Abstract
This is the first report of a tetraspanin (TSP)-like molecule in the lymphatic filarial parasites. Expressed sequence tag (EST) database search for TSP like molecules in the filarial genome resulted in three significant EST hits (two partial ESTs from Brugia malayi and one full length EST from Wuchereria bancrofti). The full length gene cloned from B. malayi showed significant similarity to Caenorhabditis elegans TSP and human TSP and hence the gene was named B. malayi TSP (BmTSP). Subsequent Genbank analysis with the predicted ORF of BmTSP showed additional homologous genes reported from Schistosoma mansoni and Taenia solium parasites. Structural analyses showed that BmTSP has four transmembrane domains and other conserved domains such as CCG and two other critical cysteine residues present within the large extracellular loop similar to other reported TSPs. In addition, putative post-translational modifications such as N-glycosylation, protein kinase c phosphorylation, casein kinase II phosphorylation and N-myristoylation sites have been found in BmTSP sequence. Further, PCR analyses showed that BmTSP is differentially transcribed, with highest level of expression being present in the adult stages followed by L3 and mf stages. This study thus describes a novel TSP cloned from B. malayi, its putative functions in cuticle biogenesis and role in protective immunity.
Keywords: Brugia malayi, TSP, cuticle, protective immunity
Introduction
Lymphatic filariasis is a mosquito borne disease caused by the parasitic worms Wuchereria bancrofti, Brugia malayi and B. timori. This debilitating disease is endemic in Asia, Africa, the Pacific, and Latin America, with an estimated 120 million people at risk (Michael and Bundy 1997). Adult worms of the parasite live inside lymphatic vessels and are responsible for major pathology associated with this infection. Diethylcarbamazine (DEC) is the drug of choice for this disease and decreasing the infection rate by 60–70% can have significant medical, social, and economic benefits (Ottesen 2000). Currently, there are no vaccines available for this infection. Mass drug treatment in lieu of controlling this infection has been unsatisfactory and unsuccessful besides increasing the risk of drug resistance. An increase in the number of new cases in endemic countries suggests the need for a more effective drug or alternate control strategy to reduce the incidence of this infection (Wang et al. 1997). In this regard, a vaccine based control strategy seems to be a viable approach for controlling lymphatic filariasis (Gregory et al. 2000).
Published evidences support the notion that development of a vaccine against this disease is feasible (Li et al. 1993; Gregory et al. 2000; Gnanasekar et al. 2004; Dabir et al. 2006). For example, protective immunity can be achieved by vaccinating animals with irradiated (Oothuman et al. 1979) or chemically abbreviated larvae (Grieve et al. 1988). However, this method of vaccination is potentially limited because of safety issues in humans. Recent developments in recombinant DNA technology have circumvented these problems. Great deal of efforts have been made to identify candidate vaccine antigens by immunoscreening expression libraries of the parasite (Gnanasekar et al. 2004), differential screening of abundantly expressed mRNAs (Gregory et al. 1997) or by expressed sequence tag (EST) approach (Lizotte-Waniewski et al. 2000). Recombinant proteins isolated through these strategies have been shown to confer varying degrees of protection in animal models (Li et al. 1993). However, the levels of protection achieved with these antigens are unsatisfactory except those reported for ALT family of proteins (Gregory et al. 2000; Gnanasekar et al. 2004). Hence, there is a need to identify more candidate antigens for prophylactic use. Cuticular antigens of nematodes have been suggested as potential targets for protective immunity (Selkirk et al. 1989). In this study, we report the identification of a novel putative surface expressed cuticular tetraspanin (TSP) from B. malayi.
