Abstract
Pkh1, -2, and -3 are the yeast orthologs of mammalian 3-phosphoinositide-dependent protein kinase-1 (PDK1). Although essential for viability, their functioning remains poorly understood. Sch9, the yeast protein kinase B and/or S6K ortholog, has been identified as one of their targets. We now have shown that in vitro interaction of Pkh1 and Sch9 depends on the hydrophobic PDK1-interacting fragment pocket in Pkh1 and requires the complementary hydrophobic motif in Sch9. We demonstrated that Pkh1 phosphorylates Sch9 both in vitro and in vivo on its PDK1 site and that this phosphorylation is essential for a wild type cell size. In vivo phosphorylation on this site disappeared during nitrogen deprivation and rapidly increased again upon nitrogen resupplementation. In addition, we have shown here for the first time that the PDK1 site in protein kinase A is phosphorylated by Pkh1 in vitro, that this phosphorylation is Pkh-dependent in vivo and occurs during or shortly after synthesis of the protein kinase A catalytic subunits. Mutagenesis of the PDK1 site in Tpk1 abolished binding of the regulatory subunit and cAMP dependence. As opposed to PDK1 site phosphorylation of Sch9, phosphorylation of the PDK1 site in Tpk1 was not regulated by nitrogen availability. These results bring new insight into the control and prevalence of PDK1 site phosphorylation in yeast by Pkh protein kinases.
Keywords: Protein Kinases, Protein Kinase A (PKA), Protein Phosphorylation, Site-directed Mutagenesis, Yeast, PDK1, PKB, Pkh1–3, Saccharomyces cerevisiae, Sch9
Introduction
The activity of a large number of protein kinases is regulated through phosphorylation of their activation loop (also known as T-loop), one of the most dynamic regions of the kinase core (1). A feature shared by almost all members of the AGC subfamily of protein kinases (to which PKA,3 PKG, and PKC belong) is the presence of a phosphorylation site in their activation loop with consensus sequence TFCGTXEY where the Thr residue in bold represents the phosphorylated residue and X represents any amino acid. Phosphorylation of this site is required for full kinase activity. In most AGC kinases, this site is targeted by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and is therefore also called the PDK1 site (2).
PDK1 is a main downstream effector of phosphatidylinositol 3-kinase (PI3K) signaling: binding of growth factors or insulin to their corresponding receptors triggers activation of phosphatidylinositol 3-kinases, which phosphorylate inositol phospholipids (PtdIns) at the 3′-OH position of their inositol ring. In this way, class I PI3K generate PtdIns3P, PtdIns(3,4)P2, and PtdIns(3,4,5)P3 (3). The latter two lipid species recruit PDK1 to the plasma membrane through binding to its C-terminal pleckstrin homology domain. This causes colocalization of PDK1 and its substrate, PKB, at the plasma membrane (4–6). In addition, binding of these phosphoinositides to PKB causes a conformational change in PKB, enabling it to be phosphorylated by PDK1 (7).
Apart from PKB, none of the other PDK1 substrates contain a pleckstrin homology domain, nor do they interact with phosphoinositides. The interaction of these substrates with PDK1 is regulated in a different way. They contain a hydrophobic motif (HM) located C-terminal of the catalytic domain with consensus sequence FXXF(S/T)(F/Y) with the Ser/Thr residue in bold indicating the phosphorylation site. This HM functions as a docking site for PDK1 since it can bind to a hydrophobic pocket in PDK1 called the PDK1-interacting fragment “(PIF) pocket” (8–11). Crystallographic studies demonstrated the existence of a phosphate binding groove adjacent to the PIF pocket in PDK1 capable of binding the phosphorylated Ser/Thr of the HM in PDK1 substrates. Binding of the phosphorylated HM to PDK1 increases PDK1 catalytic activity, resulting in phosphorylation of the substrate activation loop (12). This in turn prompts binding of the phosphorylated HM to the HM pocket of the PDK1 substrate, creating an active conformation of this kinase. Although not required for its interaction with PDK1, PKB also contains a HM whose phosphorylation further increases the catalytic activity of PKB. Recent work has shown that the mammalian target of rapamycin (mTOR), either as part of mTORC1 or mTORC2, is capable of phosphorylating the HM motif found in most AGC kinases (for a recent review, see Ref. 13).
