Abstract
A balance between angiogenesis inducers and inhibitors in the microenvironment controls the rate of new blood vessel formation. We hypothesized that fibroblasts, an important cellular constituent of the tissue stroma, secrete molecules that contribute to this balance. We further hypothesized that fibroblasts secrete molecules that promote angiogenesis when they are in a proliferative state and molecules that inhibit angiogenesis when they are not actively cycling (quiescent). Microarray analysis revealed that angiogenesis inducers and inhibitors are regulated as fibroblasts transition into a quiescent state and re-enter the cell cycle in response to changes in serum. To assess whether changes in transcript levels result in changes in the levels of secreted proteins, we collected conditioned medium from proliferating and quiescent fibroblasts and performed immunoblotting for selected proteins. Secreted protein levels of the angiogenesis inhibitor PEDF were higher in quiescent than proliferating fibroblasts. Conversely, proliferating fibroblasts secreted increased levels of the angiogenesis inducer VEGF-C. For the angiogenesis inhibitor thrombospondin-2, quiescent cells secreted a prominent 160 kDa form in addition to the 200 kDa form secreted by proliferating and restimulated fibroblasts. Using immunohistochemistry we discovered that fibroblasts surround blood vessels and that the angiogenesis inhibitor PEDF is expressed by quiescent fibroblasts in uterine tissue, supporting a role for PEDF in maintaining quiescence of the vasculature. This work takes a new approach to the study of angiogenesis by examining the expression of multiple angiogenesis genes secreted from a key stromal cell, the fibroblast.
Keywords: proliferation, quiescence, angiogenesis, fibroblasts, pigment epithelium derived factors, vascular endothelial growth factor C, thrombospondin-2
Introduction
Homeostasis, a state of equilibrium, is essential to the vitality and proper functioning of biological organisms. Following development, adult organisms must maintain the same size and cellular composition for the remainder of adulthood. A small but continuous decrease or increase in size would be incompatible with our long lifetimes. Achieving the goal of size constancy is deceptively complicated. The cells within a tissue must act in a coordinated fashion to produce new cells in order to replace cells that are damaged or lost from age. Yet, this regrowth must be limited to that required to maintain tissues of a constant size. The mechanisms by which tissues maintain size homeostasis, and the mechanisms by which different cell types within the body coordinate with each other to maintain homeostasis, are poorly understood.
A lack of size homeostasis can result from endocrine abnormalities including overexpression of growth hormone 1 or increased signaling of the insulin pathway, 2 or genetic manipulation, for instance, deletion of the cell cycle regulator p27.3 Organ size is also likely to be controlled by the vasculature.4,5 In order to receive adequate oxygen and nutrients, cells must be within 150 - 200 μm of vessel capillaries.6 Thus, any increase in body size must of necessity be associated with an increase in vasculature to properly oxygenate the new tissue. Indeed, tissue growth and remodeling associated with wound healing, exercise, reproduction and embryonic development all have an angiogenic component.7 A failure to control angiogenesis can contribute to hyperproliferation of tissues, disorganized tissue structure, and tumorigenesis. In one recent study, administration of the pro-angiogenic growth factor basic fibroblast growth factor (bFGF) to regenerating liver after partial hepatectomy resulted in increased endothelial cell proliferation and faster tissue regeneration, which suggests that new blood vessel growth is rate-limiting for tissue regeneration in adult mice.5
Under non-pathological conditions, angiogenesis is subject to tight regulation by soluble and matrix factors, cell-to-cell signaling, and the metabolic needs of the tissue. The most prevalent model proposes that angiogenesis is regulated by a balance of pro- and anti-angiogenesis signaling molecules.8 Angiogenesis inducers, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and platelet derived growth factor (PDGF), promote vessel sprouting through stimulation of matrix protease production, inhibition of endothelial cell apoptosis, and increased endothelial cell mobility.9–11 Angiogenesis inhibitors counteract the activity of angiogenesis inducers and keep new blood vessel growth in check.12 The mechanisms of action of angiogenesis inhibitors include interfering with signal transduction stimulated by angiogenesis inducers, inhibiting the proteases that generate inducers, inhibiting endothelial cell proliferation, migration, and tubule formation,13 and inducing endothelial apoptosis through stimulation of the CD36 receptor and Fas-L signaling.14,15 While a balance between pro- and anti-angiogenic molecules is hypothesized to be important for the maintenance of tissues and the regulated growth needed to regenerate damaged tissue, the molecular basis for the angiogenic balance remains unclear. For instance, of the many identified molecules, which ones are the most important for keeping vasculature quiescent or for stimulating its mobilization?
We focus here on the role of the fibroblast in regulating angiogenesis. The predominant state of fibroblasts in healthy adult tissue is a state of quiescence characterized by a lack of cell division, but the capacity to reenter the cell cycle upon stimulation by the correct signal. In response to such a signal, for example, a wounding event, fibroblasts can enter a proliferating state in which they progress through the cell cycle and generate more fibroblasts.16 When cultured fibroblasts transition from a quiescent to proliferative state in response to serum, there is a rapid upregulation of genes involved in tissue repair, including angiogenesis genes.17 We discovered that when human diploid fibroblasts are made quiescent by one of multiple different signals, transcript levels of a small number of angiogenesis inhibitors are upregulated relative to proliferating fibroblasts.18 This suggests a model in which fibroblasts secrete regulators of angiogenesis and thus help to coordinate the controlled development of new vasculature under growth conditions, and the inhibition of blood vessel growth in quiescent tissues. In unwounded adult tissue, quiescent fibroblasts may aid in the prevention of tissue growth by limiting angiogenesis, thus restricting the capacity of parenchymal cells to proliferate. We examine these hypotheses here.
Results
Angiogenesis inducers and inhibitors are regulated at the transcript level during the transition between fibroblast proliferation and quiescence
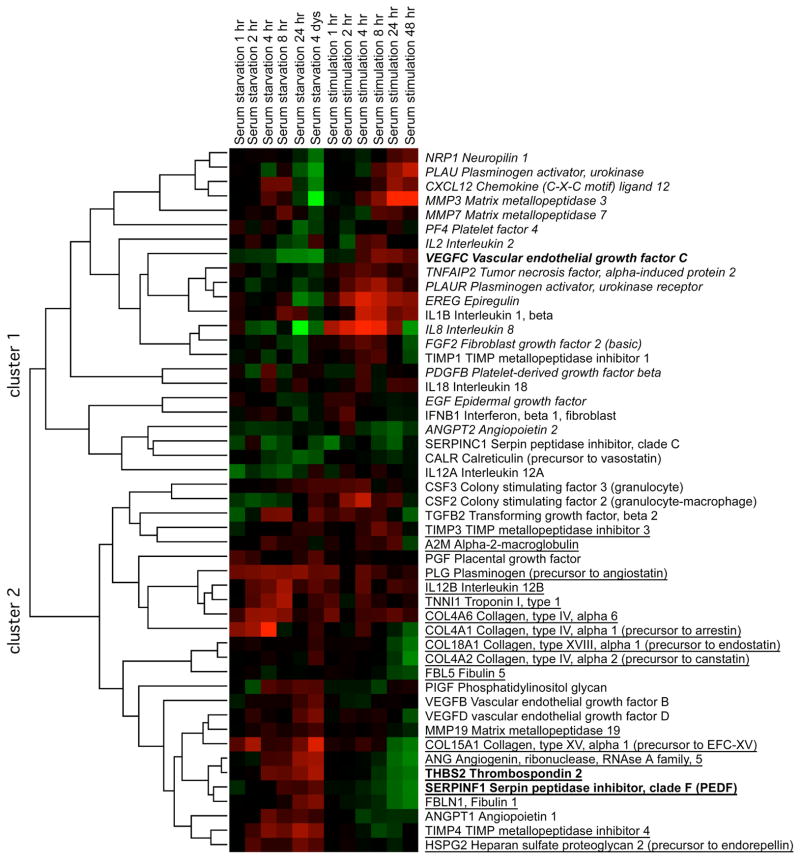
Our microarray data suggested that angiogenesis inhibitors and inducers may be differentially expressed in proliferating compared with quiescent fibroblasts.18 To test this hypothesis, we performed a detailed time-course microarray analysis of gene expression changes as fibroblasts become quiescent as a result of serum starvation and then reenter the cell cycle. We used a serum withdrawal and readdition protocol because of the physiological importance of serum for indicating the presence of a wound, and thus, triggering a proliferative response (Fig. 1). Hierarchical clustering of the angiogenesis regulators produced two main clusters that roughly separated inhibitors and inducers. The genes in cluster 1 generally decreased in expression in quiescent cells (Fig. 1, columns 1–6) and increased in expression in restimulated cells (Fig. 1, columns 7–12). Genes within this cluster are largely angiogenesis inducers (16/23 or 70%), including VEGF-C, and are indicated in italics in Fig. 1. The genes within cluster 2 generally increased in expression in quiescent cells and decreased in expression in proliferating cells. These genes are mostly angiogenesis inhibitors (18/26 or 70%), including pigment epithelium derived factor (PEDF) and thromobospondin-2. The angiogenesis inhibitors are underlined in Fig. 1. Thus, genome-wide gene expression changes support a model in which proliferating fibroblasts express multiple genes that contribue to a pro-angiogenic environment,17 while quiescent fibroblasts express higher amounts of genes that encode anti-angiogenesis factors and thus help to restrain angiogenesis in quiescent tissue.18
FIGURE 1.
