Abstract
Background & objectives
Programmatic management of MDR-TB using a standardized treatment regimen (STR) is being implemented under the Revised National Tuberculosis Control Programme (RNTCP) in India. This study was undertaken to analyse the outcomes of MDR-TB patients treated at the Tuberculosis Research Centre, Chennai, with the RNTCP recommended 24 months STR, under programmatic conditions.
Methods
Patients failed to the category II re-treatment regimen and confirmed to have MDR-TB, were treated with the RNTCP's STR in a prospective field trial on a predominantly ambulatory basis. Thirty eight patients were enrolled to the trial from June 2006 to September 2007.
Results
Time to culture conversion was two months or less for 82 per cent of patients. Culture conversion rates at 3 and 6 months were 84 and 87 per cent respectively. At the end of treatment, 25 (66%) were cured, 5 defaulted, 3 died and 5 failed. At 24 months, 30 (79%) patients, including 5 defaulters, remained culture negative for more than 18 months. Twenty two (58%) patients reported adverse drug reactions (ADRs) which required dose reduction or termination of the offending drug. No patient had XDR-TB initially, but 2 failure cases emerged as XDR-TB during treatment.
Interpretation & conclusions
Outcomes of this small group of MDR-TB patients treated with the RNTCP's STR is encouraging in this setting. Close attention needs to be paid to ensure adherence, and to the timely recognition and treatment of ADRs.
Keywords: Ambulatory treatment, India, multidrug-resistant TB, standardized regimen
Tuberculosis (TB) remains a major health problem in India accounting for more than 20 per cent of the global incident cases1. Since 1997, the Government of India has implemented under the Revised National TB Control Programme (RNTCP) the globally recommended DOTS strategy, and covered the entire country by March 2006. Having achieved the global targets for cure rates among new smear-positive pulmonary TB cases detected under the programme, RNTCP is now implementing the programmatic management of multidrug-resistant TB (MDR-TB - defined as resistance to at least isoniazid and rifampicin) cases in a phased manner and a standardized category-IV treatment regimen (STR) has been approved by the RNTCP National DOTS-Plus Committee2.
Knowledge of the feasibility and effectiveness of an STR given on an ambulatory basis for MDR-TB is very limited in India3. A prospective field trial was initiated at the Tuberculosis Research Centre (TRC), Chennai, Tamil Nadu, using the RNTCP management guidelines and STR for MDR-TB cases to test their feasibility and effectiveness. We report here the treatment outcomes of the first patient cohort.
Material & Methods
This prospective feasibility study was approved by the Institutional Ethics Committee of TRC.
Study area: Patients for this study were recruited from Tiruvallur district and the Chennai Corporation area (with a combined population of almost 7.5 million) in southern India identified for operational research activities conducted by the TRC.
Case finding strategy: As per the RNTCP policy during the period of this study (June 2006-September 2007), all patients who remain smear positive after 4 months or more of treatment with the RNTCP category II re-treatment regimen from the above mentioned study areas, were referred to TRC. Two sputum specimens were collected for drug susceptibility testing (DST). DST was done for the first line anti-TB drugs, namely rifampicin, ethambutol, streptomycin, isoniazid, and the second line anti-TB drugs (SLDs) kanamycin, ofloxacin and ethionamide by conventional methods4–6. Culture was done on L-J medium, and DST performed by proportion method on solid medium. When the results became available, the patient and the health care staff were informed of the result either by telephone or a visit by a TRC field worker.
Pre-treatment investigations: Pre-treatment investigations done included sputum for acid fast bacilli (AFB) by smear, culture and DST, chest X-ray, urine for albumin, sugar and bile pigment, pregnancy test for female patients (if 18 to 45 yr old), complete haemogram, renal and liver function tests, and ELISA for HIV antibodies.
Eligibility criteria: Patients under 18 yr of age, pregnant women, patients having a concurrent major psychiatric illness or serious medical illnesses, patients having had >1 month treatment with any second line anti-TB drugs, and HIV positive cases, were excluded from the study. These exclusions, bar HIV positive status, were as per the RNTCP guidelines current at the time of the study. Patients confirmed to have MDR-TB by the DST result and who met the inclusion criteria, were traced and enrolled into the study.
