Abstract
Uremic toxins such as indoxyl sulfate (IS) accumulate at a high level in end stage renal disease (ESRD) and can exhibit significant systemic endothelial toxicity leading to accelerated cardiovascular events. The precise molecular mechanisms by which IS causes endothelial dysfunction are unknown. We tested the hypothesis that IS negatively influences properties of endothelial cells, such as migration and tube formation, by depleting nitric oxide (NO) bioavailability, and that an NO donor can reverse these inhibitory effects. IS inhibited human umbilical vein endothelial cell (HUVEC) migration and formation of tubes on matrigel. Mechanistically, IS inhibited VEGF-induced NO release from HUVECs. An NO donor, SNAP, reversed IS-mediated inhibition of HUVEC migration as well as tube-formation. IS inhibited ERK 1/2 MAP kinase activity in a dose-dependent manner, but this was preserved by SNAP. Inhibition of ERK 1/2 with a pharmacological inhibitor (U0126) decreased HUVEC migration and tube formation; these effects too were prevented by SNAP. Further, IS stimulated activation of myosin light chain (MLC), potentially stimulating endothelial contractility, while SNAP decreased MLC activation. Thus, we conclude that the negative effects of IS on endothelial cells are prevented, to a major extent, by NO, via its divergent actions on ERK MAP kinase and MLC.
Keywords: Indoxyl sulfate, endothelial cell migration, nitric oxide, ERK MAP kinase, myosin light chain
INTRODUCTION
Cardiovascular events are the leading cause of mortality and morbidity in patients with chronic and end-stage renal disease (ESRD) [1, 2]. Patients with ESRD suffer from significant vascular complications including peripheral vascular disease, resistant hypertension, and atherosclerotic coronary artery disease; these are thought to result from endothelial dysfunction, that is prevalent in uremia [3].
Indoxyl sulfate (IS) is a uremic toxin that is derived from the metabolism of tryptophan. It is absorbed from the gut as indole and converted into IS by the liver [4]. It is highly protein-bound and thus fairly resistant to removal using conventional hemodialysis prescriptions [5–7]. IS inhibits endothelial cell proliferation and migration in vitro [8], causes oxidative stress within endothelial cells, and is linked to vascular calcification in vivo [4, 9]. Serum IS levels have been shown to correlate with worse cardiovascular outcomes in patients with CKD [10]. Additionally, IS accelerates progression of chronic kidney disease in rats [11, 12] and positively correlates with atherosclerotic disease in patients with ESRD [13]. As a result, methods to remove such protein-bound toxins, using oral adsorbent (AST-120), have been shown to improve endothelial function in patients with CKD [14]. While these studies clearly point towards a critical role of IS in the pathogenesis of endothelial dysfunction, the precise molecular pathways it affects are unclear.
Nitric oxide (NO) is a crucial regulator of endothelial cell homeostasis and angiogenesis [15–17]. NO is produced by endothelial nitric oxide synthase (eNOS) within the differentiated endothelial cells and governs endothelial cell migration, proliferation, sprouting or tube-formation as well as apoptosis [17–20]. Migration of endothelial cells is central to sprouting and capillary elongation, which are hallmarks of angiogenesis. Cell migration is a highly orchestrated process, governed by an array of intracellular signaling pathways such as ERK 1/2 MAP kinase, myosin light chain (MLC), phospholipase Cγ, Rho kinase, etc.; each governing specific and discrete biophysical processes such as lamellipodal extension, cell contraction, and membrane detachment [21–25]. While targeting any of these signaling proteins can affect cell migration, whether IS interacts with any of these is unknown. Furthermore, whether NO can modulate any of these individual biophysical events during endothelial cell motility is unclear. We hypothesized that IS inhibits endothelial cell migration and tube-formation by depleting NO, thereby affecting NO-mediated downstream signaling pathways. We show that IS is a potent inhibitor of migration, tube-formation, and ERK MAP kinase, but is a stimulator of MLC, and that these effects can be reversed by the presence of an NO donor.
MATERIALS AND METHODS
Cell culture
Human umbilical vein endothelial cells were obtained from Cambrex (East Rutherford, NJ) and maintained in endothelial basal medium (EBM-2) containing 5% fetal bovine serum (FBS) supplemented with singlequots according to manufacturer’s recommendations.
