Abstract
Background & objectives
Breast cancer is a leading cause of cancer death in women; dietary fat is the one of the factors that influences its incidence. In the present study we investigated the effect of feeding cow ghee versus soybean oil on 7,12-dimethylbenz(a)anthracene (DMBA) induced mammary cancer in rat and expression of cyclooxygenase-2 and peroxisome proliferators activated receptor- γ (PPAR-γ) in mammary gland.
Methods
Two groups of 21 day old female rats (30 each) were fed for 44 wk diet containing cow ghee or soybean oil (10%). The animals were given DMBA (30mg/kg body weight) through oral intubation after 5 wk feeding. Another two groups (8 each) fed similarly but not given DMBA served as control for the gene expression study.
Results
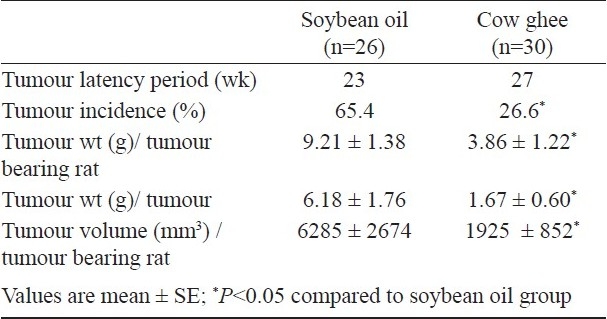
In DMBA treated groups, the animal fed soybean oil had higher tumour incidence (65.4%), tumour weight (6.18 g) and tumour volume (6285 mm3) compared to those fed cow ghee (26.6%, 1.67 g, 1925 mm3, respectively). Tumour latency period was 23 wk on soybean oil compared to 27 wk on cow ghee. Histological analysis of tumours showed that the progression of carcinogenesis was more rapid on soybean oil than on cow ghee. The expression of cyclooxygenase-2 was observed only in DMBA treated rats and it was significantly less on cow ghee than on soybean oil. The expression of PPAR-γ was significantly more on cow ghee than on soybean oil.
Interpretation & conclusions
Our results show that dietary cow ghee opposed to soybean oil attenuates mammary carcinogenesis induced by DMBA; and the effect is mediated by decreased expression of cyclooxygenase-2 and increased expression of PPAR-γ in the former group.
Keywords: Cow ghee, cyclooxygenase 2, DMBA, mammary carcinogenesis, PPAR-γ, soybean oil
Breast cancer is the most commonly diagnosed cancer in women and is the leading cause of cancer mortality in females around the world1. A strong positive correlation between fat intake and age adjusted incidence and mortality from breast cancer has been shown2. The vegetable oils have been reported to enhance 7,12-dimethylbenz(a) anthracene (DMBA) induced mammary adenocarcinomas more than butter and some other saturated fats in rodents3. It has been suggested that high dietary levels of unsaturated fatty acids enhance tumour development through increased synthesis of prostaglandins4. Cyclooxygenase (COX) that catalyzes the conversion of arachidonic acid to prostaglandins exists in two isoforms (COX-1 and COX-2). The constitutively expressed COX-1 is important for maintaining the homeostatic function, whereas COX-2 is upregulated in response to growth factors, tumour promoters and cytokines5. The overexpression of cyclooxygenase-2 is sufficient to induce mammary tumours in mice6.
Peroxisome proliferators activated receptor-gamma (PPAR-γ) is a ligand activated transcription factor and its activity is regulated by several natural ligands, including fatty acids and eicosanoids7. The PPAR-γ ligands promote differentiation and reduce growth rate of breast adenocarcinoma cell lines in vitro and promote regression of DMBA induced rat mammary tumours in vivo8,9. These studies suggest that COX-2 and PPAR-γ are the regulatory molecules in the development of mammary carcinogenesis.
