Abstract
Two major biological players in the regulation of body weight are the gut and the brain. Peptides released from the gut convey information about energy needs to areas of the brain involved in homeostatic control of food intake. There is emerging evidence that human food intake is also under the control of cortical and subcortical areas related to reward and cognition. The extent to which gut hormones influence these brain areas is not fully understood. Novel methods combining the study of neural activity and hormonal signalling promise to advance our understanding of gut–brain interactions. Here, we review a growing number of animal and human studies using neuroimaging methods (functional magnetic resonance imaging, positron emission tomography) to measure brain activation in relation to nutrient loads and infusion of gut peptides. Implications for current and future pharmacological treatments for obesity are discussed.
Keywords: brain imaging, gut hormones, functional magnetic resonance imaging, positron emission tomography, food intake, obesity
A long tradition of physiological research in animals and humans has provided a basic understanding of how gastrointestinal hormones communicate with the brain to influence nutrient digestion, absorption, transport and storage. Research is now focusing on the relationships between these gut hormones and brain areas controlling appetite, ingestion, food reward and body weight (1, 2). Despite these advances, there is still much to learn about how the gut and brain interact to influence body weight and adiposity. We review recent animal and human research concerning the role of gut peptides in appetite and body weight. We then describe novel studies combining neuroimaging approaches with experimental manipulations of hormones via feeding or direct hormone infusions to reveal the dynamic neuroendocrine pathways underlying appetite regulation in lean and obese animals and humans.
Gut, appetite and weight
The gut influences feeding behaviour by generating hunger and satiety signals secondary to acute changes in mechanical and nutritional stimuli. These signals travel primarily via the vagus nerve to the brainstem (i.e. nucleus tract solitaris), and then to the hypothalamus (i.e. the arcuate nucleus; ARC) for processing (3). Following peripheral and central signal integration, effector responses are generated to modulate energy balance based on immediate physical need and food reward (4). Peptides from the gastrointestinal tract (e.g. ghrelin) and adipose tissue (e.g. leptin) penetrate the blood–brain barrier (BBB) via transporters. Insulin alters the brain endothelial cell function in the BBB, and adiponectin promotes the secretion of substances such as interleukin-6 in the BBB to mediate effects on feeding centrally (5).
Peripheral peptides can be characterised as orexigenic [i.e. rise before meals and may help initiate food intake and then decline following meals such as ghrelin, anorexigenic [i.e. rise following meals and help to terminate food intake (e.g. cholecystokinin; CCK)], or regulate inter-meal intervals [e.g. glucagon-like peptide 1 (GLP-1) and peptideYY3–36 (PYY3–36)] (6). Gut peptides and adiposity signals (e.g. leptin and insulin) can act both locally and centrally to influence appetite control. For example, ghrelin, a hormone produced in the gastric antrum and fundus, can increase gastric acid production, gastric motility and emptying as well as activate vagal afferents and the hypothalamus to stimulate food intake in both animal models and in humans (7). Similarly, GLP-1, which is co-localised with PYY3–36 in the distal gut, acts as an ileal brake for the upper gastrointestinal tract, slowing gastric emptying of liquid and solid meals, thus inhibiting food intake via both gastric distension and vagally mediated central actions (8, 9). Leptin and insulin are long-term regulators of food intake and energy balance and can modulate the effects of a number of gut peptides (e.g. CCK). Although a small amount is secreted by the stomach, leptin is mainly produced by adipose tissue and generates feedback signals to the brain about the degree of fat storage, as does insulin (10). Key findings of obese–lean differences in basal plasma peptide levels and responsivity to experimental manipulations in animals and humans are summarised in Table 1.
Table 1.
Key Peripheral and Central Peptides Involved in Feeding and Body Weight Regulation.
| Peptides | Site of synthesis | Effect on food intakea |
Basal levels: obese versus leana | Responsivityb: obese versus leana |
|---|---|---|---|---|
| Peripheral | ||||
| Adiponectin | Adipose tissue | ↑ (80) | Lower in obese Ss (81) | Lesser postprandial ↑ in obese women post high-CHO (60%) meal (82) |
| Amylin | Pancreas | ↓ (83) | Higher in obese cats (84) | Greater postprandial ↑ in obese versus lean cats post high-fat (46%), high-protein (48%), and high-CHO (35%) meal (84) |
| Cholecystokinin (CCK) |
GI tract, mostly small intestine |
↓ (85) | Lower in obese women with metabolic syndrome (86) |
Lesser postprandial ↑ in morbidly obese women with metabolic syndrome post high-CHO (54.4%) meal (86) |
| Endocannabinoids (AEA, 2-AG) |
GI tract, adipose tissue |
↑ (12) | Higher GI tissue AEA and 2-AG and lower visceral and s.c. fat AEA in obese fa/fa versus lean rats (87) |
Post-fasting ↑ in AEA levels in visceral and s.c. fat in obese fa/fa rats versus lean rats, and ↓ during refeeding (87) |
| Ghrelin | Stomach | ↑ (88) | Lower in obese Ss (86, 89) | Lesser postprandial initial ↓ in obese women post high-CHO (60%) meal (82) |
| Glucagon-like peptide-1 (GLP-1) |
Pancreas, large intestine |
↓ (90) | Higher in ZDF rats versus NOD rats (91) |
Lesser postprandial ↑ in obese Ss post standard breakfast (45) |
| Insulin | Pancreas | ↓ (92) | Higher in obese Ss (93, 94) | Greater postprandial ↑ in obese Ss post standard liquid meal (93) and post high-CHO (60%) meal (82) |
| Leptin | Adipose tissue | ↓ (95) | Higher in obese Ss (81) | Greater postprandial ↑ in obese children post standard meal (96) and obese women post high-CHO (60%) meal (82) |
| Oxyntomodulin (OXM) |
L cells in GI tract | ↓ (97) | Unknown | Unknown |
| Pancreatic polypeptide (PP) |
Pancreas, colon, rectum |
↓ (98) | ↔ in obese Ss (99) Lower in obese versus lean children (94) |
Lesser postprandial ↑ in obese Ss during somatostatin infusion post test meal (99) Postprandial ↑ in obese, lean and post-RYGB Ss. No significant difference between groups (100) |
| Peptide YY3–36 (PYY3–36) |
L cells in lower GI tract |