Materials and methods
EST database analyses
TSP antigens expressed on the surface of several helminth parasites are shown to be promising vaccine targets because of their immunodominant nature (Hancock et al. 2006; Loukas et al. 2006; Tran et al. 2006). However, to date, there are no reports on the existence of a TSP-like molecule in the lymphatic filarial parasites. Therefore, in this study, we searched the EST databases of filarial parasites with Schistosoma mansoni TSP sequences for the presence of TSP homologues in the filarial genome at the BLAST server (www.ebi.ac.uk/blast2/parasites.html). This search revealed three ESTs, of which two were from B. malayi (EMBL accession # AA161593 and AW061610) and one from W. bancrofti (EMBL accession # CD455836). The reported ESTs from B. malayi were partial clones, whereas, the EST from W. bancrofti was a full length clone. We first decided to amplify the B. malayi TSP homologue as parasite materials and cDNA libraries were readily available in our laboratory. For PCR cloning, forward (5′ATGGTTCACGGCTGTGGTAAT3′) and reverse primers (5′TTAAGCATAATAGT ATGGTGTTTGATAGCG3′) were designed from W. bancrofti EST (CD455836). B. malayi adult cDNA library was used as the template and B. malayi TSP gene was then amplified by PCR using the following parameters: 95°C of initial denaturation for 30 s, following 45°C of primer annealing for 60 s, 72°C of primer extension for 60 s and 72°C of primer extension for 60 s. These conditions were repeated for 35 cycles and a final extension of 5 min was performed at 72°C before storing the samples at 4°C. PCR products were then cloned in TOPO TA cloning vector (Invitrogen, Carlsbad, CA) and the DNA insert was sequenced on both strands at the University of Illinois core DNA sequencing facility to confirm the authenticity of the gene. Sequences were analyzed using a software program at the GenBank (www.ncbi.nlm.nih.gov) site and multiple sequence analysis was performed by Clustal W program. Insert analysis showed significant identity with W. bancrofti full length EST. Subsequent Genbank analyses showed that the sequence had significant similarity with Caenorhabditis elegans TSP (CeTSP) and S. mansoni TSP (SmTSP) and hence the newly identified gene was designated BmTSP.
Stage-specific expression of BmTSP
BmTSP genes were then amplified from the cDNA libraries of various life cycle stages of B. malayi using insert specific primers by PCR and separated on a 1% agarose gel. After staining with ethidium bromide, band intensity was determined using NIH image software. PCR products were then normalized to the housekeeping B. malayi GAPDH (BmGAPDH) gene.
Results and discussion
Identification of a novel tetraspanin (TSP) gene from B. malayi
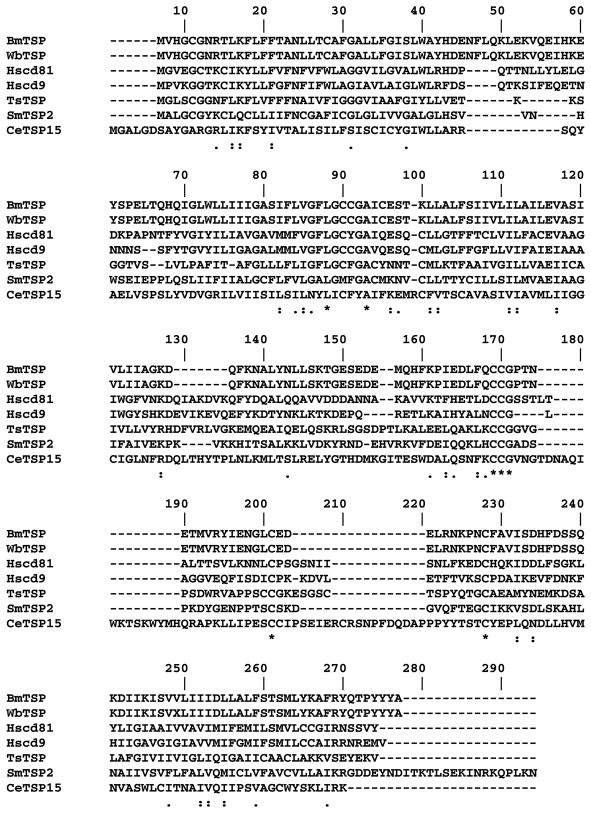
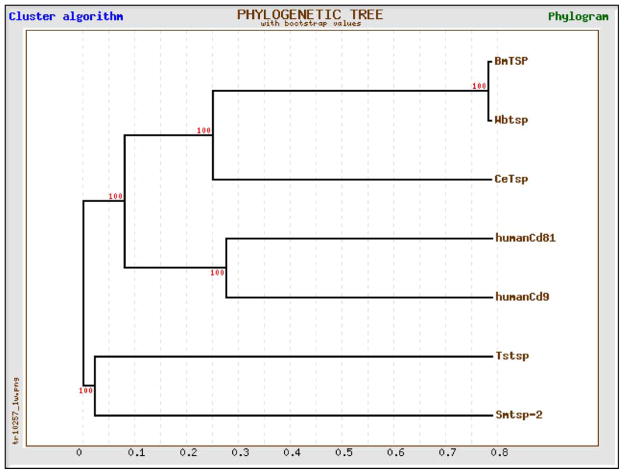
