Abstract
Medial prefrontal cortex (MPFC) is among those brain regions having the highest baseline metabolic activity at rest and one that exhibits decreases from this baseline across a wide variety of goal-directed behaviors in functional imaging studies. This high metabolic rate and this behavior suggest the existence of an organized mode of default brain function, elements of which may be either attenuated or enhanced. Extant data suggest that these MPFC regions may contribute to the neural instantiation of aspects of the multifaceted “self.” We explore this important concept by targeting and manipulating elements of MPFC default state activity. In this functional magnetic resonance imaging (fMRI) study, subjects made two judgments, one self-referential, the other not, in response to affectively normed pictures: pleasant vs. unpleasant (an internally cued condition, ICC) and indoors vs. outdoors (an externally cued condition, ECC). The ICC was preferentially associated with activity increases along the dorsal MPFC. These increases were accompanied by decreases in both active task conditions in ventral MPFC. These results support the view that dorsal and ventral MPFC are differentially influenced by attentiondemanding tasks and explicitly self-referential tasks. The presence of self-referential mental activity appears to be associated with increases from the baseline in dorsal MPFC. Reductions in ventral MPFC occurred consistent with the fact that attention-demanding tasks attenuate emotional processing. We posit that both self-referential mental activity and emotional processing represent elements of the default state as represented by activity in MPFC. We suggest that a useful way to explore the neurobiology of the self is to explore the nature of default state activity.
Functional brain imaging studies in normal human subjects with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have frequently revealed task-induced decreases in regional brain activity that appear to be largely task-independent, varying little in their location across a wide range of tasks (1). The consistency with which certain areas of the brain participate in these decreases led us to posit the existence of an organized mode of brain function that is present as a baseline or default state and attenuated during specific goal-directed behaviors (2).
Among the areas most prominently exhibiting these decreases are areas in medial prefrontal cortex (MPFC). The MPFC areas involved often include elements of both its dorsal and ventral aspects. Dorsally, when decreases are observed, they tend to spare the cingulate cortex, which usually exhibits an increase in activity during attention-demanding tasks (3, 4).
Anatomical studies in nonhuman primates provide support for a dorsal–ventral functional distinction within the MPFC (5–8). Imaging studies (for reviews see refs. 3, 4, and 9) have suggested functional distinctions between dorsal and ventral MPFC as well, with the former more associated with complex cognitive operations and the latter with emotional or affective processes. With regard to the dorsal MPFC, additional data indicate that elements of BA 8, 9, and 10 may contribute to self-referential or introspectively oriented mental activity (10–16).
The MPFC is also among those brain regions having the highest baseline metabolic activity at rest (2). Ingvar was the first to note high resting blood flow in prefrontal cortex (17). He attributed this to spontaneous self-generated mental activity of the resting human brain (18).
The specific aim of this work was to expand our knowledge of the functions instantiated in the default state with specific reference to the MPFC and to suggest that these functions include elements that are integral to aspects of the multifaceted concept known as the “self,” including a “narrative” (19) or “autobiographical” self (20). This latter term is here broadly understood to mean an ongoing phenomenon associated with an awareness of a personal past, present, and future (18–22).
Specifically, we used fMRI and a paradigm including a self-referential behavioral task previously associated with changes in dorsal MPFC (15) in an effort to more precisely define its functionality. By the addition of the control task of visual fixation, we hoped to relate these changes to the default state and differentiate dorsal MPFC behavior from that in the ventral MPFC, thereby expanding our understanding of the functions being represented throughout MPFC.
Materials and Methods
Subjects.
Twenty-four subjects (twelve male, twelve female) without significant psychiatric or neurologic history between the ages of 20 and 35 (mean age 24 ± 3 yrs) were recruited from the local Washington University community. All subjects were right-handed as judged by the Edinburgh Handedness Inventory (23) and were normal or corrected-to-normal in visual acuity. Subjects were paid $25 for each hour of their participation and gave informed consent in accordance with guidelines set by the Human Studies Committee of Washington University Medical Center.
Pictorial Stimuli.
Subjects viewed full-color pictures selected from the International Affective Picture System (IAPS) (24). Pictures were resized such that their heights were uniform, subtending a vertical visual angle of ≈9°. The horizontal visual angle subtended ranged from a minimum of ≈10° to a maximum of ≈20°. All pictures were centered on a black background.