Ghee, the clarified butterfat, has an important place in Indian dietary because of its characteristic flavour and pleasant aroma. Ayurvedic literature has held cow ghee in high esteem in terms of health benefits, and it has been used for the treatment of various ailments. However, there is no scientific literature to explain the role of milk fat in treatment of these diseases. Milk fat because of its saturated fatty acids content has received adverse publicity for its suspected role in promotion of cancer, although there is no experimental evidence to support this contention. Milk fat contains a number of micronutrients [conjugated linoleic acid (CLA), vaccenic acid, sphingolipids, butyric acid, β-carotene, etc.], which are potential therapeutic agents, while vegetable oils contain large amount of linoleic acid known to have promotional role in carcinogenesis4. In the present study, we examined the effect of feeding cow ghee versus vegetable oil (soybean oil) on DMBA induced rat mammary carcinogenesis. In order to provide further information on the mechanism by which dietary fats modulate mammary cancer development, we also examined the effect of cow ghee versus soybean oil on the expression of COX-2 and PPAR-γ genes in rat mammary gland.
Material & Methods
Animals, diet, and mammary tumour induction: Female (21 day old) albino rats (Wistar), obtained from the Small Animal House of the National Dairy Research Institute, Karnal, Haryana, were housed in metal cages and were given water and diet ad libitum. Institute's Animal Ethics Committee approved the study protocols. The experimental diet was comprised of chick pea, 56.4 per cent; wheat, 15 per cent; groundnut cake, 10 per cent; cow ghee or soybean oil, 10 per cent; skim milk powder, 6 per cent; mineral mixture, 2.16 per cent; vitamin mix, 0.2 per cent and choline chloride, 0.2 per cent. The composition of mineral and vitamin mixtures was designed so as to provide these nutrients (including those derived from above feed ingredients) in diets in accordance to AIN-9310. The animals tabulated in accordance to their body weight were divided into two groups of 30 each with mean body weight 22 g in each group, and were fed on cow ghee or soybean oil diets. After 5 wk feeding, each animal was administered through oral intubation 7,12-dimethylbenz (a) anthracene (DMBA) (30 mg/kg body weight) in soybean oil, and the feeding on respective diet continued for another 39 wk (the total period of the study was 44 wk). The animals were palpated weekly to determine the time of appearance of tumours. When the number of palpable tumours plateaued for at least a few weeks, the animals were sacrificed by cervical dislocation. Another two groups (8 rats each) fed similarly but not given DMBA served as control for the gene expression study. Body weights were recorded biweekly.
At necropsy, mammary glands were exposed and tumours were excised. Tumour incidence, volume and weight were determined. Portions of mammary tissue from no tumour bearing and uninvolved tissue of tumour bearing animals and tumour tissue were preserved in RNA later for gene expression studies. Another portion of tumour tissue was fixed in formalin (10%) for histopathological studies.
Reverse transcription (RT)-polymerase chain reaction (PCR): Total RNA was isolated from mammary tissue with TRIzol reagent (Sigma, USA) and cDNA was synthesized by using RevertAid™ first strand cDNA synthesis kit (Fermentas Inc, USA) as per the manufacturer's instructions. Polymerase chain reaction was carried by amplification of genes together with reference gene (GADPH) using cDNA template. The primers were designed using the MIT Primer3 design program (http://www.genome.wi.mit.edu//cgi-bin/primer/primer3.cgi) and were synthesized by Imperial Life Sciences (P) Ltd (Gurgaon, India). The primer sequences were: 5'-CTGTATCCCGCCCTGCTGGTG-3' (sense) and 5'-TTGCGTTGATGGTGGCTGTCTT-3' (antisense) for COX-2; 5'-CATCGAGGACATCCAAGACAAC-3' (sense) and 5' TGAAGGCTCATATCTGTCTCC-3'(antisense) for PPAR-γ; 5'-CCTTCATTGA CCTCAACTAC-3' (sense) and 5'- GGAAGGCCA TGCCAGTGAGC-3' (antisense) for GADPH. These primer pairs yield amplified products of 279 bp for COX-2, 161 bp for PPAR-γ and 574 bp for GADPH. The reaction mixture contained 2 μl of RT product, 0.5 μl each of forward and reverse gene specific primer (10 μM), 2.5 μl of dNTP mix (2 mM), 2.5 μl of 10 × Taq buffer containing 15 mM MgCl2, 0.5U Taq DNA polymerase (1U/μl) and final volume was made up to 25 μl with nuclease-free water. The cycling programme was an initial 4 min for denaturation (94°C) followed by 30 cycles of denaturation (94°C, 1 min), annealing (55°C, 1 min) and extension (72°C, 1 min), and a final extension step (72°C, 10 min). The reaction products were separated on 2 per cent agarose gel. The controls were run to rule out contamination of RNA with genomic DNA in which reverse transcriptase was omitted from the reaction mixtures. In order to rule out other sources of contamination, control PCR reactions were carried out in reaction mixtures containing no cDNA.