The predicted ORF of the sequence that we cloned from B. malayi was found to encode 229 amino acids which shared 99% identity with W. bancrofti EST (accession # CD455836). This high sequence identity is not surprising as genes cloned from B. malayi and W. bancrofti are shown to be highly conserved between these two species (Gnanasekar et al. 2002; Rathaur et al. 2003). Subsequent analyses of the amino acid sequences deposited in Genbank showed high homology with Homo sapiens CD81 (25% identity and 23% similarity), H. sapiens CD9 (23% identity and 25% similarity), S. mansoni TSP-2 (21% identity and 21% similarity), Taenia solium TSP (19% identity and 20% similarity) and C. elegans TSP-15 (12% identity and 23% similarity) antigens (Figure 1). Phylogenetic analyses showed that filarial derived TSPs are closely related but distantly separated from trematode and tape worm TSPs (Figure 2). In addition, PROSITE scan tool analyses showed that BmTSP has four different patterns of putative post translational modifications. These include two N-glycosylation sites at aa7–10 and aa158–161, three protein kinase c phosphorylation at aa9–11, aa91–93 and aa191–193, two casein kinase II phosphorylation sites at aa137–140 and aa191–194 and one N-myristoylation site at aa83–88.
Figure 1.
Multiple alignments (CLUSTAL) of the amino acid sequences of TSP family of proteins from B. malayi (BmTSP, accession no. EF397425), W. bancrofti (WbTSP, accession no. CD455836), H. sapiens (HsCD81, accession no. NP_004347), H. sapiens (HsCD9, accession no. NP_001760), S. mansoni (SmTSP-2, accession no. AF521091), T. solium (TsTSP, accession no. AY211879) and C. elegans (CeTSP-15, accession no. Q9XVM9). (★) Indicates identical amino acids, (:) indicates strongly similar amino acids and (.) indicates weakly similar amino acids.
Figure 2.
Phylogenetic tree analysis of TSP family of proteins. The tree distances were generated according to the ClustalW algorithm, and the tree was constructed using TreeTop (Phylip program). Phylogenetic tree analysis shows that filarial TSPs are closely related with distantly separated from trematode (S. mansoni) and cestode (T. solium) parasites.
TSPs are abundantly expressed transmembrane proteins ranging from 25 to 50 kDa which is present in almost all cells and tissues (Todres et al. 2000). To date 87 different TSPs have been identified from various species; 32 in mammals, 35 in flies and 20 in worms (Todres et al. 2000). In general, fewer studies have attempted to characterize TSPs compared to integrins, another membrane associated protein. Nevertheless, TSP is back in the lime light and is gaining significant momentum due to recent reports on its varied functions including its potential as a vaccine candidate (Hemler 2001; Loukas et al. 2006). Certain genetic mutations in the TSP gene are associated with mental retardation in humans and retinal dystrophy in mice (Hemler 2001). Similarly, TSP-15 gene knockout studies in C. elegans suggests that TSP are essential for epithelial integrity (Hemler 2001). Thus, some of the major cellular functions of TSP proteins include regulation of cell motility, cell morphology, invasion, fusion and cell signaling events.