Reagents and Antibodies
Indoxyl sulfate, 4,5-Diaminofluorescein diacetate (DAF-2), and the NO donor, S-nitroso-N-acetyl-DL-penicillamine (SNAP) were purchased from Sigma (St. Louis, MO). Antibodies to phospho-ERK 1/2, phosphorylated and total myosin light chain were obtained from Cell Signaling Technology (Danvers, MA). U0126, ERK 1/2 inhibitor, was obtained from Calbiochem (San Diego, CA).
DAF-2 assay for NO release
1 × 104 HUVECs were seeded on 4-well slides coated with 10 µg/ml of fibronectin for 24 hours. The medium was then changed to serum free medium containing 0.5% BSA and required concentration of IS for 4 hours. Cells were washed once with PBS and incubated with 10 µM of DAF-2 for 30 minutes followed by incubation with PBS alone for 1 minute at 37°C [26]. Cells were incubated with serum-free medium containing 100 ng/ml of VEGF for 15 minutes. The cells were washed and fixed in 2% formaldehyde; slides were mounted using Fluoromount solution and DAF-2 fluorescence was imaged using a Nikon fluorescence microscope with a standard fluorescein filter set.
Cell migration assay
Migration of HUVECs towards a chemotactic agent (VEGF) has been previously described [26, 27]. Briefly, 2.5 × 104 cells were seeded in the upper chamber of modified Boyden chamber with VEGF only in the lower chamber as the chemoattractant. IS and SNAP, where indicated, were added both to the upper and lower chambers and cells were allowed to migrate through the membranes for 6 hours. The unmigrated cells at the upper chamber were removed, and the migrated cells stuck at the bottom of the membrane were fixed in 2% formaldehyde, stained with hematoxylin, and counted in 5 random fields. The average of cell numbers counted per high power field from the 5 fields is shown with each experiment done in triplicate.
Tube formation on Matrigel
Tube formation was assessed by the ability of HUVECs to form tube-like structures on growth factor-reduced matrigel by 6 hours. 4-well glass slides were coated with reduced growth factor matrigel and were incubated for 30 minutes prior to cell seeding. 4 × 104 cells were seeded within each well of the 4-well glass slides and IS or SNAP (or both) were added to the wells where indicated. After 6 hours, medium was gently aspirated and cells/tubes were gently washed once with PBS and then fixed in 2% formaldehyde for 15 minutes. Tubes were visualized using a phase contrast microscope and representative photographs for each condition were taken using a SPOT camera.
To quantify cells participating in tube-formation, we counted the total number of cells per high power field (hpf) that were either visibly part of a multicellular tube, or that showed cytoskeletal extensions that were at least four times in length as compared to the diameter of the cell. Isolated cells that showed no cytoskeletal extensions were taken to be non-participating cells and were excluded. The number of cells/hpf participating in tube-formation were divided by total number of cells/hpf, and the percentage of participating cells was calculated. 5 high power fields for each treatment condition were analyzed using the above method.
As a surrogate quantitative measure of capillary network-formation when all cells were participating in tube formation, we quantified the number of spaces completely enclosed by the branched tube structures. We visually counted the number of empty spaces that were completely surrounded by tubes within each high power field for each treatment condition. Total number of cells within each field were also counted to verify that each field contained the same number of cells participating in tube-formation. 5 high power fields were analyzed for each condition.
Western Blotting
HUVECs were cultured in growth medium and incubated with serum free medium containing 0.5% BSA for 4 hours along with IS, SNAP, or U0126 (20 µM) where indicated followed by stimulation with VEGF at 100 ng/ml for 30 minutes. Cells were lysed in MPER protein extraction buffer (Therma Scientific, Rockford, IL) containing phosphatase and protease inhibitors and equal amount of protein lysates were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting with specific antibodies.