Statistical analysis: The values were expressed as mean ± SE. Kruskal-wallis one-way analysis of variance (ANOVA) was used for the feed intake, body weight, tumour weight, tumour volume and tumour multiplicity using Systat 7.0 software (Spss Inc., Chicago, USA). Statistical analysis of tumour incidence was done by Chi-square test using Systat 7.0 software. Statistical analysis of COX-2 and PPAR-γ gene expression data was done through analysis of variance (ANOVA), and the comparisons between means were tested by Tukey: Compare all pair of columns using PRISM 3.0 software (Graph- Pad, San Diego, CA, USA). A difference with P<0.05 was considered statistically significant.
Results
Effect of cow ghee versus soybean oil on mammary carcinogenesis: There was no significant difference between cow ghee and soybean oil fed rats in feed intake and body weight gain in carcinogen treated as well as in untreated groups (data not shown). In DMBA treated soybean oil fed group, four rats died 16 wk past carcinogen administration due to carcinogen toxicity, while no mortality was observed in ghee group. Table I summarizes the data on incidence, latency period and weight and volume of tumours in mammary gland. The incidence of tumours on soybean oil diet (65.4%) was significantly (P<0.05) higher than on cow ghee diet (26.6%). The tumour latency period was 27 wk in cow ghee group compared to 23 wk in soybean oil group. The average size of tumour was generally larger in soybean oil group than in cow ghee group. The average tumour load per tumour bearing animal or tumour weight per tumour in soybean oil group was significantly (P<0.05) higher than in cow ghee group. Similarly, average tumour volume was significantly (P<0.05) less in cow ghee group than on soybean oil group. Further the rats, which did not develop tumour showed greater degree of angiogenesis in soybean oil group than in cow ghee group.
Table I.
Effect of feeding cow ghee versus soybean oil on mammary carcinogenesis in 7, 12-dimethylbenz(a)anthracene (DMBA) administered rats
Histopathology of tumour and progression of mammary tumorigenesis: Based on histopathological analysis, tumours were classified in to 5 types: papilloma, fibroma, adenoma, fibroadenoma and adenocarcinoma (Table II). The former four were the common types of tumours found in both dietary groups. The malignant tumours (adenocarcinoma) were detected only in rats fed soybean oil diet. The papilloma was characterized by the presence 4-5 layers of epithelial cells (Fig. 1); the epithelial cells were, however, homogeneous in size and shape. The most common type tumour found was fibroma, which was characterized by the presence of increased stromal tissue element with detachment of epithelial cells, and the epithelium was composed of several layers of cells. The adenoma was characterized by the presence of numerous acini with stromal elements and nucleus of benign type. The fibroadenoma was characterized by the presence of high amount of fibrous stromal element along with acini. The adenocarcinoma was characterized by the presence of solid sheets of neoplastic epithelial cells with loss of tubular alveolar pattern.
Table II.
Tumour type (%) in rats fed with cow ghee or soybean oil
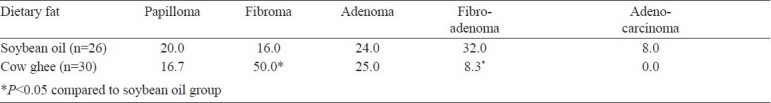
Fig. 1.