↓ (14) | Lower in obese Ss (14) | Lesser postprandial ↑ in obese Ss post buffet lunch (14) and obese women post high-CHO (54.4%) meal (86) |
| Central | ||||
| α-melanocyte stimulating hormone (α-MSH) |
Hypothalamus | ↓ (101) | Lower POMC RNA in obese fa/fa versus lean +/+, +/fa rats (102) |
Post s.c. leptin infusion, ↑ in POMC1 mRNA in wt lean and ob/ob mice (103) |
| Agouti-related protein (AgRP) |
Hypothalamus | ↑ (104) | Higher AGRP in ob/ob mice (23) | Post s.c. leptin infusion, ↑ AGRP in wt lean mice and ↓ in ob/ob mice (103) |
| β-endorphin | Pituitary, hypothalamus, brainstem |
↑ (85) ↓ (105) |
Lower POMC RNA in obese fa/fa versus lean +/+, +/fa rats (102) Higher β-endorphin levels in obese Ss versus lean Ss (106) |
Post s.c. leptin infusion, ↑ POMC1 mRNA in wt lean and ob/ob mice (103) |
| Cocaine and amphetamine- regulated transcript (CART) |
Hypothalamus | ↓ (107) ↑ (108) |
↓ CART mRNA in ob/ob versus wt lean mice (103) |
Post s.c. leptin infusion, ↔ in CART mRNA in wt lean mice and ↑ in ob/ob mice (103) |
| Corticotrophin- releasing hormone (CRH) |
Hypothalamus | ↓ (109) | Higher corticosterone in obese fa/fa rats versus lean Fa/? rats (110) |
Greater post-prandial ↑ CRH-binding protein in anterior pituitary in lean Fa/? versus obese fa/fa rats post ad libitum meal (111) |
| Endocannabinods | Hypothalamus | ↑ (12) | More striatal CB1-phosphorylated receptor cells in obese fa/fa versus lean Fa/? rats (112) |
Greater ↓ striatal and hippocampa CB1-phosphorylated receptor cells with repeated fluoxetine in fa/fa versus saline-treated rats (112) |
| GABA | CNS | ↑ (113) | Higher in medial hypothalamus of obese fa/fa versus lean Fa/Fa rats ↔ in lateral hypothalamus (114) |
Post 2-DG infusion, ↔ GABA in medial and lateral hypothalamus in obese fa/fa rats. Post 2-DG infusion, ↑ GABA in medial and ↓ GABA in lateral hypothalamus in lean Fa/Fa rats (114) |
| Galanin (GAL) | Hypothalamus, brainstem |
↑ (115) | Lower in obese fa/fa versus lean FA/– rats (116) Higher in obese versus lean female Ss (117) Greater gene expression in obese fa/fa versus lean Fa/? rats (118) |
↑ GAL mRNA expression in obese fa/fa rats versus lean Fa/? rats post 4-week high- CHO (66%) or intermediate versus high-fat (72%) diet (118) |
| Galanin-like peptide (GALP) |
Hypothalamus, median eminence |
↑ (119) | Lower mRNA expression in ob/ob mice versus wt lean mice (120) |
Post i.c.v. leptin infusion, greater ↑ in mRNA expression in ob/ob versus wt lean mice (120) |
| Glucagon-like peptide-1 (GLP-1) |
Brainstem | ↓ (8) | Higher preproglucagon mRNA levels in obese fa/fa rats versus lean Fa/? rats (121) |
Post food restriction, ↓ preproglucagon.Post over-feeding, ↑ preproglucagon in lean Fa/? rats versus ↔ in obese fa/fa rats (121) |
| Melanin- concentrating hormone (MCH) |
Hypothalamus | ↑ (122) | Higher MCH mRNA levels in obese fa/fa versus lean rats (122) |
Post 6-months high-fat (45%) then 1-month low-fat (10%) diet, ↔ MCH mRNA in DIO and diet-resistant lean rats (123) |
| Neuropeptide Y (NPY) |
Hypothalamus | ↑ (101) | Higher NPY in obese fa/fa rats (124), and obese female Ss (117) |
Post s.c. leptin infusion, ↔ in NPY mRNA in wt lean mice and ↓ in ob/ob mice (103) |
| Norepinephrine (NE) | Locus coeruleus | ↑↓ (101, 125) | ↔ in obese fa/fa and lean Fa/fa rats (126) |
Post CHO-rich (95%) meal, greater ↑ in NE in lean versus obese female Ss; post high-fat (76%) diet, ↔ between groups (127) |
| Oxyntomodulin (OXM) |
Brainstem | ↓ (13) | Higher preproglucagon mRNA in obese fa/fa rats (121) |
Post food restriction, ↓ preglucagon; and post overfeeding.↑ preproglucagon in lean Fa/? rats versus ↔ in fa/fa rats (121) |
| Serotonin (5-HT) | Dorsal raphe nucleus | ↓ (11, 101) | ↔ in lean Fa/Fa and obese fa/fa rats (128) |
Greater postprandial ↑ 5-HT at 40 min and 60 min post-meal (40% CHO, 30% protein, 30% fat) in obese fa/fa versus lean Fa/Fa rats (128) |
Illustrative examples only; not an inclusive list.
Response to experimental manipulation, including feeding, restriction, fasting, or hormone infusion; ↑, increase; ↓, decrease; ↔, no significant change; Ss, adult human subjects, all comparisons are with lean Ss, and groups include both men and women, unless otherwise specified; 2-AG, arachidonoylglycerol; 2-DG, 2-deoxy-d-glucose; AEA, anandamide; CB, cannabinod; CHO, carbohydrate; CNS, central nervous system; DIO, diet-induced obesity; fa/fa, genotype encoding obese Zucker leptin receptor deficient; Homozygous (Fa/Fa, +/+), Heterozygous (Fa/−, +/fa), Homozygous or Heterozygous (Fa/?) genotypes encoding lean Zucker; GI, gastrointestinal; mRNA, messenger RNA; NOD, non-obese diabetic; ob/ob, genetic leptin deficient obese mice; POMC, pro-opiomelanocortin (precursor of α-MSH and β-endorphin); RYGB, Roux-en-Y gastric bypass; wt, wild-type; ZDF, Zucker diabetic fatty.
Brain, appetite and weight
In addition to signalling in the periphery, a number of hormones produced in the brain also regulate appetite (Table 1). Neuropeptide Y (NPY) and agouti-related protein-producing neurones in the ARC are activated by ghrelin to stimulate food intake, whereas serotonin from the raphe nucleus inhibits feeding (10, 11). Similar to their peripheral effects, centrally-released endocannabinoids (12) increase food intake, whereas GLP-1 (8), and oxyntomodulin (13) decrease it. By contrast to ghrelin, PYY appears to have different actions according to site of administration and release. Suppression of food intake has been found by peripheral administration (14), whereas increases have been found after central administration (15, 16), highlighting the complexity of neural and hormonal signals relating to appetite control.
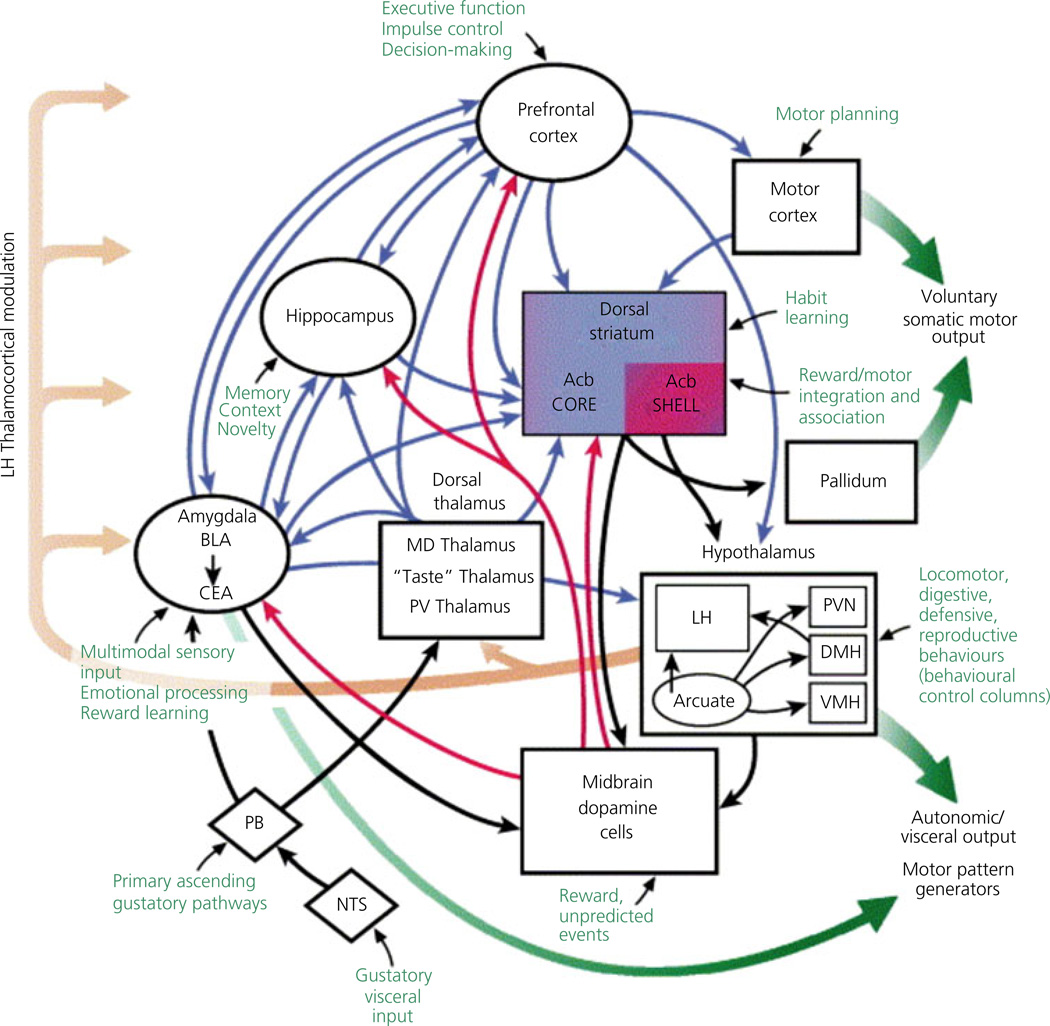
A number of brain regions regulate appetite and food intake (Fig. 1), and may be considered as either 'homeostatic' or 'hedonic' systems. The homeostatic system is comprised of the hypothalamus (10) and brainstem, and appears to drive food intake based on caloric need or energy balance (17, 18). Homeostatic areas integrate inputs from higher cortical regions involved with the perceived reward value of food (19, 20), indicating that the desire to eat palatable foods, sometimes termed 'hedonic' or reward-related hunger, is neurally mediated (2, 21, 22). The mesolimbic dopamine system, including the ventral tegmental area (VTA) of the midbrain and extending to the nucleus accumbens in the striatum, is considered the major reward pathway (23). Neural responses within the hedonic brain network to environmental food cues may override homeostatic signals, contributing to the development and maintenance of obesity (18).