Structurally, TSP family of proteins has four transmembrane domains, a highly conserved CCG motif and two other critical cysteine residues which are present within the large extracellular loop. Structural analysis of the filarial encoded TSPs show that they also carry all these conserved features to be qualified as TSP family of proteins. SOSUI analysis showed that filarial TSPs has four transmembrane (Tm) domains (Tml aa8–30, Tm2 aa64–86, Tm3 aa96–118, Tm4 aa195–217), CCG motif at aa153–155 and two additional cysteine residues in the second extracellular loop at aa170 and 180. These structural features confirm that the BmTSP gene that we identified from B. malayi is indeed a new member of the TSP family. Interestingly, a protein namely DiL3M C4 also having four transmembrane domains have been reported from the dog filarial parasite Dirofilaria immitis (Tsuji et al. 2000). Although this antigen has the characteristic transmembrane domains of TSP, it lacks the conserved domains and is thus not included in the TSP family.
Stage-specific expression of BmTSP in different life-cycle stages of B. malayi
TSP proteins of C. elegans (Moribe et al. 2004), S. mansoni (Tran et al. 2006) and T. solium (Hancock et al. 2006) are expressed exclusively on the surface of the parasite similar to the mammalian TSP proteins. Prediction of protein localization using PSORT program analyses suggest that BmTSP is also expressed on the surface. Further localization studies using anti-BmTSP antibodies will determine whether BmTSP are present on the parasite cuticle. Nevertheless, in the present study, we determined the expression of BmTSP mRNA in various life-cycle stages of the parasite. These studies showed that BmTSP is indeed expressed in all mammalian life-cycle stages of B. malayi with highest level being expressed in the adult stages followed by L3 and mf (Figure 3).
Figure 3.
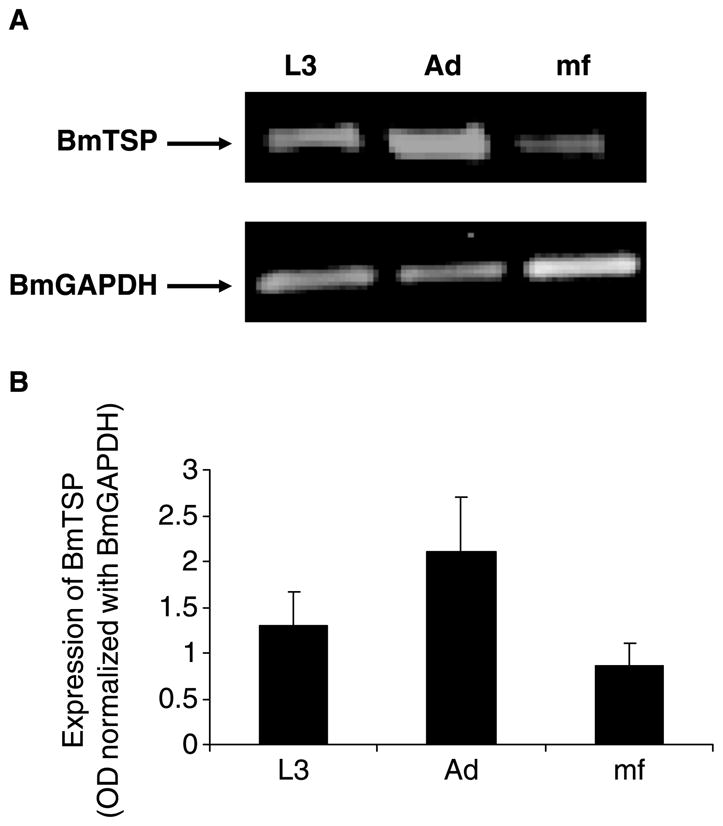
Expression of BmTSP mRNA in various life cycle stages of B. malayi. A. BmTSP and BmGAPDH transcripts were amplified by PCR from the cDNA libraries of various life cycle stages (L3, adult and Mf) of B. malayi using primers specific for BmTSP and BmGAPDH. Expression of BmGAPDH was used as an internal house keeping control gene. PCR products were resolved on a 1% agarose gel and stained with ethidium bromide. B. Band intensity was normalized to BmGAPDH PCR products and values were calculated using NIH image software. Data represented in B is average of values from three similar experiments.