Transcript levels of angiogenesis factors correlate with the proliferative state of fibroblasts
Fibroblasts growing in 5% serum were switched to 0.1% serum, sampled for microarray analysis over a time course for four days, then restimulated with 5% serum and sampled for microarrays. During the serum withdrawal time course (columns 1–6), RNA from the proliferating cells cultured in 5% serum was used as a comparison for measuring transcript levels in serum-starved cells sampled at different timepoints. The ratio of transcript levels in serum-starved to proliferating samples for each gene in each sample is indicated in color. Red indicates upregulation in serum-starved compared with proliferating cells; green indicates downregulation. For the serum restimulation time course (column 7–12), four-day serum-starved quiescent cells served as controls for comparison with stimulated cells. Red indicates elevated expression in serum-stimulated cells compared with quiescent cells; green indicates downregulation. Angiogenesis factors were identified based on published lists,70 and were filtered to include proteins for which expression changed more than 1.5-fold at a time point. The filtered list was hierarchically clustered by average linkage using Cluster 3.0 and visualized using Java Treeview. Cluster 1 contains genes downregulated with quiescence and upregulated with serum stimulation. Genes in cluster 1 are enriched for angiogenesis inducers, which are indicated in italics. Genes in cluster 2 are upregulated with quiescence and downregulated with serum stimulation. Genes in cluster 2 are enriched in angiogenesis inhibitors, which are indicated by underlining. The three genes chosen for further analysis of protein level changes are in indicated in bold.
Proteins selected for quantitative immunoblot analysis
To further test our hypothesis that fibroblasts contribute to the regulation of vasculature, we monitored the levels of three selected proteins (VEGF-C, PEDF and thrombospondin-2, indicated in bold in Fig. 1) secreted by proliferating and quiescent fibroblasts. VEGF-C, a member of the vascular endothelial growth factor family of angiogenesis inducers, is a potent inducer of blood vessels and lymphatic vessels.19–21 PEDF, a member of the serpin family of serine protease inhibitors, is one of the most potent natural inhibitors of physiological and pathological ocular vascularization.22 PEDF knock-out mice have increased numbers of blood vessels in the kidney, prostrate and pancreas.23 Previous studies have also reported increased PEDF transcript and protein level expression in quiescent as opposed to proliferating, senescent or transformed fibroblasts.24,25 Thrombospondin-2 (TSP-2) is a member of the thrombospondin family of extracellular matrix proteins that regulate cellular migration and proliferation in the context of tissue remodeling and angiogenesis.26 TSP-2 is highly expressed in the connective tissue of mice, including skin fibroblasts27 and TSP-2 knockout mice exhibit increased vascular density at the site of healed wounds.28
A model system for monitoring the levels of angiogenesis regulators in conditioned medium
To determine whether the changes in transcript abundance between proliferating and quiescent cells detected by microarray translate into changes in the levels of protein secreted into the extracellular environment, we designed an in vitro system to collect conditioned medium from proliferating, quiescent, and restimulated human dermal fibroblasts. To model a proliferating state, fibroblasts were plated at low density in the presence of mitogens to ensure that they were actively dividing. To model a quiescent state, fibroblasts were plated densely to induce quiescence by contact inhibition in the absence of a mitogenic stimulus. To generate restimulated samples, fibroblasts maintained in a quiescent state for 30 days were replated sparsely and stimulated with mitogens to induce them to re-enter the cell cycle. These conditions allowed us to assess changes in the levels of secreted proteins during transitions both from proliferation to quiescence and from quiescence to proliferation. In establishing such a system, we needed to control for the differences inherent to the three conditions, in particular, the presence of serum, changes in cell mass, and differences in cell division rates.
Serum concentrations sufficient to induce proliferation inhibited our ability to transfer protein to PVDF membranes for immunoblot analysis. We therefore maintained both proliferating and quiescent cells in basal medium containing insulin to promote survival, and added either no serum or 0.1% serum. To specifically induce fibroblasts to proliferate or exit the cell cycle, we relied on the potent mitogen platelet-derived growth factor (PDGF). Proliferating cells were plated sparsely in the presence of PDGF to stimulate proliferation. Quiescent cells were plated densely to achieve a contact inhibition-mediated cell cycle exit and were maintained in the same medium conditions as the proliferating cells, with either no serum or 0.1% serum, but in the absence of PDGF. This resulted in four conditions for analysis at four days: proliferating in no serum, proliferating in 0.1% serum, quiescent in no serum and quiescent in 0.1% serum. To monitor changes when quiescent cells were restimulated, quiescent cells in no serum and quiescent cells in 0.1% serum were replated sparsely and stimulated to proliferate in 0.1% serum and PDGF.
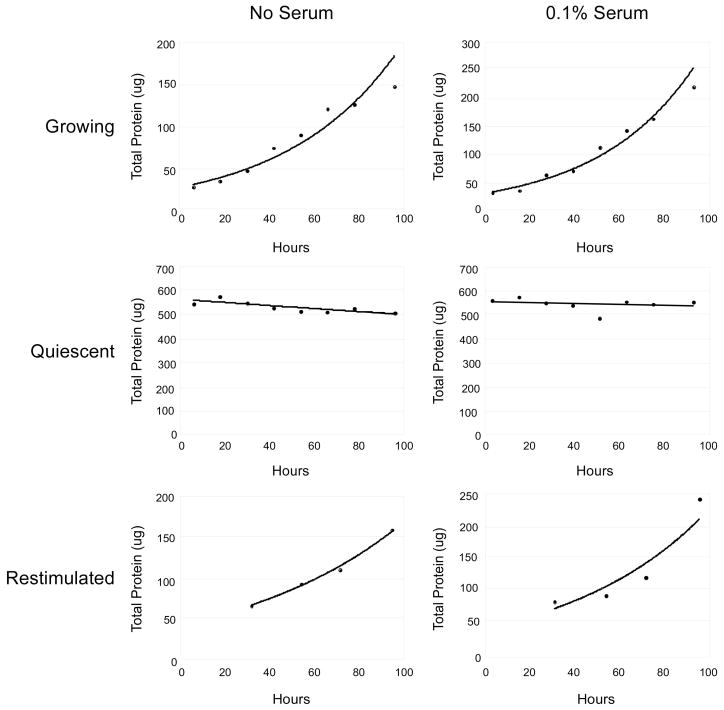
To accurately compare angiogenesis factor secretion by low-density proliferating cultures and high-density quiescent cultures, the volumes of proliferating and quiescent samples were normalized prior to precipitation and immunoblot analysis. We monitored the amount of cellular protein present over a timecourse during the conditioning of the medium and plotted the amount of protein present over time (Fig. 2). We normalized media volumes by an integral representing the total cellular mass present in a tissue culture plate during the four days of conditioning. The integral value was determined by plotting the protein content over the time of conditioning and using regression analysis to define the best fitting curve. Integral equations are given in Supplementary Table 1. We used cellular mass rather than cell number because the transition from a proliferating to quiescent state results in a decrease in cellular mass (A. Legesse-Miller, unpublished data). This method allows us to monitor the levels of the proteins of interest in comparison to the total amount of protein in the cell. By taking the integral of cellular mass over the four days of conditioning, we incorporate the fact that the number of cells and cellular mass on proliferating plates and restimulated plates changed significantly during this time, while the mass was essentially constant for quiescent cells.
FIGURE 2.
Protein concentration as a function of time in proliferating, quiescent and restimulated cells
Proliferating, quiescent, and restimulated cells were plated as described in Materials and Methods either in the absence of serum or in the presence of 0.1% serum. Protein content was determined for one plate at each of the indicated timepoints after plating. Regression curves that best fit the data are shown, and the equations are given in Supplementary Table 1. Proliferating and restimulated cells were modeled on the assumption of exponential growth while quiescent cultures were constant (0.1% serum) or decreased in protein content slightly over time (no serum). The integrated areas under these curves were used for normalization of the volume of conditioned medium for analysis and are given in Supplementary Table 1.
Localization of angiogenesis regulators in conditioned medium
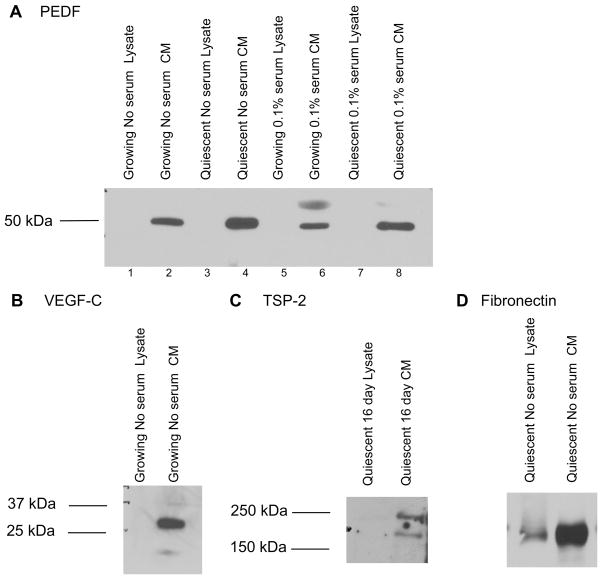
Using this model for conditioned medium, we first assessed whether the selected molecules are found in the cellular lysate, which contains cellular material and the attached extracellular matrix, or are secreted into the conditioned medium. The processed forms of all three molecules —PEDF, VEGF-C and thrombospondin-2— were observed in the conditioned medium and not in the cellular/extracellular matrix portion (Fig. 3A, B and C). As a control, we ensured the extracellular matrix protein fibronectin was present in both the cellular/extracellular matrix and the conditioned medium as expected (Fig. 3D). Secretion of these angiogenesis regulators would allow them to diffuse to nearby endothelial cells. As secreted proteins, these factors may affect the ability of nearby endothelial cells to migrate and form blood vessels.
FIGURE 3.
Angiogenesis regulators are secreted into the conditioned medium
A. Conditioned medium and cellular lysates were collected from proliferating and quiescent fibroblasts maintained in no serum or 0.1% serum for four days. Conditioned medium samples were normalized based on the integral method. Precipitated conditioned medium or 22 μg of protein lysates collected from the same cultures were separated on SDS-PAGE gels and immunoblotted for PEDF. PEDF was detected in the conditioned medium but not the lysate under all tested conditions. A background band at about 75 kDa was consistently observed in samples with serum.
B. Conditioned medium and cellular lysates were collected and separated as in A. Membranes were immunoblotted with an anti-VEGF-C antibody. Processed VEGF-C was observed in conditioned medium but not cellular lysates. In some samples, preprocessed VEGF-C of approximately 58 kDa was detected in the cellular lysate (not shown).