Treatment regimen: The standardized regimen consisted of an intensive phase (IP) of 6-9 months with 6 drugs, namely kanamycin (Km), ofloxacin (Ofx), ethionamide (Eto), pyrazinamide (Z), ethambutol (E), and cycloserine (Cs) given daily. This was followed by a continuation phase (CP) of 18 months of 4 drugs, namely Ofx, Eto, E and Cs2. At the end of 6 months of treatment, if the fourth month culture remained positive, the IP was extended for a further 3 months. All patients enrolled to the study were treated with a daily supervised regimen.
Patient management and drug logistics: The policy under RNTCP is to give ambulatory treatment after initial hospitalization for a period of 2 to 4 wk. Hence all patients were motivated to get admitted for this initial short period. However, unwillingness for hospitalization was not an exclusion criterion provided the patient was willing to initially attend TRC for daily supervised treatment. Intense health education was given to the patients, along with family members, by a medical officer and a medical social worker. Patients were monitored closely during the first few days of treatment for any adverse events. Subsequent treatment was arranged at the nearest health centre according to the patient's choice.
The drugs were delivered to the health centre by a TRC field worker. Each day's drugs were packed in individual polythene bags to make administration of the drugs easier for the DOT providers (Governmental health care providers, private medical practitioners, and friends/relatives staying close by). The DOT providers were trained on a one to one basis in administering the drugs under supervision, to counsel the patient for drug regularity, to identify and refer them to the medical officer in the event of any adverse drug reactions, and to send them for follow up as per the schedule. Patients were given the phone numbers of the medical officer and TRC field workers so that they could contact them in case of any emergency.
Patients attended TRC once a month during treatment for monitoring, and for other investigations. Two sputum specimens were collected for smear microscopy and culture every month up to 12 months, and thereafter at months 15, 18, 21 and 24. Chest radiography was repeated at the end of IP and at the end of treatment. Complete haemogram, liver and renal function tests were done every month for the first 6 months and whenever indicated. The field worker visited the local health centre at least once a month for supervision and supply of drugs.
Outcome measures: Outcome measures analysed were: (i) time to sputum smear and culture conversion: defined as the duration from the initiation of treatment to the date of the first of the two consecutive negative smears or cultures, taken at least one month apart, irrespective of the subsequent results, (ii) smear and culture conversion rates at 3 and 6 months, (iii) treatment outcomes: such as cure, default, failure and death as per the RNTCP definitions2. In addition, patients who remained persistently sputum culture positive even after 9 months of regular treatment were declared as failures7.
Results
A total of 138 MDR-TB suspects were referred to TRC for DST between June 2006 and September 2007. Of them, 38 were admitted to study (Fig.).
Fig.

Flow chart showing admission of MDR-TB patients to treatment. DST, drug susceptibility testing; SLD, second line anti-TB drugs; Rx,
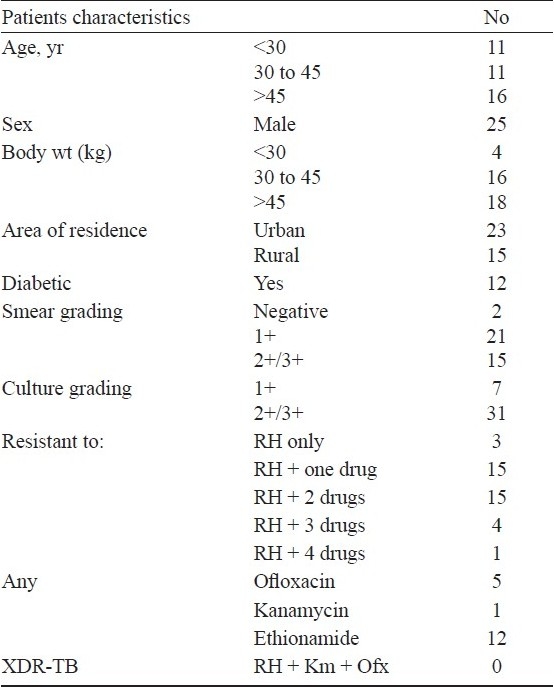
Twenty five were males, 4 weighed less than 30 kg and 12 had associated diabetes mellitus. None of the patients were found at the start of treatment to have extensively drug resistant TB (XDR-TB), defined as MDR-TB patients who are also resistant to one of the injectable SLDs and any one of the quinolones (Table I). Twenty five (64%) patients were hospitalized, with half staying for less than 14 days, and only 2 patients stayed for more than one month.
Table I.
