Abstract
Rationale
Aripiprazole (Abilify) is an atypical antipsychotic drug characterized by partial agonist activity at dopamine (DA) D2/D3 receptors and a low side-effect profile. While we previously demonstrated that acute aripiprazole blocked the reinstatement of cocaine seeking in an animal model of relapse, clinical treatment of relapse prevention necessitates testing the effects of aripiprazole following prolonged abstinence, as well as after repeated administration during withdrawal from cocaine.
Objectives
We assessed the effects of repeated aripiprazole treatment on cocaine seeking after abstinence and during conditioned cue-induced and cocaine-primed reinstatement in rats.
Materials and methods
Rats self-administered intravenous cocaine paired with a light + tone stimulus for 10–14 days, followed by 2 weeks of abstinence. Following post-abstinence relapse testing, lever responding was allowed to extinguish, with subsequent reinstatement testing occurring either in the presence of the conditioned stimulus, or after a cocaine-priming injection (10 mg/kg, intraperitoneal (IP)). Following 3 or 7 days of pretreatment, rats received an injection of aripiprazole (0.25, 0.5, and 1.0 mg/kg, IP) or vehicle prior to post-abstinence relapse and reinstatement testing.
Results
Vehicle-pretreated animals showed robust cocaine seeking during relapse and reinstatement testing, an effect that was significantly attenuated by aripiprazole pretreatment, although no lasting effects were found in the absence of acute injection.
Discussion
These findings support the possibility that repeated aripiprazole may be an effective therapeutic agent for the prevention of relapse in abstinent cocaine users. Based on its antipsychotic profile, aripiprazole may be particularly useful for individuals diagnosed with comorbid psychoses, such as schizophrenia or bipolar disorder.
Keywords: Aripiprazole, Chronic, Cocaine, Reinstatement, Relapse, Abstinence
Introduction
Drug addiction is a chronic relapsing disorder, characterized by compulsive drug-seeking and drug-taking behaviors, despite negative consequences. In particular, psychostimulant abuse and dependence (especially cocaine and amphetamines) constitutes a major psychiatric disorder, both in terms of severity and incidence (O’Brien and Anthony 2005; Sloboda 2002). While several therapeutic compounds are in various stages of testing for the treatment of psychostimulant addiction (Bergman 2008; Vocci et al. 2005), no medications are currently approved by the Food and Drug Administration for the treatment of cocaine or amphetamine addiction. However, one therapeutic approach involves the use of dopamine (DA) receptor partial agonists (Childress and O’Brien 2000; Kosten et al. 2002; Platt et al. 2002).
Given its efficacy and low side-effect profile, the atypical antipsychotic aripiprazole has been identified as one of the best DA receptor partial agonist treatment options for schizophrenia (DeLeon et al. 2004; Lieberman 2004) and bipolar disorder (Keck et al. 2003). While having some affinity for serotonin 5HT1A (i.e., partial agonist (Jordan et al. 2002a; Jordan et al. 2002b) and 5HT2A (i.e., antagonist (Davies et al. 2004; Grunder et al. 2003) receptors, aripiprazole’s primary mechanism of action involves both postsynaptic DA D2/D3 receptors and presynaptic DA autoreceptors (Lawler et al. 1999). For example, an examination of the in vivo receptor occupancy profile in rat brain revealed an ED50 of 0.44 mg/kg for D2 receptors, while the ED50 was 7.2 and 8.8 mg/kg for 5HT1A and 5HT2A receptors, respectively (Langlois et al. 2005). Furthermore, in vivo binding studies in animal models (Natesan et al. 2006) and humans (Mamo et al. 2007) have demonstrated relatively selective and unique DA D2 partial agonist properties of aripiprazole. Compared with other antipsychotic drugs, aripiprazole is generally safe and well tolerated by patients, and produces fewer side effects, including less extrapyramidal symptoms, reduced incidence of weight gain and sedation, and a minimal risk for diabetes and hyperlipidemia (DeLeon et al. 2004; Kane et al. 2002; Newcomer 2005). The antipsychotic effects of aripiprazole are hypothesized to involve the “stabilization” of mesocorticolimbic DA neurotransmission (Burris et al. 2002; Stahl 2001; Yokoi et al. 2002), a pathway that has been extensively implicated in drug addiction and relapse (Robinson and Berridge 2000; White and Kalivas 1998). Since DA receptor partial agonists do not produce the physiological and behavioral effects of full agonists, while simultaneously acting as partial antagonists when DA activity is increased (Ariens 1983), it has been hypothesized that DA partial agonists, such as aripiprazole, may be effective for the prevention of relapse in cocaine-dependent individuals (Platt et al. 2002).
Treatment management of addiction is hampered by the high incidence of relapse to drug-seeking and drug-taking behaviors following prolonged periods of abstinence (Dackis and O’Brien 2001; Wagner and Anthony 2002). A number of factors contribute to drug craving and relapse, including drug-associated stimuli and exposure to a small amount of the previously self-administered drug. For example, abstinent cocaine users report an increase in drug craving following exposure to cocaine-associated stimuli (Childress et al. 1993) or in response to a noncontingent dose of cocaine (Jaffe et al. 1989). These trigger factors have been extensively applied in animal models of relapse, primarily the extinction-reinstatement paradigm following chronic cocaine self-administration, in which previously cocaine-paired stimuli or noncontingent cocaine administration results in a robust reinstatement of cocaine seeking (i.e., relapse) as indexed by responding on a previously cocaine-paired operandum (Shaham et al. 2003).