The picture sets and their organization were identical to those used in a prior PET study (15). The picture sets were organized into six types of emotional picture sets, which varied from 10% to 90% neutral pictures within each set. Neutral stimuli included neutral faces, household objects, and complex scenes such as a freeway. Remaining stimuli (i.e., the emotional pictures) in each picture set were equally divided between pleasant and unpleasant. Pleasant pictures included stimuli such as smiling babies, flowers, and skydivers. Unpleasant pictures included stimuli such as burn victims, snakes, and guns pointed at others as well as at the viewer. Two versions of each picture set had been created and the two versions were counterbalanced across subjects with respect to their occurrence in either of the two experimental conditions.
Stimuli were generated by a Macintosh G3 computer using the psyscope software program (25) and rear projected (Ampro Model LCD-150, Melbourne, FL) onto a projection screen positioned at the head end of the MRI scanner bore. Subjects viewed the screen through a mirror mounted on the head coil. Subjects responded by pushing a fiber-optic light sensitive keypress connected to a PsyScope button box (Carnegie Mellon University, Pittsburgh).
Experimental Design.
The fMRI design was “blocked” with picture blocks alternating with visual fixation blocks of equal length (36 s). Ten pictures varying along the dimensions of valence and arousal were presented within each block, with a total of 30 pictures being presented during each run. Each picture was displayed for a duration of 500 ms with an ISI of 3,600 ms, during which period subjects made their keypress responses. A fixation crosshair was displayed in the center of the screen before the first picture block for a duration of four frames, between the picture blocks, and during a visual fixation block at the end of each run. Twelve runs of functional data were acquired in a 2.25-hour session.
Subjects were instructed to fixate on the crosshair when it was present. They were told that when a picture appeared, they could move their eyes freely and that they were to perform one of two tasks. For one task [the internally cued condition (ICC)], they were to decide how the picture made them feel and note that if the feeling evoked was pleasant, to press the keypress button under the index finger of their right hand, if it was unpleasant, to press the button under the middle finger of their right hand, and if the feeling was uncertain or neutral, to press the button under the index finger of their left hand. For the other task [the externally cued condition (ECC)], they were to judge an aspect of the picture itself and note that if the picture depicted a scene that was indoors, to press the keypress button under the index finger of their right hand, if it was outdoors, to press the button under the middle finger of their right hand, and if they couldn't tell whether it was indoors or outdoors, to press the button under the index finger of their left hand. The two tasks were performed on alternate runs and the order was counterbalanced across subjects. Subjects were informed immediately before each run which task they were to perform. Responses and response times were recorded by the psyscope program. Response times were calculated from stimulus onset to the keypress response.
Subjects became familiar with the performance of both tasks in a practice session before the first run of the actual study. None of the pictures used in this familiarization session were duplicated in the subsequent experimental runs. As in the prior PET study (15), all subjects indicated during a debriefing session that they had noted emotional responses during the runs for both task conditions, but that they had a more enduring awareness of the response in the internal condition.
Functional Imaging.
Imaging was performed on a Siemens 1.5-Tesla Magnetom Vision System (Erlangen, Germany). Structural magnetic resonance (MR) images were acquired by using a sagittal magnetization prepared rapid gradient echo (MP-RAGE) three-dimensional T1-weighted sequence (repetition time [TR] = 9.7 ms, echo time [TE] = 4 ms, flip angle α = 12°, inversion time [TI] = 300 ms, voxel size = 1.25 × 2 × 1 mm), as well as a T2-weighted spin-echo sequence (TR = 3,800 ms, TE = 90 ms, α = 90°, voxel size = 0.94 × 1.85 × 8 mm). Functional MR (fMRI) images were collected in runs by using an asymmetric spin-echo echo-planar sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (T2*) (TR = 2,160 ms, T2* evolution time = 50 ms, α = 90°). During each functional run, 106 sets (“frames”) of 16 contiguous, 8-mm-thick slices were acquired (3.75 × 3.75 mm in-plane resolution), allowing complete brain coverage. Functional images were acquired parallel to the plane containing the anterior and posterior commissures (AC–PC plane) in each subject after prescribing slice position based on automatic measurements of rotation, translation, and tilt of the structural images relative to an averaged (n = 12) MP-RAGE anatomical image representative of the atlas of Talairach and Tournoux (26). This procedure also centered the brain in the multislice volume and the MR scanner field of view.
Each run of functional imaging data were postprocessed to remove artifacts due to properties of the MR scanning system and subject movement (27). A six-parameter rigid-body realignment mutually registered all frames in all runs for each subject (28–30). Reslicing was by 3D cubic spline interpolation (31).
For each subject, an atlas transformation was computed based on an average of the first frame of each functional run and the T2 and MP-RAGE structural images. An eight-parameter (rigid-body plus in-plane stretch) cross-modal registration similar to the method of Andersson et al. (32) aligned the averaged first frame data to the T2 image. A similar procedure registered the T2 image to the MP-RAGE image. The MP-RAGE image was registered to the atlas representative target by using a twelve-parameter general affine transformation. Finally, all of the involved images were brought into mutual register by matrix multiplication. Functional data were interpolated to 2-mm cubic voxels in the atlas space.