Fig. 1.
Brain regions involved in feeding behaviour. Acb, nucleus accumbens; BLA, basolateral amygdala; CEA, central nucleus of the amygdala; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; MD, mediodorsal thalamic nucleus; NTS, nucleus of the solitary tract; PB, parabrachial nucleus; PV, paraventricular; PVN, paraventricular nucleus of the hypothalamus; VMH, ventromedial nucleus of the hypothalamus. Image reproduced with permission (129).
Functional neuroimaging has recently been employed to learn more about the neural basis of appetite and weight regulation. One of the advantages of functional neuroimaging is the ability to conduct in vivo assessments of human brain function. However, it is also possible to use small scanners for live laboratory animals, reducing the need for invasive techniques (e.g. single-cell recording), and also providing a view of whole brain activity.
In positron emission tomography (PET), a radioactive tracer commonly labeled with 15O (to measure regional cerebral blood flow) is injected. The tracer is absorbed by active regions as neuronal activity leads to an increase in blood flow (24). As the radioactive tracer decays, two gamma photons are emitted in opposite directions and simultaneously recorded by detectors, producing high resolution, topographically-accurate images (24). Neurotransmitter release and changes in the radiotracer binding potential at neuroreceptor sites can also be measured using PET (25).
By comparison, functional magnetic resonance imaging (fMRI) detects changes in the magnetic properties of haemoglobin, resulting from neural demands for oxygenated blood. The fMRI scanner detects changes in this hemodynamic response and records the blood oxygen level-dependent (BOLD) signal. The higher the proportion of oxyhaemoglobin (diamagnetic) relative to deoxyhaemoglobin (paramagnetic), the less interference to the radio frequency pulse generated by the scanner, and the stronger the BOLD signal and the brighter the image (26). Although specific biomarkers are not easily identified using fMRI in comparison to a radioligand-PET study (27), the relative safety, absence of radioactivity, and high spatial and temporal resolution of fMRI, has resulted in its eclipsing PET (28) as the dominant approach in neuroimaging research.
Functional neuroimaging can be used to study the whole brain in exploratory fashion or to map a priori brain regions of interest (27). In addition, analyses of functional connectivity can provide insight into how neural networks interact with each other to carry out cognitive and behavioural functions (29). Conclusions about the exact circuitry and causal mechanisms are limited by spatiotemporal resolution and, frequently, by the cross-sectional, descriptive nature of the data generated (27, 30).
Neuroimaging studies of ingestion and gut hormones
Homeostatic pathways, and to some degree hedonic pathways, have been mapped to a large extent in the brains of lean and obese animals. However, attempts to do this in humans are more difficult and complex, and the pathways involve many higher-order cortical areas. Functional neuroimaging has become key for understanding the appetitive pathways in the human brain. The effects of gut peptides on appetite and weight are relatively well studied in both animals and humans, and neuroimaging studies have advanced our understanding of human brain areas. However, the interaction between hormonal activity and brain activation has been little studied. Combining these approaches should reveal the relative contribution of specific appetite-related hormones to activity in brain regions of interest.
Animal studies
Neuroimaging experiments in small animals are evolving; however, there are several caveats and limitations. There are currently few structural brain atlases created for mouse models and no concordance on which three-dimensional mapping brain atlas system to use (31). Also, in contrast to humans, rodents are often anaesthetised, which could affect the BOLD-fMRI signal by alterations in brain perfusion (32). To reduce potential confounding, imaging paradigms in animals should ideally include concurrent monitoring of heart rate, respiratory rate, carbon dioxide levels, temperature and blood pressure. In non-anaesthetised animals and humans, studies of the brainstem should consider movement artefacts introduced by respiratory and cardiac motion and adjust for this by using respiratory and cardiac monitoring. Finally, few institutions have appropriate small animal MR scanners and instead rely on clinical MR scanners, which have much lower field strengths (1.0–3.0 T) than small-bore scanners (4.7–16.4 T) compromising image quality (33). Nevertheless, a number of important findings have emerged from neuroimaging in animals.
PET
Thanos et al. (34) compared brain activation during presentation of a highly palatable food stimulus (bacon scent) in 20 male adult Zucker (leptin-deficient) obese and lean rats, following a 24-h fast. Four groups [obese ad libitum fed, obese food restricted (70% of amount of ad libitum fed), lean ad libitum fed, and lean food restricted] rats, were scanned twice in a 2-week span for 40 min, once with bacon scent only (pre-diet) and once with bacon scent after the mice were fed 5 g of bacon for five consecutive days (post-diet). Obese rats showed greater activation of the medial thalamus (goal-directed behaviour) and deactivation of hippocampus (memory) than lean rats in response to the bacon scent at pre-diet compared to post-diet. They also showed deactivation in the frontal cortex (higher level functions), which was not present in the lean rats. The ad libitum fed rats (both obese and lean) demonstrated greater activation in the right insular/parietal cortex (integrates multimodal sensory input) and medial olfactory bulb post-diet, whereas restricted animals (both obese and lean) showed greater activation in the medial thalamus, right and left olfactory bulb, and right hippocampal fissure in the post-diet compared to pre-diet.
These findings suggest that obese or restricted animals may experience more activation of areas involved in goal-directed behaviour and thus be more motivated to seek out a food reward after exposure to a highly palatable food stimulus. Lower-order brain regions responsive to food cues (e.g. olfactory nucleus) may also be modulated by access to food, suggesting that an extensive brain network is recruited to promote feeding in conditions of perceived caloric need (34). Because the obese rats were leptin-deficient, the study suggests that leptin plays a key role in shaping neural responses to recognisable or rewarding food stimuli. In support of this, recent studies have shown that leptin-deficient humans demonstrate changes in hedonic [i.e. ventral striatum, pre-frontal cortex (PFC)] brain areas after repeated exposure to palatable food cues following leptin replacement (35, 36).
fMRI
A number of studies have now used fMRI to directly assess whole brain or region of interest activation parallel with or following changes in gut hormone levels brought about by either direct hormone infusion or glucose infusion or ingestion. For example, Chen et al. (37) studied the hypothalamic response after glucose ingestion in 24 lean and 24 overweight rats with fMRI. Three midsagittal slices through the hypothalamus were obtained with echo-planar imaging for 60 min, and NPY and serotonin (5-HT) expression was detected with immunohistochemistry following image acquisition. As a control, a second fMRI scan was conducted in six lean and six overweight rats after ingestion of water on an alternate day. The hypothalamic fMRI signal was transiently lowered in all rats within 19.5–25.5 min of oral glucose consumption, although the decrease was greater in the lean than the overweight rats, with no change observed in the control animals. Both NPY and 5-HT concentrations were reduced to a greater degree in the overweight than the lean rats, but only the reduction in 5-HT levels was significant.