Analysis of C. elegans genome reveal the presence of 20 distinct TSP genes (Moribe et al. 2004). Although our EST database search did not result in any additional hits, at this time, we do not know whether B. malayi expresses other variants of TSP. RNA interference (RNAi) analyses on each of the 20 TSP genes of C. elegans showed that out of 20 TSP proteins only CeTSP-15 had any conclusive function (Moribe et al. 2004). C. elegans lacking the CeTSP-15 gene had impaired barrier function of the hypodermal membrane, degeneration of the hypodermis and blistered cuticle. Interestingly, BmTSP shares 23% sequence similarity with CeTSP-15. Although at present we do not know the putative function of BmTSP, we believe that BmTSP may have a possible role in cuticular biogenesis and maintenance of cuticle integrity similar to CeTSP-15.
The nematode cuticle is a complex extracellular structure, which provides many important basic functions such as protection from dehydration, abrasion and immune attack (Maizels et al. 1993). In addition to serving as a protective covering, the cuticle also has important roles in absorptive, enzymatic and secretory activities (Maizels et al. 1993). Several immunological studies reiterate the importance of cuticular and surface antigens as targets of protective immunity (Maizels et al. 1989; Selkirk et al. 1989; Selkirk and Blaxter 1990). However, little is known about the protective capabilities of these cuticular antigens. Recent studies by an Australian group of investigators have raised hopes that the surface expressed TSP of S. mansoni can be developed into a vaccine against schistosomiasis (Tran et al. 2006). Similarly, TSP from T. solium has also been shown to be a potent immunodominat antigen in the infected individuals (Hancock et al. 2006). Since parasite TSPs appear to play an important role in maintaining the extracellular integrity and these molecules are recognized by the host immune system suggests that BmTSP may be a potential target for vaccine or drug development against lymphatic filariasis.
Acknowledgments
B. malayi cDNA libraries used in this study were obtained from Dr Steven Williams, Smith College, Northampton, MA. This study was supported by NIH grant AI064745.
Footnotes
Note: Nucleotide sequence data reported in this paper is submitted to Genbank under the accession number: BmTSP: EF397425
References
- Dabir P, Dabir S, Krithika KN, Goswami K, Reddy MV. Immunoprophylactic evaluation of a 37-kDa Brugia malayi recombinant antigen in lymphatic filariasis. Clin Microbiol Infect. 2006;12:361–368. doi: 10.1111/j.1469-0691.2006.01362.x. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Rao KV, Chen L, Narayanan RB, Geetha M, Scott AL, Ramaswamy K, Kaliraj P. Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol. 2002;121:107–118. doi: 10.1016/s0166-6851(02)00027-0. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, Ramaswamy K. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun. 2004;72:4707–4715. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory WF, Blaxter ML, Maizels RM. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- Gregory WF, Atmadja AK, Allen JE, Maizels RM. The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect Immun. 2000;68:4174–4179. doi: 10.1128/iai.68.7.4174-4179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve RB, Abraham D, Mika-Grieve M, Seibert BP. Induction of protective immunity in dogs to infection with Dirofilaria immitis using chemically-abbreviated infections. Am J Trop Med Hyg. 1988;39:373–379. doi: 10.4269/ajtmh.1988.39.373. [DOI] [PubMed] [Google Scholar]
- Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, Gonzalez AE, Oilman RH, Tsang VC. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol. 2006;147:109–117. doi: 10.1016/j.molbiopara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Specific tetraspanin functions. J Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BW, Chandrashekar R, Weil GJ. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. J Immunol. 1993;150:1881–1885. [PubMed] [Google Scholar]
- Lizotte-Waniewski M, Tawe W, Guiliano DB, Lu W, Liu J, Williams SA, Lustigman S. Identification of potential vaccine and drug target candidates by expressed sequence tag analysis and immunoscreening of Onchocerca volvulus larval cDNA libraries. Infect Immun. 2000;68:3491–3501. doi: 10.1128/iai.68.6.3491-3501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2006 doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Gregory WF, Kwan-Lim GE, Selkirk ME. Filarial surface antigens: The major 29 kilodalton glycoprotein and a novel 17–200 kilodalton complex from adult Brugia malayi parasites. Mol Biochem Parasitol. 1989;32:213–227. doi: 10.1016/0166-6851(89)90072-8. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Blaxter ML, Selkirk ME. Forms and functions of nematode surfaces. Exp Parasitol. 1993;77:380–384. doi: 10.1006/expr.1993.1096. [DOI] [PubMed] [Google Scholar]
- Michael E, Bundy DA. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13:472–476. doi: 10.1016/s0169-4758(97)01151-4. [DOI] [PubMed] [Google Scholar]
- Moribe H, Yochem J, Yamada H, Tabuse Y, Fujimoto T, Mekada E. Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J Cell Sci. 2004;117:5209–5220. doi: 10.1242/jcs.01403. [DOI] [PubMed] [Google Scholar]
- Oothuman P, Denham DA, McGreevy PB, Nelson GS, Rogers R. Successful vaccination of cats against Brugia pahangi with larvae attenuated by irradiation with 10 krad cobalt 60. Parasite Immunol. 1979;1:209–216. doi: 10.1111/j.1365-3024.1979.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Ottesen EA. The global programme to eliminate lymphatic filariasis. Trap Med Int Health. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- Rathaur S, Fischer P, Domagalski M, Walter RD, Liebau E. Brugia malayi and Wuchereria bancrofti: Gene comparison and recombinant expression of pi-class related glutathione S-transferases. Exp Parasitol. 2003;103:177–181. doi: 10.1016/s0014-4894(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Selkirk ME, Blaxter ML. Cuticular proteins of Brugia filarial parasites. Acta Trop. 1990;47:373–380. doi: 10.1016/0001-706x(90)90038-2. [DOI] [PubMed] [Google Scholar]
- Selkirk ME, Nielsen L, Kelly C, Partono F, Sayers G, Maizels RM. Identification, synthesis and immunogenicity of cuticular collagens from the filarial nematodes Brugia malayi and Brugia pahangi. Mol Biochem Parasitol. 1989;32:229–246. doi: 10.1016/0166-6851(89)90073-x. [DOI] [PubMed] [Google Scholar]
- Todres E, Nardi JB, Robertson HM. The tetraspanin superfamily in insects. Insect Mol Biol. 2000;9:581–590. doi: 10.1046/j.1365-2583.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, Don TA, McManus DP, Correa-Oliveira R, Loukas A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- Tsuji N, Morales TH, Ozols VV, Carmody AB, Chandrashekar R. Cloning and preliminary characterization of a novel cuticular antigen from the filarial parasite Dirofilaria immitis. Parasitol Int. 2000;49:321–325. doi: 10.1016/s1383-5769(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Wang SH, Zheng HJ, Dissanayake S, Cheng WF, Tao ZH, Lin SZ, Piessens WF. Evaluation of recombinant chitinase and SXP1 antigens as antimicrofilarial vaccines. Am J Trop Med Hyg. 1997;56:474–481. doi: 10.4269/ajtmh.1997.56.474. [DOI] [PubMed] [Google Scholar]