Statistical Analysis of fMRI Data.
The fMRI data were analyzed by using the general linear model (28, 33–35). For each subject, the tasks (e.g., the ICC) were modeled with an assumed hemodynamic response. This response was created by convolving a gamma function with a rectangle function representing the duration of the task. Control periods were defined as those intervals where this modeled response was less than 10% of its maximum. The magnitudes of the BOLD responses during these periods were then estimated by a linear regression model in each subject. The regression model also included terms for linear trends, intercepts, and a high pass filter for each scan. The high-pass filter had a cutoff frequency of 0.009 Hz.
The magnitudes estimated from the model were then analyzed by voxel-based t tests in which the subject was treated as a random effect. t tests were conducted on three comparisons: ICC vs. fixation control; ECC vs. fixation control; and the interaction [i.e., (ICC vs. fixation control) vs. (ECC vs. fixation control), where (ECC vs. fixation control) becomes the control condition for the comparison]. Each statistical map was corrected for multiple comparisons at the 0.05 level by using a Monte Carlo simulation (36) that takes into account the magnitude and size of clusters of contiguous voxels.
The locations of responses in the multiple-comparison corrected maps were identified by using an automated peak-search algorithm (37) that searched for local maxima and minima.
Results
Reaction times did not differ between performances of the two tasks (ICC 1.14 s and ECC 1.12 s; t test, P = 0.36).
Image analysis revealed multiple significant foci along the anterior midline of the cerebral hemispheres running from BA 6/32 dorsally to BA 24/25/32 ventrally. These are summarized in Fig. 1 and Table 1. For current explanatory purposes, we place them into two groups related to their location (i.e., dorsal vs. ventral) and direction of change (i.e., increase vs. decrease). We recognize that such an approach may blur subtle functional distinctions among the areas within each group.
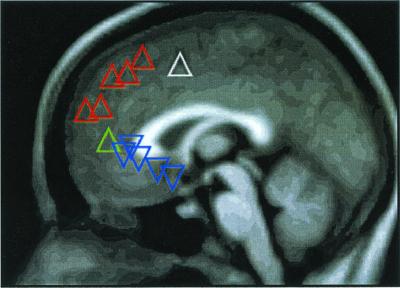
Figure 1.
The distribution of significant regions of increased (▵) and decreased (▿) activity along the medial prefrontal cortex, the coordinates and Z scores of which are listed in Table 1. The triangle colors relate to image data shown in Figs. 2 (white), 3 (red), 4 (blue), and 5 (green).
Table 1.
Coordinates and Z scores for regions of interest in MPFC
| BA | Coordinates
(x,y,z)
|
Z score
|
|||||
|---|---|---|---|---|---|---|---|
| ICC | ECC | ICC > ECC | ICC | ECC | ICC > ECC | ||
| Increases | |||||||
| 6/32 | −5, 3, 48 | −3, 3, 48 | 6.63 | 6.92 | 1.43 | ||
| 8/9 | −9, 39, 42 | 5.31 | 0.80 | 5.41 | |||
| 10 | −3, 53, 24 | 3.92 | −1.45 | 5.29 | |||
| 6/8 | −11, 23, 52 | 3.95 | 1.01 | 4.74 | |||
| 8 | −11, 30, 44 | 4.76 | 2.95 | 4.46 | |||
| 9 | 7, 45, 25 | 3.48 | −0.11 | 4.20 | |||
| Relative increase | 32 | −3, 41, 8 | −0.60 | −5.03 | 4.20 | ||
| Decreases | |||||||
| 24 | 3, 33, 0 | 3, 33, 0 | −4.83 | −6.61 | 2.68 | ||
| 32 | −13, 27, 6 | −9, 29, 4 | −4.09 | −6.00 | 0.70 | ||
| 24/32 | −7, 25, 0 | −3, 25, −2 | −4.53 | −5.85 | 1.73 | ||
| 25 | 7, 13, −4 | 7, 15, −8 | −4.12 | −4.82 | 2.25 | ||
| 25 | −5, 3, −8 | −1, 7, −12 | −2.93 | −4.77 | 0.34 | ||
Coordinates are in the space of Talairach and Tournoux (26). BA, Brodmann areas. ICC and ECC refer to the internally and externally cued conditions vs. fixation control. ICC > ECC refers to the interaction [i.e., (ICC vs. fixation control) vs. (ECC vs. fixation control)]. Bold Z scores refer to areas illustrated in Figs. 2–5.