RESULTS
Indoxyl sulfate inhibits endothelial cell migration and formation of tube-like structures on matrigel
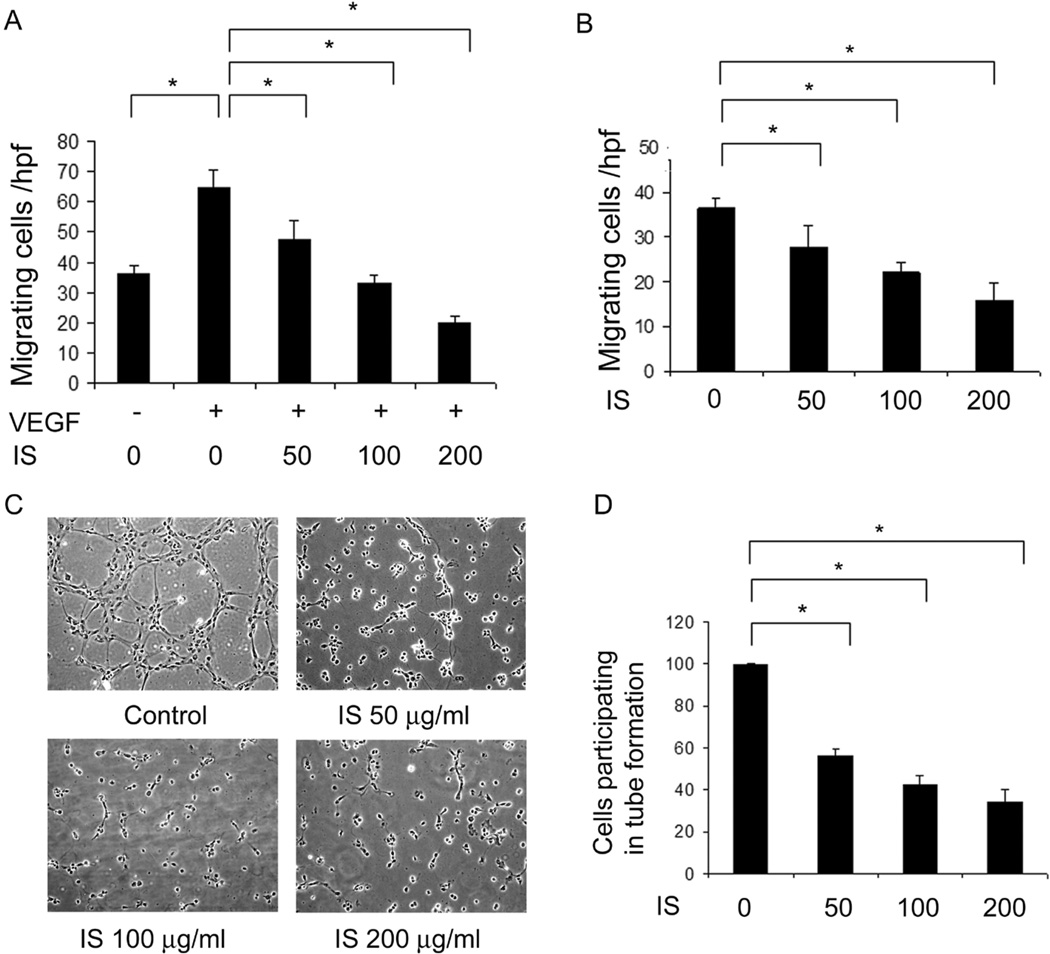
Previous studies have shown that a variety of uremic toxins inhibit migration of endothelial cells using a “scratch” assay [8]. To confirm if IS also affects VEGF-induced chemotactic endothelial cell migration, we assessed migration of HUVECs against a VEGF concentration gradient in the presence of IS using a modified Boyden chamber. IS, significantly and dose dependently, decreased both chemotactic (VEGF-induced) (figure 1A) and chemokinetic (non VEGF-induced) (figure 1B) migration.
Figure 1. IS inhibits endothelial cell migration and formation of tube-like structures on matrigel.
Migration of HUVECs for 6 hours, in the presence of increasing concentrations of IS (50–200 µg/ml) was studied using a modified Boyden chamber assay with VEGF as the chemoattractant. IS inhibited VEGF-induced (A) and basal migration of HUVECs (B) in a dose-dependent manner. C) IS also inhibited formation of tube-like structures by HUVECs. Tube formation was studied by seeding HUVECs on matrigel for 6 hours in the presence of IS at the indicated concentrations. A representative phase-contrast photograph from each condition is shown. D) Quantification of the percentage of HUVECs participating in tube formation in each of the above treatment conditions in 1C. * indicates p<0.05. Error bars denote SEM.
Since endothelial cell migration is critical for tube formation in vitro and angiogenesis in vivo, we assessed if IS could inhibit the formation of tube-like structures on matrigel. As expected, tube formation on matrigel was almost entirely inhibited with IS with a maximum inhibition at or above 200 µg/ml (figure 1C). Quantitatively, IS significantly and dose-dependently, reduced the number of cells that were involved in formation of tube-like structures (figure 1D).