C. Medium conditioned for four days and cellular lysates were collected from cells maintained in quiescence in the absence of serum for 16 days. Precipitated conditioned medium and lysates were separated and immunoblotted with an anti-thrombospondin-2 antibody raised in goats. TSP-2 was detected in conditioned medium but not cellular lysates.
D. Conditioned medium and lysates were collected from quiescent cells grown in the absence of serum. Samples were separated and immunoblotted with an anti-fibronectin antibody. Fibronectin was detected in both the conditioned medium and cellular lysates as expected for an extracellular matrix protein.
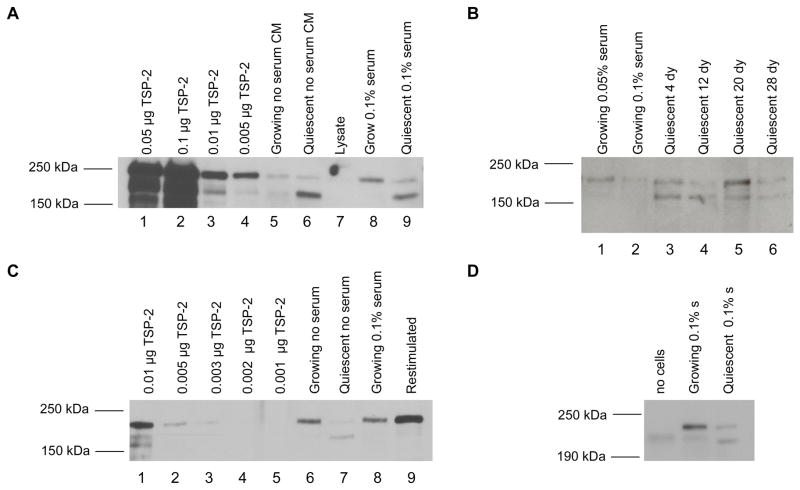
Protein levels of the angiogenesis inhibitor PEDF are upregulated in quiescent conditioned medium
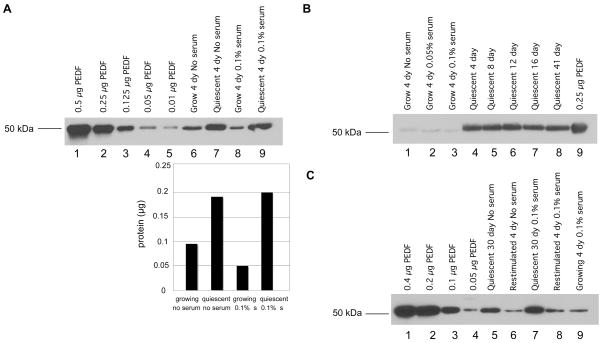
We then determined the levels of secreted PEDF in conditioned medium from proliferating and quiescent fibroblasts (Fig. 4A). Using the integral method for normalization, we precipitated proliferating and quiescent conditioned medium from cells cultured in no serum or 0.1% serum and compared protein levels to a standard curve of different amounts of recombinant PEDF. Comparing lanes 6 and 7 of Fig. 4A illustrates that increased levels of PEDF were secreted from quiescent as opposed to proliferating cells under conditions of no serum. Lanes 8 and 9 indicate that PEDF is secreted at higher levels from quiescent than proliferating cells under conditions of 0.1% serum. Based on comparison with the standard curve, the ratio of PEDF in quiescent versus proliferating cells was estimated as 2 (0.18 μg for quiescent/ 0.09 μg for proliferating) in the absence of serum, and 4 (0.2 μg for quiescent/ 0.05 μg for proliferating) in the presence of 0.1% serum. These results are consistent with the elevated PEDF expression levels observed at the transcript level in cells made quiescent by serum withdrawal. They are also consistent with previous reports of higher PEDF expression in quiescent cells.24,25 To assess whether PEDF continues to be expressed at high levels as cells are maintained in a quiescent state for longer periods of time (Fig. 4B), medium was conditioned in cells maintained in quiescence for as long as 41 days. PEDF levels continued to be elevated in these cells. These results are consistent with a potential role for PEDF secreted by quiescent fibroblasts in adult tissue in maintaining a quiescent vasculature.
FIGURE 4.
Quiescent fibroblasts secrete increased levels of the angiogenesis inhibitor PEDF
A. Medium was conditioned for 4 days with proliferating or quiescent fibroblasts with no serum or 0.1% serum. Conditioned medium was normalized based on the integral method. Samples and recombinant protein were separated on SDS-PAGE gels and analyzed by immunoblotting for PEDF levels. The graph below indicates band intensity in the proliferating and quiescent lanes based on analysis with the ImageJ software. In no serum or 0.1% serum, quiescent fibroblasts secreted higher levels of PEDF than proliferating fibroblasts.
B. PEDF is elevated in fibroblasts maintained in quiescence up to 41 days. Conditioned medium was collected from cells growing in no serum, 0.05% serum, or 0.1% serum. Conditioned medium was also collected from cells that had been quiescent with no serum for 4, 8, 12, 16 or 41 days. Medium was conditioned for four days for each sample. In this experiment, volumes of precipitated media were normalized by the protein content ratio of 16-day quiescent cultures versus 4-day growing cultures. Medium was precipitated, separated and immunoblotted for PEDF as in A.
C. PEDF levels decrease when quiescent cells are stimulated to reenter the cell cycle. Cells maintained in a quiescent state by contact inhibition in the absence of serum for 30 days were replated sparsely and stimulated to reenter the cell cycle by addition of PDGF. Medium conditioned in quiescent cells or restimulated cells for four days was collected and normalized by the integral method. Conditioned medium from quiescent and restimulated cells was precipitated, separated by SDS-PAGE, and analyzed by immunoblotting as in Fig. 4A.
To determine whether the upregulation of PEDF that occurs when proliferating cells enter quiescence is reversible, fibroblasts maintained in a quiescent state for 30 days were then stimulated to reenter the cell cycle. As shown in Fig. 4C lanes 5–9, PEDF levels decrease to the levels in proliferating cells when quiescent cells are stimulated to reenter the cell cycle. We conclude that the increased transcript levels of PEDF in quiescent cells results in higher secreted levels of the angiogenesis inhibitor PEDF. Secreted PEDF protein levels are upregulated when proliferating cells enter quiescence, are maintained at a high level during quiescence, and are downregulated when quiescent cells reenter the cell cycle.
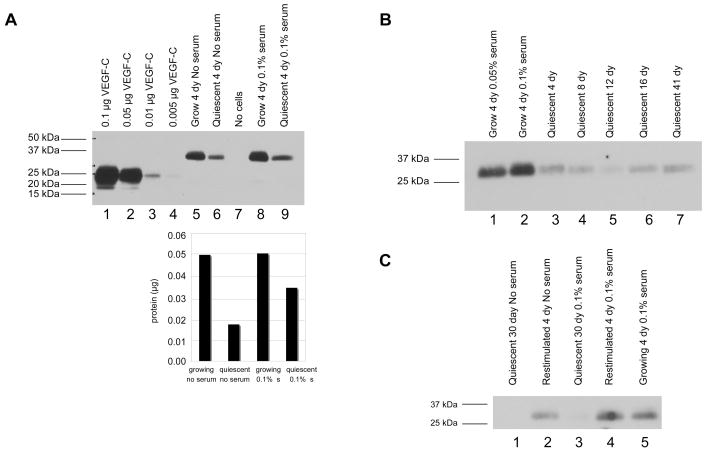
Angiogenesis inducer VEGF-C is secreted at higher levels by proliferating fibroblasts
We also monitored the levels of the pro-angiogenic regulator VEGF-C in the proliferating and quiescent fibroblasts. Conditioned medium was collected from proliferating and quiescent fibroblasts in no serum and in 0.1% serum, and from medium with serum that had not been exposed to cells. As shown in Fig. 5A, VEGF-C from conditioned medium migrated as a 31 kDa band while the recombinant protein migrated as a 21 kDa band. The 21 kDa band is expected to be the fully processed form of the protein while the 31 kDa band is likely a propeptide, as VEGF-C is proteolytically cleaved in order to achieve its final processed form.29 Levels of the VEGF-C 31 kDa propeptide were higher in conditioned medium collected from proliferating fibroblasts than conditioned medium from quiescent fibroblasts (Fig. 5A). Proliferating cells secreted more VEGF-C than quiescent cells whether the cells were cultured in no serum or in 0.1% serum. For cells maintained with no serum, the ratio of proliferating to quiescent protein levels was 2.9 (0.049 μg for proliferating/0.017 μg for quiescent) (lanes 5 and 6 of Fig. 5A). In conditioned medium from cells cultured in 0.1% serum, we observed not only the 31 kDa preprocessed form of VEGF-C, but also a small amount of the fully processed 21 kDa form (lane 8 of Fig. 5A) and the ratio of the VEGF-C levels in proliferating to quiescent conditioned medium was approximately 1.5. VEGF-C was not observed in the same volume of medium with 0.1% serum that had never been exposed to cells (lane 7 of Fig. 5A).
FIGURE 5.
Proliferating fibroblasts secrete increased levels of the angiogenesis inducer VEGF-C
A. Samples of proliferating and quiescent conditioned medium, and medium that was not incubated with cells, were prepared as in Fig. 4A. Volumes were normalized according to the integral method. Medium and recombinant proteins were separated on SDS-PAGE gels and analyzed by immunoblotting for VEGF-C levels. Recombinant protein migrated as the fully processed 21 kDa form while the predominant species in the conditioned medium was an approximately 31 kDa partially processed form. For conditioned medium from cells growing in 0.1% serum, both preprocessed and fully processed forms of VEGF-C were detected. The amount of VEGF-C in each sample was determined by comparison with the standard curve and is indicated in the graph below.
B. VEGF-C levels are reduced in cells maintained in quiescence for a long period of time. Conditioned medium was collected and precipitated from samples as in Fig. 4B. Medium was precipitated, separated and immunoblotted for VEGF-C as in Fig. 5A.
C. VEGF-C levels increased in conditioned medium from quiescent cells induced to reenter the cell cycle. Conditioned medium was collected from proliferating cells, quiescent cells and cells maintained in a quiescent state for 30 days and then stimulated to reenter the cell cycle. Samples were normalized according to the integral method. Precipitated conditioned medium and recombinant protein were separated and immunoblotted with an anti-VEGF-C antibody.
