead parasites ÷ number of total parasites recovered x 100.
Cytokine analysis in mice
The percent of rBmHSP and rBmALT-2 specific interferon- (IFN- ) and interleukin-4 (IL-4) secreting cells were determined in the spleen of control and vaccinated mice using an ELISPOT assay. Briefly, Millipore MultiScreen HTS Filter plates were coated with monoclonal rat anti mouse IFN- or monoclonal rat anti-mouse IL-4 antibodies (BD Pharmigen, San Diego, CA) at a concentration of 10 g/ml in PBS buffer. After washing the plates, non-specific sites were blocked by incubating the wells in complete RPMI with 10% foetal calf serum for one hour at room temperature. Approximately 3 × 106 spleen cells suspended in complete RPMI1640 medium supplemented with 10% heat inactivated FBS were then added to each wells. Cells were stimulated with rBmHSP or rBmALT-2 (1 g/ml). Unstimulated cells served as controls. 48 hours after incubation at 37 °C in humidified 5% CO2, plates were washed and further incubated for 1 hour at room temperature with 2μg of biotinylated rat anti mouse IFN- or biotinylated rat anti mouse IL-4 antibody (BD Pharmigen). After washing the plates, streptavidin conjugated horse radish peroxidase (Thermo Fisher Scientific) was added (1:800) to each well and incubated at room temperature for one hour. Plates were washed and colour developed using DAB substrate (Thermo Fisher Scientific). Total numbers of spots were counted under a dissection microscope.
Statistical Analysis
Statistical analysis was performed using XL STAT software v.7.5.2 (Kovach Computing Services, Anglesey, UK). Statistical significance between comparable groups was estimated using appropriate non-parametric tests, with the level of significance set at p<0.05.
Results and Discussion
Antibody responses in mice
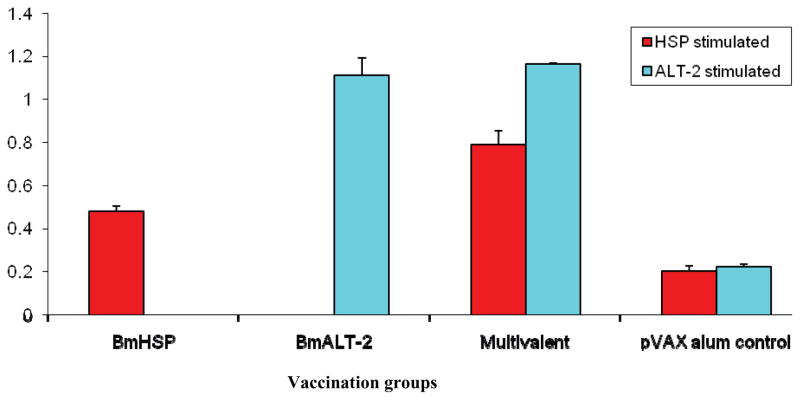
One of the first questions we asked was does the multivalent vaccine elicit significant antibodies against each of the antigenic components. Previous studies from our laboratory showed that mice similarly vaccinated with B. malayi antigens elicited significant host protective IgG antibodies [14]. Therefore, in this study, we focused only in measuring IgG antibody titres. As expected, the monovalent immunization with Bmhsp + rBmHSP and Bmalt-2 + rBmALT-2 did elicit significant (p< 0.005) titres of anti-BmHSp and anti-BmALT-2 IgG antibodies (Figure 1). The multivalent vaccine also elicited significant IgG antibody titres. Following multivalent vaccine the mice produced IgG antibodies against both BmHSP and BmALT-2 equally suggesting that the antigens do not interfere or compete for dominance. An interesting finding was that the multivalent vaccine elicited 1.5 to 1.75 fold higher (p< 0.005) titres of IgG antibodies compared to the monovalent vaccine (Figure 1). These finding suggested that the two antigens in the multivalent formulation can act synergistically by increasing the vaccine-induced antibody responses against each antigens in the vaccinated mice. The findings also suggested that combining these two antigens in the vaccine formulation has a great advantage. Given the robust IgG antibody responses induced following vaccination, it is also possible that the concentration of the component antigens in the multivalent preparation can be reduced. However, further optimization of the vaccine formulation is clearly needed.
Figure 1.