Indoxyl sulfate inhibits release of nitric oxide from endothelial cells
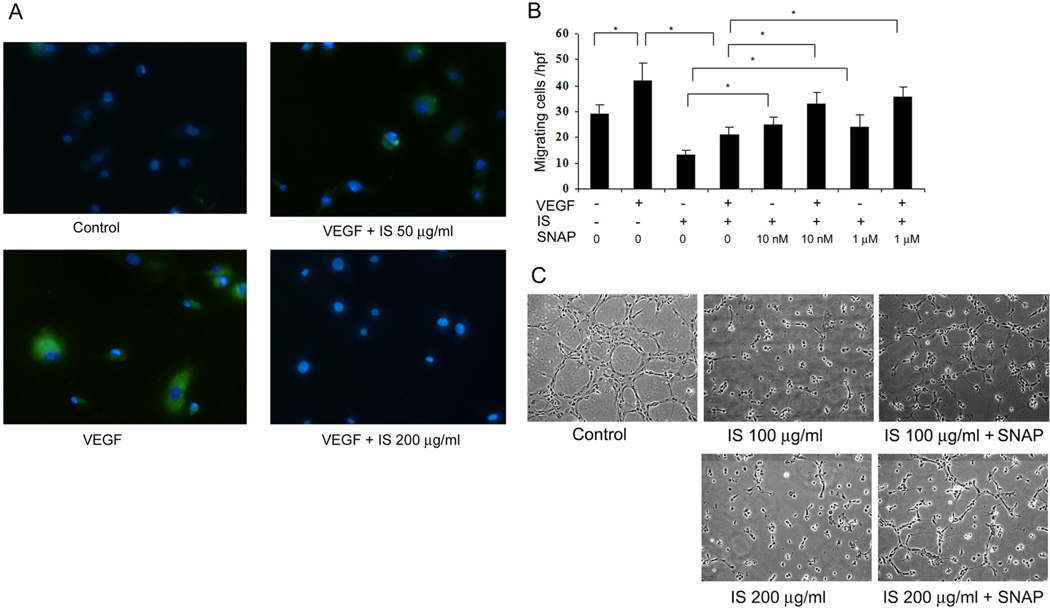
NO is a key mediator of endothelial cell function and angiogenesis. IS has previously been shown to induce production of reactive oxygen species leading to oxidative stress within endothelial cells [9]. To assess if the inhibitory effects of IS on endothelial cell migration are due to deficient NO bioavailability, we assessed the release of NO from endothelial cells upon stimulation with VEGF by staining with the fluorescent NO indicator dye, DAF-2 [28], which we have previously confirmed to be specific to NO under these conditions [26, 29]. IS dose-dependently inhibited VEGF-stimulated DAF-2 signal from endothelial cells, indicating that NO production was impaired (figure 2A).
Figure 2. NO donor, SNAP, reverses IS-mediated inhibition of endothelial cell migration and tube-formation.
A) IS inhibits VEGF-induced NO release from HUVECs, as shown by DAF-2 staining (green). Nuclei are stained blue with Hoechst 33258. Representative image from three independent experiments is shown. B) Migration of HUVECs towards VEGF was studied in the presence of IS (100 µg/ml) and SNAP (10 nM and 1 µM). Average cells migrating per high power field in each condition are shown. Each experiment was repeated thrice with each condition done in triplicate. As shown, IS reduces both the basal and VEGF-induced migration of HUVECs, which is reversed by SNAP. * indicates p < 0.05. Error bars denote SEM. C) Tube formation by HUVECs on matrigel was studied for 6 hours in presence of IS (100 and 200 µg/ml) and 1 µM of SNAP where indicated. SNAP visibly reversed tube-formation that was inhibited by IS.
NO donor, SNAP, prevents indoxyl sulfate-mediated inhibition of endothelial cell migration and tube formation
If the inhibitory effects of IS on endothelial cell migration and tube formation are due to depletion of NO, we asked if addition of an NO donor can overcome these inhibitory effects. SNAP is a sustained NO donor that releases NO in a continuous and stable concentration [30]. Clearly, addition of SNAP, even at low doses (10 nM), could prevent the inhibitory effects of IS on endothelial cell migration (figure 2B). Higher doses (1 µM) of SNAP did not have any additional stimulatory effect on HUVEC migration, indicating that even low doses of sustained NO donor could overcome inhibitory effects of IS. Interestingly, SNAP could prevent inhibition by IS of both basal (chemokinetic) and VEGF-induced (chemotactic) endothelial migration. Furthermore, SNAP could prevent, to a major extent, the IS-mediated inhibition of endothelial tube-like structures (figure 2C).