Cells maintained in quiescence for up to 41 days secreted low amounts of VEGF-C, confirming that VEGF-C levels are reduced when cells become quiescent and remain low during quiescence (Fig. 5B). When cells maintained in quiescence for 30 days were stimulated to re-enter the cell cycle, VEGF-C levels were induced in the restimulated cells (Fig. 5C) to a level similar to that observed in proliferating cells. These findings demonstrate that the VEGF-C levels are high in proliferating cells, are downregulated in quiescent cells, are maintained at a low level for the duration of quiescence, and are induced when quiescent cells reenter the cell cycle. We conclude that VEGF-C is secreted by actively proliferating fibroblasts and downregulated when fibroblasts enter quiescence, suggesting that it may be an important mediator of fibroblast-mediated angiogenic and/or lymphangiogenic induction under growth or repair conditions.
Proliferating and quiescent cells secrete different forms of thrombospondin-2
When we analyzed the conditioned medium from proliferating and quiescent cells for the levels of TSP-2, we discovered that the most prominent form of TSP-2 in conditioned medium from proliferating cells has an apparent molecular weight of ~200 kDa (Fig. 6A). Although TSP-2’s primary sequence predicts a mass of 130 kDa under reducing conditions, previous studies have shown that TSP-2 often migrates at a molecular weight of 180–200 kDa.30,31 The difference in TSP-2’s predicted molecular weight and its apparent weight under reducing conditions may partially result from glycosylation of thrombospondin-2.32,33 In medium conditioned by quiescent cells, TSP-2 migrated as two bands, a ~200 kDa band and another ~160 kDa band (Fig. 6A).
FIGURE 6.
Quiescent and proliferating fibroblasts secrete different forms of thrombospondin-2
A. TSP-2 migrates differently in conditioned medium from proliferating and quiescent fibroblasts. Conditioned medium was collected from proliferating and quiescent cells and normalized according to the integral method. Conditioned media samples were precipitated, separated on SDS-PAGE gels and immunoblotted with an anti-thrombospondin-2 antibody raised in goats. The 160 kDa band was prominent in conditioned medium from quiescent cells in no serum or 0.1% serum, but not in conditioned medium from proliferating cells.
B. Long-term quiescent cells continue to express the 160 kDa TSP-2 band. Conditioned medium was collected from fibroblasts growing in 0.05% serum or 0.1% serum, or from fibroblasts maintained in a quiescent state with no serum for 4, 12, 20 or 28 days. A mouse monoclonal anti-TSP2 antibody was used for immunoblotting. The quiescence-specific 160 kDa band was present in samples maintained in quiescence for up to 28 days. Samples in this blot were normalized based on the amount of protein present at the time of collection.
C. Expression of the 160 kDa band is reduced when quiescent fibroblasts are restimulated to enter the cell cycle. Conditioned medium was precipitated from fibroblasts in proliferating, quiescent and restimulated conditions according to the integral method. Samples and indicated amounts of recombinant protein were separated on SDS-PAGE gels and immunoblotted with a goat anti-thrombospondin-2 antibody.
D. Medium that had not been conditioned with cells and medium conditioned in proliferating or quiescent cells was normalized, precipitated and separated on an SDS-PAGE gel. Membranes were immunoblotted with an antibody raised against the N-terminal 40 amino acids of thrombospondin-2. The band pattern observed with the N-terminal antibody is similar to the pattern observed with antibodies against the full-length form. The 160 kDa band is therefore unlikely to reflect proteolytic processing of the TSP-2 N-terminal end.
The presence of both the 200 kDa and 160 kDa bands in glycosylated, recombinant TSP-2, which was expressed in a mouse myeloma cell line (Fig. 6A lane 3), suggests that the bands detected in our samples are in fact TSP-2. To ensure that the shorter form was specifically secreted by quiescent fibroblasts, we probed with two different antibodies, a goat polyclonal antibody (Fig. 6A) and a mouse monoclonal (Fig. 6B), both of which were raised against the full-length TSP-2 protein. Both antibodies detected primarily a 200 kDa band in conditioned medium from proliferating samples, and both a 200 kDa and a 160 kDa band in conditioned medium from quiescent samples. When cells were maintained in quiescence for as long as 28 days, the 160 kDa band continued to be present (Fig. 6B). When quiescent cells were restimulated to enter the cell cycle, the 200 kDa band was once again the predominant band (Fig. 6C). We conclude that the relative amount of the 200 kDa and 160 kDa bands is altered when cells transition between a proliferative and quiescent state, and the 160 kDa form is secreted in higher amounts by quiescent cells.
To define further the molecular basis for the differences in TSP-2 migration, we tested whether the two forms of TSP-2 might reflect quiescence-specific proteolytic cleavage of TSP-2. The ADAMTS1 metalloproteinase has been shown to cleave the N-terminus of TSP-2 to generate a 42 kDa peptide.34 To test whether differences in metalloproteinase cleavage could account for the change in migration between proliferating and quiescent cells, we immunoblotted conditioned medium with an antibody generated against a region within the first 40 amino acids of TSP-2’s N-terminus. If the N-terminus of TSP-2 is cleaved to generate the smaller fragments, we reasoned that blotting with an N-terminal antibody would result in the 200 kDa band and possibly a smaller 40 kDa band, but not the 160 kDa fragment that represents the C-terminal portion of the protein. When conditioned medium from proliferating and quiescent fibroblasts was immunoblotted with an antibody raised against the N-terminus, however, the 160 kDa band was still present and the banding pattern was similar to the banding pattern with the full-length antibodies (Fig. 6D). These results make it unlikely that the 160 kDa band reflects cleavage of the N-terminal region.
Our results thus demonstrate that proliferating fibroblasts, as compared with quiescent fibroblasts, secrete lower levelsof the angiogenesis inhibitor PEDF, elevated levels of the angiogenesis inducer VEGF-C, and mostly the 200 kDa form of thrombospondin-2. Quiescent fibroblasts, in contrast, secrete elevated levels of the angiogenesis inhibitor PEDF, decreased levels of the angiogenesis inducer VEGF-C, and an extra 160 kDa version of the angiogenesis inhibitor thrombospondin-2. These expression patterns persisted when cells were maintained in quiescence for thirty days, and were reversed when quiescent cells were restimulated to reenter the cell cycle. The findings support the hypothesis that fibroblasts affect the proliferative status of endothelial cells.
Fibroblasts surround blood vessels
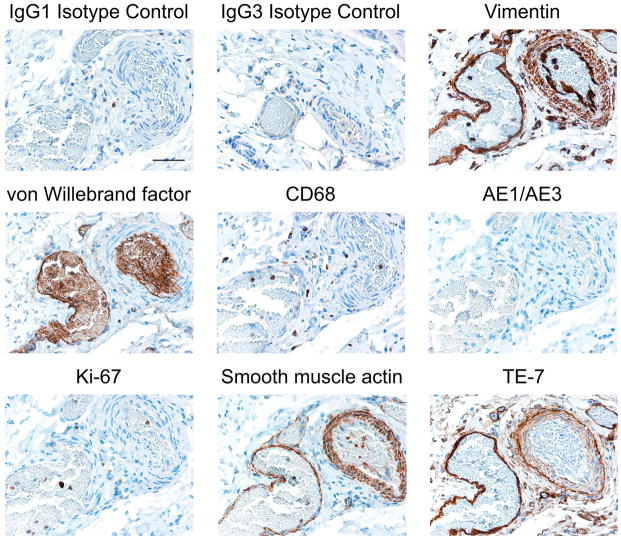
To modulate angiogenesis, the proteins secreted by fibroblasts would need to be secreted into the extracellular environment (Fig. 3) and interact with nearby endothelial cells. We therefore asked whether fibroblasts are physically localized near endothelial cells. We performed immunohistochemistry to identify fibroblasts in regions near blood vessels using a monoclonal antibody, TE-7,35 that we have shown recognizes a fibroblast-associated protein.36 Immunohistochemical staining was performed to identify specific cell types in a section of smooth muscle containing a vein, an artery, and multiple smaller vessels and capillaries (Fig. 7). Isotype-control antibodies (IgG1 and IgG3) resulted in little staining as expected. An antibody to vimentin stained the smooth muscle cells and fibroblasts in the tunica media and adventitia surrounding the artery, and mesenchymal cells surrounding the vein and capillaries. An antibody to von Willebrand factor recognized the endothelial cells lining the vessels and the cells within the vessels.
FIGURE 7.
Fibroblasts surround blood vessels in smooth muscle
Immunostaining of fibroblasts surrounding an artery (right hand side of the image) and a vein (left hand side of the image) in smooth muscle is shown. Smooth muscle tissue was collected in 10% neutral buffered formalin and paraffin-embedded. Serial sections were stained with antibodies that recognize specific cell types. Antibody staining is shown in brown, counterstain is in blue. IgG1 and IgG3 isotype controls display little staining as expected. An antibody to vimentin stains mesenchymal cells surrounding the vein, the artery and smaller vessels and capillaries. An antibody to von Willebrand factor recognizes endothelial cells lining both the vein and artery and the blood cells within the vessels. An antibody to CD68 identifies macrophages scattered throughout the tissue. The AE1/AE3 antibody that recognizes epithileal cytokeratins does not stain this smooth muscle tissue, as expected. An antibody to Ki-67 recognizes few cells within this tissue, indicating that most cells within the tissue are not actively dividing. An antibody to smooth muscle actin recognized a thin layer of cells surrounding the vein (left) and a thicker layer of cells surrounding the artery (right). TE-7, an antibody that recognizes fibroblasts, recognized cells surrounding the large vein (left) and the large artery (right), as well as smaller vessels. A scale bar indicating 10 microns is shown in the first panel.