We previously demonstrated that acute doses of aripiprazole blocked both conditioned-cued and cocaine-primed reinstatement of cocaine seeking at doses that failed to affect other behaviors, including reinstatement of food-seeking behavior or basal locomotor activity (Feltenstein et al. 2007). Similar reductions in drug reinforcement and/or drug-seeking behavior following aripiprazole pretreatment have been reported both preclinically and clinically for alcohol (Janiri et al. 2007), opiates (Li et al. 2009), nicotine (Liu et al. 2009), and psychostimulants (Beresford et al. 2005; Brown et al. 2005; Sørensen et al. 2008), although not in a methamphetamine-dependent population (Newton et al. 2008). However, in order to assess the potential clinical utility of aripiprazole in relapse prevention, it is necessary to test cocaine seeking following prolonged abstinence in the absence of extinction, as well as the effects of repeated aripiprazole administration during withdrawal from cocaine. To test the hypothesis that repeated aripiprazole treatment may be an effective pharmacotherapy for preventing relapse, we assessed the effects of repeated administration of various doses of aripiprazole on relapse behavior after forced abstinence. In addition, we also examined the effects of repeated aripiprazole administration on conditioned-cued and cocaine-primed reinstatement of cocaine seeking following explicit extinction trials.
Materials and methods
Subjects
Male, Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA; initial weight 275–300 g) were individually housed in a temperature- and humidity-controlled vivarium on a 12-h reverse light cycle with the lights on at 18:00. Animals were given water ad libitum and maintained on 20–25 g of standard rat chow (Harlan, Indianapolis, IN, USA) per day for the duration of the experiment. Rats were habituated to the vivarium for at least 4 days prior to the start of the experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and conformed to federal guidelines as described in the “Guide for the Care and Use of Laboratory Rats” of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
Surgery
Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, intraperitoneal (IP)), followed by equithesin (sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution, IP) and chronic indwelling catheters were implanted into the right jugular vein using previously described methods (Feltenstein et al. 2007). Catheter patency was maintained by flushing with 0.1 ml of 10 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ, USA) immediately prior to self-administration sessions with a 0.1 ml antibiotic solution of cefazolin (Schein Pharmaceuticals, Florham Park, NJ, USA; 10 mg/ml dissolved in 70 U/ml heparinized saline) and 0.1 ml 70 U/ml heparinized saline regimen following each session. Stylets were inserted into the catheters when the rats were not connected to the infusion pumps. If lever responding became erratic during self-administration, rats received a 0.12 ml intravenous (IV) infusion of methohexital sodium (Eli Lilly, Indianapolis, IN, USA; 10 mg/ml dissolved in 0.9% physiological saline), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously in order to verify catheter patency.
Cocaine self-administration
Rats lever pressed for cocaine in standard self-administration chambers (30 cm × 20 cm × 20 cm) linked to a computerized data collection program (MED-PC, Med Associates Inc., St. Albans, VT, USA). The chambers were equipped with two retractable levers, a white stimulus light above each lever, a tone generator, and a white house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan.
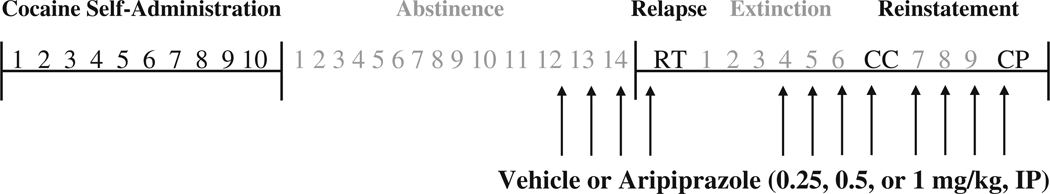
Figure 1 illustrates the phases of cocaine self-administration, abstinence, extinction, and reinstatement, and the times when aripiprazole or vehicle treatment occurred. Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% physiological saline; cocaine provided by the National Institute on Drug Abuse, Research Park Triangle, NC, USA) during daily 2-h sessions according to an FR1 schedule of reinforcement. At the start of each session, the catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). The house light signaled the initiation of the session and remained illuminated throughout the entire session. Lever presses on the active (i.e., cocaine-paired) lever resulted in a 2-s activation of the infusion pump (0.2 mg cocaine per 50 µl infusion) and a 5-s presentation of a stimulus complex, consisting of illumination of the white stimulus light above the active lever and activation of the tone generator (4.5 kHz, 78 dB). After each infusion, responses on the active lever resulted in no consequences during a 20-s time-out period. During the sessions, responses on the inactive lever were recorded, but had no programmed consequences. All self-administration sessions occurred during the animals’ dark cycle and were conducted 6 days/week until the animals achieved the self-administration criterion of ten sessions with at least ten infusions per session (i.e., 10–14 days).
Fig. 1.