NO donor, SNAP, preserves the activity of ERK MAP kinase in endothelial cells that is inhibited by IS
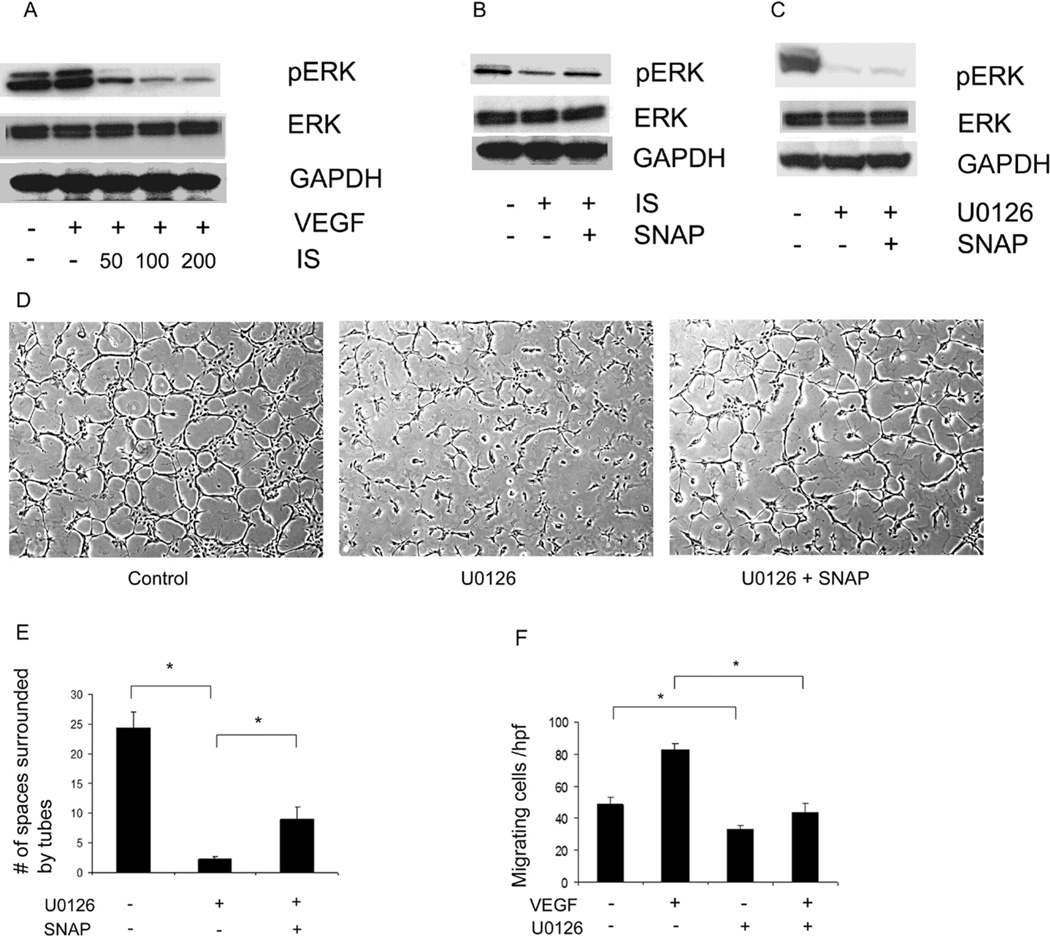
Since IS could inhibit endothelial tube formation by decreasing cell motility, we investigated specific signal transduction pathways affected by IS within endothelial cells. ERK 1/2 MAP kinase is required for migration of a variety of epithelial cells as well as endothelial cells [23, 31]. We found that IS dose-dependently inhibited ERK 1/2 activity without affecting total ERK level (figure 3A). However, addition of SNAP could restore the activity of ERK 1/2 that was inhibited by IS (figure 3B). ERK 1/2 activation was required for endothelial cell migration and tube formation as U0126, a specific MEK inhibitor that prevents ERK 1/2 activation (figure 3C), could decrease both processes in HUVECs (figure 3D, E, F). (Note that unlike IS, which prevented the cells from forming tubes or cords of any appreciable length, U0126 inhibition of ERK still allowed virtually all cells to participate in tube formation, but the normally-resulting network was disrupted. For this reason, these tube formation assays were quantitated by counting empty spaces completely enclosed by tubes, which was substantially different in these conditions. Measurement of tube length between branch points was not felt to be a meaningful quantitation under these conditions). SNAP restored, at least in part, U0126-mediated inhibition of endothelial tube-formation (figure 3D, E), indicating that IS mediates its effects by down-regulating ERK 1/2, and SNAP could counter these effects by reactivating ERK.
