Abstract
Introduction
The gold standard in organ preservation is static cold storage (SCS) using University of Wisconsin solution (UW). While it’s known that there is a finite limit to SCS preservation, and that there is a correlation between the ATP levels and organ function post preservation, a quantitative relationship has not been established, which is important in understanding the fundamental limitations to preservation, minimizing cold ischemic injury and hence maximizing utilization of the donor organ pool.
Aim
To determine the time limits of cellular viability and metabolic function during SCS, and to characterize the relationship between cellular viability and energetic state, using clinically relevant techniques in organ preservation.
Methods
Rat livers were procured and stored using conventional storage in UW solution at 4°C. Viability was assessed by determining the amount of viable hepatocytes and intracellular ATP content after 0, 24, 48, 72 and 120 hours of storage.
Results
Numbers of viable hepatocytes that were isolated from these livers decreased steadily during SCS. After 5 days, viable hepatocytes decreased from 25.95×106 to 0.87×106 cells/gram tissue. Intracellular ATP content decreased from 9.63 to 0.93 μmoles/gram tissue. Statistical analysis (ANOVA) established a linear relation for both parameters as a function of time (P<0.05).
Conclusion
The linear correlation between hepatocyte viability, ATP content, and storage time suggests a shared physiological foundation. These findings confirm ATP as direct predictor for organ quality in the context of liver preservation, which will aid quantitative assessment of donor organs for various applications.
Introduction
The shortage of donor organs for transplantation is a major crisis in treatment of organ failure, with the gap between demand and supply growing rapidly. Attempts to address this gap include development of superior storage solutions and machine perfusion [1–3], enabling longer organ storage times and recovery of discarded donor livers [4]. Through these progressions, transplantation surgery will advance from an urgent and precarious practice towards being an elective procedure. However, evaluation of the organ post-storage and prior to transplantation remains subjective, with assessment generally limited to gross morphology and donor criteria [5]. Histology is performed occasionally, although reports in kidney transplantation indicate its use does not significantly increase the likelihood of graft survival[6–8]. Even machine perfusion has yet to provide definitive quantitative criteria of organ viability, despite the availability of multiple dynamic measurements [9]. The establishment of markers for organ viability would assuage uncertainty for the surgical team, and reduce the number of organs discarded due to poor and often conservative assessment of organ viability. Furthermore, such a method would be of immediate use in the procurement of hepatocytes from discarded organs, which serve as a finite resource for cell transplantation and bio-artificial livers.
Obvious parameters to consider as being correlated with graft survival are those associated with the energy state of the organ. It is well known that as the duration of organ storage increases, the levels of metabolic substrates ATP, adenosine and ADP, decreases [10]. However, the dynamics of energy level depletion during storage have not been quantified in detail; hence it is unknown whether this correlation can be leveraged for accurate viability assessment. Therefore, in this preliminary study we analyzed the transient changes in ATP content during static cold storage. In order to gauge the quality of the organ, we measured the amount of viable hepatocytes in a liver graft after cell isolation, which is a known measure of function for cell transplantation [11, 12].
Experimental Procedures
Procurement of rat livers
Experiments were performed using female Lewis rats (n=30) weighing 150–200 g (Charles River Labs, Wilmington, MA). The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the Subcommittee on Research Animal Care, Massachusetts General Hospital. Subjects were anesthetized with isoflurane (Forane, Baxter, Deerfield, IL) using a Tech 4 vaporizer (Surgivet, Waukesha, WI). The liver was harvested using the technique previously described by Delrivière and Kamada [13, 14]. During the last stage of surgery, livers were flushed with 5ml of cold UW using an 18G intravenous catheter introduced into the portal vein and secured with a 6-0 silk ligation, marking the start of cold storage time. Livers were washed and weighed (average weight 7.87±2.3g), and then flushed with an additional 5ml of UW immediately after procurement.
Static Cold Storage of rat livers
Livers were stored in UW solution at 4°C for 0, 24, 48, 72, and 120 hours (n=6 livers per time point). Literature indicates that rat livers can be stored and transplanted after an average SCS time of 18–24 hours [15–21] with one report extending the storage time to 48 hours [22], hence this design incorporates two storage durations that would very likely result in successful transplantation (0 and 24 hours), two with expected negative outcomes (72 and 120 hours), and one with marginal results (48 hours), providing a relevant time span to assess the value of ATP in evaluation of graft viability.