Schematic representing the phases of cocaine self-administration, abstinence, relapse testing (RT), extinction, and conditioned-cued (CC) and cocaine-primed (CP) reinstatement testing. Arrows indicate when vehicle or aripiprazole was administered (i.e., immediately following the last 3 days of abstinence or extinction trials and 30 min prior to relapse and reinstatement testing)
Abstinence, relapse, and reinstatement testing
After chronic self-administration, rats were made abstinent from cocaine for a period of 14 days. For the first 7 days of abstinence, animals remained in their home cages in the colony room. For the final 7 days of abstinence, animals were transported to a testing room where they were placed in an alternate, nondrug-paired environment (i.e., a polycarbonate cage covered with a filter top) for 2-h at the same time of day. This procedure was designed to mitigate the potential stress of not being handled for an extended period of time. On the 15th day following the last cocaine self-administration session, animals were returned to the self-administration chamber to assess cocaine seeking during a 2-h test session (i.e., relapse testing). We use the term “relapse” rather than “reinstatement” for this initial test day, because rats did not experience extinction learning before this post-abstinence test day. During testing, lever presses were recorded but had no scheduled consequences (i.e., within-session extinction).
Following relapse testing and before the first reinstatement test, animals underwent additional daily 2-h extinction sessions. Once active lever responding extinguished to a criterion of a minimum of six extinction sessions with ≤25 active lever responses per session for two consecutive days (i.e., 6–9 days), animals underwent conditioned-cued and cocaine-primed reinstatement testing. During conditioned-cued reinstatement testing, active lever presses resulted in the presentation of the previously drug-paired light + tone conditioned stimulus (CS) in the absence of cocaine reinforcement. Immediately prior to the cocaine-primed reinstatement test, animals received an injection of cocaine hydrochloride (10 mg/kg dissolved in 0.9% physiological saline, IP), a dose that has been shown to produce robust reinstatement in previous studies (Cornish et al. 1999; Feltenstein et al. 2007; Fuchs et al. 2004). During cocaine-primed reinstatement testing, lever presses had no programmed consequences. Further extinction sessions occurred between reinstatement testing until the extinction criteria were reestablished (i.e., a minimum of 3 days with ≤25 active lever presses per session for two consecutive days).
Aripiprazole treatment
Table 1 illustrates the different treatment groups. To assess the effects of repeated aripiprazole treatment on cocaine seeking following abstinence, animals received an injection of aripiprazole hydrochloride (0.25, 0.5, or 1.0 mg/kg, IP) or vehicle (0.9% physiological saline and Tween 80; 20:1) in a volume of 1.0 ml/kg on the last 3 days of abstinence (i.e., immediately following the 2-h alternate environment session). Although not a chronic regimen, the 3-day pretreatment period was selected based on consultation with addiction psychiatrists aimed at matching clinical laboratory trials, while the doses of aripiprazole were selected based on prior evidence that acute injections at these doses effectively reduced conditioned-cued and cocaine-primed reinstatement without significantly affecting locomotor activity (Feltenstein et al. 2007). Thirty minutes before relapse testing, each rat received the same drug treatment (aripiprazole/aripiprazole groups) or vehicle injections (vehicle/vehicle or aripiprazole/vehicle groups) prior to being placed back in the self-administration chamber.
Table 1.
Experimental treatment groups indicating the injections given during the 3-day pre treatment phase and on the test day
| Group | 3-day pretreatment | Test day |
|---|---|---|
| Vehicle/vehicle | Vehicle | Vehicle |
| Aripiprazole/aripiprazole | Aripiprazole (0.25, 0.5, or 1.0 mg/kg) | Aripiprazole (0.25, 0.5, or 1.0 mg/kg) |
| Aripiprazole/vehicle | Aripiprazole (1.0 mg/kg) | Vehicle |
Following additional extinction sessions, animals received the same drug or vehicle treatment regimen that occurred during abstinence immediately following the last 3 days of extinction training, with the same drug treatment or vehicle injections occurring 30 min prior to conditioned-cued and cocaine-primed reinstatement testing.
Data analysis
To determine if any preexisting group differences existed, analysis of variance (ANOVA) or independent t tests were used to analyze lever responses and number of cocaine infusions during the last 2 days of cocaine self-administration, as well as the number of days of cocaine self-administration, with drug treatment as the between-subjects factor. Similar analyses were used for lever responding during relapse and reinstatement testing, as well as the number of extinction sessions, with repeated measures ANOVA used to analyze lever responses during extinction with drug and session as the between- and within-subjects factors, respectively. Data points were eliminated if they were 2.5 standard deviations beyond the group mean. All post-hoc analyses were conducted using Tukey’s tests with the alpha set at 0.05, and only significant F or t values are presented.
Results
Rats readily acquired cocaine self-administration, responded preferentially on the active lever, and displayed stable lever responding and drug intake during the self-administration maintenance phase. Statistical analyses on active and inactive lever responding (Fig. 2) and the mean number of cocaine infusions across the last 2 days of cocaine self-administration, as well as total number of days of cocaine self-administration (Table 2), failed to reveal any preexisting differences between the groups subsequently pretreated with aripiprazole or vehicle. In addition, no significant group differences were noted for inactive lever responding.
Fig. 2.