Figure 3. NO donor, SNAP, restores the activity of ERK MAPkinase in endothelial cells that is inhibited by IS.
A) ERK 1/2 activity was analyzed using western blotting of HUVEC lysates treated with indicated concentrations of IS and VEGF. As shown, IS dose-dependently inhibited ERK activity. B) Immunoblot showing phosphorylated ERK was reduced by presence of IS (100 µg/ml) which was restored in the presence of SNAP (1 µM). C) Inhibition of ERK 1/2 using MEK inhibitor, U0126. SNAP does not reactivate ERK inhibited by U0126. D) Tube formation is reduced by U0126 but is re-stimulated in presence of SNAP. E) Quantitation of number of spaces surrounded by tube-like structures as a surrogate measure of network formation. F) HUVEC migration is inhibited by U0126 indicating ERK 1/2 is required for HUVEC migration. * indicates p<0.05. Error bars in E, F denote SEM.
NO donor, SNAP, downregulates indoxyl sulfate-mediated myosin light chain activation in endothelial cells
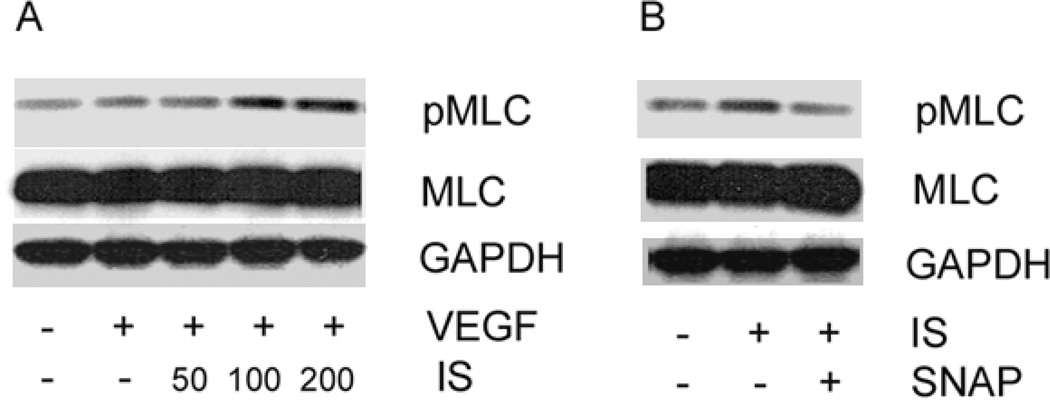
Since cell contractility is another key event that governs cell migration, we assessed if IS could modulate the regulatory MLC within endothelial cells. Interestingly, IS dose-dependently stimulated MLC activation with highest activity tested at 200 µg/ml of IS (figure 4A), potentially stimulating cell contractility. However, SNAP could downregulate IS-mediated phosphorylation of MLC (figure 4B), suggesting that IS/SNAP regulation of migration and tube formation may involve regulation of myosin function.
Figure 4. NO donor, SNAP, downregulates IS-mediated myosin light chain (MLC) activation in endothelial cells.
A) Activation of MLC was assessed using immunoblotting of HUVEC lysates treated with VEGF and indicated concentrations of IS (50–200 µg/ml). IS dose-dependently stimulates phosphorylation of MLC in HUVECs. B) SNAP downregulates IS-mediated activation of MLC in HUVECs. Cells were incubated with IS (100 µg/ml) and SNAP (1 µM) where indicated and phosphorylated MLC levels were detected using immunoblotting. Total MLC levels were unaffected. GAPDH served as a loading control. Representative immunoblot from three independent experiments is shown.
DISCUSSION
Patients with advanced renal disease suffer from significant cardiovascular complications such as myocardial infarction, sudden cardiac death and ischemic stroke. In majority of these events, immediate revascularization can lead to significant improvement in patient outcomes. Thus, factors that lead to impaired angiogenic response can lead to insufficient tissue preservation and hence, fibrosis. CKD patients are at a high risk of further progression of their renal disease due to the burden of uremic toxins that can significantly reduce angiogenesis. Thus understanding and targeting the underlying mechanisms by which uremic toxins such as IS inhibit, may significantly improve the ability to salvage tissue by augmenting re-vascularization.