Saccharomyces cerevisiae contains three PDK1 orthologs, Pkh1–3. Combined deletion of PKH1 and PKH2 is lethal due to a cell wall defect, and expression of human PDK1 rescues this lethality (14). Pkh3 was isolated as a multicopy suppressor of the cell wall defect in a pkh1ts pkh2Δ strain (15), but so far no data are available that could reveal a functional role for Pkh3. Pkh substrates identified to date include mostly protein kinases involved in cell integrity signaling, such as the yeast serum and glucocorticoid inducible kinase homologs Ypk1 and Ypk2 and the yeast PKC1 homolog Pkc1, all of which are phosphorylated on their conserved PDK1 site (15–17). A PDK1 site is also present in the protein kinase Sch9. Sch9 was first isolated as a multicopy suppressor of the growth arrest of cAMP-PKA signaling-defective strains (18) and has since then been shown to play a role in nitrogen signaling (19), regulation of cell size (20), ribosome biogenesis (21, 22), stress resistance (20, 23), and longevity (24, 25). Although originally considered as the yeast PKB ortholog (26), recent work suggests that Sch9 might also function analogously to mammalian S6K (27).
Although these data underscore the importance of Sch9 in cellular signaling, its precise activation mechanism is still elusive. Urban et al. (27) showed that six amino acids in the C terminus of Sch9 are directly phosphorylated by the rapamycin-sensitive TORC1 complex, and this phosphorylation is sensitive to the nutrient status of the medium. One of these residues, Thr-737, is located in a HM C-terminal to the catalytic domain and is conserved in PKB and S6K. mTORC1 phosphorylates the HM in S6K, whereas mTORC2 targets the HM in PKB. Phosphorylation of this HM is essential for Sch9 activity. The presence of a PDK1 site in Sch9 indicates a possible role for Pkh kinases as regulators of Sch9. Previous work has shown that PDK1 site phosphorylation in Sch9 is dependent in vivo on Pkh1–2 activity (27) and that Pkh1 can phosphorylate this site in vitro (28). However, the physiological role of Pkh-dependent phosphorylation of Sch9 has remained unclear.
Other yeast kinases containing a PDK1 site are the catalytic subunits of PKA (encoded by TPK1, TPK2, and TPK3). Yeast Tpk1 is a phosphoprotein, and the PDK1 site in Tpk1 was identified as one of the phosphorylated residues (29). Which protein kinase targets this site in S. cerevisiae has not been explored. In mammalian cells, PDK1-mediated phosphorylation of PKB is well-established, but phosphorylation of PKA by PDK1 remains enigmatic (30–32).
In this work, we show that both Sch9 and Tpk1 physically interact with Pkh1 in vitro and that this interaction depends on a hydrophobic pocket in the kinase domain of Pkh1. We demonstrate that yeast Pkh kinases target the PDK1 site of Sch9 both in vitro and in vivo. Moreover, this site is no longer phosphorylated in nitrogen-deprived cells, and addition of all nitrogen compounds required for growth results in rephosphorylation. We also demonstrate for the first time direct Pkh-dependent phosphorylation of the PDK1 site in the yeast PKA catalytic subunit Tpk1 in vitro and absence of this phosphorylation in newly synthesized Tpk1 in a strain lacking Pkh activity in vivo. Contrary to PDK1 site phosphorylation of Sch9, the PDK1 site in Tpk1 is not nitrogen-responsive.