Baseline characteristics of 38 MDR-TB patients treated with the standardized treatment regimen under RNTCP
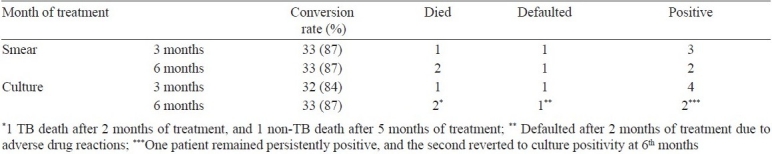
Time to culture conversion (Table II) was 2 months or less for 31 (82%) patients. By 6 months, 35 (92%) patients had culture converted, 1 had persistent culture positivity, 1 patient had defaulted and one had died after 2 months of treatment with positive cultures. Four of the 35 patients who showed culture conversion at different time points (1, 2 months and two at 5 months) reverted to persistent positivity from the 6th to 8th months onwards.
Table II.
Time to smear and culture conversion in months of MDR-TB patients treated with the RNTCP STR (n=38)

The smear and culture conversion rates (Table III) at 3 months were 87 per cent (33/38) and 84 per cent (32/38) respectively and at 6 months, they were 87 per cent (33/38) for both smear and culture. At the end of treatment, 25 (66%) were cured, 5 (13%) had defaulted, 3 (8%) had died and 5 (13%) had failed treatment. Thirty (79%) patients, including 5 defaulters, remained culture negative for more than 18 months. Of the 5 defaulted cases, one defaulted after 2 months of treatment due to drug intolerance, was retrieved after 6 months of default, and re-treated with the STR with reduced dosages for 24 months successfully. Another two defaulted due to drug intolerance after 11 and 18 months of treatment, and two due to personnel reasons after 15 and 20 months. All these 4 patients were followed up with sputum examinations as required and remained culture negative for more than 18 months at 24 months. The response of 12 patients with associated diabetes was equally good, however, two died of reasons other than TB.
Table III.
Smear and culture conversion rates at 3 and 6 months (n=38)
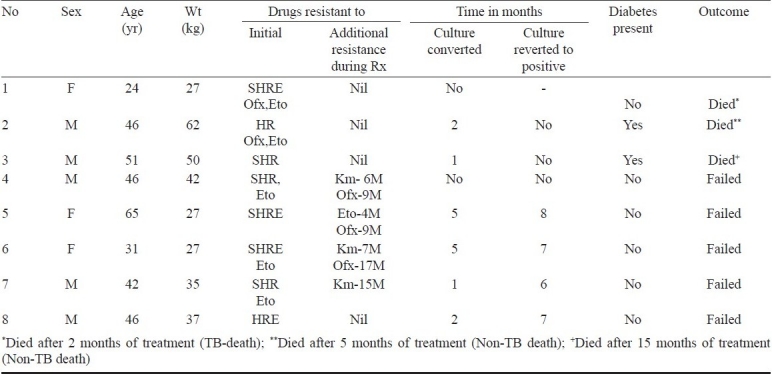
The details of patients who died or failed treatment are given in Table IV. Of the three patients who died, 2 were diabetic and died of causes other than TB. Of the five failures, four had culture converted early, but then reverted to positivity soon afterwards and did not show any bacteriological response thereafter. Two of the five failures showed XDR pattern on follow up DSTs during treatment. Of the four patients with a body weight of <30 kg, three had an unfavourable response (1 died, 2 failures).
Table IV.
Details of patients who died (3) or failed treatment (5)
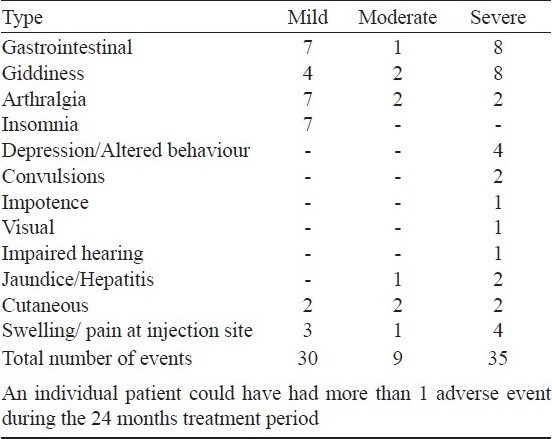
Adverse drug reactions: Only five patients did not complain of any adverse drug reactions (ADR) (Table V). ADRs were considered mild if the patient made 1 or 2 complaints during the 12 month period and only required symptomatic treatment, moderate if the complaint was repeated or of prolonged duration but still could be managed with symptomatic drugs, and severe if either a reduction of dosage or termination of the offending drug(s) was warranted. Severe ADR was observed in 22 (58%) patients. Of these, 13 had termination of ethionamide, 4 of ofloxacin, and 1 each had kanamycin, ethambutol, pyrazinamide or cycloserine terminated. In addition, ofloxacin dosage was reduced in 1 patient, pyrazinamide in 2 others, and kanamycin injections changed to 3 days a week for 5 patients.