Titer of anti-BmHSP and anti-BmALT-2 IgG antibodies in the sera of vaccinated mice. 6-week-old balb/c mice were immunized using a prime boost approach with a monovalent vaccine (Bmhsp prime and rBmHSP boost or Bmalt-2 prime and rBmALT-2 boost) and multivalent vaccine (Bmhsp/Bmalt-2 prime and rBmHSP and rBmALT-2 boost). Titer of IgG antibodies were measured in the sera using an indirect ELISA. Data presented here is the antibody titer 2 weeks after the last booster. Results show that both bivalent and multivalent vaccines induce significant IgG antibodies against each of the component antigens. The findings also show that the antigens in the monovalent and multivalent formulations act synergistically in boosting the immune responses. N=5. Statistically significant ** p <0.001, * p < 0.05. Values represented are mean + SD.
Multivalent vaccine induces significant protection in mice
Above results show that significant IgG antibodies are elicited following vaccination with monovalent and multivalent vaccine preparations. To test if the immune responses elicited following vaccination is protective, we challenged vaccinated animals with live third stage infective larvae (L3) of B. malayi. Since the parasites do not reach to maturity in these animals, a better recovery of worms is obtained if the parasites are surgically implanted into the animals. We used a standard micropore chamber challenge method as described previously by [16]. These studies showed that close to 61% protection can be achieved in mice immunized with a monovalent vaccine (Table 1). This is highly significant (p <0.001) compared to negative controls. This finding also shows that rBmHSP and rBmALT-2 are promising vaccine candidates for lymphatic filariasis. Challenge experiments in mice immunized with multivalent vaccine showed that significantly (p <0.005) higher protection can be achieved compared to monovalent vaccination (Table 1). These findings also clearly correlated with the higher IgG antibody titre in these animals and support the above finding that rBmALT-2 and rBmHSP can synergistically enhance the protective immune responses in vaccinated animals when given as a prime boost regimen (Table 1).
Table 1.
Percent protection in vaccinated mice after a challenge infection.
| Vaccination regimen | Percent Larval deatha | Groups |
|---|---|---|
| Bmhsp DNA prime and rBmHSP protein boost | 61 ± 4.24 | Monovalent |
|
| ||
| Bmalt-2 DNA prime and rBmALT-2 protein boost | 76 ± 8.21 | Monovalent |
|
| ||
| Bmhsp+Bmalt-2 prime and rBmHSP and rBmALT- | Multivalent | |
| 2 protein boost | 90 ± 7.53 | |
|
| ||
| pVAX plus alum control | 22 ± 10.41 | Control |
Values are mean + SD. N=5. Data is from one of two similar experiments showing comparable results.
Cytokine responses
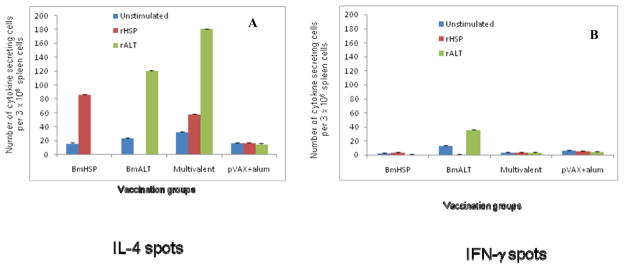
We then evaluated the immunological characteristics of the protective responses in vaccinated mice by evaluating the secreted cytokine responses of spleen cells in response to the vaccine antigens. When spleen cells were stimulated with rBmHSP or rBmALT there was significant antigen-specific proliferation of spleen cells (data not shown) suggesting a strong recall cellular response to the antigens. To identify the cytokine profile of these antigen-responding cells, we counted the IFN-γ and IL-4 secreting cells using an ELISPOT assay. Results from these studies showed that spleen cells from mice vaccinated with multivalent vaccine were predominantly secreting IL-4 (Figure 2). The numbers of IFN-γ secreting cells were very low. Overall, these findings suggested that vaccine-induced protection is largely mediated by Th2 type responses.
Figure 2.
Number of IL-4 (A) and IFN- (B) secreting cells were measured in the spleen of mice vaccinated with monovalent (BmHSP or BmALT-2) or multivalent vaccine. An ELISPOT assay was performed after stimulating the cells with rBmHSP or rBmALT (1 μg/ml). Single cell preparations of spleen cells were stimulated with respective antigens for 48 hours and spot forming cells were counted. Results show that both monovalent and multivalent vaccine promoted IL-4 secreting cells. Multivalent vaccination induced the higher number of IL-4 producing cells than controls. IFN-γ producing cells were comparatively low. These findings further confirm that BmHSP and BmALT-2 synergistically boost the immune responses in vaccinated animals following a multivalent vaccination. N=5. Results are expressed as mean number of spot forming units per 3 × 106 cells ± SD.
In conclusion, results presented in the study show that a multivalent vaccine formulation consisting of BmHSP and BmALT-2 is an excellent vaccine against lymphatic filariasis. The studies also showed that a DNA prime protein boost regimen is more robust in eliciting the protective immune responses. Finally, the vaccine-induced protection appears to be mediated by Th2 driven responses.