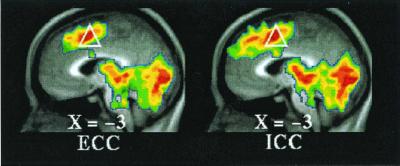
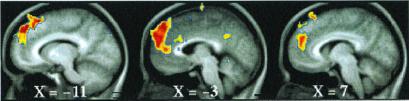
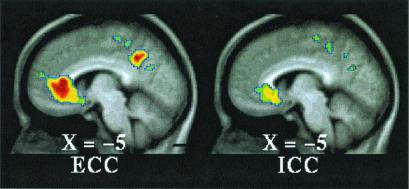
Dorsally, both task conditions (ICC and ECC) exhibited increased activity in the SMA/dorsal anterior cingulate (BA6/32) when each of these conditions was compared with their respective fixation control condition (Fig. 2 and Table 1, ICC = ECC). When the two conditions were directly compared [i.e., (ICC vs. fixation control) vs. (ECC vs. fixation control), where (ECC vs. fixation control) becomes the control condition for the comparison], multiple regions of increased activity were identified running from dorsal to midsections of MPFC (Fig. 3 and Table 1, ICC > ECC). Ventrally, both task conditions (ICC and ECC) exhibited decreased activity in BA 24/25/32 when each of these conditions was compared with their respective fixation control condition (Fig. 4 and Table 1). Near the mid-MPFC at the transition between the increases shown in Fig. 3 and the decreases shown in Fig. 4, there was another region that showed increased activity in the comparison shown in Fig. 3. In this case, however, the increase can be explained by a decrease in (ECC vs. fixation control), whereas there was no significant change in the (ICC vs. fixation control) condition (Fig. 5 and Table 1). The effect of the percentage of neutral pictures in the picture sets will be reported separately.
Figure 2.
T-statistic images of increases common to both active tasks (ECC, Left; ICC, Right) when compared with visual fixation. The white triangle (as in Fig. 1) is located in the vicinity of dorsal anterior cingulate and adjacent pre-SMA and SMA (Table 1, ICC = ECC).
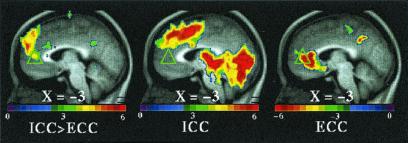
Figure 3.
T-statistic images of increases in ICC greater than in ECC in dorsal MPFC. The two conditions were directly compared [i.e., (ICC vs. fixation control) vs. (ECC vs. fixation control), where (ECC vs. fixation control) became the control condition for the comparison]. The approximate locations of significant regions within BA 8, 9, and 10 are listed in Table 1 (ICC > ECC).
Figure 4.
T-statistic images of decreases common to both tasks (ECC, Left; ICC, Right) in ventral MPFC. The approximate locations of significant regions within BA 24, 25, and 32 are listed in Table 1.
Figure 5.
Three T-statistic images comparing: ICC to ECC (see Fig. 3), and the individual task conditions, ICC and ECC, relative to their own controls (Left to Right). The green triangle (as in Fig. 1) denotes a region (Table 1) of so-called increased activity (Left) that arises entirely as the result of a decrease in ECC relative to its control (Right). Note that there is no change in the ICC relative to its control in this region (Center).
While not the primary focus of the paper, we note that our study identified responses outside of medial prefrontal cortex in the comparison between the two active tasks that corresponded to the frontal operculum/left insula (ICC > ECC) and right and left parieto-occipital cortices (ECC > ICC) responses found in the prior PET study (15). The corresponding coordinates (26) found in the present study were the following: −43, 21, −6 (z = 4.44); 39, −77, 22 (z = 3.45); and, −31, −79, 32 (z = 3.25). We note that our focus in the frontal operculum/left insula, while having a lower center of mass, did extend dorsally to include the exact coordinates specified by Lane and colleagues (15). Additionally, we identified increases (ICC > ECC) in the right cerebellum (25, −75, −30; z = 3.59) and the right insula (37, 21, −2; z = 3.43). We did not observe any change in activity in the vicinity of the right temporal pole at the level specified by Lane and colleagues (15).
Discussion
The results of our fMRI experiment are generally consistent with those of the prior PET study (15), which used the same two tasks (i.e., ICC and ECC) in ten male subjects. With regard to the MPFC, an increase in activity in anterior cingulate cortex (BA 32), extending into medial prefrontal cortex (BA 9), was found in their study. In our study, however, the responses in dorsal MPFC that distinguish ICC from ECC were largely confined to the medial extensions of Brodmann areas 8, 9, and 10, with only limited involvement of adjacent anterior cingulate (Fig. 3). The clearer definition of these regions may be related to the use of magnetic resonance imaging with its higher spatial resolution as compared with PET and the increased statistical power afforded by a much greater number of subjects (24 compared with 10). Our data would suggest the possibility of more than one area of increased activity in Brodmann areas 8, 9, and 10 as well (Table 1, ICC > ECC).