Manganese-enhanced MRI (MEMRI)
An alternative imaging method is MEMRI, a contrast-based technique in which manganese ions enter excitable cells and accumulate in active areas of the brain. MEMRI can be used to enhance measurement of neural activation following gut hormone administration (38). Kuo et al. (39) used MEMRI in mice fed ad libitum to track gut-peptide central nervous system interactions and found enhanced signal intensity in the ARC, paraventricular (PVN), ventromedial (VMH) and periventricular nuclei of the hypothalamus following ghrelin intraperitoneal infusions. Significant signal differences were also found in the periventricular and fourth ventricle after PYY3–36 administration in fasted mice versus fasted vehicle-treated mice. The signal changes in the ARC, induced by the hormone administration, preceded the effects of ghrelin and PYY on food intake, providing support for mediation by the hypothalamic/homeostatic pathway.
Using similar techniques, Chaudhri et al. (40) examined changes in hypothalamic signalling following intraperitoneal administration of the anorexigenic hormones GLP-1 and oxyntomodulin (OXM). In fasted mice, injections of 900 nmol/kg of GLP-1 led to a decrease in the PVN and an increase in the VMH signal compared to saline-treated fasted and ad libitum fed controls. After 900 and 5400 nmol/kg OXM injections, there was decreased hypothalamic activity in the ARC, PVN and supraoptic nuclei in fasted mice compared to fasted saline treated controls. Cellular toxicity currently precludes the use of MEMRI in humans. However, future animal studies using this technique promise to help further elucidate the neural mechanisms underlying the effects of gut hormones on appetite.
Human studies
In addition to the innovative animal studies described above, there are also a number of human studies combining neuroimaging methods with experimental manipulations of hormone levels via feeding or direct hormone infusions. These are described in more detail below and are summarised in Table 2.
Table 2.
Summary of Animal and Human Neuroimaging Studies of Appetite-Related Peptides.
| Study [similar studies] |
Peptides (effect on food intake) |
Method | Protocol | Key findings |
|---|---|---|---|---|
| Animal studies | ||||
| Thanos et al. (34) |
Leptin (↓) | µPET | PET activation in 20 obese Zucker fa/fa and 20 lean rats at baseline and after 5 days of bacon feeding (5 g/daily) in both ad libitum and food restricted rats |
↓ Hippocampal and ↑ medial thalamic activity to bacon scent in obese versus lean rats. ↑ in right insular/ parietal cortex, and medial olfactory bulb in ad libitum fed rats and ↓ in restricted rats following bacon feeding (5 g/daily) |
| Chen et al. (37) |
NPY (↑) 5-HT (↓) |
fMRI | Hypothalamic responses following glucose in 24 lean rats (365 ± 76.5 g) and 24 overweight rats (714 ± 83.5 g) and water in six lean and six overweight rats |
↓ hypothalamic activation after glucose load in all rats. BOLD signal delayed following glucose in overweight versus lean rats. ↔ in hypothalamic activity after water control. ↓ 5-HT levels in overweight versus lean rats |
| Kuo et al. (39) |
Ghrelin (↑) | MEMRI | Hypothalamic responses following i.p. ghrelin administration in male (C57BL/6) mice |
↑ in the ARC, VMH, PVN and Pe and ↔ in fourth ventricle, anterior and posterior pituitary activation after 0.06 nmol/g and 0.3 nmol/g ghrelin injections in ad libitum fed mice versus vehicle-treated controls |
| Kuo et al. (39) |
PYY3–36 (↓) | MEMRI | Hypothalamic responses following i.p. PYY3–36 in male (C57BL/6) mice |
↓ in the Pe and ↑ in the fourth ventricle, and ↔ in VMH PVN, anterior or posterior pituitary activation after 0.025 nmol/g PYY3–36 in fasted mice versus vehicle- treated controls |
| Chaudhri et al. (40) [Parkinson et al. (130)] |
GLP-1 (↓) | MEMRI | Hypothalamic responses following i.p. GLP-1 administration in male (C57BL/6) mice |
↑ in the VMH, ↓ in the PVN and ↔ in ARC and SON activation after 900 nmol/kg GLP-1 versus saline in fasted and ad libitum fed control mice |
| Chaudhri et al. (40) [Parkinson et al. (130)] |
OXM (↓) | MEMRI | Hypothalamic responses following i.p. OXM administration in male (C57BL/6) mice |
↓ in the ARC, PVN, SON after 5400 nmol/kg OXM in fasted mice versus fasted controls. ↔ in ARC activation after OXM injection versus saline-treated ad libitum fed controls.↑ in VMH activity after 5400 nmol/kg in fasted mice versus ad libitum fed and fasted controls |
| Human Studies | ||||
| Pannacciulli et al. (41) |
GLP-1 (↓) | PET | GLP-1 levels and brain activity in 22 male and 20 female Ss (BM 31 ± 9) post 25 min liquid formula mea |
Postprandial ↑ in plasma GLP-1 correlated with ↑ in the left dorsolateral prefrontal cortex and hypothalamus |
| Matsuda et al. (48) [Liu et al. (49), Smeets et al. (50)] |
Insulin (↓) | fMRI | Hypothalamic activity after oral (75 g) glucose load in ten obese versus lean male and female Ss |
↑ in delayed and attenuated inhibitory responses in obese versus lean group in PVN and VMH. Correlation with inhibitory responses and fasting insulin and glucose in the obese and lean groups |
| Batterham et al. (51) |
PYY (↓) | fMRI | Brain activation, subjective feelings, and hormone levels during 100 min of continuous PYY and saline infusion in eight lean male Ss (BMI 21.7 ± 0.7); ad libitum intake 30 min post scan |
↑ in left orbitofrontal cortex, brainstem, parabrachial nucleus, midbrain, VTA, insula, anterior cingulate cortex, ventral striatum (globus pallidus and putamen), regions in frontal, parietal, temporal and cerebellar cortex, posterior hypothalamus, right substania nigra covaried positively with plasma PYY |
| Malik et al. (55) |
Ghrelin (↑) | fMRI | Brain activation to food and nonfood cues following single- blinded ghrelin infusions (1 µg/kg) in 20 lean (BM 22.3 ± 0.7) male Ss |
↑ in the bilateral amygdala, left orbitofrontal cortex, right substantia nigra/VTA, left caudate, right hippocampus, anterior insular cortex, and visual areas (including pulvinar and fusiform gyrus) following ghrelin to food versus non-food cues |
| Rosenbaum et al. (61) [Baicy et al. (36), Farooqi et al. (35)] |
Leptin (↓) | fMRI | Brain activation to visual food cues in two male, four female inpatient obese (BMI > 30 kg/m2) Ss at their usual weight and 10% reduced body weight, when receiving either twice daily s.c. injections of leptin or placebo |
After weight loss, leptin-reversible ↑s were found in the brainstem, culmen, parahippocampal gyrus, inferior and middle frontal gyri, mid temporal gyrus, and lingual gyrus to visual food cues. There were also leptin-reversible ↓s elicited by food-related cues in the hypothalamus cingulate gyrus, and middle frontal gyrus |
↑, increase; ↓, decrease; ↔, no significant difference; ARC, arcuate nucleus; BMI, body mass index (kg/m2); BOLD, blood oxygen level-dependent; fa/fa, gene-type encoding obese Zucker leptin receptor deficient; GLP-1, glucagon-like peptide 1; 5-HT, serotonin; fMRI, functional magnetic resonance imaging; MEMRI manganese-enhanced magnetic resonance imaging; NPY, neuropeptide Y; OXM, oxyntomodulin; Pe, periventricular hypothalamic nucleus; µPET, micro positron emission tomography; PET, positron emission tomography; PVN, paraventricular nucleus; PYY3–36, peptide YY3–36; SON, supraoptic nucleus; Ss, subjects; VMH ventromedial hypothalamus; VTA, ventral tegmental area.