EXPERIMENTAL PROCEDURES
Strains and Growth Media
Yeast strains used in this study are listed in supplemental Table 1. Standard rich yeast peptone (YP) and defined minimal media (synthetic complete (SC)) containing either 2% glucose, 2% raffinose, or 2% galactose as the carbon source and supplemented with the appropriate nutrients to maintain selection for plasmids were used for yeast cultivation. Cells were routinely grown at 30 °C with the exception of temperature-sensitive strains, which were grown at 24 °C. For experiments with nitrogen-starved cells, cells were first grown to exponential phase (A600 nm = 1.5–2.0), harvested, and resuspended in nitrogen starvation medium (0.17% yeast nitrogen base without amino acids and without ammonium sulfate) containing 4% glucose. Cultures were then incubated with shaking for 24 h unless stated otherwise; care was taken that the glucose level remained high (2%) throughout the entire period of incubation.
Plasmids
Plasmids used in this study are listed in supplemental Table 2. Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit (Stratagene), and all constructs were sequenced to verify the presence of the desired mutation(s) as well as the absence of any erroneous mutations.
Expression and Purification of GST-tagged Proteins from Escherichia coli
Proteins were expressed in E. coli strain BL21, and expression was induced by addition of 0.3 mm isopropyl 1-thio-β-d-galactopyranoside (final concentration). Cells were harvested and washed once with ice-cold PBS buffer. Cells were then resuspended in 5 ml of lysis buffer (1× PBS, 0.4% Triton X-100, 2 mm MgCl2, 1 mm EDTA, pH 8.0, 2 mm DTT, 0.2 mg/ml lysozyme, and protease inhibitor mixture (Complete EDTA-free, Roche Applied Science)) and incubated on ice for 15 min. Lysis was completed by 3 × 15-s pulses of sonication. Lysates were clarified by centrifugation for 10 min at 12,000 × g at 4 °C. The resulting supernatant fraction was incubated with 400 μl of a 50:50 slurry of glutathione-Sepharose beads (GE Healthcare) (pre-equilibrated in wash buffer (1× PBS, 0.1% Triton X-100, 2 mm MgCl2, 1 mm EDTA, 1 mm DTT)) in a roller drum for 1 h at 4 °C. Beads were collected by centrifugation at 500 × g for 2 min at 4 °C and washed five times with wash buffer.
Expression and Purification of HA-tagged Proteins from S. cerevisiae
For the induction of Pkh1-HA expression from the GAL promoter, plasmids were transformed into the protease-deficient yeast strain BJ2168. Cultures were grown to midlog phase on SC-raffinose medium, 2% galactose was added to the cultures, and cells were harvested and washed once with ice-cold PBS buffer 4 h later. Cells were resuspended in 500 μl of ice-cold lysis buffer (1× PBS, 0.1% Triton X-100, 10% glycerol, 2.5 mm MgCl2, 1 mm EDTA, 1 mm DTT, 10 mm NaF, 0.4 mm Na3VO4, 0.1 mm β-glycerophosphate containing protease inhibitor mixture (Complete EDTA-free, Roche Applied Science)). Glass beads were added, and cells were lysed by vigorous vortexing (4 × 1 min with cooling on ice in between). Lysates were clarified by centrifugation at 14,000 × g at 4 °C for 10 min. The supernatant was transferred to a new Eppendorf tube and centrifuged for a second time at 14,000 × g. Clarified extracts were incubated with 100 μl of a 50:50 slurry of protein G-agarose beads (washed with lysis buffer) and 5 μl of anti-HA antibody (Roche Applied Science) for 1 h at 4 °C. Bead-bound immunocomplexes were collected by centrifugation, and beads were extensively washed with lysis buffer. Finally, beads were resuspended in SDS sample buffer, boiled for 5 min at 95 °C, and analyzed via SDS-PAGE and Western blotting. Signal intensity was quantified with AIDA software.