It is important to emphasize that the addition of a simple baseline condition, in our case visual fixation, provided significant additional information about changes occurring in other areas within MPFC using the same active task conditions that were not able to be identified in the prior study (15). Dorsally, increased activity was observed in the vicinity of the anterior cingulate equally in both tasks (Fig. 2). This activity broadly appeared to include the adjacent pre-SMA and SMA consistent with the motor activities associated with task performance (38) and its associated attentional demands (39). Accompanying these increases in dorsal MPFC were decreases in ventral MPFC in both task conditions (Fig. 4).
Others have posited functional differences between dorsal and ventral MPFC (e.g., refs. 4, 3, 9, and 40). Although most investigators have approached these areas separately, our experimental data invite a consideration of them together.
In considering a possible functional interpretation of our data, we begin by noting that the areas of MPFC exhibiting task-specific changes in our study [except for the motor cingulate/pre-SMA point mentioned above (38)] are among those prominently seen to decrease their activity in a wide variety of cognitive activation paradigms (1). Researchers have encountered these task-induced decreases in regional brain activity even when the control state consists of lying quietly with eyes closed or passively viewing a stimulus. The consistency with which these areas of the brain participate in task-associated decreases despite the wide variety of tasks with which they have been associated has led us to posit that there exists an organized mode of brain function that is an active default state whose functions are attenuated during specific goal-directed behaviors (2, 41).
A unique feature of the default state we posit (2) is that it embodies a functionally significant, long-term modal level of neuronal activity. We derive this definition from the strikingly uniform relationship between blood flow and oxygen utilization that exists across the human brain in the resting but awake state and includes those areas regularly exhibiting decreases. Under such circumstances, the discrepancies between local blood flow and oxygen utilization that characterize areas of so-called activation and lead to the BOLD signal in fMRI studies (for review, see ref. 42) are conspicuous by their absence. This uniformity suggests that equilibrium has been reached between the local metabolic requirements necessary to sustain such a long-term modal level of neural activity, and the level of blood flow in that region. We believe that clues to the functional nature of this baseline or default state are revealed through a consideration of the changes observed in areas participating in decreases from this baseline state (1). In this communication, we focus our discussion on the MPFC.
Decreases in activity from a passive control state (e.g., visual fixation) in the ventral MPFC are some of the most frequently observed in functional imaging studies (1). In both of our task states, significant decreases were observed again in this area (Fig. 4). How might this be understood given what is currently known about the functionality of this region?
Anatomically, the ventral MPFC is composed of cytoarchitectonically discrete areas that receive a wide range of sensory information from the body and the external environment via the orbital prefrontal cortex (43–45) and are heavily interconnected with limbic structures such as the amygdala, ventral striatum, hypothalamus, midbrain periaqueductal gray region, and brainstem autonomic nuclei (46–52). Such anatomical relationships suggest a role for these medial areas in the integration of the visceromotor aspects of emotional processing with information gathered from the internal and external environments. Some have extended this idea to suggest that the ventral MPFC plays a role in the integration of emotional and cognitive processes by incorporating emotional biasing signals or markers into decision-making processes (53–55). A related suggestion has been made that ventral MPFC is involved in regulating other limbic structures (e.g., amygdala) on the basis of the “current meaning” of stimuli (56). As a corollary of our model of a default mode of brain function, we would posit that these activities are ongoing unless attenuated during the performance of an attentionally demanding cognitive task.
It has, in fact, been shown that the reductions in activity in ventral MPFC as seen in this and other experiments (1) often occur in the setting of attention-demanding cognitive task performance. This is consistent with the observation that cognitive activity can attenuate aspects of emotional processing such as the experience and expression of distress (57–59).
Although our data do not reveal a statistically significant difference in the degree to which ventral MPFC activity is reduced in our two tasks, visual inspection of the images in Fig. 4 (see also Table 1) suggests that reductions are less in the ICC. This observation prompted us to examine the data from the individual subjects (to be published separately), which revealed greater variability in this region associated with the ICC task than the ECC task. That the level of emotional processing might be greater in the ICC task, at least for some individuals, is intuitively appealing. That there may be less of a decrease in this area when emotional processing co-occurs with an attention-demanding cognitive task is consistent with our previous findings (60); so, also, is the individual variability seen under such circumstances (61). Finally, individual variability coupled with a small sample may well account for Lane and colleagues (15) noting increased activity in ventral MPFC (BA25) in ICC vs. ECC contrast in their PET study.