Effects of 3 days of aripiprazole pretreatment on relapse responding in animals given aripiprazole (ARI/ARI; top panel) or vehicle (ARI/VEH; bottom panel) on test day. The number in parentheses equals the dose of aripiprazole in mg/kg. Left panels show active (AL) and inactive (IL) lever responding (mean ± SEM) for the last 2 days of self-administration and during relapse following 14 days of abstinence. Right panels show active (large symbols) and inactive (small symbols) lever responding for the six subsequent extinction trials. Significant differences relative to vehicle/vehicle (VEH/VEH) treatment are indicated (*p < 0.05)
Table 2.
Mean number of infusions across the last 2 days of cocaine self-administration, the total number of cocaine self-administration sessions, and the total number of extinction sessions prior to reinstatement testing. Data are expressed as mean ± SEM
| Self-administration | Extinction | ||
|---|---|---|---|
| Mean # of cocaine infusions | Total # of sessions | Total # of sessions | |
| Aripiprazole/aripiprazole | |||
| VEH/VEH (n = 13) | 35.19±2.76 | 11.00±0.32 | 7.62±0.39 |
| 0.25 mg/kg ARI/ARI (n = 12) | 30.33±2.83 | 11.83±0.42 | 7.42±0.19 |
| 0.5 mg/kg ARI/ARI (n = 12) | 29.87±2.91 | 11.17±0.39 | 7.75±0.28 |
| 1.0 mg/kg ARI/ARI (n = 13) | 34.00±2.27 | 11.69±0.57 | 7.54±0.24 |
| Aripiprazole/vehicle | |||
| VEH/VEH (n = 13) | 34.46±2.16 | 12.39±0.56 | 8.23±0.58 |
| 1.0 mg/kg ARI/VEH (n = 12) | 34.50±2.32 | 11.83±0.61 | 7.83±0.51 |
Similar to previous research (Fuchs et al. 2006; Pickens and Thompson 1968; Schuster and Johanson 1981; See et al. 2007), reexposure to the cocaine-paired environment in the absence of cocaine reinforcement resulted in robust active lever responding in vehicle-pretreated rats (i.e., vehicle/vehicle groups; Fig. 2). However, animals pretreated with aripiprazole during abstinence and on the test day (i.e., aripiprazole/aripiprazole groups) showed significantly lower active lever responding during the relapse test (F3,41 = 8.36, p < 0.001), with post-hoc analyses revealing a significant attenuation in responding for all three aripiprazole/aripiprazole groups when compared with the vehicle/vehicle group (ps < 0.05). In contrast, this reduction in relapse responding did not occur for animals pretreated with aripiprazole (1.0 mg/kg) during abstinence, but not the day of test (i.e., aripiprazole/vehicle group).
Following relapse testing, animals underwent additional extinction trials prior to reinstatement testing. While all groups demonstrated normal extinction curves, animals in the 1.0 mg/kg aripiprazole/aripiprazole group exhibited slightly higher responding on the first post-relapse extinction day when compared with the vehicle/vehicle group (Fig. 2). A two-way repeated measures ANOVA of active lever responding showed significant main effects for group (F3,46 = 4.67, p < 0.01) and session (F5,230 = 64.82, p < 0.001), as well as a significant group × session interaction (F15,230 = 1.92, p < 0.05). One-way ANOVA for each session only revealed a significant main effect for the first post-relapse extinction day (F3,49 = 3.81, p < 0.05), with significantly higher responding for the 1.0 mg/kg aripiprazole group (p < 0.05). Similar analyses for the aripiprazole/vehicle groups only revealed a significant main effect for session (F5,115 = 29.65, p < 0.001). No significant group differences were noted for responding at the end of extinction, or in the number of extinction sessions (Table 2), prior to reinstatement testing.
Compared with responding at the end of extinction, animals in the vehicle/vehicle groups exhibited a significant increase in active lever responding during the conditioned-cued reinstatement test (ts8–24 = 3.39–5.16, ps < 0.01–0.001; Fig. 3). This effect was significantly attenuated by aripiprazole/aripiprazole treatment (F3,46 = 3.55, p < 0.05), with post-hoc analyses revealing a significant reduction in conditioned-cued reinstatement for all three aripiprazole/aripiprazole groups relative to the vehicle/vehicle group (ps < 0.05). However, this reduction in cue-induced reinstatement did not occur following aripiprazole/vehicle treatment (Fig. 3). Similar to conditioned-cued reinstatement testing, animals in the vehicle/vehicle groups exhibited a significant increase in active lever responding (ts8–24 = 3.53–3.76, ps < 0.001) during cocaine-primed reinstatement (Fig. 3). Moreover, this effect was significantly attenuated by aripiprazole/aripiprazole (F3,46 = 3.19, p < 0.05), but not aripiprazole/vehicle treatment, with post-hoc analyses revealing a significant reduction in cocaine-primed reinstatement responding for animals in the 0.5 and 1.0 mg/kg aripiprazole/aripiprazole groups (ps < 0.05).
Fig. 3.