Copper-inducible Expression of HA3-Tpk1
Yeast strains were transformed with a plasmid containing HA3-GFP-Tpk1 under control of the CUP1 promoter (derived from plasmid pPHY2203 (33)). Transformants were grown to midexponential phase (A600 nm = 1.5) at 24 °C on synthetic medium lacking uracil to select for plasmid maintenance. 1.2 m sorbitol was included in the growth medium to compensate for cell wall defects caused by Pkh inactivation. After Pkh inactivation by shifting cells to 35 °C for 30 min, Tpk1 expression was induced (for a period of 2 h) by the addition of CuSO4 to a final concentration of 100 μm. As a control, the same experiment was performed at the permissive temperature of 24 °C. Cells were harvested by centrifugation, and proteins were purified as described.
GST Pulldown Assay
GST fusion proteins were extracted from BL21 E. coli cells as described. Beads were finally resuspended in 500 μl of binding buffer (1× PBS, 0.05% Triton X-100, 0.1 mm DTT). Yeast extracts were prepared as described, and clarified extracts were incubated for 30 min at 4 °C with 50 μl of glutathione-Sepharose beads (GE Healthcare) to reduce aspecific binding. Beads were collected with a brief spin at 500 × g, and the resulting supernatant was incubated with equal amounts of bead-bound purified GST fusion proteins prepared as described. After a 2-h incubation at 4 °C, samples were allowed to stand for 5 min on ice. The sedimented beads were washed three times with PBS-T (1× PBS, 0.1% Triton X-100). Finally, proteins were solubilized by adding SDS sample buffer, separated by SDS-PAGE, and visualized by Coomassie staining or immunoblotting with anti-HA antibody.
In Vitro Kinase Assay
Yeast extracts were prepared as described. After incubation with the appropriate antibody, bead-bound immunocomplexes were collected by centrifugation and washed once with lysis buffer and twice with kinase buffer (50 mm Tris, pH 8.0, 1 mm EGTA, 1 mm DTT, 5 mm MgCl2, 0.5 mm Na3VO4, 10 mm β-glycerophosphate). Beads were resuspended in kinase buffer containing 10 μm ATP, 1 μCi of [γ-32P]ATP, and the appropriate substrate (purified as bead-bound GST fusion proteins from E. coli as described). Reaction mixtures were incubated for 45 min at 30 °C, and the reaction was terminated by addition of SDS sample buffer followed by boiling for 5 min at 95 °C. Proteins were resolved by SDS-PAGE. Gels were stained by Coomassie and analyzed by autoradiography using a PhosphorImager and by immunoblotting using anti-HA antibody.
Dot Blot Assay
To test specificity of the phosphospecific antibodies, a dot blot assay was performed. 100 μg of the synthetic phosphopeptides used to generate the antibodies (Eurogentec) was spotted on a nitrocellulose membrane (Hybond, Amersham Biosciences) and incubated for 1 h at room temperature with blocking buffer (5% (w/v) BSA in TBS-Tween buffer (25 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.05% (v/v) Tween 20). Membranes were incubated overnight at 4 °C with the appropriate primary antibody in blocking buffer. After this, blots were washed three times with TBS-Tween buffer and incubated with the appropriate secondary antibody in blocking buffer. After washing three times with TBS-Tween, membranes were incubated with SuperSignal chemiluminescence substrate (Pierce) for visualization.
Antibodies Used
HA-tagged proteins were immunoprecipitated using rat monoclonal antibodies (3F10) (Roche Applied Science) and detected using rat monoclonal anti-HA-peroxidase high affinity antibodies (Roche Applied Science). GST-PIFtide was detected using GST antibody from GE Healthcare. Anti-Bcy1 antibody was custom-made by Eurogentec. Sch9 Thr-570 phosphospecific antibodies and Tpk1 Thr-241 phosphospecific antibodies were generated by Eurogentec using synthetic phosphopeptides (with sequences H2N-KDRTNT(PO3H2)FCGTTEY-CONH2 and H2N-VPDVTYT(PO3H2)LCGTPD-CONH2, respectively) as immunizing reagent. The antiserum was depleted of antibodies that recognized the non-phosphorylated form before phosphospecific antibodies were purified. Secondary antibodies were from GE Healthcare.