Table V.
Frequency of adverse drug events observed during treatment
Discussion
This study, although based on a small number of patients, found that the standardized regimen recommended by RNTCP for the treatment of MDR-TB cases in India appears to be effective in terms of high culture conversion, high cure (66%) and low death (8%) in this setting. In all, 79 per cent of cases remained culture negative for 18 months or more. However, the high incidence of adverse drug reactions observed in the study, highlights the need for adequate clinical back up for managing ADRs while decentralizing treatment for MDR-TB. The initial absence of XDR-TB and low levels of mono-quinolone resistance in this group of patients may have been important factors for the relatively good outcomes observed. Adherence to treatment was attained by strong health education to the patient and their family members prior to starting treatment and at periodic intervals, decentralized DOT supplemented with rigorous supervision by experienced health care staff, intense monitoring, prompt identification and management of adverse drug reactions, and involvement of community and family in providing DOT.
Our findings are similar to recently published reports from New Delhi3 and Nepal8 where cure rates of 61 and 70 per cent have been reported. These results contrast to the 48 per cent favourable response reported from Peru using a standardized regimen for chronic TB patients with MDR-TB9. However the STR used in Peru contained kanamycin for only 3 months, along with ciprofloxacin, ethionamide, pyrazinamide and ethambutol. Similarly, previous reports from TRC have shown poor success rates of 37-50 per cent for the treatment of MDR-TB10,11. These lower success rates could have been due to the lower dosages of drugs used in the regimen at that time.
Sputum culture conversion of up to 74 per cent at 6 months have been reported from other studies in India on treatment of MDR-TB cases3,12–14.Patients in our study treated with the RNTCP recommended regimen, appear to have had a more rapid clearance of bacilli from sputum (82% by two months) and showed better treatment outcomes. None of the patients who remained culture negative consistently from 6th to 12th month failed to treatment.
The finding that the time to smear and culture conversion was two months or less for 82 per cent of patients indicates that effective management of MDR-TB with an adequate regimen with high end dosing, can make the patient noninfectious rapidly and probably give a better treatment outcome. Similar findings have been reported from Latvia by Holtz et al15. At the end of 24 months, 79 per cent of patients remained culture negative for more than 18 months including 4 patients who had defaulted before completing the prescribed duration of treatment and one patient who was re-treated with the same regimen with lower dosage after default.
It has been previously reported that initial resistance to either kanamycin, ofloxacin or pyrazinamide is significantly associated with a longer time to initial sputum culture conversion, and that resistance to ofloxacin and low body mass index are risk factors for poor treatment outcomes16. In this study, of the four patients with a body weight of <30 kg, three had an unfavourable response (1 death and 2 failures). Of the five patients with quinolone resistance, two died and three responded to treatment. Although based on small numbers, the response of patients with associated diabetes to the RNTCP STR was good, although two patients died of causes other than TB. Patients with diabetes on ofloxacin containing regimens may, however, require more close monitoring. Low body weight appeared to be associated with an unfavourable response.
Another important finding was that two treatment failure cases had extended their initial resistance patterns to XDR-TB during treatment. Whether this was because of amplification of the initial resistance pattern or external re-infection, could not be confirmed.
Though severe adverse reactions were frequent, treatment could be continued in most cases with modification of the treatment regimen. Other studies have reported major adverse reaction ranging from 19-72 per cent3,8,17. Thus close monitoring, prompt and timely administrative actions are essential for treatment adherence.
Patients in this study were managed under field research conditions with the close oversight and monitoring of TRC, which may possibly have impacted on the delivery of the treatment. Caution needs to be taken to extrapolate the findings of this small group of study patients to the much larger group of MDR-TB patients in India.