In our studies, we found that IS, at concentrations found in the serum of ESRD patients [13], dose-dependently inhibited migration of endothelial cells. Its inhibitory effects on endothelial tube formation can be attributed, to a major extent, to its effects on cell migration due to the short incubation of 6 hours of IS with the cells during our migration and tube-formation experiments, during which the cell viability and number was relatively unchanged (data not shown). IS at very high doses (approximately 1000 µg/ml) has been shown to cause apoptosis, likely due to direct toxic effects [8]. However, these dosages are substantially higher than those found in the serum of uremic patients. Moreover, IS is known to circulate in serum in a highly protein-bound state. Dou et al. have previously shown that IS can still exert its effects on endothelial migration in medium containing human albumin, albeit at slightly higher dosages [8]. However, the precise kinetics of IS (protein-bound vs. free) during various conditions such as acidosis, low serum albumin, etc., which are prevalent in ESRD patients, is unknown. Thus, it is possible that despite being highly protein-bound, under certain conditions, sufficient free IS is still available to exert its toxic effects on endothelial cells.
Interestingly, IS inhibited ERK MAP kinase, which is required in endothelial migration and tube-formation. These effects were, to a large extent, reversed by the NO donor, SNAP, indicating that NO is required for ERK 1/2-mediated effects on endothelial cells. SNAP could reactivate ERK 1/2 that was inhibited by IS. Mechanistically, this could be hypothesized to stem from its control over protein tyrosine phosphatases that are critically involved in maintaining activity of a variety of intracellular protein kinases [32]. In contrast, SNAP could not reactivate ERK 1/2 that was inhibited by U0126, which deactivates MEK-mediated downstream signaling, but could still partially reverse tube-formation that was inhibited by U0126, indicating that SNAP mediates these effects by acting on multiple signaling proteins within endothelial cells other than ERK 1/2, such as MLC.
Cellular contractility is a prerequisite property for motility. Excess contractility can lead to a contracted or “stuck” cell phenomenon, whereas too little contractility may limit the force generated for migration [33]. In our studies, IS dose-dependently increased MLC, possibly leading to a “stuck” cell situation especially at high doses, further leading to decreased motility; this was reversed by SNAP, likely mimicking the vasorelaxing effects of NO in smooth muscle cells. Our studies indicate that NO has a similar effect on MLC within endothelial cells. Interestingly, SNAP did not totally inhibit MLC activation in the presence of IS, but only reduced it enough to stimulate maximal motility, likely by providing optimal cell contraction. How IS could directly stimulate MLC activation is unclear and is a focus of further investigation. MLC is under a tight regulatory control of MLC kinase (MLCK), rho kinase and MLC phosphatase, each of which could be potentially activated/inhibited by IS. Additionally, how SNAP can directly dephosphorylate IS-stimulated MLC is unclear; it is likely, that SNAP (like NO) mediates these effects by increasing soluble guanylyl cyclase activity [34].
In summation, we have identified a novel molecular mechanism by which the circulating uremic toxin IS can mediate its deleterious effects on endothelial cells. Furthermore, we show for the first time, that a sustained NO donor can counter these inhibitory effects by differentially modulating specific intracellular signaling pathways. These findings open interesting avenues for further research in cardiovascular biology, particularly, in patients with CKD/ESRD. Whether nitrates or NO donors can improve cardiovascular mortality by stimulating angiogenesis after tissue injury would be an exciting and promising further avenue for research.