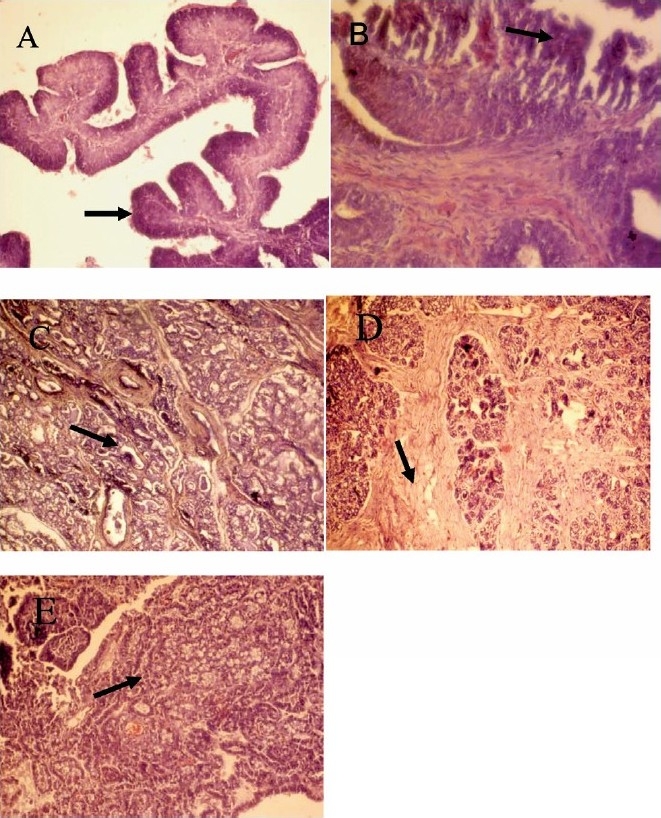
Histopathological section of mammary tumours. A, Papilloma; B, Fibroma; C, Adenoma; D, Fibroadenoma and E, Adenocarcinoma. H&E; ×400.
Expression of COX-2 and PPAR-γ: The effect of dietary fat on expression of COX-2 and PPAR-γ was investigated in normal mammary gland, tumour tissue and uninvolved tissue of tumour bearing rats. The COX-2 was not expressed in normal mammary tissue but its expression was induced in response to DMBA treatment (Fig. 2). In DMBA treated rats, the expression of COX-2 was significantly greater in tumour bearing than in no tumour bearing rats. Further, the expression of COX-2 was greater in tumour tissue than in uninvolved adjoining tissue. In carcinogen treated rats wherein no tumour appeared, the expression of COX-2 on soybean oil diet was 2.8 fold of that on cow ghee diet. Similarly, expression of COX-2 on soybean oil diet was 1.7 to 1.8 fold of that on cow ghee diet in tumour tissue as well as in uninvolved adjoining mammary tissue of tumour bearing rats.
Fig. 2.
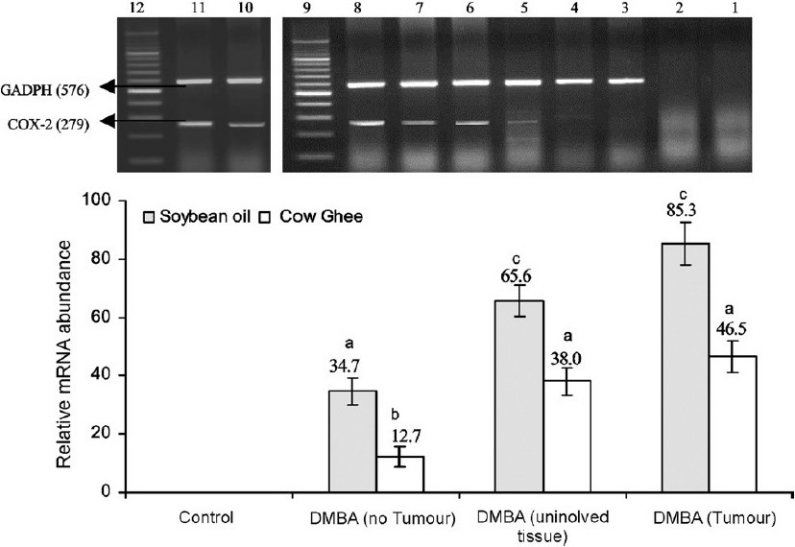
Effect of feeding cow ghee versus soybean oil on expression of COX-2 in mammary tissue of control and DMBA treated rats. Lane-1, -ve RT-PCR; Lane-2, -ve PCR; Lane-3, cow ghee control; Lane-4, soybean oil control; Lane-5, cow ghee + DMBA (no tumour); Lane- 6, soybean oil + DMBA (no tumour); Lane-7, cow ghee + DMBA (uninvolved tissue); Lane-8, soybean oil + DMBA (uninvolved tissue); Lane-9, DNA ladder; Lane-10, cow ghee + DMBA (tumour); Lane-11, soybean oil +DMBA (tumour); Lane-12, DNA ladder. The relative mRNA abundance is calculated as per cent of reference gene (GADPH) expression. Values (mean ± SE for n=8) with different letters are significantly different (P<0.05).