Effects of 3 days of aripiprazole pretreatment on reinstatement responding in animals given aripiprazole (ARI/ARI; top panel) or vehicle (ARI/VEH; bottom panel) on test day. The number in parentheses equals the dose of aripiprazole in mg/kg. Panels show active (AL) and inactive (IL) lever responding (mean ± SEM) for the last 2 days of extinction training and during conditioned-cued and cocaine-primed reinstatement testing. Significant differences relative to vehicle/vehicle (VEH/VEH) treatment are indicated (*p < 0.05)
In order to further investigate whether the lack of effect for the aripiprazole/vehicle group was due to the relatively short 3 day pretreatment period, we conducted an additional experiment in which animals were pretreated with 1.0 mg/kg aripiprazole for 7 days prior to relapse and reinstatement testing in the absence of the drug (i.e., 7 day aripiprazole/vehicle group). Similar to the 3 day group, no significant reductions in cocaine-seeking behavior were found after 7 days of pretreatment (Fig. 4). Additionally, in order to verify the effects of an acute injection of aripiprazole in the absence of pretreatment, we administered aripiprazole (0.5 mg/kg) following vehicle pretreatment (3 days). Similar to results previously reported for acute aripiprazole reduction of reinstatement responding (Feltenstein et al. 2007), we saw a significant decrease in cocaine seeking (t6 = 2.77, ps < 0.05; Fig. 5).
Fig. 4.
Effects of 7 days of aripiprazole pretreatment (1.0 mg/kg) on relapse (top panel) and reinstatement (bottom panel) responding in animals given vehicle on test day (ARI (1.0)/VEH). Top left panel shows active (AL) and inactive (IL) lever responding (mean ± SEM) for the last 2 days of self-administration and during relapse following 14 days of abstinence. Top right panel shows active (large symbols) and inactive (small symbols) lever responding for the six subsequent extinction trials. Bottom panel shows active (AL) and inactive (IL) lever responding (mean ± SEM) for the last 2 days of extinction training and during conditioned-cued and cocaine-primed reinstatement testing
Fig. 5.
Effects of aripiprazole (0.5 mg/kg) on relapse responding in animals given 3 days of vehicle pretreatment [VEH/ARI (0.5)]. Panel shows active (AL) and inactive (IL) lever responding (mean ± SEM) for the last 2 days of self-administration and during relapse following 14 days of abstinence. Significant differences relative to vehicle/vehicle (VEH/VEH) treatment are indicated (*p < 0.05)
Discussion
The current results demonstrate that repeated aripiprazole attenuates cocaine-seeking behavior following 2 weeks of forced abstinence and after exposure to cocaine-paired cues or a priming injection of cocaine, stimuli that have been shown to induce craving in humans (Childress et al. 1993; Jaffe et al. 1989) and drug seeking in animals (Cornish et al. 1999; Fuchs et al. 2004; Stewart 2000). In our previous study, acute aripiprazole dose-dependently attenuated conditioned-cued and cocaine-primed reinstatement of cocaine seeking (Feltenstein et al. 2007). The current study importantly shows that tolerance to the effects of aripiprazole does not develop with repeated treatment, in that the degree of reduced responding seen in the current study is comparable to the effects of single treatment at these same doses (Feltenstein et al. 2007). Moreover, repeated administration of aripiprazole is not necessary to reduce relapse responding following forced abstinence, in that an acute dose of 0.5 mg/kg produced a similar reduction in cocaine-seeking behavior. While other DA receptor partial agonists have demonstrated some efficacy in various models of cocaine seeking (Cervo et al. 2003; Gal and Gyertyan 2006; Gilbert et al. 2005; Khroyan et al. 2000; Pilla et al. 1999), these effects do not appear to be specific to drug-seeking behavior. For example, effective doses of the D2 partial agonist, terguride, significantly reduced food-seeking behavior (Platt et al. 2003), while the D3 partial agonist, BP-897, reduced cocaine seeking at doses that also elicited conditioned place aversion (Duarte et al. 2003; Gyertyan and Gal 2003). In contrast, effective doses of aripiprazole in the current study do not affect spontaneous locomotor activity, cocaine self-administration, food self-administration, or reinstatement of food-seeking behavior (Feltenstein et al. 2007). Similar effects have been noted following chronic aripiprazole treatment, in that a 5-day continuous aripiprazole infusion regimen of 0.32 or 0.56 mg/kg/h was found to have no significant effect on food self-administration in rats (Thomsen et al. 2008), suggesting that decreases in cocaine seeking in the current experiment were not due to locomotor inhibitory or aversive properties of the drug. Taken together, these results suggest that aripiprazole may be an effective therapeutic for reducing cocaine craving and preventing relapse in abstinent users.
In contrast to significantly reduced cocaine seeking after repeated aripiprazole treatment, cocaine seeking was unaffected in animals repeatedly given the highest dose of aripiprazole (1.0 mg/kg) but not administered the drug at the time of testing. While it could be argued that a longer pretreatment may be necessary to produce an effect, 7 days of pretreatment at this higher dose failed to affect drug seeking. Taken together, these results suggest that aripiprazole needs to be bioavailable at the time of testing in order to reduce cocaine seeking. As previous research has shown that the half-life of aripiprazole is around 2 h in rats (Shimokawa et al. 2005), sufficient aripiprazole levels were not likely present 1 day after final administration. While aripiprazole has a half-life of 60 h in humans (McGavin and Goa 2002), abstinent users would most likely need to be on a maintenance aripiprazole regimen in order to achieve maximal efficacy for preventing relapse.