PET
A post-hoc analysis of a cross-sectional PET study was performed assessing brain activation with postprandial GLP-1 levels. Forty-two lean adult males and females were given continuous oral administration of a liquid formula meal until satiated as determined by visual analogue scale ratings. The meal was delivered via peristaltic pump for over 25 min, following a 36-h fast (41). Brain activation was measured before and after the meal. Correlational analyses revealed associations between postprandial GLP-1 levels and increases in activation in the dorsolateral PFC (dlPFC) and hypothalamus. The dlPFC is involved in controlling inappropriate behavioural responses (42) and has been associated with both food reward and satiety (41, 43, 44), making it unclear whether activation in this area reflects motivation to eat or inhibition of intake. An impairment in dlPFC's response to food stimuli may be associated with the blunted postprandial rise in GLP-1 seen in obese vs. lean individuals (45, 46). Examination of areas of co-activation may shed further light on the role of the dlPFC in eating behaviour (47).
fMRI
Imaging studies investigating neuronal responses to oral glucose administration in humans have provided data on the relationship between plasma glucose and insulin and appetite regulation. Matsuda et al. (48) administered a 75 g oral glucose load to ten obese versus ten lean male and female adults after a 12-h fast. Oral glucose ingestion started 10 min after subjects were placed in the scanner. Following ingestion, lean participants showed deactivation in the lower posterior quadrant of the hypothalamus including the VMH, and obese participants showed a slower and smaller response. In the upper anterior hypothalamic region including the PVN, there was a slight deactivation and a relative delay in hypothalamic inhibition in obese versus lean participants. The decrease (4–8%) in BOLD signal in the lower posterior hypothalamus started 4 min after ingestion and lasted approximately 10 min in all subjects, providing information about the lag time of homeostatic neural responses. There was a positive correlation between the time to reach the maximum response in the lower posterior hypothalamus and upper anterior hypothalamus and fasting glucose and insulin concentrations in both obese and lean subjects. The findings of Matsuda et al. (48) suggest that delayed activation of satiety centers (e.g. VMH) following glucose consumption may contribute to excessive intake in obese individuals.
In a similar fMRI study by Liu et al. (49), 21 healthy adults were given a 75 g oral glucose load after a 12-h fast. A reduction in hypothalamic activity (up to 4%) was observed initially at 1–2 min and then again at 7–12 min following ingestion. Smeets et al. (50) recently extended these findings by varying the glucose load and adding a control water condition to rule out the possibility that hypothalamic signal decreases independently over time. In this study of 15 lean healthy males, 25 g or 75 g of glucose was administered in 300 ml of orange-flavoured water, and the hypothalamic signal (mostly in the upper anterior hypothalamus) was shown to be significantly lower (1–2.5%) than in the water condition (300 ml)) for up to 30 min post-ingestion. This decrease was significantly greater for the 75 g than the 25 g glucose load, supporting a dose–response pattern. By contrast to the findings of Matsuda et al. (48), there was no effect found in the lower hypothalamus. It was postulated that the difference could be the result of a decrease in activity within the lateral hypothalamic area in which glucose sensitive neurones are activated by hypoglycaemia.
Other studies have combined direct hormone infusions with fMRI. In a double-blind placebo-controlled crossover study by Batterham et al. (51), eight lean healthy males were infused with physiologically relevant doses of PYY3–36 to mimic the hormone profile after satiation, whereas, on another day, they received saline infusions designed to simulate the fasted state. All participants were scanned throughout both infusions, and blood draws were taken every 10 min throughout the 100-min procedure to measure hormone levels, with visual analogue scale ratings made every minute to assess subjective appetite. Thirty minutes following the scan, participants consumed a mixed buffet meal and caloric intake was measured. Correlational analyses revealed increased activation with PYY3–36 infusion and corresponding decreases with saline infusion in the left orbitofrontal cortex, parabrachial nucleus, ventral tegmental area, insula, anterior cingulate cortex, ventral striatum (globus pallidus, putamen), regions of the frontal, parietal, temporal and cerebellar cortices and posterior hypothalamus (including VMH), providing evidence for tight yoking between gut hormones and brain activity. Furthermore, caloric intake in the buffet meal was predicted by activation in the hypothalamus in the saline condition, and by deactivation in higher-order reward areas (i.e. orbitofrontal cortex) in the PYY condition. This was interpreted as indicating a switch from homeostatic determination of feeding in the fasted state to hedonic determination of feeding in the satiated state (52).
An interesting distinction should be noted between the findings of Matsuda et al. (48) and Batterham et al. (51). Although the increase in hypothalamic signal with PYY3–36 (51) suggests that the posterior hypothalamus is activated during satiation, the inhibition of signal with glucose ingestion suggests the posterior hypothalamus is deactivated with feeding (48). This distinction could reflect differences in mechanisms and brain areas involved in response to glucose compared to PYY3–36. In addition, the hypothalamus is surrounded by a vascular network and in close proximity to a sinus cavity, potentially limiting spatial resolution in imaging studies (53). Furthermore, although there are more established atlases and parcellation protocols (e.g. Talairach space) for human compared to animal brains, there is still no widely accepted standard. Imprecise labelling can be a limitation when imaging micro-anatomical structures, such as the hypothalamus and brainstem (54). The difference between the findings of Matsuda et al. (48) and Batterham et al. (51) could therefore also be a result of activation of different anatomical areas within the posterior hypothalamus (e.g. ARC or VMH) and/or error attributable to differences in imaging techniques.
Neuroimaging methods have been used to examine brain activation in response to food stimuli following manipulation of appetite-related hormones. For example, in a study combining i.v. infusions of ghrelin with fMRI, Malik et al. (55) measured brain activation in response to pictures of highly palatable foods (e.g. pizza, hamburgers) versus nonfood stimuli (e.g. scenery pictures) following a 3-h fast in lean healthy males (n = 12) before and after a 20-min ghrelin infusion. They compared the results with those from similarly timed scans conducted in a control group (n = 8) receiving no ghrelin. Post-infusion increases in response to the food (versus non-food) pictures were observed in the amygdala, orbitofrontal cortex, insula and striatum, comprising areas that are associated with encoding the reward value of stimuli (56) and have shown activation to appetising food images (57, 58). These findings are consistent with other studies showing that ghrelin activates dopamine neurones in the VTA and increases dopamine turnover in the nucleus accumbens of the ventral striatum (59, 60). They suggest that the orexigenic effect of ghrelin is associated with an up-regulation of mesolimbic dopaminergic activity accompanied by an increase in motivational salience of high-energy foods (4, 55).
Two studies have also combined hormones with neuroimaging of responses to food stimuli to examine the effect of long-term or genetic alterations of signalling on appetite and body weight in humans. In a study of leptin replacement in genetically leptin-deficient adults, Baicy et al. (36) reported reduced fMRI activation in the leptin supplemented group in areas involved with hunger (insula, parietal and temporal cortex) and greater activation in regions linked to inhibition and satiety (PFC) in response to visual pictures of food (e.g. fried chicken, cheeseburgers) compared to neutral stimuli (e.g. brick walls). Another group extended this to leptin-deficient adolescents, demonstrating marked fMRI activation in the ventral striatum in response to food images presented in both the fed and fasted states, which was markedly reduced following 1 week of leptin administration (35). Rosenbaum et al. (61) have applied this methodology to individuals with common polygenic obesity. In their study, six obese inpatients that had lost 10% of their initial weight on a liquid diet were given twice-daily s.c. injections of leptin or saline for 5 weeks (Table 2). Responses to visual presentation of actual foods were assessed at baseline, after weight loss, and after leptin and saline administration. Post-administration, the saline group showed significantly greater activation to food cues compared to the leptin group in areas including the insula, parahippocampal gyrus, and middle and superior frontal gyri, consistent with a relatively greater appetitive responsivity and drive to eat (61). Taken together, these studies are consistent with a model in which leptin down-regulates 'hedonic' activation in reward areas in response to food stimulation, and simultaneously up-regulates homeostatic control by enhancing the central response to peripheral satiety signals (35, 62).