In carcinogen treated rats wherein no tumour appeared, the expression of PPAR-γ in both cow ghee and soybean oil groups was almost of the same magnitude as observed in their respective untreated conterparts (Fig. 3). However, the PPAR-γ expression in cow ghee fed rats was 2.2 fold of that in soybean oil fed rats, in untreated controls as well as in DMBA treated rats wherein no tumour appeared. The expression of PPAR-γ in tumour bearing rats on soybean oil diet decreased by 47.2 and 20.8 per cent in uninvolved tissue and tumour tissue, respectively, compared to soybean oil fed control group. In cow ghee group, no decline in PPAR-γ expression was observed in uninvolved tissue of tumour bearing rats, compared to cow ghee fed control rats. In tumour tissue, however, the expression of PPAR-γ decreased significantly in comparison with cow ghee fed control group. The expression of PPAR-γ in mammary gland on cow ghee diet was 2.3, 4.4 and 1.4 fold in no tumour bearing, uninvolved tissue of tumour bearing and tumour tissue compared to corresponding tissue in soybean oil fed DMBA treated rats.
Fig. 3.
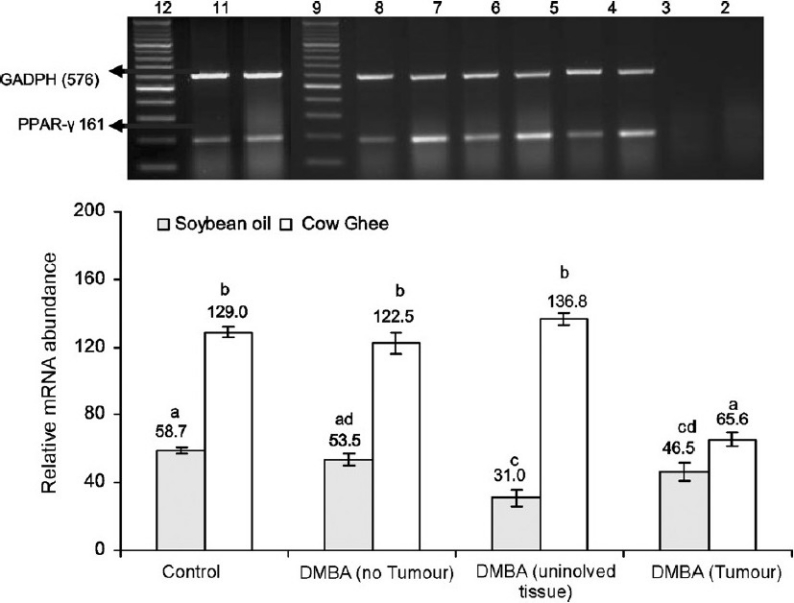
Effect of feeding cow ghee versus soybean oil on the expression of PPAR-γ in rat mammary tissue of control and DMBA treated rats. Lane-1, -ve RT-PCR; Lane-2, -ve PCR; Lane-3, cow ghee control; Lane-4, soybean oil control; Lane-5, cow ghee + DMBA (no tumour); Lane- 6, soybean oil + DMBA (no tumour); Lane-7, cow ghee + DMBA (uninvolved tissue); Lane-8, soybean oil + DMBA (uninvolved tissue); Lane-9, DNA ladder; Lane-10, cow ghee + DMBA (tumour); Lane-11, soybean oil + DMBA (tumour); Lane-12, DNA ladder. The relative mRNA abundance is calculated as per cent of reference gene (GADPH) expression. Values (mean ± SE for n=8) with different letters are significantly different (P<0.05).