-
➢
Indoxyl sulfate (IS) inhibits nitric oxide (NO) release in endothelial cells
-
➢
IS inhibits endothelial cell migration and tube formation in vitro
-
➢
NO donor, SNAP, reverses the inhibitory effects of IS on endothelial cells
-
➢
IS inhibits ERK MAP kinase activity in endothelial cells which is prevented by SNAP
-
➢
IS activates myosin light chain in endothelial cells which is down-regulated by SNAP
ACKNOWLEDGEMENTS
We thank Qiumei Chen for her insight and helpful discussions. This work was supported by NIH (NHLBI) R01 HL086917 to MLS, and by a T32 training grant from the NIH to SK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fort J. Chronic renal failure: a cardiovascular risk factor. Kidney Int. Suppl. 2005:S25–S29. doi: 10.1111/j.1523-1755.2005.09906.x. [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Mallamaci F, Tripepi G. Inflammation and atherosclerosis in end-stage renal disease. Blood Purif. 2003;21:29–36. doi: 10.1159/000067852. [DOI] [PubMed] [Google Scholar]
- 3.Niwa T. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 2010;72:1–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010;20:S2–S6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Fagugli RM, De Smet R, Buoncristiani U, Lameire N, Vanholder R. Behavior of non-protein-bound and protein-bound uremic solutes during daily hemodialysis. Am. J. Kidney Dis. 2002;40:339–347. doi: 10.1053/ajkd.2002.34518. [DOI] [PubMed] [Google Scholar]
- 6.Raff AC, Meyer TW, Hostetter TH. New insights into uremic toxicity. Curr. Opin. Nephrol. Hypertens. 2008;17:560–565. doi: 10.1097/MNH.0b013e32830f45b6. [DOI] [PubMed] [Google Scholar]
- 7.Vanholder R, De Smet R, Lameire N. Protein-bound uremic solutes: the forgotten toxins. Kidney Int. Suppl. 2001;78:S266–S270. doi: 10.1046/j.1523-1755.2001.59780266.x. [DOI] [PubMed] [Google Scholar]
- 8.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 9.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007;5:1302–1308. doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 10.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int. Suppl. 1997;62:S15–S22. [PubMed] [Google Scholar]
- 12.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 13.Taki K, Tsuruta Y, Niwa T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am. J. Nephrol. 2007;27:30–35. doi: 10.1159/000098542. [DOI] [PubMed] [Google Scholar]
- 14.Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011;6:30–39. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goligorsky MS, Noiri E, Tsukahara H, Budzikowski AS, Li H. A pivotal role of nitric oxide in endothelial cell dysfunction. Acta Physiol. Scand. 2000;168:33–40. doi: 10.1046/j.1365-201x.2000.00636.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation. 2002;105:2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- 17.Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr. Pharm. Des. 2003;9:521–530. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- 18.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am. J. Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem. Biophys. Res. Commun. 2006;343:848–856. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 23.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell. Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwabu A, Smith K, Allen FD, Lauffenburger DA, Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J. Biol. Chem. 2004;279:14551–14560. doi: 10.1074/jbc.M311981200. [DOI] [PubMed] [Google Scholar]
- 25.Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- 26.Heiss C, Wong ML, Block VI, Lao D, Real WM, Yeghiazarians Y, Lee RJ, Springer ML. Pleiotrophin induces nitric oxide dependent migration of endothelial progenitor cells. J. Cell Physiol. 2008;215:366–373. doi: 10.1002/jcp.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiss C, Schanz A, Amabile N, Jahn S, Chen Q, Wong ML, Rassaf T, Heinen Y, Cortese-Krott M, Grossman W, Yeghiazarians Y, Springer ML. Nitric oxide synthase expression and functional response to nitric oxide are both important modulators of circulating angiogenic cell response to angiogenic stimuli. Arterioscler. Thromb. Vasc. Biol. 2010;30:2212–2218. doi: 10.1161/ATVBAHA.110.211581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 1998;(70):2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 29.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J. Am. Coll. Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Jansen A, Drazen J, Osborne JA, Brown R, Loscalzo J, Stamler JS. The relaxant properties in guinea pig airways of S-nitrosothiols. J. Pharmacol. Exp. Ther. 1992;261:154–160. [PubMed] [Google Scholar]
- 31.Tang FY, Chiang EP, Shih CJ. Green tea catechin inhibits ephrin-A1-mediated cell migration and angiogenesis of human umbilical vein endothelial cells. J. Nutr. Biochem. 2007;18:391–399. doi: 10.1016/j.jnutbio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Lander HM, Sehajpal P, Levine DM, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J. Immunol. 1993;150:1509–1516. [PubMed] [Google Scholar]
- 33.Kharait S, Hautaniemi S, Wu S, Iwabu A, Lauffenburger DA, Wells A. Decision tree modeling predicts effects of inhibiting contractility signaling on cell motility. BMC Syst. Biol. 2007;1:9. doi: 10.1186/1752-0509-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivero-Vilches FJ, de Frutos S, Saura M, Rodriguez-Puyol D, Rodriguez-Puyol M. Differential relaxing responses to particulate or soluble guanylyl cyclase activation on endothelial cells: a mechanism dependent on PKG-I alpha activation by NO/cGMP. Am. J. Physiol. Cell Physiol. 2003;285:C891–C898. doi: 10.1152/ajpcell.00590.2002. [DOI] [PubMed] [Google Scholar]