Neurohormonal treatments for obesity
Neuroimaging studies of gut–brain interactions offer new avenues for developing more efficacious pharmacological agents for weight control. Such treatments may become essential given the growing rates of severe obesity and the limited success of behavioural weight loss interventions (63). However, to date, orlistat (lipase inhibitor), sibutramine and phentermine-like sympathomimetics are the only approved anti-obesity agents available worldwide, whereas exenatide (GLP-1 analogue) and sitagliptin (DPP-IV inhibitor) are licensed adjuncts to current diabetes treatments (52). Leptin is a potential therapeutic agent; however, its administration in obese humans has not been nearly as efficacious in reducing food intake and body weight as in animals, probably because of the greater development of leptin resistance in humans (62, 64). Pharmaceutical companies are testing ghrelin antagonist drugs (NOX-B11), synthetic forms of amylin (pramlintide, davalinitide), PP (TM-30339), leptin (metreleptin) and oxyntomodulin (TKS1225) in clinical trials, although none of these are yet approved for weight loss.
Existing neuroimaging and gut hormone studies already give clues to mechanisms for current and future gut hormone-like drugs. For example, PYY3–36 and GLP-1 have been shown to induce concomitant changes in the orbitofrontal cortex and PFC, respectively (41, 51), suggesting that drug analogues that promote the anorexigenic properties of PYY3–36 (obinepitide, s.c.) and GLP-1 (exenatide, liraglutide) may be altering the perceived reward value of certain foods and responsivity to food cues, via activation of appetitive neural networks (52). Neuroimaging studies of the mechanisms of Roux-en-Y gastric bypass, which produces dramatic therapeutic changes in gastrointestinal hormones (65, 66), could also assist in the development of nonsurgical interventions to mimic its effects (67). Animal studies have already demonstrated hyperphagia and increased preferences for high-energy foods following pharmacological activation of hedonic brain networks (68, 69), suggesting that pharmacological agents with the opposite effect may be plausible for inducing weight loss.
The disadvantage of using gut hormones as therapeutic agents include the need for peptides to be injected to avoid being digested following oral administration by gut enzymes. Furthermore, efficacy may be short-lived, requiring repeated injections to maintain weight control. A number of adverse psychiatric and physiological side effects have also been associated with the use of neuromodulatory agents, which led to the removal from the market of drugs such as rimonabant, a cannabinoid receptor antagonist (52).
Discussion
Feeding behaviour and body weight regulation are largely under the control of hunger and satiety signals resulting from gut–brain interactions, triggered in response to appetitive stimuli and ingested nutrients (70). Lesion studies, genetic knockouts and single cell recording in animals have improved our knowledge of the underlying neuroendocrine pathways, and new studies using PET and fMRI are now providing insights into in vivo functioning of gut–brain pathways in humans. For example, neuroimaging studies have shown that exposure to appetising food cues results in preferential activation of hedonic regions of the human brain, and that obese individuals show greater activation in these areas (30, 71, 72). It is also known that obese and lean animals and humans differ in basal and meal-stimulated levels of appetite-related hormones (Table 1). Currently, studies combining neuroimaging with hormone manipulations are revealing that hedonic brain activation may be accentuated through the actions of gut peptides (51, 55) and also by a deficiency of regulatory hormones, such as leptin (35, 36, 61).
These findings are consistent with a model in which hormones released by the gut and adipose tissue modulate perceived food reward. Abnormalities in hormonal signalling may sometimes precede abnormalities in brain responses in regions involved with food reward, emotion, satiation and inhibitory control. Gut peptides may influence brain activation by mediating a switch from need-based when hungry to reward-based regulation of intake when sated (41, 51). The ease with which gastrointestinal satiety signals are over-ridden by changes in neural influences on perceived reward value of food may be related to genetically-influenced differences in responsivity to food cues (73–75) in the environment (76).
There are a number of promising avenues to take this research forward. Functional connectivity studies examining differences in dynamic neural pathways between homeostatic and hedonic brain structures may help to elucidate mechanisms underlying weight gain in obese individuals. Meanwhile, the identification of the peripheral signals that interact with these brain pathways will be key for future development of gut–hormone-derived therapies and brain-imaging biomarkers (77). Studies of bariatric surgery will provide an opportunity to explore potential neurohormonal targets, aiming to achieve less invasive nonsurgical appetite reductions (78, 79). There is little doubt that relationships exist between the obesogenic food environment, neural activity and appetite-related peptide hormone levels. Studies combining neuroimaging and hormone methodologies may help to elucidate causal models describing the interaction between these major influences on appetite regulation and obesity.
Acknowledgement
Supported in part by R01DK080153 (A.G.).
References
- 1.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. 2008;70:239–255. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC. 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16:S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks WA. The blood-brain barrier: connecting the gut and the brain. Regul Pept. 2008;3:11–14. doi: 10.1016/j.regpep.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellstrom PM, Geliebter A, Naslund E, Schmidt PT, Yahav EK, Hashim SA, Yeomans MR. Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. Br J Nutr. 2004;92:S47–S57. doi: 10.1079/bjn20041142. [DOI] [PubMed] [Google Scholar]
- 7.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin - a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;2:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 9.Naslund E, Hellstrom PM. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol Behav. 2007;2:256–262. doi: 10.1016/j.physbeh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Arora S, Chitkara A. Role of neuropeptides in appetite regulation and obesity - a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Leibowitz SF, Weiss GF, Walsh UA, Viswanath D. Medial hypothalamic serotonin: role in circadian patterns of feeding and macronutrient selection. Brain Res. 1989;503:132–140. doi: 10.1016/0006-8993(89)91713-7. [DOI] [PubMed] [Google Scholar]
- 12.Wenger T, Moldrich G. The role of endocannabinoids in the hypothalamic regulation of visceral function. Prostaglandins Leukot Essent Fatty Acids. 2002;3:301–307. doi: 10.1054/plef.2001.0353. [DOI] [PubMed] [Google Scholar]
- 13.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 14.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 15.Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol. 1990;2:R317–R323. doi: 10.1152/ajpregu.1990.259.2.R317. [DOI] [PubMed] [Google Scholar]
- 16.Hagan MM. Peptide YY: a key mediator of orexigenic behavior. Peptides. 2002;23:377–382. doi: 10.1016/s0196-9781(01)00614-3. [DOI] [PubMed] [Google Scholar]
- 17.Nisbett RE. Eating behavior and obesity in men and animals. Adv Psychosom Med. 1972;71:73–93. doi: 10.1159/000393300. [DOI] [PubMed] [Google Scholar]
- 18.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci. 2006;361:1275–1280. doi: 10.1098/rstb.2006.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kampe J, Tschop MH, Hollis JH, Oldfield BJ. An anatomic basis for the communication of hypothalamic, cortical and mesolimbic circuitry in the regulation of energy balance. Eur J Neurosci. 2009;30:415–430. doi: 10.1111/j.1460-9568.2009.06818.x. [DOI] [PubMed] [Google Scholar]
- 21.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20:1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 22.Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammertsma AA. PET/SPECT: functional imaging beyond flow. Vision Res. 2001;11:1277–1281. doi: 10.1016/s0042-6989(00)00262-5. [DOI] [PubMed] [Google Scholar]