In Vitro PKA Assay
Yeast extracts were prepared as described. Extracts were diluted to a final concentration of 0.125 μg/μl. The kinase reaction mixture (50-μl final volume) contained 0.5 μg of protein, 300 μm kemptide (Sigma), 5 μg of BSA in kinase buffer (25 mm Tris, pH 8.0, 2 mm Na3VO4, 1 mm β-glycerophosphate), and the indicated cAMP concentrations. Kinase reactions were initiated by addition of [γ-32P]ATP (to a final concentration of 0.1 μCi/μl) in the presence of 0.1 mm ATP and 1.5 mm Mg(CH3COO)2 (final concentrations). After incubation for 10 min at 30 °C, reactions were stopped by spotting 10 μl onto a phosphocellulose filter (Whatman P81). Filters were washed three times with phosphoric acid for 10 min and once with acetone. Filter papers were then dried at 60 °C, and radioactivity was measured using a scintillation counter.
Cell Size Measurements
Yeast strains containing GST-SCH9 behind the GAL1 promoter, integrated in the genome, were transformed with plasmids expressing HA3-tagged mutant versions of Sch9. Cells were diluted to a start A600 nm of 0.02, grown for 6 h in rich medium containing 2% galactose, and analyzed by flow cytometry. Cells were harvested, resuspended in rich medium containing 2% glucose as the carbon source, and allowed to grow for 9 h before being analyzed by flow cytometry. Forward light scattering was used as a measure of cell size. Standard deviations were below 6% in each case.
Reproducibility of Results
All experiments were repeated at least twice.
RESULTS
Deletion of PKH3 Exacerbates Growth Defect of pkh1ts pkh2Δ Strain
PKH3 was originally isolated as a multicopy suppressor of the growth defect of a pkh1D398G pkh2Δ strain (hereafter referred to as pkh1ts pkh2Δ). Unlike a pkh1Δ pkh2Δ strain, combined deletion of PKH3 and PKH1 or of PKH3 and PKH2 is viable (15). Because of this, studies on the functional role of yeast Pkh kinases have focused on Pkh1 and Pkh2. We have now created a pkh1ts pkh2Δ pkh3Δ strain to investigate the relative importance of all three PDK1 orthologs. Additional deletion of PKH3 exacerbated the growth defect of a pkh1ts pkh2Δ strain at the restrictive temperature of 35 °C (Fig. 1). Moreover, although the presence of 1.2 m sorbitol (acting as osmosupport and thus preventing cell lysis) could rescue the growth defect of a pkh1ts pkh2Δ strain at 35 °C, it did not fully restore growth in a pkh1ts pkh2Δ pkh3Δ strain. This could indicate a more severe cell wall defect in the latter strain that could not be entirely overcome by sorbitol. Alternatively, it is plausible that inactivation of all three Pkh protein kinases prevents activation of proteins involved in cell growth. In this regard, it is interesting to note that cells lacking Sch9 or cells with low PKA activity display a slow growth phenotype. Together with the presence of a putative PDK1 site in these kinases, this prompted us to investigate the precise connection between Pkh1–3, Sch9, and PKA.
FIGURE 1.
Deletion of PKH3 exacerbates growth defect of pkh1ts pkh2Δ strain at 35 °C. 10-fold serial dilutions (starting A600 nm = 1.0) of wild type, pkh1ts pkh2Δ, and pkh1ts pkh2Δ pkh3Δ strains were spotted on YPD (yeast, peptone, D-glucose) plates or YPD plates containing 1.2 m sorbitol and incubated for 2 days at 24 °C or 35 °C.
Pkh1 Interacts with Sch9 in Vitro
Using a GST pulldown assay, we could demonstrate binding between Sch9 and Pkh1. An N-terminal GST fusion of the Sch9 protein was expressed in E. coli, purified, and incubated with yeast extract containing Pkh1-HA. After isolation of GST-Sch9, Pkh1-HA could be recovered, indicating that these proteins interact (Fig. 2A). Our results are consistent with a recent report documenting interaction between Sch9 and Pkh2 (27).