Despite both preclinical (Feltenstein et al. 2007; Jerlhag 2008; Li et al. 2009; Schwabe and Koch 2007; Sørensen et al. 2008; Wee et al. 2007) and clinical (Beresford et al. 2005; Brown et al. 2005; Lile et al. 2005; Stoops et al. 2006) reports suggesting that aripiprazole may be an effective pharmacotherapy for the treatment of addiction, several recent studies have indicated possible limitations. For example, a recent study found that while acute aripiprazole administration reduced cocaine self-administration and shifted responding towards an alternate food reinforcer, this effect was not maintained with chronic treatment (Thomsen et al. 2008). Several recent clinical studies have reported that chronic aripiprazole was either without effect or even increased drug use and desire in addicts exposed to acute cocaine or amphetamines (Haney and Spealman 2008; Newton et al. 2008; Stoops et al. 2007; Tiihonen et al. 2007). Interestingly, a number of preclinical (Kleven and Woolverton 1990; Kosten 1997; LeDuc and Mittleman 1993) and clinical (Haney et al. 2001; Romach et al. 1999) studies examining the effectiveness of various DA receptor antagonists for attenuating drug-seeking behaviors have reported a similar loss in acute efficacy with chronic treatment, an effect hypothesized to be due to an enhancement in DA receptor upregulation and/or sensitivity (Creese and Chen 1985; Hess et al. 1986). Given the ability of aripiprazole to act as an antagonist in the presence of increased DA levels, repeated aripiprazole treatment may enhance sensitivity to psychostimulants by senzitization of mesocorticolimbic DA neurons, although no evidence to date has shown such an effect. While this potential effect could be problematic in some individuals, the key feature of aripiprazole of attenuated responsiveness to stimuli that trigger craving during periods of no drug (i.e., abstinence) supports the use of the drug as an antirelapse medication. Furthermore, the efficacy of aripiprazole in comorbid patients (Beresford et al. 2005; Brown et al. 2005) suggests particular utility in targeted subpopulations of addicted individuals.
While aripiprazole possesses affinity for serotonin 5-HT1A (partial agonist) and 5-HT2A (antagonist) receptors (Jordan et al. 2002b; Lawler et al. 1999), the primary mechanism of action for reducing relapse likely involves partial agonist activity at DA D2 receptors (Bowles and Levin 2003; Stahl 2001). Aripiprazole has been shown to inhibit DA neurons in the ventral tegmental area (Momiyama et al. 1996) and the substantia nigra (Matsubayashi et al. 1999), suggesting that the drug alters mesocortical and mesostriatal pathways implicated in relapse to drugs of abuse (Feltenstein and See 2008). Microdialysis studies have demonstrated increases in hippocampal (Li et al. 2004) and prefrontal (Li et al. 2004; Zocchi et al. 2005) DA with doses as low as 0.1 mg/kg, while higher doses reduced DA release in the nucleus accumbens (Li et al. 2004). However, extracellular levels of other monoamines were generally unaffected, in that no changes in 5-HT (Assié et al. 2005; Zocchi et al. 2005) or norepinephrine (Zocchi et al. 2005) were found with similar treatment.
Further evidence for the selectivity of aripiprazole comes from the in vivo receptor occupancy profile of aripiprazole relative to other receptor subtypes (e.g., D1, D3, 5-HT1A, 5-HT2A, and 5-HT2c receptors), whereby aripiprazole demonstrated 10–20 times higher affinity for D2 receptors (Langlois et al. 2005). Despite evidence for 5-HT2A antagonist reduction of cocaine seeking (Filip 2005; Fletcher et al. 2002), 5-HT2A receptor occupancy by aripiprazole does not exceed 50%, even at doses as high as 30 mg/kg, while D2 receptor occupancy shows an ED50 of 0.7 mg/kg (Natesan et al. 2006). It would be premature to rule out a role for other receptor targets of aripiprazole, however, as some of the effects of aripiprazole on DA may involve interactions with 5-HT1A receptors (Bortolozzi et al. 2007), as well as modulation of glutamate activity in the prefrontal cortex (Yang and Wang 2008), a pathway well implicated in relapse (Kalivas et al. 2005).
Similar to evidence indicating efficacy of other partial agonist-based therapeutics for treating opioid (e.g., buprenorphine (Gonzalez et al. 2004; Wilcox and Erikson 2004) and nicotine (e.g., varenicline (Cahill et al. 2008; Tonstad 2007) dependence, an abundance of preclinical (Feltenstein et al. 2007; Jerlhag 2008; Li et al. 2009; Schwabe and Koch 2007; Sørensen et al. 2008; Wee et al. 2007) and clinical (Beresford et al. 2005; Brown et al. 2005; Lile et al. 2005; Stoops et al. 2006) data indicates that DA partial agonist compounds such as aripiprazole may be effective therapeutic agents for preventing relapse in abstinent cocaine users. Despite some evidence of limited efficacy under certain test conditions (Newton et al. 2008; Stoops et al. 2007; Tiihonen et al. 2007), further study of aripiprazole and similar partial receptor agonists is warranted. As compared with other antipsychotic drugs, aripiprazole produces far fewer side effects that often lead to cessation of treatment (Davies et al. 2004; McQuade et al. 2004; Newcomer 2005). Additionally, given the chronic nature of addiction, it is noteworthy that aripiprazole possesses superior safety and tolerability than most drugs with long-term treatment (Kasper et al. 2003). Thus, aripiprazole may offer a viable choice for pharmacotherapy in select populations of addicted individuals, particularly during periods of abstinence from active drug use.