- 25.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Amaro E, Jr, Barker GJ. Study design in fMRI: basic principles. Brain Cogn. 2006;60:220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Del Parigi A. Promise and limitations of functional neuroimaging in the study of obesity: is it time for a consortium and a multicenter trial? Int J Obes (Lond) 2009;33:607–610. doi: 10.1038/ijo.2009.55. [DOI] [PubMed] [Google Scholar]
- 28.Herholz K, Krieg JC, Emrich HM, Pawlik G, Beil C, Pirke KM, Pahl JJ, Wagner R, Wienhard K, Ploog D, Heiss WD. Regional cerebral glucose metabolism in anorexia nervosa measured by positron emission tomography. Biol Psychiatry. 1987;22:43–51. doi: 10.1016/0006-3223(87)90128-4. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;1:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- 30.Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res Bull. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage. 2008;42:60–69. doi: 10.1016/j.neuroimage.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging. 2001;19:821–826. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 33.Brockmann MA, Kemmling A, Groden C. Current issues and perspectives in small rodent magnetic resonance imaging using clinical MRI scanners. Methods. 2007;43:79–87. doi: 10.1016/j.ymeth.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Thanos PK, Michaelides M, Gispert JD, Pascau J, Soto-Montenegro ML, Desco M, Wang R, Wang GJ, Volkow ND. Differences in response to food stimuli in a rat model of obesity: in-vivo assessment of brain glucose metabolism. Int J Obes (Lond) 2008;32:1171–1179. doi: 10.1038/ijo.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA. 2007;104:18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen M, Zhang TM, Luo SL, Zhou C, Wu XM, Zhou NN, Cai K, Yang ZH, Wang WC, Zhao WF, Li SY, Wang Z, Zhang YT, Li GZ. Functional magnetic resonance imaging and immunohistochemical study of hypothalamic function following oral glucose ingestion in rats. Chin Med J (Engl) 2007;120:1232–1235. [PubMed] [Google Scholar]
- 38.Parkinson JR, Chaudhri OB, Bell JD. Imaging appetite-regulating pathways in the central nervous system using manganese-enhanced magnetic resonance imaging. Neuroendocrinology. 2009;89:121–130. doi: 10.1159/000163751. [DOI] [PubMed] [Google Scholar]
- 39.Kuo YT, Parkinson JR, Chaudhri OB, Herlihy AH, So PW, Dhillo WS, Small CJ, Bloom SR, Bell JD. The temporal sequence of gut peptide CNS interactions tracked in vivo by magnetic resonance imaging. J Neurosci. 2007;27:12341–12348. doi: 10.1523/JNEUROSCI.2391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhri OB, Parkinson JR, Kuo YT, Druce MR, Herlihy AH, Bell JD, Dhillo WS, Stanley SA, Ghatei MA, Bloom SR. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun. 2006;350:298–306. doi: 10.1016/j.bbrc.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Pannacciulli N, Le DS, Salbe AD, Chen K, Reiman EM, Tataranni PA, Krakoff J. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage. 2007;35:511–517. doi: 10.1016/j.neuroimage.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 45.Adam TC, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr. 2005;93:845–851. doi: 10.1079/bjn20041335. [DOI] [PubMed] [Google Scholar]
- 46.Le DS, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch J, Moreno DR, Kim KH. Interconnected large-scale systems for three fundamental cognitive tasks revealed by functional MRI. J Cogn Neurosci. 2001;13:389–405. doi: 10.1162/08989290151137421. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48:1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Gao JH, Liu HL, Fox PT. The temporal response of the brain after eating revealed by functional MRI. Nature. 2000;405:1058–1062. doi: 10.1038/35016590. [DOI] [PubMed] [Google Scholar]
- 50.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage. 2005;24:363–368. doi: 10.1016/j.neuroimage.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 51.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 52.Neary MT, Batterham RL. Gut hormones: implications for the treatment of obesity. Pharmacol Ther. 2009;124:44–56. doi: 10.1016/j.pharmthera.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Tataranni PA, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obes Rev. 2003;4:229–238. doi: 10.1046/j.1467-789x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 54.Bohland JW, Bokil H, Allen CB, Mitra PP. The brain atlas concordance problem: quantitative comparison of anatomical parcellations. PLoS ONE. 2009;4:e7200. doi: 10.1371/journal.pone.0007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 57.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 59.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 60.Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 61.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 63.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;2:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 64.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 65.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 66.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33:S33–S40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 67.Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–317. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Uneyama H, Torii K. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology. 2009;137:262–273. doi: 10.1053/j.gastro.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 71.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH, Wardle J. Continuity and stability of eating behaviour traits in children. Eur J Clin Nutr. 2007;62:985–990. doi: 10.1038/sj.ejcn.1602855. [DOI] [PubMed] [Google Scholar]
- 74.Carnell S, Wardle J. Appetite and adiposity: a behavioral susceptibility model of obesity American Journal of Clinical Nutrition. Am J Clin Nutr. 2008;88:22–30. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 75.Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. Int J Obes (Lond) 2008;32:1468–1473. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- 76.Wadden TA, Brownell KD, Foster GD. Obesity: responding to the global epidemic. J Consult Clin Psychol. 2002;70:510–525. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- 77.Goldstone AP. The hypothalamus, hormones, and hunger: alterations in human obesity and illness. Prog Brain Res. 2006;153:57–73. doi: 10.1016/S0079-6123(06)53003-1. [DOI] [PubMed] [Google Scholar]
- 78.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 2008;69:173–179. doi: 10.1111/j.1365-2265.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 79.Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes (Lond) 2009;33:S28–S32. doi: 10.1038/ijo.2009.14. [DOI] [PubMed] [Google Scholar]
- 80.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 82.Carlson JJ, Turpin AA, Wiebke G, Hunt SC, Adams TD. Pre- and postprandial appetite hormone levels in normal weight and severely obese women. Nutr Metab (Lond) 2009;6:32. doi: 10.1186/1743-7075-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav. 1995;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 84.Martin LJ, Siliart B, Lutz TA, Biourge V, Nguyen P, Dumon HJ. Postprandial response of plasma insulin, amylin and acylated ghrelin to various test meals in lean and obese cats. Br J Nutr. 2010;103:1610–1619. doi: 10.1017/S000711450999359X. [DOI] [PubMed] [Google Scholar]
- 85.Baile CA, McLaughlin CL, Della-Fera MA. Role of cholecystokinin and opioid peptides in control of food intake. Physiol Rev. 1986;66:172–234. doi: 10.1152/physrev.1986.66.1.172. [DOI] [PubMed] [Google Scholar]
- 86.Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, Adamczyk-Sowa M, Kukla M, Berdowska A, Rehfeld JF, Bielanski W, Brzozowski T. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol. 2007;58:13–35. [PubMed] [Google Scholar]
- 87.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 89.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 90.Meier JJ, Gallwitz B, Schmidt WE, Nauck MA. Glucagon-like peptide 1 as a regulator of food intake and body weight: therapeutic perspectives. Eur J Pharmacol. 2002;3:269–279. doi: 10.1016/s0014-2999(02)01434-6. [DOI] [PubMed] [Google Scholar]
- 91.Berghofer P, Peterson RG, Schneider K, Fehmann HC, Goke B. Incretin hormone expression in the gut of diabetic mice and rats. Metabolism. 1997;46:261–267. doi: 10.1016/s0026-0495(97)90251-1. [DOI] [PubMed] [Google Scholar]