FIGURE 2.
Pkh1 interacts with Sch9, interaction depends on hydrophobic motif in Sch9 and on matching hydrophobic pocket in Pkh1, and this pocket is important for growth. A, B, and C, the indicated GST fusion proteins were purified from bacteria onto glutathione-Sepharose beads and incubated with cell extracts from strain BJ2168 expressing Pkh1-HA. A GST pulldown assay demonstrates interaction between Pkh1-HA and GST-Sch9 (A) and between Pkh1-HA and GST-PIFtide (B). No Pkh1 was recovered when the assay was performed with GST alone. Mutagenesis of the hydrophobic motif (FXXF) in GST-PIFtide (B) and in GST-Sch9 (C) abolishes or strongly reduces the interaction as does mutagenesis of the hydrophobic PIF pocket in Pkh1-HA (A). The input fraction represents 10% of the total amount added to each binding reaction. The amount of Pkh1-HA that is pulled down by GST-Sch9 (FXXA) and GST-Sch9 (AXXF) represents 21 and 16%, respectively, of the amount pulled down by GST-Sch9. D, the hydrophobic pocket mutant of Pkh1 partially rescues the temperature-sensitive phenotype of a pkh1ts pkh2Δ strain but is unable to rescue the temperature-sensitive phenotype of a pkh1ts pkh2Δ pkh3Δ strain as measured by growth at 35 °C. 10-fold serial dilutions (start A600 nm = 1.0) of the different strains were spotted on SD-Trp plates and incubated for 2 days. As a control, growth on SD-Trp at 24 °C is shown.
Sch9 belongs to the class of AGC kinases whose members contain both a PDK1 phosphorylation site and a C-terminal HM. The yeast Pkh proteins display high sequence similarity to mammalian PDK1 in the region comprising the hydrophobic PIF pocket, which contributes to PDK1-substrate binding through interaction with the HM in substrates (10, 34). To check whether Pkh1 has a functional equivalent of the PIF pocket found in PDK1 and thus perhaps a substrate binding mechanism similar to PDK1, a binding assay using GST-PIFtide was performed. This peptide of 24 amino acids encompasses the HM found in the PDK1 substrate PRK2 and shows high similarity to the HM found in PKB except that the residue equivalent to Ser-473 in PKB is an acidic Asp. GST-PIFtide interacted with Pkh1, and mutagenesis of the FXXF motif (part of the HM) of PIFtide into FXXA almost completely abolished interaction (Fig. 2B). Substitution of Phe by Ala in the FXXF motif of Sch9 reduced the amount of bound Pkh1 as shown by a GST pulldown assay (Fig. 2C).
We also modified the PIF pocket of Pkh1 by mutagenesis of a conserved, crucial hydrophobic residue into a glutamate residue (L199E), thus disrupting the hydrophobic nature of the pocket. Although the expression of the Pkh1L199E allele was somewhat reduced compared with the wild type allele, the results clearly showed that mutagenesis of the Leu residue largely abolished Pkh1-Sch9 binding (Fig. 2A). In addition, the L199E mutant allele cannot substitute for wild type Pkh1 in vivo as judged by its inability to rescue the temperature-sensitive phenotype of a pkh1ts pkh2Δ pkh3Δ strain (Fig. 2D). The pkh1ts pkh2Δ strain was partially rescued by this allele, suggesting a function for Pkh3 in support of growth under certain conditions.
Pkh1 Phosphorylates Sch9 on Its PDK1 Site Both in Vitro and in Vivo
All known substrates of Pkh1 (the cell integrity signaling proteins Ypk1, Ypk2, and Pkc1) are phosphorylated on their PDK1 site (14, 15, 17). Sch9 too contains a PDK1 site, and we could demonstrate Pkh1-dependent phosphorylation of Sch9 in an in vitro kinase assay (Fig. 3A). This phosphorylation was strongly reduced in a Sch9T570A mutant protein, confirming that Pkh1 phosphorylates Sch9 primarily on its PDK1 site (Fig. 3A). While our work was in progress, consistent results were published by Urban et al. (27). Taken together, this demonstrates that yeast Pkh proteins are capable of phosphorylating the PDK1 site in Sch9, similar to PDK1-dependent activation of PKB and S6K (35, 36). Mutagenesis of the hydrophobic pocket in Pkh1 prevented PDK1 site phosphorylation of Sch9 (Fig. 3B).