Hepatocyte isolation
The protocol used is adapted from the enzyme-based hepatocyte isolation protocol developed by Kreamer et al [23]. The livers were perfused with Krebs Ringer’s Lactate with a flow rate of 17ml/min. Digestion was achieved with collagenase (Type IV, Sigma, St Louis, MO). The hepatocytes were isolated from the non-parenchymal fraction by filtration and centrifugation steps and live cells were segregated through Percoll gradient purification. Viability was >90% in all experiments, as assessed by Tryphan blue staining using a hemocytometer.
ATP assay
After 0, 24, 48, 72, and 120 hours, tissue biopsies (20–40mg each) were taken from each specimen (threefold to decrease sampling error) and flash-frozen using liquid nitrogen. After thawing, samples were kept at 4°C. The tissue samples were weighed and homogenized in perchloric acid (Biovision, Mountain View, CA) in order to neutralize phosphatases present in the tissue. Samples were centrifuged, resuspended and deproteinated using a Deproteinizing Sample Prep Kit (Biovision, Mountain View, CA). Concentration of ATP in each sample was determined using an ATP colorimetric/Fluorometric Assay Kit (Biovision, Mountain View, CA) and fluorescence was detected using an fmax microplate reader (Molecular Devices, Sunnyvale, California).
Statistical analysis
Pearson correlation coefficient (R) and linear regression were used to analyze the relationship of cell yield, ATP level, and storage time. Analysis of variance and post-hoc Tukey test were performed for comparisons between groups with different storage times. P <0.05 was considered to be significant.
Results
Cell yield during SCS
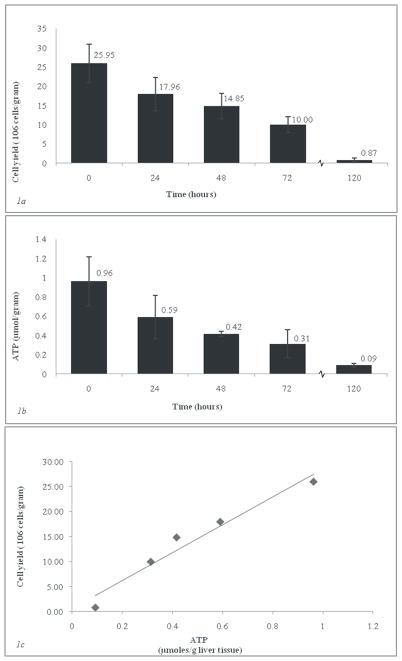
In order to assess the viability of each graft, the live hepatocyte fraction was determined at various SCS storage times. Figure 1a shows the results of the hepatocyte isolations for each time point. Cell yields declined consistently with the increase in storage time; the 120 hour control time point showed a yield of 0.87±0.42 ×106 hepatocytes per gram of liver tissue, or 3.3% of the amount of freshly isolated hepatocytes (25.95 ×106 cells/gram liver). Linear regression analysis revealed a correlation between the viable cell numbers and storage time in UW (R 0.96 with a significance of P=0.0081). We analyzed independent differences between the individual groups (P<0.05) using analysis of variance and post-hoc Tukey test. There was no significant difference between any two groups stored for less than 24 hours. However, significant variation existed in the comparison of 0 hours vs. ≥48 hours, 24 hours vs. 120 hours s, and 48 hours vs. 120 hours of SCS.
Fig. 1. Hepatocyte isolations and ATP assay for various periods of SCS. Error bars reflect standard deviation.
Fig. 1a) Cell yield after percoll-purification (>90% viability, n=3 per time point). Depicted are numbers of viable hepatocytes (>90% viability) per time point (106 cells/g).
Fig. 1b) Average ATP per gram of liver tissue (n=3 livers per time point, 3 sections per liver.
Fig. 1c) Cell yield in 106 cells/gram vs. Average ATP per gram of liver tissue.
ATP levels during SCS
ATP was measured at each storage time. Figure 1b depicts the intracellular content of ATP per gram of liver tissue. A linear relationship between the amount of ATP isolated from each sample and the amount of time the sample had been stored was determined by regression analysis (R calculated at 0.95 with a significance of P=0.011). Individual group differences were significant in the comparison of 0 hours vs. ≥48 hours and 24 hours vs. 120 hours of SCS. As was the case for cell yield, no differences were significant between groups stored less than 24 hours. The 120 hour control group showed ATP levels averaging 0.93 μmoles/gram tissue (<10% of fresh ATP levels).