Acknowledgments
This study was funded by an NIH RO1 grant (AI064745). We would also like to thank NIAID/NIH Filariasis Research Reagent Resource Center (FR3) at the University of Georgia, Athens, GA for providing us with parasite materials for this study.
References
- 1.WHO. Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. World Health Organ Tech Rep Ser. 1992;821:1–71. [PubMed] [Google Scholar]
- 2.Hotez PJ. Mass drug administration and integrated control for the world's high-prevalence neglected tropical diseases. Clin Pharmacol Ther. 2009 Jun;85(6):659–64. doi: 10.1038/clpt.2009.16. [DOI] [PubMed] [Google Scholar]
- 3.Babu BV, Mishra S. Mass drug administration under the programme to eliminate lymphatic filariasis in Orissa, India: a mixed-methods study to identify factors associated with compliance and non-compliance. Trans R Soc Trop Med Hyg. 2008 Dec;102(12):1207–13. doi: 10.1016/j.trstmh.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 4.El-Setouhy M, Abd Elaziz KM, Helmy H, Farid HA, Kamal HA, Ramzy RM, et al. The effect of compliance on the impact of mass drug administration for elimination of lymphatic filariasis in Egypt. Am J Trop Med Hyg. 2007 Dec;77(6):1069–73. [PMC free article] [PubMed] [Google Scholar]
- 5.Horton J. The development of albendazole for lymphatic filariasis. Ann Trop Med Parasitol. 2009;103(1):S33–40. doi: 10.1179/000349809X12502035776595. [DOI] [PubMed] [Google Scholar]
- 6.Schwab AE, Churcher TS, Schwab AJ, Basanez MG, Prichard RK. An analysis of the population genetics of potential multi-drug resistance in Wuchereria bancrofti due to combination chemotherapy. Parasitology. 2007 Jul;134(Pt 7):1025–40. doi: 10.1017/S0031182007002363. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux DH. Filaria control and elimination: diagnostic, monitoring and surveillance needs. Trans R Soc Trop Med Hyg. 2009 Apr;103(4):338–41. doi: 10.1016/j.trstmh.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Bottazzi ME, Miles AP, Diemert D, Hotez PJ. An ounce of prevention on a budget: a nonprofit approach to developing vaccines against neglected diseases. Expert Rev Vaccines. 2006 Apr;5(2):189–98. doi: 10.1586/14760584.5.2.189. [DOI] [PubMed] [Google Scholar]
- 9.Chenthamarakshan V, Reddy MV, Harinath BC. Immunoprophylactic potential of a 120 kDa Brugia malayi adult antigen fraction, BmA-2, in lymphatic filariasis. Parasite Immunol. 1995 Jun;17(6):277–85. doi: 10.1111/j.1365-3024.1995.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 10.Dissanayake S, Perler FB, Xu M, Southworth MW, Yee CK, Wang S, et al. Differential recognition of microfilarial chitinase, a transmission-blocking vaccine candidate antigen, by sera from patients with Brugian and Bancroftian filariasis. Am J Trop Med Hyg. 1995 Sep;53(3):289–94. [PubMed] [Google Scholar]
- 11.Li BW, Chandrashekar R, Weil GJ. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. J Immunol. 1993 Mar 1;150(5):1881–5. [PubMed] [Google Scholar]
- 12.Maizels RM, Gomez-Escobar N, Gregory WF, Murray J, Zang X. Immune evasion genes from filarial nematodes. Int J Parasitol. 2001 Jul;31(9):889–98. doi: 10.1016/s0020-7519(01)00213-2. [DOI] [PubMed] [Google Scholar]
- 13.Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Gnanasekar M, Nandakumar K, et al. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Exp Parasitol. 2007 Aug;116(4):483–91. doi: 10.1016/j.exppara.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veerapathran A, Dakshinamoorthy G, Gnanasekar M, Reddy MV, Kalyanasundaram R. Evaluation of Wuchereria bancrofti GST as a vaccine candidate for lymphatic filariasis. PLoS Negl Trop Dis. 2009;3(6):e457. doi: 10.1371/journal.pntd.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, et al. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun. 2004 Aug;72(8):4707–15. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham D, Grieve RB, Holy JM, Christensen BM. Immunity to larval Brugia malayi in BALB/c mice: protective immunity and inhibition of larval development. Am J Trop Med Hyg. 1989 Jun;40(6):598–604. doi: 10.4269/ajtmh.1989.40.598. [DOI] [PubMed] [Google Scholar]