Although we have noted the frequent occurrence of activity reductions in ventral MPFC, others have reported increases (55). Here we wish to emphasize the importance of the control state used in any imaging study. Our data illustrate the issue. Employing an attention-demanding control task as the baseline, as is so often done (55), is likely to be associated with activity reductions in ventral MPFC. When this is coupled with a task of interest, which also incorporates an element of emotional processing (with the term “emotional” being understood very broadly), the difference between the baseline established by this control task and the task of interest will, in all likelihood, appear as an increase. This is precisely what we observed in one area of MPFC in the comparison of the ICC to the ECC task condition (see Fig. 5). The implications of this issue have been explored in greater depth elsewhere (2, 60–63). Thus, what others report as an increase should on occasion more properly be regarded as a decrease, which more accurately reflects the local task-related changes in neural activity.
In contrast to the ventral MPFC, observed changes in the dorsal MPFC (specifically, BA 8, 9, and 10) have included both increases and decreases. At least two studies, including the present experiment and a mood-induction study by Pardo and colleagues (12), demonstrate true increases from a passive baseline state, whereas a large metaanalysis (1) noted consistent decreases from a passive baseline state. Other studies (for review, see ref. 14) usually involve the use of a complex baseline state (control task), thus making it impossible to determine whether the reported increases arose from comparison to a decrease engendered by the performance of the control task or a true increase in the task of interest. Nevertheless, the dynamic range of activity in BA 8, 9, and 10 appears to include both increases as well as decreases from its baseline or default mode of operation.
The recent report and review by Castelli and colleagues (14) summarizes many of the functional imaging experiments that have reported increases in activity in BA 8, 9, and 10 and the adjacent paracingulate sulcus. The cognitive processes covered in their review fell into two general categories. The first category was monitoring or reporting of one's own mental states, such as self-generated thoughts (16) and intended speech (64), as well as emotions (15). An extension of this latter category includes mood-induction experiments (10, 12, 65) that have generally involved the recollection of personal affect-laden life events. These have demonstrated increases in activity in this area as well. A second category of experiments engaging this region involved attributing mental states to others (14, 66).
On the basis of these imaging results, the Friths have postulated that dorsal “medial prefrontal regions are concerned with explicit representations of states of the self.” Our results (Fig. 3) are consistent with this formulation and suggest that activity within dorsal MPFC is increased when attention is directed specifically toward self-referential or introspectively oriented mental activity.
It has been noted by many that similar mental activity arises spontaneously when subjects are not actively engaged in the processing of externally generated information. Discussions of uncontrolled self-referential or introspectively oriented mental activity occurring during rest conditions have largely focused on its content or psychological character. It has been referred to, for example, as “stimulus independent thoughts” (SITs) or daydreams (67, 68), “task unrelated imagery and thought” (69), and “free association” or “stream of consciousness” (70).
Ingvar (18) was the first to attribute spontaneous self-generated mental activity of the resting human brain to prefrontal cortex activity. This was based on his discovery of a high resting blood flow in the prefrontal area (17). A more recent experiment using PET (16) indicated that activity in BA 8, 9, and 10 correlated with the number of SITs and was highest in the rest condition. This is consistent with the fact that increasing externally directed cognitive load reduces SITs as well as activity in BA 8, 9, and 10 (1). This would be consistent with the view that BA 8, 9, and 10 are involved in the production of SITs as a function of their default state.
Thus, functional imaging studies would suggest that BA 8, 9, and 10 might be necessary for spontaneous as well as task-related self-referential or introspectively oriented mental activity. Lesion studies of dorsal MPFC (71) (see also refs. 72 and 73) and its connections (74, 75) provide support for this hypothesis as well.
We would emphasize that this spontaneous activity does not simply represent “noise” (62, 63), but as David Ingvar first posited (18, 76), it might imply a continuous “simulation of behavior,” an inner rehearsal as well as an optimization of cognitive and behavioral serial programs for the individual's future. Tulving and colleagues have extended this thinking in their work on episodic memory and autonoetic consciousness (for recent review see ref. 22). We would propose that dorsal MPFC participates in the processing of such representations that embody aspects of the self, particularly the temporally extended “narrative” (19) or “autobiographical” (20) self.