Discussion
We examined the effect of feeding cow ghee versus soybean oil on DMBA induced mammary carcinogenesis and on the expression on COX-2 and PPAR-γ genes. Fewer tumour incidence, smaller tumour size and greater tumour latency period on cow ghee than on soybean oil were suggestive of protection conferred by cow ghee or the promotional role of soybean oil in mammary gland carcinogenesis. Cow ghee also protected from the progression of tumour to malignancy since adenocarcinoma was developed only in soybean oil fed rats.
The role of cow ghee or soybean oil in mammary carcinogenesis may be explained by their ability to modulate pathway of prostaglandin synthesis. Mammary carcinogenesis is triggered by inappropriate induction and upregulation of COX-2. It was hypothesed11 that the expression of normally silent COX-2 gene results in excess production of prostaglandin E2 and increase in local estrogen biosynthesis by aromatse. This results in to three major forces that drive the process of mammary carcinogenesis: (i) mutagenesis by creation of free radical involved in sustained prostaglandin biosynthesis; (ii) angiogenesis by stimulation of vascular endothelial growth factor by prostaglandin E2; and (iii) mitogenesis without natural apoptosis due to estrogen production by aromatase. In addition, COX activity may also be linked to the metabolic activation and metabolism of DMBA and other polycyclic aromatic hydrocarbons through the cytochrome P-450 system12.
In the present study, COX-2 was undetectable in normal mammary tissue, and its expression induced by DMBA treatment was significantly higher in tumour tissue as compared to uninvolved mammary tissue. The reduced expression of COX-2 in mammary tissue in cow ghee fed rats compared to soybean oil fed rats was associated with decreased tumour incidence in the former group. This finding is supported by the observations that the overexpression of COX-2 in mice induces mammary tumours6, and specific COX-2 inhibitors such as nimesulide13 and celecoxib14 prevent mammary tumour from developing in experimental animals. Increased expression of COX-2 in HER2/neu-transformed human mammary epithelial cells has been observed15.
Peroxisome proliferators activated receptor-γ, a key component in regulation of growth and progression of mammary cancer, is expressed in normal as well as in malignant mammary epithelial cells and its activation by ligands induces cellular differentiation16,17. The activation of PPAR-γ induces proapoptotic caspase-3 protein in human liver cancer cell lines18 and reduces antiapoptotic Bcl-2 protein level in human colon cancer cell19. Ligands for PPAR-γ also inhibit growth and induce apoptosis in human breast cancer cell in vitro and in BAX mice8. Further, PPAR-γ ligand (GW 7854) inhibits nitrosomethylurea induced mammary carcinogenesis9 in rats.
An inverse relationship between COX-2 and PPAR-γ expression was observed in the present study and it was associated with decreased mammary tumour incidence in cow ghee fed rats compared to soybean oil fed ones. Similarly, an inverse correlation between PPAR-γ and COX-2 expressions was observed in colon adenocarcinomas20. The PPAR-γ down-regulation in colon adenocarcinomas enhances AP-1 transcriptional activity leading to up-regulation of COX-2 expression. In the genesis of breast cancer, evidences suggest that induction of COX-2 and downregulation of PPAR-γ can be the key components21,22. Simultaneous targeting with COX-2 inhibitor (celecoxib) and PPAR-γ agonist [N - (9-fluorenyl-methyloxycarbonyl)-L-leucine] has been reported to inhibit mammary gland carcinogenesis in rats21,22. Further, activation of PPAR-γ by cigiltazone (PPAR-γ ligand) decreases the COX-2 expression23, and the inhibition of COX-2 induces PPAR-γ expression24.
While vegetable oils contain large amount of linoleic acid known to have promotional role in carcinogenesis25, milk fat contains CLA, which has been shown unequivocally to inhibit mammary carcinogenesis26. In the present study, feeding cow ghee started during mammary gland development period led to 39 per cent lower cancer incidence than in soybean oil fed rats. Feeding CLA during pubescent mammary gland development period lowers the population and proliferating activity of the terminal end buds cells26, which are the target sites for development of adenocarcinomas in response to carcinogenic stimulus. In the present study, the feeding of cow ghee started during the pubescent period of mammary gland development might have resulted in the decreased tumour incidence and progression to malignancy.