- 92.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL. Influence of BMI and gender on postprandial hormone responses. Obesity (Silver Spring) 2007;15:2974–2983. doi: 10.1038/oby.2007.355. [DOI] [PubMed] [Google Scholar]
- 94.Reinehr T, Enriori PJ, Harz K, Cowley MA, Roth CL. Pancreatic poly-peptide in obese children before and after weight loss. Int J Obes (Lond) 2006;30:1476–1481. doi: 10.1038/sj.ijo.0803393. [DOI] [PubMed] [Google Scholar]
- 95.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 96.Gil-Campos M, Aguilera CM, Ramirez-Tortosa MC, Canete R, Gil A. Fasting and postprandial relationships among plasma leptin, ghrelin, and insulin in prepubertal obese children. Clin Nutr. 2010;29:54–59. doi: 10.1016/j.clnu.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 97.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 98.Ueno N, Inui A, Iwamoto M, Kaga T, Asakawa A, Okita M, Fujimiya M, Nakajima Y, Ohmoto Y, Ohnaka M, Nakaya Y, Miyazaki JI, Kasuga M. Decreased food intake and body weight in pancreatic polypeptide-over-expressing mice. Gastroenterology. 1999;117:1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 99.Schusdziarra V, Lawecki J, Ditschuneit HH, Lukas B, Maier V, Pfeiffer EF. Effect of low-dose somatostatin infusion on pancreatic and gastric endocrine function in lean and obese nondiabetic human subjects. Diabetes. 1985;34:595–601. doi: 10.2337/diab.34.6.595. [DOI] [PubMed] [Google Scholar]
- 100.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008;32:1640–1646. doi: 10.1038/ijo.2008.157. [DOI] [PubMed] [Google Scholar]
- 101.Ramos EJ, Meguid MM, Campos AC, Coelho JC. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition. 2005;21:269–279. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 102.Korner J, Chua SC, Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology. 1999;70:377–383. doi: 10.1159/000054499. [DOI] [PubMed] [Google Scholar]
- 103.Duan J, Choi YH, Hartzell D, Della-Fera MA, Hamrick M, Baile CA. Effects of subcutaneous leptin injections on hypothalamic gene profiles in lean and ob/ob mice. Obesity (Silver Spring) 2007;15:2624–2633. doi: 10.1038/oby.2007.314. [DOI] [PubMed] [Google Scholar]
- 104.Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, Aizawa-Abe M, Yoshimasa Y, Nakao K. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 1999;48:2028–2033. doi: 10.2337/diabetes.48.10.2028. [DOI] [PubMed] [Google Scholar]
- 105.Low MJ, Hayward MD, Appleyard SM, Rubinstein M. State-dependent modulation of feeding behavior by proopiomelanocortin-derived beta-endorphin. Ann NY Acad Sci. 2003;994:192–201. doi: 10.1111/j.1749-6632.2003.tb03180.x. [DOI] [PubMed] [Google Scholar]
- 106.Giugliano D, Cozzolino D, Torella R, Lefebvre PJ, Franchimont P, D'Onofrio F. Persistence of altered metabolic responses to beta-endorphin after normalization of body weight in human obesity. Acta Endocrinol (Copenh) 1991;124:159–165. doi: 10.1530/acta.0.1240159. [DOI] [PubMed] [Google Scholar]
- 107.Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res. 2002;957:298–310. doi: 10.1016/s0006-8993(02)03640-5. [DOI] [PubMed] [Google Scholar]
- 108.Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic. 2005;4:95–111. doi: 10.1093/bfgp/4.2.95. [DOI] [PubMed] [Google Scholar]
- 109.Drescher VS, Chen HL, Romsos DR. Corticotropin-releasing hormone decreases feeding, oxygen consumption and activity of genetically obese (ob/ob) and lean mice. J Nutr. 1994;124:524–530. doi: 10.1093/jn/124.4.524. [DOI] [PubMed] [Google Scholar]
- 110.Richard D, Rivest R, Naimi N, Timofeeva E, Rivest S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology. 1996;137:4786–4795. doi: 10.1210/endo.137.11.8895348. [DOI] [PubMed] [Google Scholar]
- 111.Timofeeva E, Deshaies Y, Picard F, Richard D. Corticotropin-releasing hormone-binding protein in brain and pituitary of food-deprived obese (fa/fa) Zucker rats. Am J Physiol. 1999;2:R1749–R1759. doi: 10.1152/ajpregu.1999.277.6.R1749. [DOI] [PubMed] [Google Scholar]
- 112.Zarate J, Churruca I, Pascual J, Casis L, Salles J, Echevarria E. Brain endocannabinoid system is involved in fluoxetine-induced anorexia. Nutr Neurosci. 2008;11:111–118. doi: 10.1179/147683008X301496. [DOI] [PubMed] [Google Scholar]
- 113.Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl) 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- 114.Specter SE, Horwitz BA, Beverly JL. Basal and glucoprivic-induced changes in extracellular GABA in the ventral hypothalamus of Zucker rats. Am J Physiol. 1996;2:R388–R392. doi: 10.1152/ajpregu.1996.271.2.R388. [DOI] [PubMed] [Google Scholar]
- 115.Gundlach AL. Galanin/GALP and galanin receptors: role in central control of feeding, body weight/obesity and reproduction? Eur J Pharmacol. 2002;3:255–268. doi: 10.1016/s0014-2999(02)01433-4. [DOI] [PubMed] [Google Scholar]
- 116.Beck B. Hypothalamic galanin and early state of hyperphagia in obese Zucker rats. Appetite. 2007;48:206–210. doi: 10.1016/j.appet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Baranowska B, Wasilewska-Dziubinska E, Radzikowska M, Plonowski A, Roguski K. Neuropeptide Y, galanin, and leptin release in obese women and in women with anorexia nervosa. Metabolism. 1997;46:1384–1389. doi: 10.1016/s0026-0495(97)90136-0. [DOI] [PubMed] [Google Scholar]
- 118.Mercer JG, Lawrence CB, Atkinson T. Regulation of galanin gene expression in the hypothalamic paraventricular nucleus of the obese Zucker rat by manipulation of dietary macronutrients. Brain Res Mol Brain Res. 1996;2:202–208. doi: 10.1016/s0169-328x(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 119.Kuramochi M, Onaka T, Kohno D, Kato S, Yada T. Galanin-like peptide stimulates food intake via activation of neuropeptide Y neurons in the hypothalamic dorsomedial nucleus of the rat. Endocrinology. 2006;147:1744–1752. doi: 10.1210/en.2005-0907. [DOI] [PubMed] [Google Scholar]
- 120.Jureus A, Cunningham MJ, Li D, Johnson LL, Krasnow SM, Teklemichael DN, Clifton DK, Steiner RA. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology. 2001;142:5140–5144. doi: 10.1210/endo.142.12.8542. [DOI] [PubMed] [Google Scholar]
- 121.Vrang N, Larsen PJ, Jensen PB, Lykkegaard K, Artmann A, Larsen LK, Tang-Christensen M. Upregulation of the brainstem preproglucagon system in the obese Zucker rat. Brain Res. 2008;1187:116–124. doi: 10.1016/j.brainres.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 122.Stricker-Krongrad A, Dimitrov T, Beck B. Central and peripheral dysregulation of melanin-concentrating hormone in obese Zucker rats. Brain Res Mol Brain Res. 2001;2:43–48. doi: 10.1016/s0169-328x(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 123.Gao J, Ghibaudi L, van Heek M, Hwa JJ. Characterization of diet-induced obese rats that develop persistent obesity after 6 months of high-fat followed by 1 month of low-fat diet. Brain Res. 2002;2:87–90. doi: 10.1016/s0006-8993(02)02493-9. [DOI] [PubMed] [Google Scholar]
- 124.Beck B, Burlet A, Nicolas JP, Burlet C. Hypothalamic neuropeptide Y (NPY) in obese Zucker rats: implications in feeding and sexual behaviors. Physiol Behav. 1990;47:449–453. doi: 10.1016/0031-9384(90)90107-f. [DOI] [PubMed] [Google Scholar]
- 125.Swiergiel AH, Wieczorek M. Noradrenaline-induced feeding responses in the rat do not depend on food characteristics. Acta Neurobiol Exp (Wars) 2008;68:354–361. doi: 10.55782/ane-2008-1701. [DOI] [PubMed] [Google Scholar]
- 126.Svec F, Thompson H, Corll C, Porter J. Levels of hypothalamic neurotransmitters in lean and obese Zucker rats. Nutr Neurosci. 2002;5:321–326. doi: 10.1080/1028415021000033785. [DOI] [PubMed] [Google Scholar]
- 127.Tentolouris N, Tsigos C, Perea D, Koukou E, Kyriaki D, Kitsou E, Daskas S, Daifotis Z, Makrilakis K, Raptis SA, Katsilambros N. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism. 2003;52:1426–1432. doi: 10.1016/s0026-0495(03)00322-6. [DOI] [PubMed] [Google Scholar]
- 128.De Fanti BA, Hamilton JS, Horwitz BA. Meal-induced changes in extracellular 5-HT in medial hypothalamus of lean (Fa/Fa) and obese (fa/fa) Zucker rats. Brain Res. 2001;902:164–170. doi: 10.1016/s0006-8993(01)02371-x. [DOI] [PubMed] [Google Scholar]
- 129.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 130.Parkinson JR, Chaudhri OB, Kuo YT, Field BC, Herlihy AH, Dhillo WS, Ghatei MA, Bloom SR, Bell JD. Differential patterns of neuronal activation in the brainstem and hypothalamus following peripheral injection of GLP-1, oxyntomodulin and lithium chloride in mice detected by manganese-enhanced magnetic resonance imaging (MEMRI) Neuroimage. 2009;44:1022–1031. doi: 10.1016/j.neuroimage.2008.09.047. [DOI] [PubMed] [Google Scholar]