Emerging is an expanded view of the neural instantiation of the multifaceted “self.” In our previous work (2), we have suggested that the default state of the brain instantiates functions that are integral to the self, and may be available or unavailable to awareness. These include surveillance of the internal and external environments and some assessment of salience of stimuli for the individual. We believe that the ventral MPFC contributes to the latter. However, this obviously represents a very incomplete view of the self. As Ingvar noted, “the brain cannot produce normal conscious awareness without the ‘self’ having a ‘total’ somehow simultaneous access to the information in the neuronal systems subserving the experience of a past, a present, and a future” (18). Here we believe that dorsal MPFC is particularly important. We recognize, however, that the neural instantiation of the self in all its dimensions is likely to be widely distributed.
Finally, the oft-observed presence of spontaneous mental activity has frequently been regarded as something of a “problem” (e.g., refs. 62 and 63) that needs to be controlled in functional imaging experiments. We would suggest that a useful way to approach the study of an important concept such as the self is to further explore the nature of default state activity.
Acknowledgments
We thank Richard Lane for details of stimulus selection and John Ollinger for assistance with statistical analysis. This work was supported by National Institutes of Health Grants NS06833 and DA07261.
Abbreviations
- MPFC
medial prefrontal cortex
- ICC
internally cued condition
- ECC
externally cued condition
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- BOLD
blood oxygenation level-dependent
References
- 1.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle M E, MacLeod A M, Snyder A Z, Powers W J, Gusnard D A, Shulman G L. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paus T, Koski L, Caramanos Z, Westbury C. NeuroReport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bush G, Luu P, Posner M I. Trends Cognit Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 5.Morris R, Pandya D N, Petrides M. J Comp Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Petrides M, Pandya D N. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 7.Ongur D, Price J L. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. New York: Springer; 1988. [Google Scholar]
- 9.Drevets W C, Raichle M E. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- 10.Reiman E M, Lane R D, Ahern G L, Schwartz G E, Davidson R J, Friston K J, Yun L-S, Chen K. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 11.Lane R D, Reiman E M, Bradley M M, Lang P J, Ahern G L, Davidson R J, Schwartz G E. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 12.Pardo J V, Pardo P J, Raichle M E. Am J Psychiatry. 1993;150:713–719. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- 13.George M S, Ketter T A, Parekh P I, Horwitz B, Herscovitch P, Post R M. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 14.Castelli F, Happe F, Frith U, Frith C. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 15.Lane R D, Fink G R, Chau P M-L, Dolan R J. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 16.McGuire P K, Paulesu E, Frackowiak R S J, Frith C D. NeuroReport. 1996;7:2095–2099. [PubMed] [Google Scholar]
- 17.Ingvar D H. Acta Neurol Scand. 1979;60:12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- 18.Ingvar D H. Hum Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- 19.Gallagher S. Trends Cognit Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- 20.Damasio A R. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 21.Metzinger T. In: Neural Correlates of Consciousness: Empirical and Conceptual Questions. Metzinger T, editor. Cambridge, MA: MIT Press; 2000. pp. 285–306. [Google Scholar]
- 22.Wheeler M A, Stuss D T, Tulving E. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- 23.Raczkowski D, Kalat J W, Nebes R. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- 24.Lang P J, Bradley M M, Cuthbert B N. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: National Institute of Mental Health Center for the Study of Emotion and Attention; 1997. [Google Scholar]
- 25.Cohen J D, MacWhinney B, Flatt M, Provost J. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- 26.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 27.Simpson, J. R., Jr., Ongur, D., Akbudak, E., Conturo, T. E., Ollinger, J. M., Snyder, A. Z., Gusnard, D. A. & Raichle, M. E. (2000) J. Cognit. Neurosci., in press. [DOI] [PubMed]
- 28.Friston K J, Jezzard P, Turner R. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- 29.Friston K J, Williams S, Howard R, Frackowiak R S, Turner R. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 30.Snyder A Z. In: Quantification of Brain Function Using PET. Myers R, Cunningham V, Bailey D, Jones T, editors. San Diego: Academic; 1995. pp. 131–137. [Google Scholar]
- 31.Hajnal J V, Saeed N, Soar E J, Oatridge A, Young I R, Bydder G M. J Comput Assist Tomogr. 1995;19:289–296. doi: 10.1097/00004728-199503000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Andersson J L, Sundin A, Valind S. J Nucl Med. 1995;36:1307–1315. [PubMed] [Google Scholar]