The anticarcinogenic effect of CLA may be partly explained by its effect on the COX-2. Conjugated linoleic acid affects the COX-2 at the level of mRNA as well as protein in cultured macrophage cell line27. It represses AP-1 mediated activation of COX-2 transcription in MCF-7 breast cancer cells28. McCarty29 hypothesised that activation of PPAR-γ may mediate a portion of the anticancer activity of CLA. The treatment of colon cancer cells with CLA inhibits cell proliferation; increases expression of PPAR-γ and downregulates APC and c-myc proteins30,31. The higher tumour incidence and faster progression of DMBA induced mammary carcinogenesis in rats fed on soybean oil compared to cow ghee fed ones could be due, partly, to high content of linoleic acid in soybean oil. The promotion of mammary carcinogenesis in rats by n-6 polyunsaturated fatty acids is associated with enhanced expression of COX-225.
We conclude from this study that cow ghee opposed to soybean oil protects against DMBA induced mammary carcinogenesis and the effect is mediated through decreased expression of COX-2 and increased expression of PPAR-γ. Further work is needed to understand the regulation of COX-2 and PPAR-γ, apoptotic singling, cell proliferation and prostaglandin synthesis in response to dietary fat.
Acknowledgments
The authors acknowledge the Indian Council of Agricultural Research (ICAR), New Delhi for financial support.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of world wide burden of cancer in 2008: Globocan 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Wynder EL, Rose DP, Cohen LA. Diet and breast cancer in causation and therapy. Cancer. 1986;58:1804–13. doi: 10.1002/1097-0142(19861015)58:8+<1804::aid-cncr2820581404>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Yanagi S, Yamashita M, Sakamoto M, Kumazawa K, Imai S. Comparative effects of butter, margarine, safflower oil and dextrin on mammary tumorigenesis in mice and rats. In: Kabara JJ, editor. The pharmacological effects of lipids III: The role of lipids in cancer research. Galena, IL: Lauricidin Inc; 1989. pp. 159–69. [Google Scholar]
- 4.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res. 1992;52(Suppl 7):2040S–8S. [PubMed] [Google Scholar]
- 5.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 6.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 7.Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–91. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 8.Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator activated receptor gamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BAX mice. Proc Natl Acad Sci USA. 1998;95:8806–11. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, et al. A new ligand for the peroxisome proliferators-activated receptor-γ (PPAR-γ), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671–3. [PubMed] [Google Scholar]
- 10.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 11.Harris RE, Robertson FM, Abou-Issa HM, Farrar WB, Brueggemeier R. Genetic induction and upregulation of cyclooxygenase (COX) and aromatase (CYP19): an extension of the dietary fat hypothesis of breast cancer. Med Hypotheses. 1999;52:291–2. doi: 10.1054/mehy.1998.0009. [DOI] [PubMed] [Google Scholar]
- 12.Shou M, Korzekwa KR, Krausz KW, Buters JT, Grogan J, Goldfarb I, et al. Specificity of cDNA-expressed human and rodent cytochrome P450s in the oxidative metabolism of the potent carcinogen 7,12-dimethylbenz(a)anthracene. Mol Carcinogen. 1996;17:241–9. doi: 10.1002/(SICI)1098-2744(199612)17:4<241::AID-MC8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsugi S, Ohta T, Kawamori T, Mutoh M, Tanigawa T, Watanabe K, et al. Chemoprevention by nimesulide, a selective cyclo-oxygenase-2 inhibitor of 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP)-induced mammary gland carcinogenesis in rats. Jpn J Cancer Res. 2000;91:886–92. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:2101–3. [PubMed] [Google Scholar]