FIGURE 3.
Pkh1 phosphorylates Sch9 in vitro and in vivo on its PDK1 site (Thr-570), and this phosphorylation is required for cell size control. A, Pkh1-HA, but not a kinase-dead (KD) mutant version thereof, phosphorylates recombinant Sch9 on Thr-570. A Western blot shows similar levels of both active and inactive Pkh1-HA, and Coomassie staining of the same gel as that used to obtain the autoradiogram shows equal levels of GST-Sch9 and GST-Sch9T570A. B, hydrophobic pocket mutagenesis of Pkh1 prevents PDK1 site phosphorylation of Sch9 in vitro. Pkh1-HA, but not Pkh1L199E-HA, phosphorylates recombinant Sch9. A Western blot shows similar levels of the different mutants of Pkh1-HA, and Coomassie staining of the same gel as that used to obtain the radiogram shows equal levels of GST-Sch9 in the different kinase reactions. The different small blots in A and B come from the same original autoradiogram for each experiment. C, phosphospecific antibodies directed against the Thr-570 site specifically recognize HA3-Sch9 phosphorylated on its PDK1 site, and in vivo, this phosphorylation requires Pkh1–3 activity. Upper left panel, 100 μg of the phosphorylated (left) and unphosphorylated (right) peptide containing the PDK1 site of Sch9 were spotted on a nitrocellulose membrane. The phosphospecific antibody only recognizes the phosphorylated peptide. Upper right panel, the antibody does not recognize HA3-Sch9T570A. Lower panels, cells of the different mutant strains (each expressing HA3-Sch9 from plasmid pRS316-HA3-Sch9) were grown to midexponential phase, harvested, and resuspended in medium containing 1.2 m sorbitol. Cultures were split in half: whereas control strains were incubated at 24 °C, the others were shifted to 35 °C for 30 min. A Western blot shows levels of immunoprecipitated HA3-Sch9 from each of the strains. Values represent the intensity of phospho-Thr-570 signal over HA signal with wild type at 24 °C taken as reference. D, mutagenesis of the Thr-570 phosphorylation site in Sch9 reduces cell size (see also Table 1). Forward light scattering (FSC) was used as a measure of cell size. Experiments were performed with strain LW201.
To check phosphorylation of the PDK1 site of Sch9 in vivo, phosphospecific antibodies were raised against phospho-Thr-570. A synthetic peptide comprising the phosphorylated PDK1 site in Sch9 was used for immunization. Dot blot assays showed that the antibody is specific for the phosphorylated peptide (Fig. 3C, upper left panel). The antibody recognized the wild type Sch9 protein but not a mutant allele in which Thr-570 was replaced with Ala (Fig. 3C, upper right panel). The nature of the modifications responsible for the two or sometimes multiple bands visible on the HA blots is currently not known. Urban et al. (27) showed that a pkh1ts pkh2Δ strain still displayed PDK1 site phosphorylation of Sch9 at the permissive temperature. We now show that incubating the pkh1ts pkh2Δ pkh3Δ strain at the permissive temperature already abolished all detectable phosphorylation on the Sch9 PDK1 site (Fig. 3C, lower panels), whereas phosphorylation of this site was still seen in a pkh1ts pkh2Δ strain at 24 °C in our experiments. Incubating this strain at 35 °C abolished this phosphorylation. These results show that the PDK1 site of Sch9 is phosphorylated in vivo and that all three Pkh protein kinases, including Pkh3, can sustain this phosphorylation to some extent.