- 33.Friston K, Holmes A, Poline J, Grasby P, Williams S, Frackowiak R, Turner R. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 34.Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Hum Brain Mapp. 1995;2:189–210. doi: 10.1002/(SICI)1097-0193(1996)4:2<140::AID-HBM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Worsley K J, Marrett S, Neelin P, Vandal A C, Friston K J, Evans A C. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 36.Forman S D, Cohen J D, Fitzgerald M, Eddy W F, Mintun M A, Noll D C. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 37.Mintun M A, Fox P T, Raichle M E. J Cereb Blood Flow Metab. 1989;9:96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- 38.Picard N, Strick P L. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 39.Posner M I, Petersen S E. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 40.Whalen P J, Bush G, McNally R J, Wilhelm S, McInerney S C, Jenike M A, Rauch S L. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 41.Raichle M E. Philos Trans R Soc London B. 1998;353:1–14. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raichle M E. Proc Natl Acad Sci. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbas H. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- 44.Carmichael S T, Price J L. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 45.Rolls E T, Baylis L L. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmichael S T, Price J L. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 47.Barbas H. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 48.Haber S N, Kunishio K, Mizobuchi M, Lynd-Balta E. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morecraft R J, Geula C, Mesulam M-M. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 50.Neafsey E J, Terreberry R R, Hurley K M, Ruit K G, Frysztak R J. In: The Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Boston: Birkhauser; 1993. pp. 206–223. [Google Scholar]
- 51.Sesack S R, Deutch A Y, Roth R H, Bunney B S. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 52.Bandler R, Shipley M T. Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 53.Damasio A R. Descartes' Error. New York: G. P. Putnam's Sons; 1994. [Google Scholar]
- 54.Bechara A, Damasio A R, Damasio H, Anderson S W. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 55.Elliott R, Dolan R J, Frith C D. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 56.LeDoux J E. In: Cognitive Neuroscience of Emotion. Lane R D, Nadel L, editors. New York: Oxford Univ. Press; 2000. pp. 129–155. [Google Scholar]
- 57.Harman C, Fox N A. In: Development of the Prefrontal Cortex. Krasnegor N A, Lyon G R, Goldman-Rakic P S, editors. Baltimore: Paul Brookes Publishing; 1997. pp. 191–208. [Google Scholar]
- 58.Derryberry D, Rothbart M K. J Personality Soc Psychol. 1988;55:958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- 59.Posner M I, Rothbart M K. Philos Trans R Soc London B. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson J R J, Snyder A Z, Gusnard D A, Raichle M E. Proc Natl Acad Sci USA. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simpson J R J, Drevets W C, Snyder A Z, Gusnard D A, Raichle M E. Proc Natl Acad Sci USA. 2001;98:688–691. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christoff K, Gabrieli J D E. Pyschobiology. 2000;28:168–186. [Google Scholar]
- 63.Binder J R, Frost J A, Hammeke T A, Bellgowan P S F, Rao S M, Cox R W. J Cognit Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 64.McGuire P K, Silbersweig D A, Frith C D. Brain. 1996;119:907–917. doi: 10.1093/brain/119.3.907. [DOI] [PubMed] [Google Scholar]
- 65.George M S, Ketter T A, Parekh P I, Herscovitch P, Post R M. Biol Psychiatry. 1996;40:859–871. doi: 10.1016/0006-3223(95)00572-2. [DOI] [PubMed] [Google Scholar]
- 66.Goel V, Grafman J, Tajik J, Gana S, Danto D. Brain. 1998;120:1805–1822. doi: 10.1093/brain/120.10.1805. [DOI] [PubMed] [Google Scholar]
- 67.Antrobus J S, Singer J L. Trans NY Acad Sci. 1970;32:242–252. doi: 10.1111/j.2164-0947.1970.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 68.Teasdale J D, Dritschel B H, Taylor M J, Proctor L, Lloyd C A, Nimmo-Smith I, Baddeley A D. Memory and Cognition. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 69.Giambra L M. Consciousness and Cognition. 1995;4:1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- 70.Andreasen N C, O'Leary D S, Cizadlo T, Arndt S, Rezai K, Watkins G L, Ponto L L B, Hichwa R D. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 71.Damasio A R, Van Hoesen G W. In: Neuropsychology of Human Emotion. Heilman K M, Satz P, editors. New York: The Guilford Press; 1983. pp. 85–110. [Google Scholar]
- 72.Duffy J D, Campbell J J I. J Neuropsychiatry. 1994;6:379–387. doi: 10.1176/jnp.6.4.379. [DOI] [PubMed] [Google Scholar]
- 73.Mochizuki H, Saito H. Tohoku J Exp Med. 1990;161,Suppl.:231–239. [PubMed] [Google Scholar]
- 74.Foltz E L, White L E., Jr J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson H A, Davidson K M, Davidson R I. Neurosurgery. 1999;45:1129–1136. doi: 10.1097/00006123-199911000-00023. [DOI] [PubMed] [Google Scholar]
- 76.Ingvar D H. In: Brain Work and Mental Activity. Lassen N A, Ingvar D H, Raichle M E, Friberg L, editors. Copenhagen: Munksgaard; 1991. pp. 346–359. [Google Scholar]