Acknowledgements
This research was supported by NIDA Grant Nos. DA015369 and DA016511 (RES), HD055885 (MWF), and NIH grant C06 RR015455. The authors thank Sara Deptula for technical assistance and Dr. C. Anthony Altar for the generous gift of aripiprazole.
References
- Ariens EJ. Intrinsic activity: partial agonists and partial antagonists. J Cardiovasc Pharmacol. 1983;5:S8–S15. [PubMed] [Google Scholar]
- Assié MB, Ravailhe V, Faucillon V, Newman-Tancredi A. Contrasting contribution of 5-hydroxytryptamine 1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain. J Pharmacol Exp Ther. 2005;315:265–272. doi: 10.1124/jpet.105.087163. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Clapp L, Martin B, Wiberg JL, Alfers J, Beresford HF. Aripiprazole in schizophrenia with cocaine dependence: a pilot study. J Clin Psychopharmacol. 2005;25:363–366. doi: 10.1097/01.jcp.0000169419.38899.5b. [DOI] [PubMed] [Google Scholar]
- Bergman J. Medications for stimulant abuse: agonist-based strategies and preclinical evaluation of the mixed-action D-sub-2 partial agonist aripiprazole (Abilify) Exp Clin Psychopharmacol. 2008;16:475–483. doi: 10.1037/a0014398. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Toth M, Celada P, Artigas F. In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology (Berl) 2007;191:745–758. doi: 10.1007/s00213-007-0698-y. [DOI] [PubMed] [Google Scholar]
- Bowles TM, Levin GM. Aripiprazole: a new atypical antipsychotic drug. Ann Pharmacother. 2003;37:687–694. doi: 10.1345/aph.1C297. [DOI] [PubMed] [Google Scholar]
- Brown ES, Jeffress J, Liggin JD, Garza M, Beard L. Switching outpatients with bipolar or schizoaffective disorders and substance abuse from their current antipsychotic to aripiprazole. J Clin Psychiatry. 2005;66:756–760. doi: 10.4088/jcp.v66n0613. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006103.pub2. CD006103. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Childress AR, O’Brien CP. Dopamine receptor partial agonists could address the duality of cocaine craving. Trends Pharmacol Sci. 2000;21:6–9. doi: 10.1016/s0165-6147(99)01422-4. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Creese I, Chen A. Selective D-1 dopamine receptor increase following chronic treatment with SCH 23390. Eur J Pharmacol. 1985;109:127–128. doi: 10.1016/0014-2999(85)90549-7. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Davies MA, Sheffler DJ, Roth BL. Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004;10:317–336. doi: 10.1111/j.1527-3458.2004.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26:649–666. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2007;61:582–590. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep. 2005;57:35–46. [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT (2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Expert Opin Pharmacother. 2004;5:713–725. doi: 10.1517/14656566.5.4.713. [DOI] [PubMed] [Google Scholar]
- Grunder G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60:974–977. doi: 10.1001/archpsyc.60.10.974. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Gal K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. NeuroReport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Albers LJ, Le H, Creese I. Effects of chronic SCH23390 treatment on the biochemical and behavioral properties of D1 and D2 dopamine receptors: potentiated behavioral responses to a D2 dopamine agonist after selective D1 dopamine receptor upregulation. J Pharmacol Exp Ther. 1986;238:846–854. [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Di Nicola M. Aripiprazole for relapse prevention and craving in alcohol-dependent subjects: results from a pilot study. J Clin Psychopharmacol. 2007;27:519–520. doi: 10.1097/JCP.0b013e318150c841. [DOI] [PubMed] [Google Scholar]
- Jerlhag E. The antipsychotic aripiprazole antagonizes the ethanol- and amphetamine-induced locomotor stimulation in mice. Alcohol. 2008;42:123–127. doi: 10.1016/j.alcohol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Jordan S, Chen R, Johnson J, Regardie K, Tadori Y, Kikuchi T. Aripiprazole is a potent, partial agonist at cloned human D2L and native rat 5-HT1A receptors. Eur Neuropsychopharmacol. 2002a;12:S293. [Google Scholar]
- Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002b;441:137–140. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63:763–771. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]
- Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, Archibald D, Ingenito G, Marcus R, Pigott T. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychoharmacol. 2003;6:325–337. doi: 10.1017/S1461145703003651. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, Ingenito G. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160:1651–1658. doi: 10.1176/appi.ajp.160.9.1651. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine- seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of continuous infusions of SCH 23390 on cocaine- or food-maintained behavior in rhesus monkeys. Behav Pharmacol. 1990;1:365–373. doi: 10.1097/00008877-199000140-00010. [DOI] [PubMed] [Google Scholar]