Scattered macrophages, monocytes, neutrophils, basophils and NK-cells were visible based on staining with an antibody to CD68. The smooth muscle tissue was essentially negative for staining with the AE1/AE3 antibody against epithelial cells. Very few cells within the tissue were positive for Ki-67, indicating that most cells within the tissue are not actively proliferating. Smooth muscle actin staining was present in smooth muscle cells, myofibroblasts, and myoepithelial cells surrounding the artery, the vein and some smaller vessels. Finally, we stained the same tissue with the TE-7 antibody that recognizes a protein synthesized by fibroblasts.35,36 TE-7 staining clearly surrounds both the vein and the artery, demonstrating the close proximity of fibroblasts and proteins synthesized by fibroblasts to the endothelial cells that control angiogenesis. TE-7 staining is also visible surrounding smaller capillaries further supporting a possible role for proteins synthesized by fibroblasts in controlling vasculature. The close proximity of fibroblasts to the endothelium is consistent with our hypothesis that fibroblasts may engage in the heterotypic signaling interactions that coordinate the overall growth and function of tissues.
PEDF is expressed by stromal cells in normal tissue
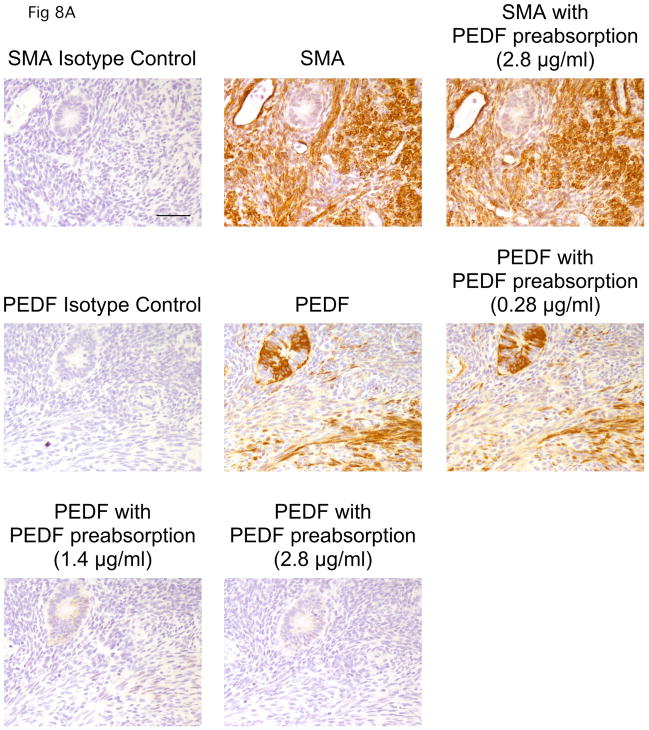
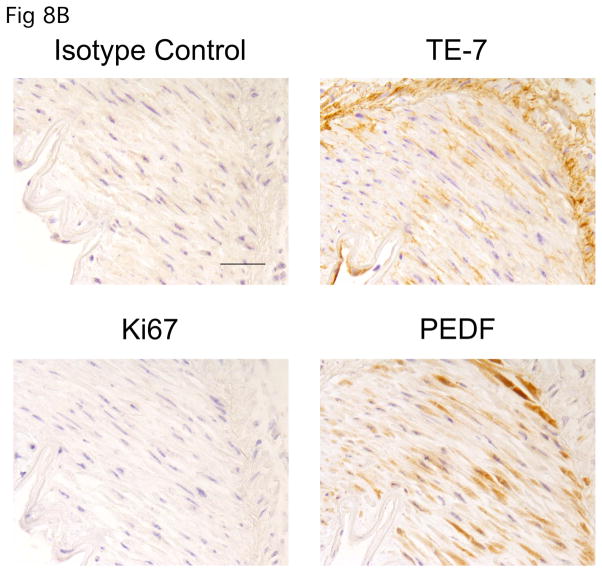
Our analysis of conditioned medium revealed the angiogenesis inhibitor PEDF is secreted by quiescent fibroblasts in vitro. To better assess whether PEDF secreted by quiescent fibroblasts could contribute to tissue quiescence, we asked whether PEDF is expressed in vivo. Using immunohistochemistry, we monitored normal tissue for PEDF expression. We first tested validated the specificity of the anti-PEDF antibody for immunohistochemistry. Preabsorption with recombinant PEDF resulted in a dose-dependent competitive inhibition of PEDF staining (Fig. 8A), while preabsorption with PEDF did not affect staining with an antibody to smooth muscle actin, thus demonstrating that staining with the anti-PEDF antibody is specific for PEDF. Using this antibody, we monitored the presence of PEDF in a region of kidney tissue surrounding a blood vessel. As shown in Fig. 8B, the stromal region surrounding the blood vessel was stained by the fibroblast-associated antibody TE-7. These stomal cells were not stained with Ki-67 indicating that they are quiescent. In this region, we observed easily detectable PEDF. Thus, in normal tissue, quiescent fibroblasts express PEDF. The results support a model in which PEDF plays a role in maintaining tissue in a quiescent state, consistent with other recent reports.37
FIGURE 8.
PEDF is expressed by stromal cells in normal tissue
A. Specificity of the anti-PEDF antibody. Human uterine tissue was formalin-fixed, paraffin-embedded, and stained. In the top row, samples were stained with an isotype control, an antibody to smooth muscle actin, or with an antibody to smooth muscle actin after preabsorption with the PEDF protein (2.8 μg/ml). Preabsorption with PEDF did not affect the staining pattern of an anti-smooth muscle actin antibody. The second and third rows indicate staining with an isotype control, an anti-PEDF antibody or an anti-PEDF antibody after preabsorption with one of several concentrations of recombinant PEDF (0.28 μg/ml, 1.4 μg/ml or 2.8 μg/ml). A dose-dependent competitive inhibition of the anti-PEDF antibody with addition of recombinant PEDF is observed, demonstrating the specificity of the staining for PEDF. A scale bar indicating 10 microns is shown in the first panel.
B. PEDF stains quiescent fibroblasts in the stromal region surrounding a blood vessel in normal kidney. Human kidney tissue was stained with an antibody to PEDF, an antibody to Ki-67, TE-7 (an antibody that recognizes a fibroblast-associated protein), or an isotype control. PEDF was easily detected in the Ki-67 negative cells within the fibroblast-rich stroma. A scale bar indicating 5 microns is shown in the first panel.
Discussion
We report herein a series of experiments designed to test the possibility that fibroblasts function to maintain the angiogenic balance in tissues via their production of angiogenesis signaling molecules. Previous studies had suggested that fibroblasts respond to serum with changes in transcription patterns, including an increase in neoangiogenesis.17 We demonstrate that proliferating fibroblasts have higher transcript levels of a number of angiogenesis inducers while quiescent fibroblasts express higher transcript levels of multiple angiogenesis inhibitors. In a model system designed to monitor the levels of angiogenesis regulators secreted by proliferating and quiescent fibroblasts, we discovered that elevated levels of the angiogenesis inhibitor PEDF are associated with a quiescent state, while levels of the angiogenesis inducer VEGF-C are consistently elevated in proliferating fibroblasts. We further demonstrated that the angiogenesis inhibitor thrombospondin-2 is present in a distinct 160 kDa form in quiescent cells. In order to assess the potential physiological significance of proteins secreted by fibroblasts, we performed immunohistochemistry with an antibody that recognizes a fibroblast-associated antigen and discovered that fibroblasts are physically located adjacent to endothelial cells lining veins, arteries and capillaries. Further, the angiogenesis inhibitor PEDF is detectable in quiescent fibroblasts surrounding a blood vessel within normal kidney tissue.
Our results demonstrate that PEDF is a signature quiescence molecule. It is strongly upregulated at the transcript level and at the protein level when fibroblasts become quiescent, and downregulated when cells begin to divide. In conjunction with previous studies demonstrating that PEDF is not expressed by dividing, senescent, or transformed cells,25 we also observed that increased PEDF expression is closely associated with a reversibly quiescent state in fibroblasts. Our microarray data revealed rapid changes in the transcript levels of PEDF after 24 hours of serum starvation, which, combined with its potent antiangiogenic activity,22 suggests that PEDF may play a role in re-setting the angiogenic balance following a pro-angiogenic stimulus. PEDF secreted by fibroblasts in a quiescent state may help to maintain the entire tissue in a quiescent state, and thus to organize “tissue quiescence.”37,38 Indeed, in PEDF-deficient mice, the pancreas and prostate exhibited increased stromal vasculature and mass, demonstrating a critical role for PEDF in the maintenance of organ size.23
The anti-angiogenic properties of PEDF may also be important for inhibiting the growth of tumor neovasculature.39 PEDF levels are inversely correlated with the metastatic potential and tumor grade of prostate adenocarcinoma,40 pancreatic adenocarcinoma,41 glioblastoma,42 hepatocellular carcinoma,43 and Wilm’s tumor.44 Reintroduction of PEDF has shown promise as a tumor treatment. Overexpression of PEDF in pancreatic adenocarcinoma cells45 and melanoma cell lines46,47 caused reduced tumor microvessel density, resulting in an antitumor effect.
PEDF protein levels in proliferating versus quiescent fibroblasts are inversely correlated with the levels of VEGF-C. Our earlier studies uncovered a strong and rapid downregulation of VEGF-C transcript levels within 14 hours after fibroblasts receive a signal to enter quiescence.18 We show here that both transcript and protein levels of VEGF-C are elevated in actively proliferating fibroblasts as compared with quiescent cells. VEGF-C may therefore represent a key signaling molecule through which fibroblasts coordinate the migration and proliferation of both the vascular and lymphatic endothelium in response to injury.19–21,48 VEGF-C binds both VEGFR-2, expressed by vascular endothelial cells, and VEGFR-3, which is expressed on blood vessels and lymphatic endothelial cells in skin wounds. The predominant VEGF-C isoform detected in conditioned medium of quiescent and proliferating fibroblasts is likely a precursor of the fully processed peptide. This form is also able to bind to and activate VEGFR-2 and VEGFR-3,29 suggesting a possible role for VEGF-C secreted by fibroblasts in both angiogenesis and lymphangiogenesis in response to injury.49
Excessive VEGF-C secretion by activated fibroblasts could represent part of the molecular basis for an association between the response of fibroblasts to serum and tumorigenesis.50,51 VEGF-C expression in cancer tissues has a positive correlation with the risk of lymphatic metastasis52–54 and angiogenesis.55–57 In esophageal squamous cell carcinoma, VEGF-C expression levels are associated with microvessel density and lymph node metastases.58 VEGF-C expression correlated with levels of metastasis when four prostate cancer cell lines were introduced into mice, and VEGF-C overexpression stimulated tumor lymphangiogenesis causing a shift from a low-metastatic to high-metastatic tumor.59.= The VEGFR-3 receptor is expressed by tumor-associated endothelial cells,55,60 leading to the suggestion that VEGF-C binding to VEGFR-3 may represent an important interaction for tumor progression and may be a good target for anti-angiogenesis cancer therapy.49 Thus, while controlled and reversible VEGF-C secretion by fibroblasts in the context of a proliferative environment may help to coordinate tissue regeneration, an uncontrolled wound healing response could result in excessive VEGF-C secretion, which would be expected to promote tumorigenesis and metastasis.