Relationship of cell yield and ATP
A scatter plot of cell yields against ATP levels is shown in Figure 1c. The cell yields and ATP level had a linear relationship with each other across all storage times (R=0.98; P=0.004). It’s therefore possible to predict the amount of hepatocytes in a rat liver from the ATP content, at the ratio of 26.94 × 106 hepatocytes / μmoles ATP · gr liver−1.
Discussion
In this study we have demonstrated that when a healthy liver is stored ex vivo there is a strong association between the amount of viable hepatocytes and the ATP level, both of which decline linearly with time. The dynamic correlation of this process supports further exploration into the use of cellular energy levels as a quantitative criterion of graft viability. For example, previous studies have demonstrated that rat livers can be stored and transplanted after an average SCS time of 18–24 hours [15–21] with one report extending the storage time to 48 hours [22], while no success has been shown at 72 and 120 hours. Our results indicate that during a storage time of 24 hours, both the viable cell yield and ATP level decline. It is notable that there is no statistical difference in ATP content or cell viability during the first 24 hours of SCS, which is in accordance with the established preservation periods in rat liver transplantation. Significant differences between individual groups surfaced when storage time was extended beyond 24 hours. After 24 hours of SCS, ATP levels decline to 70% of initial levels, and at 72 hours, this value is only 30%. These results suggest then that 70% of fresh (or 0.59/ μmoles ATP · g liver−1) might be a relevant value to indicate the limit of viability, independent of preservation time. Furthermore, we propose that an ATP-based criterion can serve in early detection of pathologies such as ischemic injury, steatosis and other donor factors, although experimental confirmation will be necessary. The one potential disadvantage of this concept is that it requires a biopsy, however in the case of machine perfusion a non-invasive indicator of energy could be developed.
Confirmation of these results in an animal transplantation model is necessary to verify this approach, and should include the viability analysis of non-parenchymal cell types such as biliary epithelial cells, which are crucial for long-term graft function. For applications where primary hepatocyte isolation is required, such as bio-artificial livers, the ATP status can be a direct predictor for the expected viable cell yield per gram of liver tissue [24, 25], and the values determined in this work can provide an initial guideline for studies with human organs.
Acknowledgments
Funding: Professor Michael – van Vloten foundation, National Institutes of Health (R01 DK59766, R01 EB 008678, R00 DK080942), Shriners Hospitals for Children
Tim Berendsen was supported by the Prof. Michaël-van Vloten foundation. Funding from the National Institutes of Health (R01 DK59766, R01 EB 008678, R00 DK080942), and the Shriners Hospitals for Children are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
TA Berendsen, Email: timberendsen@gmail.com.
ML Izamis, Email: yiapani@gmail.com.
H Xu, Email: hxu3@partners.org.
Q Liu, Email: qiang.lui.med@gmail.com.
M Hertl, Email: mhertl@partners.org.
F Berthiaume, Email: francois_berthiaume@hms.harvard.edu.
ML Yarmush, Email: ireis@sbi.org.
K Uygun, Email: kuygun@partners.org.