From this study, it appears feasible to treat MDR-TB patients effectively in India on the predominantly ambulatory RNTCP standardized regimen, with certain additional inputs into the existing health care system. Close attention needs to be paid to ensure adherence, and to the timely recognition and treatment of adverse drug reactions.
Acknowledgments
The authors acknowledge support given by Dr Santha, former d0 eputy d0 irector of TRC. Authors thank the Tamil Nadu State TB Officer and programme managers and staff of Tiruvallur district and Chennai Corporation for all the support in case referrals and arrangement of treatment supervision, and the staff of TRC specially bacteriology, statistics, clinic and biochemistry divisions for the support in conducting the study. Authors also thank all the patients for their co-operation in participating in the trial. The study was supported in part by the WHO, with financial assistance provided by the United States Agency for International Development under the Model DOTS Project.
References
- 1.Geneva: WHO; 2009. World Health Organization (WHO). Global tuberculosis control 2009: epidemiology, strategy, financing. WHO/HTM/TB/2009.411. [Google Scholar]
- 2.DOTS-plus guidelines. New Delhi: CTD; 2006. Central TB Division (CTD), Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 3.Singla R, Sarin R, Khalid UK, Mathuria K, Singla N, Jaiswal A, et al. Seven-year DOTS-Plus pilot experience in India: Results, constraints and issues. Int J Tuberc Lung Dis. 2009;13:976–81. [PubMed] [Google Scholar]
- 4.Allen B, Baker FJ. London: Butterworth; 1968. Mycobacteria: isolation, identification and sensitivity testing. [Google Scholar]
- 5.Canetti G, Fox W, Khomenko A, Mahler HT, Menon AK, Mitchison DA, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Tuberculosis Research Centre, Madras. Study of chemotherapy regimens of 5 and 7 months’ duration and the role of corticosteroids in the treatment of sputum positive patients with pulmonary tuberculosis in south India. Tubercle. 1983;64:73–91. doi: 10.1016/0041-3879(83)90032-6. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CY, Caminero JA, Enarson DA. Reporting on multi-drug resistant tuberculosis: a proposed definition for the treatment outcome ‘failed’. Int J Tuberc Lung Dis. 2009;13:548–50. [PubMed] [Google Scholar]
- 8.Malla P, Kanitz EE, Akthar M, Falzon D, Feldmann K, Gunneberg C, et al. Ambulatory-based standardised therapy for multi-drug resistant tuberculosis: experience from Nepal, 2005-2006. PLoS One. 2009;4:08313. doi: 10.1371/journal.pone.0008313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez PG, Floyd K, Portocarrero J, Alarcón E, Rapiti E, Ramos G, et al. Feasibility and cost-effectiveness of standardized second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–9. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]
- 10.Chemotherapy of drug resistant tuberculosis: The Tuberculosis Research Centre experience over 40 years. Indian J Tuberc. 2000;47:201–10. [Google Scholar]
- 11.Thomas A, Ramachandran R, Rehaman F, Jaggarajamma K, Santha T, Selvakumar N, et al. Management of multi-drug resistant tuberculosis in the field - Tuberculosis Research Centre experience. Indian J Tuberc. 2007;54:117–24. [PubMed] [Google Scholar]
- 12.Katiyar K, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12:139–45. [PubMed] [Google Scholar]
- 13.Prasad R, Verma SK, Sahai S, Kumar S, Jain A. Efficacy and safety of kanamycin, Ethionamide, PAS, and Cycloserine in multidrug-resistant pulmonary tuberculosis patients. Indian J Chest Dis Allied Sci. 2006;48:183–6. [PubMed] [Google Scholar]
- 14.Arora VK, Sarin R, Singla R, Khalid UK, Mathuria K, Singla N, Myneedu VP. DOTS-Plus for patients with multidrug-resistant tuberculosis in India: Early results after three years. Indian J Chest Dis Allied Sci. 2007;49:75–9. [Google Scholar]
- 15.Holtz TH, Sternberg M, Kammerer S, Kayla FL, Vija R, Evija Z, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis and relationship to treatment outcome. Ann Intern Med. 2006;144:650–9. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 16.Leimane V, Riekstina V, Holtz TH, Zarovska E, Skripconoka V, Thorpe TE, et al. Clinical outcome of individualized treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–26. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 17.Nathanson E, Gupta R, Huamani P, Leimane V, Pasechnikov AD, Tupasi TE, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: Results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–4. [PubMed] [Google Scholar]