- 15.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649–57. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 16.Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, et al. Expression of peroxisome proliferator activated receptor mRNA in normal and tumorigenic rodent mammary glands. Biochem Biophys Res Commun. 1998;253:813–7. doi: 10.1006/bbrc.1998.9858. [DOI] [PubMed] [Google Scholar]
- 17.Yee LD, Guo Y, Bradbury J, Suster S, Clinton SK, Seewaldt VL. The antiproliferative effects of PPAR-γ ligands in normal human mammary epithelial cells. Breast Cancer Res Treat. 2003;78:179–92. doi: 10.1023/a:1022978608125. [DOI] [PubMed] [Google Scholar]
- 18.Toyoda M, Takagi H, Horiguchi N, Kakizaki S, Sato K, Takayama H, et al. A ligand for peroxisome proliferator activated receptor gamma inhibits cell growth and induces apoptosis in human liver cancer cells. Gut. 2002;50:563–7. doi: 10.1136/gut.50.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631–46. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 20.Konstantinopoulos PA, Vandoros GP, Sotiropoulou-Bonikou G, Kominea A, Papavassiliou AG. NF-ΚB/PPARγ and/or AP-1/PPARγ ‘on/off’ switches and induction of CBP in colon adenocarcinomas: correlation with COX-2 expression. Int J Colorectal Dis. 2007;22:57–68. doi: 10.1007/s00384-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 21.Badawi AF, Badr MZ. Chemoprevention of breast cancer by targeting cyclooxygenase-2 and peroxisome proliferator-activated receptor-.γ. Int J Oncol. 2002;20:1109–22. [PubMed] [Google Scholar]
- 22.Badawi AF, Badr MZ. Expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-γ and levels of prostaglandin E2 and 15-deoxy-∆12,14 -prostaglandin J2 in human breast cancer and metastasis. Int J Cancer. 2003;103:84–90. doi: 10.1002/ijc.10770. [DOI] [PubMed] [Google Scholar]
- 23.Yang WL, Frucht H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis. 2001;22:1379–83. doi: 10.1093/carcin/22.9.1379. [DOI] [PubMed] [Google Scholar]
- 24.Clay CE, Namen AM, Atsumi G, Willingham MC, High KP, Kute TE, et al. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20:1905–11. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]
- 25.Badawi AF, EL-Sohemy A, Stephen LL, Ghoshal AK, Archer MC. The effect of dietary n-3 and n-6 polyunsaturated fatty acids on the expression of cyclooxygenase1 and 2 and level of p21ras in rat mammary glands. Carcinogenesis. 1998;19:905–10. doi: 10.1093/carcin/19.5.905. [DOI] [PubMed] [Google Scholar]
- 26.Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, et al. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr. 1999;129:2135–42. doi: 10.1093/jn/129.12.2135. [DOI] [PubMed] [Google Scholar]
- 27.Cheng WL, Lii CK, Chen HW, Lin TH, Liu KL. Contribution of conjugated linoleic acid to the suppression of inflammatory responses through the regulation of the NF-kappaB pathway. J Agric Food Chem. 2004;52:71–8. doi: 10.1021/jf0348626. [DOI] [PubMed] [Google Scholar]
- 28.Degner SC, Kemp MQ, Bowden GT, Romagnolo DF. Conjugated linoleic acid attenuates cyclooxygenase-2 transcriptional activity via an anti-AP-1 mechanism in MCF-7 breast cancer cells. J Nutr. 2006;136:421–7. doi: 10.1093/jn/136.2.421. [DOI] [PubMed] [Google Scholar]
- 29.McCarty MF. Activation of PPAR gamma may mediate a portion of the anticancer activity of conjugated linoleic acid. Med Hypotheses. 2000;55:187–8. doi: 10.1054/mehy.1999.1010. [DOI] [PubMed] [Google Scholar]
- 30.Bozzo F, Bocca C, Colombatto S, Miglietta A. Antiproliferative effect of conjugated linoleic acid in caco-2 cells: involvement of PPAR gamma and APC/beta-catenin pathways. Chem Biol Interact. 2007;169:110–21. doi: 10.1016/j.cbi.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Yasui Y, Hosokawa M, Sahara T, Suzuki R, Ohgiya S, Kohno H, et al. Bitter gourd seed fatty acid rich in 9c,11t,13t-conjugated linolenic acid induces apoptosis and up-regulates the GADD45, p53 and PPARgamma in human colon cancer Caco-2 cells. Prostaglandins Leukot Essent Fatty Acids. 2005;73:113–9. doi: 10.1016/j.plefa.2005.04.013. [DOI] [PubMed] [Google Scholar]