References
- 1.St Peter SD, Imber, Friend PJ. Liver and kidney preservation by perfusion. The Lancet. 2002;359:604–13. doi: 10.1016/S0140-6736(02)07749-8. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Lee CY, Clemens MG, Zhang JX. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77:1676–82. doi: 10.1097/01.tp.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 3.Tolboom H, Pouw R, Izamis M, et al. Recovery of Warm Ischemic Rat Liver Grafts by Normothermic Extracorporeal Perfusion. Transplantation. 2009;87:170–7. doi: 10.1097/TP.0b013e318192df6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 5.Makowka L, Gordon RD, Todo S, et al. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19:2378–82. [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YW, Shah T, Cho ES, et al. Factors associated with discard of recovered kidneys. Transplant Proc. 2008;40:1032–4. doi: 10.1016/j.transproceed.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Akioka K, Okamoto M, Ushigome H, et al. An attempt to extend the donor criteria for successful living-related kidney transplantation from a donor with membranous nephropathy. Transplant Proc. 2009;41:446–9. doi: 10.1016/j.transproceed.2008.08.141. [DOI] [PubMed] [Google Scholar]
- 8.Kozakowski N, Regele H. Biopsy diagnostics in renal allograft rejection: from histomorphology to biological function. Transpl Int. 2009;22:945–53. doi: 10.1111/j.1432-2277.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 9.Uygun K, Tolboom H, Izamis ML, et al. Diluted blood reperfusion as a model for transplantation of ischemic rat livers: alanine aminotransferase is a direct indicator of viability. Transplant Proc. 42:2463–7. doi: 10.1016/j.transproceed.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurada M, Okochi N, Kato H, et al. Mitochondrial energy synthesis during cold preservation and after reperfusion in liver transplantation. Nippon Geka Gakkai Zasshi. 1992;93:709–15. [PubMed] [Google Scholar]
- 11.Arkadopoulos N, Papalois A, Pataryas TH, Golematis B, Papadimitriou J. Experimental transplantation of hepatocytes in cases of toxic acute liver failure. An allograft model. Transpl Int. 1994;7 (Suppl 1):S171–4. doi: 10.1111/j.1432-2277.1994.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhawan A, Strom SC, Sokal E, Fox IJ. Human hepatocyte transplantation. Methods Mol Biol. 2010;640:525–34. doi: 10.1007/978-1-60761-688-7_29. [DOI] [PubMed] [Google Scholar]
- 13.Delriviere L, Kamada N, Kobayashi E, Enosawa S, Goto S. Portosystemic shunt for orthotopic liver transplantation in the rat. J Surg Res. 1994;56:457–60. doi: 10.1006/jsre.1994.1072. [DOI] [PubMed] [Google Scholar]
- 14.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 15.Olschewski P, Hunold G, Eipel C, et al. Improved microcirculation by low-viscosity histidine- tryptophan-ketoglutarate graft flush and subsequent cold storage in University of Wisconsin solution: results of an orthotopic rat liver transplantation model. Transpl Int. 2008;21:1175–80. doi: 10.1111/j.1432-2277.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 16.Theruvath TP, Zhong Z, Pediaditakis P, et al. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–46. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann S, Caldwell-Kenkel JC, Currin RT, et al. Protection by pentoxifylline against graft failure from storage injury after orthotopic rat liver transplantation with arterialization. Transpl Int. 1992;5 (Suppl 1):S345–50. doi: 10.1007/978-3-642-77423-2_106. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann S, Caldwell-Kenkel JC, Oleksy I, Steffen R, Thurman RG, Lemasters JJ. Warm Carolina rinse solution prevents graft failure from storage injury after orthotopic rat liver transplantation with arterialization. Transpl Int. 1992;5:108–14. doi: 10.1007/BF00339225. [DOI] [PubMed] [Google Scholar]
- 19.Gores GJ, Ferguson DM, Ludwig J, Steffen R, Krom RA. Effect of acidosis during cold ischemic storage on liver viability following transplantation in the rat. Transplant Proc. 1990;22:488–9. [PubMed] [Google Scholar]
- 20.Puhl G, Olschewski P, Schoning W, et al. Low viscosity histidine-tryptophan-ketoglutarate graft flush improves subsequent extended cold storage in University of Wisconsin solution in an extracorporeal rat liver perfusion and rat liver transplantation model. Liver Transpl. 2006;12:1841–9. doi: 10.1002/lt.20913. [DOI] [PubMed] [Google Scholar]
- 21.Vairetti M, Ferrigno A, Bertone R, et al. Exogenous melatonin enhances bile flow and ATP levels after cold storage and reperfusion in rat liver: implications for liver transplantation. J Pineal Res. 2005;38:223–30. doi: 10.1111/j.1600-079X.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 22.Marshall VC, Howden BO, Jablonski P. Effect of storage temperature in rat liver transplantation: 4 degrees C is optimal and gives successful 48-hour preservation. Transplant Proc. 1994;26:3657–8. [PubMed] [Google Scholar]
- 23.Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986;22:201–11. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- 24.Takesue M, Maruyama M, Shibata N, et al. Maintenance of cold-preserved porcine hepatocyte function with UW solution and ascorbic acid-2 glucoside. Cell Transplant. 2003;12:599–606. doi: 10.3727/000000003108747208. [DOI] [PubMed] [Google Scholar]
- 25.Corps CL, Shires M, Crellin D, et al. Influence on energy kinetics and histology of different preservation solutions seen during cold ischemia in the liver. Transplant Proc. 2009;41:4088–93. doi: 10.1016/j.transproceed.2009.07.107. [DOI] [PubMed] [Google Scholar]