While the changes in the levels of secreted PEDF and VEGF correlated with transcript level changes between proliferating and quiescent cells, TSP-2 showed a different pattern. We and others have observed an increase in transcript levels of thrombospondin-2 in quiescent fibroblasts (Fig. 1).18,61 However, in our model system, we did not detect a significant increase in the total amount of TSP-2 protein in the conditioned medium from quiescent compared with proliferating fibroblasts (Fig. 6A, B). The conditioned medium from quiescent cells did contain higher levels of a 160 kDa form of TSP-2 compared with medium from proliferating cells. Further experimentation makes it unlikely that the shorter form reflects truncation at the N-terminal end. Quiescence-specific differences in glycosylation may contribute to the different forms of TSP-2 detected in proliferating versus quiescent cell conditioned medium. Thrombospondin-2 is N-glycosylated at multiple sites within the C-terminal signature domain.32 Thrombospondin-1 also contains two unusual glycosylation patterns —C-mannosylation at five sites and an O-linked disaccharide — within the properdin modules of its signature thrombospondin domain.33 It is not known whether thrombospondin-2 is modified in the same way. As the TSR-1 repeats of both TSP-1 and TSP-2 are anti-angiogenic domains, differences in glycosylation at these sites could affect TSP-2’s angiogenic activity.
Microarray analysis revealed that there are approximately 50 angiogenesis regulators that may be secreted at different levels in proliferating versus quiescent fibroblasts. We focus here on expanding our analysis from transcript to proteins for a small, carefully selected subset of these molecules. Our findings with regard to TSP-2 reinforce the importance of analysis at the protein level, including an assessment of post-transcriptional and post-translational processing events. Further, our results demonstrate that these processing events can occur differently in proliferating versus quiescent cells.
The known contributors to angiogenic homeostasis include the hypoxic state of the tissue, local inflammatory cells, circulating chemokines, and the availability of platelet alpha granules known to sequester pro and anti-angiogenic molecules.62 Our results suggest that the proliferative state of fibroblasts may also be one of several factors determining the angiogenic balance within tissues. In resting tissues, quiescent fibroblasts secrete angiogenesis inhibitors that maintain a primarily anti-angiogenic environment, including PEDF. Under these conditions, the quiescent fibroblasts signal to the endothelial cells not to undergo new blood vessel growth, which limits the proliferative capacity of the parenchymal cells that rely on blood vessels to provide oxygen and eliminate wastes. Indeed, normal fibroblastic stroma has been shown to inhibit the hyperplastic growth of abnormal mammary epithelial cells.63 Under conditions in which fibroblasts should proliferate, for instance, in response to a wound during which PDGF is released from platelet granules, quiescent fibroblasts exit their resting state to re-enter the cell cycle. In these conditions, fibroblasts upregulate their production and secretion of angiogenesis inducers including VEGF-C, while decreasing their production of inhibitors. The resulting shift in angiogenesis molecule production is likely an effective initiator of the angiogenic process.
This controlled and reversible process is likely to be distorted in tumor-associated angiogenesis so that it is no longer possible to reset the angiogenic balance to an anti-angiogenic level, thus “flipping” the “angiogenic switch”.12,64 Fibroblasts and the angiogenic molecules they secrete may contribute to this process. Fibroblasts associated with tumors have been shown to have distinct proliferative capacity and gene expression patterns, and to actively promote tumorigenesis.65–69 Further, cancer-associated fibroblasts may mimic the serum-activated state.51 VEGF-C may represent an important molecular controller of this process, as it is highly expressed by proliferating fibroblasts and in tumors. By inducing both angiogenesis and lymphangiogenesis, VEGF-C may promote both tumor growth and metastasis to lymph nodes.49
Maintaining body size and appropriate tissue morphology through numerous endogenous and exogenous challenges over a lifetime, while avoiding cancerous growths, is a complex and daunting challenge. Our results support the hypothesis that fibroblasts, by secreting angiogenesis regulators, play a role in establishing and maintaining tissue stability. By secreting different repertoires of angiogenesis regulators in proliferative versus quiescent conditions, fibroblasts may coordinate the growth or growth arrest of adjacent vasculature to correspond with their own proliferative state. In this way, fibroblasts may facilitate the heterotypic signaling interactions critical to maintaining the proper size, organization and function of tissues and organs over many years.
Materials and Methods
Microarray analysis
To compare proliferating versus quiescent states, 91FS5 dermal fibroblasts (generous gift of Dr. Thomas Norwood, University of Washington) were cultured in DMEM with 5% fetal bovine serum (FBS). To induce quiescence, cells were plated at approximately 5 × 105 cells per 10 cm plate and allowed to adhere overnight at 37°C. Cells were transferred to DMEM with 0.1% serum and samples were collected 1, 2, 4, 8, and 24 hours later. On the third day after inducing quiescence, cells were split 1:2 in DMEM with 0.1% FBS. A final sample was taken at 96 hours after serum withdrawal. Quiescent samples were re-stimulated with 5% FBS and samples were taken at 1, 2, 4, 8, 24 and 48 hours after stimulation. To collect samples for RNA isolation, cells were trypsinized from the plate, pelleted and stored at −80°C. Total RNA was isolated using the mirVana miRNA Isolation kit (Ambion, Austin, TX) according to the manufacturer’s instructions. RNA quality was verified using a Bioanalyzer 2100 (Agilent Technology, Santa Clara, CA) and the amount was determined with a Nanodrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). 325 ng total RNA was amplified using Low RNA Input Fluorescent Labeling Kit (Agilent Technologies) according to the manufacturer’s protocol. Cyanine 3-CTP (Cy-3) or Cyanine 5-CTP (Cy-5) (Perkin Elmer, Waltham, MA) were directly incorporated into the cRNA during in vitro transcription. For the serum withdrawal time course, Cy-3 labeled cRNA was used as reference RNA and compared with Cy-5 labeled cRNA generated for each individual serum withdrawal time point. For the serum re-stimulation time course, Cy-3 labeled cRNA from a 96-hour serum withdrawal sample was used as reference RNA and compared with Cy-5 labeled cRNA from individual serum re-stimulated samples over the time course. Mixtures of Cy-3 labeled and Cy-5 labeled cRNA were co-hybridized to Whole Human Genome Oligo Microarray slides (Agilent Technologies) at 60°C for 17 hrs and subsequently washed according to the Agilent standard hybridization protocol. Slides were scanned with a dual laser scanner (Agilent Technologies). Images were monitored for quality control. The Agilent feature extraction software, in conjunction with the Princeton University Microarray database (PUMAdb http://puma.princeton.edu/), was used to compute the log ratio of the two samples for each gene after background subtraction and dye normalization.
Microarray analysis of angiogenesis regulators
Angiogenesis factors were identified based on a previously published annotated list.70 The list was filtered to focus on genes that show differential expression in proliferating and quiescent states of at least 1.5-fold in an array over the serum withdrawal/restimulation time course. Average linkage hierarchical clustering of this limited gene set was performed using Cluster 3.0. The resulting data were visualized using Java TreeView.
Conditioned medium collection
91FS5 fibroblasts were expanded in Fibroblast Growth Medium (FGM-2) (Lonza Inc., Allendale, NJ), which contains the Fibroblast Basal Medium (FBM-2), 2% FBS, insulin and FGF. For proliferating conditions, fibroblasts were plated at a density of 7000 cells/cm2 in FGM-2 medium. The following day, cells were washed twice with phosphate buffered saline and cultured for four days in FBM-2 supplemented with insulin (Lonza Inc.), 0.025 mg/ml insulin growth factor (IGF-1) (R&D Systems, Minneapolis, MN), 0.03μg/ml platelet derived growth factor-BB (PDGF-BB) (Prepro Tech, Rocky Hill, NJ), gentamycin (Lonza Inc.) and either no serum or 0.1% serum. To generate quiescent cultures, fibroblasts were plated at high density (approximately 5 × 104 to 105 cells/cm2) and incubated overnight in FGM-2. The following day, cells were washed twice with phosphate buffered saline (PBS) and plated in FBM-2 containing insulin, 0.025 mg/ml IGF-1, gentamycin, and no serum or 0.1% FBS. Long-term quiescent cultures were maintained up to 30–45 days, and media was collected and replaced every 4 days. At the end of 30 days, cells were restimulated by plating them at a density of 9,400 cells/cm2 in FBM-2 containing 0.1% serum, 0.025 μg/ml IGF-1 and 0.03 μg/ml PDGF-BB.
Preparation of cellular lysates
Proliferating or quiescent fibroblasts were scraped into RIPA buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitors. The protease inhibitor cocktail was either 0.1% aprotinin (Sigma-Aldrich, St. Louis, MO), 0.1% leupeptin (Sigma-Aldrich), 0.05% pepstatin (Sigma-Aldrich), and 0.1% PMSF (VWR) or complete minitablet protease inhibitors (Roche, Basel Switzerland). Lysates were sonicated with five pulses for fifteen seconds each at 60 J/W. Lysates were then incubated for thirty seconds on ice with periodic vortexing and cleared by centrifugation at 10,0000 rpm. Total protein amount was assessed by the Lowry method using the BioRad-DC Protein Assay Kit II (BioRad Inc., Hercules, California) as described by the manufacturer. Spectrophotometer readings taken at 650 nm were compared against a standard curve to determine lysate concentration. Total protein content was determined as the product of lysate concentration and lysate volume.