- Kosten TA. Enhanced neurobehavioral effects of cocaine with chronic neuroleptic exposure in rats. Schizophr Bull. 1997;23:203–213. doi: 10.1093/schbul/23.2.203. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin Investig Drugs. 2002;11:491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- Langlois X, te Riele P, Ashton D. In vivo receptor occupancy profile of aripiprazole in rat brain. Society for Neuroscience Abstracts. 2005;444:3. [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- LeDuc PA, Mittleman G. Interactions between chronic haloperidol treatment and cocaine in rats: an animal model of intermittent cocaine use in neuroleptic treated populations. Psychopharmacology (Berl) 1993;110:427–436. doi: 10.1007/BF02244649. [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Dai J, Meltzer HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Li SX, Zou Y, Liu LJ, Wu P, Lu L. Aripiprazole blocks reinstatement but not expression of morphine conditioned place preference in rats. Pharmacol Biochem Behav. 2009;92:370–375. doi: 10.1016/j.pbb.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18:251–267. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of D-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun HQ, Bao YP, Li SX, Beveridge TJ, Di XL, Yang FD, Lu L. Subjective, cognitive/psychomotor, and physiological effects of aripiprazole in Chinese light and heavy smokers. Drug Alcohol Depend. 2009;101:42–52. doi: 10.1016/j.drugalcdep.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164:1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- Matsubayashi H, Amano T, Sasa M. Inhibition by aripiprazole of dopaminergic inputs to striatal neurons from substantia nigra. Psychopharmacology (Berl) 1999;146:139–143. doi: 10.1007/s002130051099. [DOI] [PubMed] [Google Scholar]
- McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16:779–786. doi: 10.2165/00023210-200216110-00008. discussion 787–778. [DOI] [PubMed] [Google Scholar]
- McQuade RD, Stock E, Marcus R, Jody D, Gharbia NA, Vanveggel S, Archibald D, Carson WH. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry. 2004;65 Suppl 18:47–56. [PubMed] [Google Scholar]
- Momiyama T, Amano T, Todo N, Sasa M. Inhibition by a putative antipsychotic quinolinone derivative (OPC-14597) of dopaminergic neurons in the ventral tegmental area. Eur J Pharmacol. 1996;310:1–8. doi: 10.1016/0014-2999(96)00350-0. [DOI] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Nobrega JN, Fletcher PJ, Kapur S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31:1854–1863. doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19 Suppl 1:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- Newton TF, Reid MS, De La Garza R, Mahoney JJ, Abad A, Condos R, Palamar J, Halkitis PN, Mojisak J, Anderson A, Li SH, Elkashef A. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2008;11:1037–1045. doi: 10.1017/S1461145708009097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rodefer JS, Rowlett JK, Spealman RD. Suppression of cocaine- and food-maintained behavior by the D2-like receptor partial agonist terguride in squirrel monkeys. Psychopharmacology (Berl) 2003;166:298–305. doi: 10.1007/s00213-002-1347-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive- sensitization view. Addiction. 2000;95 Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56:1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. An analysis of drug-seeking behavior in animals. Neurosci Biobehav Rev. 1981;5:315–323. doi: 10.1016/0149-7634(81)90026-9. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Koch M. Effects of aripiprazole on operant responding for a natural reward after psychostimulant withdrawal in rats. Psychopharmacology (Berl) 2007;191:759–765. doi: 10.1007/s00213-006-0520-2. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shimokawa Y, Akiyama H, Kashiyama E, Koga T, Miyamoto G. High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: application to the pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;821:8–14. doi: 10.1016/j.jchromb.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Sloboda Z. Changing patterns of "drug abuse" in the United States: connecting findings from macro- and microepidemiologic studies. Subst Use Misuse. 2002;37:1229–1251. doi: 10.1081/ja-120004181. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Sager TN, Petersen JH, Brennum LT, Thogersen P, Hee Bengtsen C, Thomsen M, Wortwein G, Fink-Jensen A, Woldbye DP. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology (Berl) 2008;199:37–46. doi: 10.1007/s00213-008-1069-z. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry. 2001;62:923–924. doi: 10.4088/jcp.v62n1201. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Stoops W, Lile J, Glaser P, Rush C. A low dose of aripiprazole attenuates the subject-rated effects of D-amphetamine. Drug Alcohol Depend. 2006;84:206–209. doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Lofwall MR, Rush CR. The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. Am J Drug Alcohol Abuse. 2007;33:769–776. doi: 10.1080/00952990701651556. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DP, Wortwein G, Sager TN, Holm R, Pepe LM, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology (Berl) 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–160. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Tonstad S. Varenicline for smoking cessation. Expert Rev Neurother. 2007;7:121–127. doi: 10.1586/14737175.7.2.121. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton W, Pulvirenti L, Koob G. Effects of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wilcox RE, Erikson CK. Prevention of relapse to addiction: information for the practitioner. Tex Med. 2004;100:52–61. [PubMed] [Google Scholar]
- Yang TT, Wang SJ. Aripiprazole and its human metabolite OPC14857 reduce, through a presynaptic mechanism, glutamate release in rat prefrontal cortex: possible relevance to neuroprotective interventions in schizophrenia. Synapse. 2008;62:804–818. doi: 10.1002/syn.20548. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27:248–259. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett. 2005;387:157–161. doi: 10.1016/j.neulet.2005.06.035. [DOI] [PubMed] [Google Scholar]