Collection and normalization of conditioned medium
For cultures in growing or quiescent conditions, lysates were collected to determine the cellular protein content every 12 hours during the four-day media collection period. The protein content of the cell lysates was plotted against the time of lysate collection and regression analysis was performed to generate a curve that best described the data. The generated equations were used to integrate the area under the curve from 0 to 96 hours. The integrated protein-hour quantity was then divided by the volume of media collected from the proliferating or quiescent plate. The total protein-hour/volume for proliferating and quiescent cells were compared and the resulting ratios were used to adjust the volume of conditioned medium used for immunoblotting. While the integral method was used for experiments in which protein amounts were quantified, in some earlier experiments, the volume of conditioned medium precipitated was normalized by the amount of lysate on the plate at the time of collection.
Immunoblotting
Conditioned medium was mixed with a 25% volume of TCA-DOC [100% trichloroacetic acid (Sigma-Aldrich), 0.1% sodium deoxycholate (Sigma-Aldrich)] and incubated for thirty minutes on ice. Following centrifugation, samples were washed five times with ice-cold acetone and resuspended in SDS-PAGE sample buffer (0.0625M Tris HCl pH 6.8, 2% SDS, 10% glycerol, 0.25% β-mercaptoethanol, 0.015% bromophenol blue). Alternatively, recombinant proteins—VEGF-C (R&D Systems), thrombospondin-2 (R&D Systems), or PEDF (Chemicon International, Temecula, CA) —were mixed with sample buffer and loaded. Samples were separated under reducing conditions on 12% or 5% SDS-polyacrylmide gels using a miniProtean 3 apparatus (Bio-Rad, Hercules, CA). Proteins were transferred for 1 hour at 100 volts to Westran polyvinylidene fluoride (PVDF) membranes (Perkin Elmer, Waltham, MA) using a vertical transfer apparatus (Bio-Rad). Membranes were blocked for 1 hour at room temperature in 5% non-fat dried milk dissolved in PBS containing 0.1% Tween-20 (PBS-T). Membranes were then incubated overnight at 4°C with primary antibodies diluted in 2.5% milk/PBS-T. Primary antibodies were used at the following final concentrations: 0.2 μg/ml mouse anti-PEDF (Chemicon Int.), 1 μg/ml goat anti-VEGF-C (R&D Systems), 0.25 μg/ml goat anti-TSP-2 (R&D Systems), 0.25 μg/ml mouse anti-TSP-2 (R&D Systems), 0.25 μg/ml goat anti-TSP-2 N-20 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:2000 dilution of mouse monoclonal anti-fibronectin clone HFN7.1 (generous gift of Jean Schwarbauer, Princeton University). Following overnight incubation in the primary antibody, membranes were washed three times in PBS-T and incubated for 1 hour in a 1:5000 dilution of species-specific HRP-conjugated secondary antibody in 2.5% milk/PBS-T. The secondary antibody for primary antibodies raised in mice was HRP-conjugated anti-mouse IgG from sheep (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The secondary antibody for primary antibodies raised in goat was HRP-conjugated anti-goat (AnaSpec, San Jose, CA). Membranes were exposed to x-ray film, and film was scanned with a Hewlett-Packard Scanjet 4890 using Hewlett-Packard software.
Images were processed using imaging software Image J. The total band intensity was determined as the mean intensity of pixels per band area multiplied by the band area. The background intensity was defined as the average background intensity for the blot multiplied by the band area. The net band intensity (total band intensity minus the background intensity) was plotted against the amount of recombinant protein loaded to generate a standard curve. Linear regression was performed to define the equation describing the relationship between band intensity and protein quantity. The regression equation was used to determine the amount of protein in each sample.
Immunohistochemistry
Smooth muscle tissue and uterine tissue were collected through the Fred Hutchinson tissue retrieval service. Informed consent was obtained as described in the Institutional Review Board protocols. Tissue samples were fixed in 10% neutral buffered formalin and paraffin-embedded. Four-micron sections were cut, baked at 60°C for 30 minutes and cooled. Sections were deparaffinized and rehydrated through graded alcohols to water. Antigen retrieval was performed as specified for each antibody below. Slides were placed in Tris-buffered saline with 0.1% Tween-20 and loaded onto a DakoCytomation Autostainer. Endogenous peroxidase was blocked with a ten-minute incubation with 3% hydrogen peroxide. When using the Vectastain Universal Elite ABC kit (Vector Laboratories, Burlingame, CA), endogenous biotin was blocked using the DAKO Biotin Blocking System (DakoCytomation, Glostrup, Denmark). Slides were washed and a serum block of 15% normal goat or swine serum plus 5% human serum (Jackson Immunoresearch, West Grove, PA) in antibody diluent (TBS, 0.1% Tween-20, 1% bovine serum albumin) was added for ten minutes. The serum block was blown off and the primary antibody was applied for 30 minutes at room temperature. A biotinylated goat anti-mouse IgG F(ab')2 (Jackson Immunoresearch) was used as a secondary followed by the Vectastain ABC kit for 30 minutes for detection. Slides were then washed with wash buffer, incubated with liquid DAB plus substrate (DakoCytomation) for 7 minutes, and rinsed with water. They were counterstained for two minutes with hematoxylin (DakoCytomation), blued with wash buffer, rinsed with water, dehydrated, cleared and coverslipped. Images were visualized with a Nikon Eclipse 50i microscope with Nikon Plan Fluor lenses. Images were taken with a Nikon Digital DS-L1 camera using a 20X objective.
Isotype control antibodies— mouse IgG1 (Chemicon Int.) and mouse IgG3 (Sigma-Aldrich) —were used at the same concentration as the antibody of interest. To identify mesenchymal cells, we used an antibody to vimentin (Clone V9 DakoCytomation mouse monoclonal IgG1 1.2 μg/ml) with pH 8 EDTA antigen retrieval (TrilogyTM). To identify endothelial cells, megakaryocytes and platelets, we used an antibody to von Willebrand Factor (DakoCytomation, rabbit polyclonal IgG at 15.5 μg/ml) with pH 8 EDTA antigen retrieval (TrilogyTM, Cell MarqueTM Corporation, Rocklin, CA) followed by the Envision Rabbit Polymer (DakoCytomation) instead of the Vectastain ABC kit. To identify macrophages, monocytes, neutrophils, basophils and NK-cells, we used an antibody to the cell surface protein CD68 (PG-M1 DakoCytomation mouse monoclonal IgG3 1.9 μg/ml) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify epithelial and glandular cells, we used clone AE1/AE3 (DakoCytomation mouse monoclonal IgG1 at 1.27 μg/ml) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify proliferating cells, we used an antibody against Ki67 clone MIB1 (DakoCytomation mouse monoclonal IgG1 0.8 μg/ml) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify smooth muscle cells, myofibroblasts, and myoepithelial cells, we used clone 1A4 against smooth muscle actin (DakoCytomation mouse monoclonal IgG2a 1.4 μg/ml) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify fibroblasts, we used clone TE-7 (Chemicon mouse monoclonal IgG1 1.3 μg/ml) with pH 8 EDTA antigen retrieval (TrilogyTM).
Peptide blocking of antibody staining
To determine PEDF expression, we used an anti-PEDF antibody (Chemicon mouse monoclonal IgG2a used at 1.7 μg/ml) with pH 9.0 Dako Antigen Retrieval solution for 20 minutes. Endogenous peroxidase was blocked with 30% H2O2 diluted to 3% in methanol for 20 min. An Avidin/Biotin block (Dako) was performed, followed by Dako’s Serum Free Protein Block for 10 min. before application of antibody/protein solution. The secondary was a biotinylated goat anti-mouse IgG F(ab’)2 diluted 1:200 (Jackson Immunoresearch). Vector elite ABC and DAB were used for detection as described above. For pre-absorption experiments, the anti-PEDF antibody and recombinant full-length human PEDF protein (Upstate Cell Signaling Solutions) were combined at the desired concentration and allowed to bind at room temperature overnight. The next day, all reagents were allowed to come to room temperature before proceeding with immunohistochemistry. An isotype control antibody at a concentration of 1.7 μg/ml was combined with PEDF protein concentration at 2.8 μg/ml and analyzed under the same conditions. As another control, an antibody to smooth muscle actin was also preabsorbed with recombinant PEDF (2.8 μg/ml) and processed as described above. Positive control slides for both the PEDF antibody and isotype were run at the same time to serve as a positive and negative run control.
Supplementary Material
Acknowledgments
The authors wish to acknowledge funding from the New Jersey Commission on Cancer Research, the Leukemia Lymphoma Society and the Avon Opportunity Breast Cancer Fund to H.A.C. H.A.C. is the Milton E. Cassel scholar of the Rita Allen Foundation. H.A.C. and A.L.-M. are supported by NIGMS Center of Excellence grant P50 GM071508. E.P. acknowledges a summer undergraduate fellowship from the New Jersey Commission on Cancer Research. The authors are grateful for technical assistance and materials from Christina DeCoste (Princeton University), Lee Fischer (Princeton University), Curtis Huttenhower (Princeton University), Peter Wei (Princeton University), Patricia Robinson (Princeton University), Dai Wang (Princeton University), Jean Schwartzbauer (Princeton University), Ashley Moses (Oregon Health Science University) and Thomas Shenk (Princeton University). They also wish to thank Courtney Williams (Princeton University), Amy Caudy (Princeton University), Jim Roberts (Fred Hutchinson Cancer Research Center), Leonid Kruglyak (Princeton University), Barry Coller (Rockefeller University), Alison Gammie (Princeton University), Daniel Notterman (Princeton University), Jonathan Eggenschwiler (Princeton University), Yibin Kang (Princeton University) and Joshua Forman (Princeton University) for helpful discussions.
References
- 1.Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300:611–5. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL, Palmiter RD. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123:2827–33. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- 3.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–80. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene AK, Wiener S, Puder M, Yoshida A, Shi B, Perez-Atayde AR, Efstathiou JA, Holmgren L, Adamis AP, Rupnick M, Folkman J, O'Reilly MS. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003;237:530–5. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimbrone MA, Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413–27. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis. In: Braumwald E, editor. Harrison's textbook of internal medicine. New York: McGraw-Hill; 2001. pp. 517–30. [Google Scholar]
- 8.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 9.Pepper MS, Sappino AP, Montesano R, Orci L, Vassalli JD. Plasminogen activator inhibitor-1 is induced in migrating endothelial cells. Journal of cellular physiology. 1992;153:129–39. doi: 10.1002/jcp.1041530117. [DOI] [PubMed] [Google Scholar]
- 10.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. The Journal of biological chemistry. 1998;273:13313–6. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 11.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. The Journal of biological chemistry. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–51. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 13.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer research. 2005;65:3967–79. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nature medicine. 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 15.Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 17.Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science (New York, NY. 1999;283:83–7. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 18.Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biology. 2006;4:e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14389–94. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circulation research. 2004;94:664–70. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 21.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. The EMBO journal. 1996;15:290–98. [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science (New York, NY. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 23.Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J, Crawford SE. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nature medicine. 2003;9:774–80. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 24.Pignolo RJ, Cristofalo VJ, Rotenberg MO. Senescent WI-38 cells fail to express EPC-1, a gene induced in young cells upon entry into the G0 state. The Journal of biological chemistry. 1993;268:8949–57. [PubMed] [Google Scholar]
- 25.Pignolo RJ, Francis MK, Rotenberg MO, Cristofalo VJ. Putative role for EPC-1/PEDF in the G0 growth arrest of human diploid fibroblasts. Journal of cellular physiology. 2003;195:12–20. doi: 10.1002/jcp.10212. [DOI] [PubMed] [Google Scholar]
- 26.Frazier WA. Thrombospondins. Curr Opin Cell Biol. 1991;3:792–9. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- 27.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. The Journal of cell biology. 1998;140:419–30. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. The Journal of investigative dermatology. 1999;113:782–7. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 29.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. The EMBO journal. 1997;16:3898–911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liska DJ, Hawkins R, Wikstrom K, Bornstein P. Modulation of thrombospondin expression during differentiation of embryonal carcinoma cells. Journal of cellular physiology. 1994;158:495–505. doi: 10.1002/jcp.1041580314. [DOI] [PubMed] [Google Scholar]
- 31.Lafeuillade B, Pellerin S, Keramidas M, Danik M, Chambaz EM, Feige JJ. Opposite regulation of thrombospondin-1 and corticotropin-induced secreted protein/thrombospondin-2 expression by adrenocorticotropic hormone in adrenocortical cells. Journal of cellular physiology. 1996;167:164–72. doi: 10.1002/(SICI)1097-4652(199604)167:1<164::AID-JCP19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 32.Carlson CB, Bernstein DA, Annis DS, Misenheimer TM, Hannah BL, Mosher DF, Keck JL. Structure of the calcium-rich signature domain of human thrombospondin-2. Nature structural & molecular biology. 2005;12:910–4. doi: 10.1038/nsmb997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. The Journal of biological chemistry. 2001;276:6485–98. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- 34.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. The EMBO journal. 2006;25:5270–83. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes BF, Scearce RM, Lobach DF, Hensley LL. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. The Journal of experimental medicine. 1984;159:1149–68. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodpaster T, Legesse-Miller A, Hameed M, Aisner A, Randolph-Habecker J, Coller H. An immunohistochemical method for identifying fibroblasts in formalin-fixed paraffin-embedded tissue. Journal of Histochemistry and Cytochemistry. 2007 doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pina AL, Kubitza M, Brawanski A, Tombran-Tink J, Kloth S. Expression of pigment-epithelium-derived factor during kidney development and aging. Cell Tissue Res. 2007;329:329–38. doi: 10.1007/s00441-007-0420-8. [DOI] [PubMed] [Google Scholar]
- 38.Sawant S, Aparicio S, Tink AR, Lara N, Barnstable CJ, Tombran-Tink J. Regulation of factors controlling angiogenesis in liver development: a role for PEDF in the formation and maintenance of normal vasculature. Biochemical and biophysical research communications. 2004;325:408–13. doi: 10.1016/j.bbrc.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Schmitz V, Perez-Mediavilla A, Izal I, Prieto J, Qian C. Suppression of angiogenesis and tumor growth by adenoviral-mediated gene transfer of pigment epithelium-derived factor. Mol Ther. 2003;8:72–9. doi: 10.1016/s1525-0016(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 40.Halin S, Wikstrom P, Rudolfsson SH, Stattin P, Doll JA, Crawford SE, Bergh A. Decreased pigment epithelium-derived factor is associated with metastatic phenotype in human and rat prostate tumors. Cancer research. 2004;64:5664–71. doi: 10.1158/0008-5472.CAN-04-0835. [DOI] [PubMed] [Google Scholar]
- 41.Uehara H, Miyamoto M, Kato K, Ebihara Y, Kaneko H, Hashimoto H, Murakami Y, Hase R, Takahashi R, Mega S, Shichinohe T, Kawarada Y, Itoh T, Okushiba S, Kondo S, Katoh H. Expression of pigment epithelium-derived factor decreases liver metastasis and correlates with favorable prognosis for patients with ductal pancreatic adenocarcinoma. Cancer research. 2004;64:3533–7. doi: 10.1158/0008-5472.CAN-03-3725. [DOI] [PubMed] [Google Scholar]
- 42.Guan M, Yam HF, Su B, Chan KP, Pang CP, Liu WW, Zhang WZ, Lu Y. Loss of pigment epithelium derived factor expression in glioma progression. J Clin Pathol. 2003;56:277–82. doi: 10.1136/jcp.56.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto K, Ishikawa H, Nishimura D, Hamasaki K, Nakao K, Eguchi K. Antiangiogenic property of pigment epithelium-derived factor in hepatocellular carcinoma. Hepatology. 2004;40:252–9. doi: 10.1002/hep.20259. [DOI] [PubMed] [Google Scholar]
- 44.Abramson LP, Browne M, Stellmach V, Doll J, Cornwell M, Reynolds M, Arensman RM, Crawford SE. Pigment epithelium-derived factor targets endothelial and epithelial cells in Wilms' tumor. Journal of pediatric surgery. 2006;41:1351–6. doi: 10.1016/j.jpedsurg.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 45.Hase R, Miyamoto M, Uehara H, Kadoya M, Ebihara Y, Murakami Y, Takahashi R, Mega S, Li L, Shichinohe T, Kawarada Y, Kondo S. Pigment epithelium-derived factor gene therapy inhibits human pancreatic cancer in mice. Clin Cancer Res. 2005;11:8737–44. doi: 10.1158/1078-0432.CCR-05-1323. [DOI] [PubMed] [Google Scholar]
- 46.Garcia M, Fernandez-Garcia NI, Rivas V, Carretero M, Escamez MJ, Gonzalez-Martin A, Medrano EE, Volpert O, Jorcano JL, Jimenez B, Larcher F, Del Rio M. Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer research. 2004;64:5632–42. doi: 10.1158/0008-5472.CAN-04-0230. [DOI] [PubMed] [Google Scholar]
- 47.Abe R, Shimizu T, Yamagishi S, Shibaki A, Amano S, Inagaki Y, Watanabe H, Sugawara H, Nakamura H, Takeuchi M, Imaizumi T, Shimizu H. Overexpression of pigment epithelium-derived factor decreases angiogenesis and inhibits the growth of human malignant melanoma cells in vivo. The American journal of pathology. 2004;164:1225–32. doi: 10.1016/s0002-9440(10)63210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science (New York, NY. 1997;276:1423–5. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 49.Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490–7. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]
- 50.Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van't Veer LJ, Bartelink H, van de Rijn M, Brown PO, van de Vijver MJ. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3738–43. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. British journal of cancer. 1999;80:309–13. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413–9. doi: 10.1016/s0959-8049(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 54.Kishimoto K, Sasaki A, Yoshihama Y, Mese H, Tsukamoto G, Matsumura T. Expression of vascular endothelial growth factor-C predicts regional lymph node metastasis in early oral squamous cell carcinoma. Oral Oncol. 2003;39:391–6. doi: 10.1016/s1368-8375(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 55.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. The American journal of pathology. 1999;154:1381–90. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furudoi A, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Chayama K, Shimamoto F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157–66. doi: 10.1159/000048262. [DOI] [PubMed] [Google Scholar]
- 57.Li Q, Dong X, Gu W, Qiu X, Wang E. Clinical significance of co-expression of VEGF-C and VEGFR-3 in non-small cell lung cancer. Chin Med J (Engl) 2003;116:727–30. [PubMed] [Google Scholar]
- 58.Ding MX, Lin XQ, Fu XY, Zhang N, Li JC. Expression of vascular endothelial growth factor-C and angiogenesis in esophageal squamous cell carcinoma. World J Gastroenterol. 2006;12:4582–5. doi: 10.3748/wjg.v12.i28.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brakenhielm E, Burton JB, Johnson M, Chavarria N, Morizono K, Chen I, Alitalo K, Wu L. Modulating metastasis by a lymphangiogenic switch in prostate cancer. Int J Cancer. 2007 doi: 10.1002/ijc.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarijs R, Schalkwijk L, Hofmann UB, Ruiter DJ, de Waal RM. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer research. 2002;62:7059–65. [PubMed] [Google Scholar]
- 61.Bein K, Ware JA, Simons M. Myb-dependent regulation of thrombospondin 2 expression. Role of mRNA stability. The Journal of biological chemistry. 1998;273:21423–9. doi: 10.1074/jbc.273.33.21423. [DOI] [PubMed] [Google Scholar]
- 62.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature reviews. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 63.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–87. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 65.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83. [PubMed] [Google Scholar]
- 66.van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988;50:159–96. doi: 10.1016/s0065-230x(08)60437-6. [DOI] [PubMed] [Google Scholar]
- 67.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer research. 1999;59:5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–46. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 70.Weinberg RA. The Biology of Cancer. New York, NY: Garland Science; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.