Mutagenesis of PDK1 Site in Sch9 Reduces Cell Size
A well-established phenotype of inactivation of Sch9 is a smaller cell size (20). PDK1-dependent phosphorylation of the PKB and S6K protein kinases is necessary for their full activity in mammalian cells (35, 36), and we reasoned that this might also be the case for Sch9 in S. cerevisiae. Because strains deleted for SCH9 easily accumulate suppressor mutations (21), we first constructed a strain with conditional expression of SCH9. We replaced the endogenous copy of SCH9 with an allele expressing GST-SCH9 from the GAL1 promoter. Induction of Sch9 on galactose medium and repression on glucose medium was confirmed by immunoblotting experiments (results not shown). Moreover, similar to what is seen for an sch9Δ strain, asparagine-induced RPL25 gene expression was absent when Sch9 was not expressed (supplemental Fig. S1 and Ref. 19). To evaluate the effect of different PDK1 site mutant alleles of Sch9 on yeast cell size, the different mutant alleles were transformed into this strain. Next, their ability to support the wild type cell size on glucose medium was examined. These conditions cause repression of the endogenous GAL-SCH9 construct, making the mutant alleles the only form of Sch9 present. All these strains displayed a somewhat smaller cell size compared with a strain expressing functional Sch9 (Table 1 and Fig. 3D). This indicates a requirement for PDK1 site phosphorylation in Sch9 for cell size regulation and thus likely also for catalytic activity. To our knowledge, these results are the first to imply PDK1 site phosphorylation of Sch9 in the proper control of yeast cell size.
TABLE 1.
Effect of PDK1 site mutagenesis of Sch9
The indicated plasmids were transformed into strain LW201 (sch9::promGAL1-GST-SCH9). Cells were analyzed by flow cytometry after 9 h of growth on glucose-containing medium. Forward scattering was used as a measure of cell size. Standard deviations were below 6% in each case.
| Plasmid (pRS316) | Cell size |
|---|---|
| % of WT | |
| HA3-Sch9 | 100 |
| Empty | 87 |
| HA3-Sch9T570A | 88 |
| HA3-Sch9T570D | 84 |
| HA3-Sch9T570E | 88 |
| HA3-Sch9K441A | 89 |
Nitrogen Levels Affect Phosphorylation of PDK1 Site in Sch9
Sch9 is thought to function in a nitrogen-responsive signaling pathway, and its activity is partially regulated by the nitrogen-sensitive TORC1 complex.
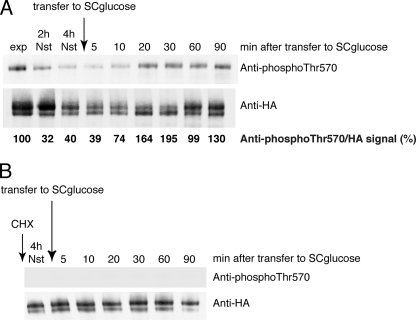
Thus, in a next step, we checked whether PDK1 site phosphorylation in Sch9 is sensitive to changes in nitrogen availability in the medium. Shifting cells from complete, nitrogen-containing SC-glucose medium to medium lacking any nitrogen source resulted in reduced PDK1 site phosphorylation already 2 h after the start of nitrogen deprivation. Transferring cells back to complete SC-glucose medium containing all nitrogen compounds required for sustained growth led to rapid rephosphorylation within 10 min (Fig. 4A). Resupplementing cells with a single nitrogen source (10 mm l-asparagine or 10 mm NH4+) resulted in a much lower level of Thr-570 rephosphorylation (results not shown). Moreover, this level never reached the original level observed in exponentially growing cells. These data indicate that PDK1 site phosphorylation in Sch9 is sensitive to the nitrogen status of a medium that is appropriate for growth and that it responds rapidly to deprivation and resupplementation. Intriguingly, the addition of cycloheximide 30 min prior to nitrogen resupplementation completely abolished the nitrogen-induced rephosphorylation of Sch9 and inhibited growth (Fig. 4B). Cells expressing a PDK1 site mutant of Sch9 resumed growth much more slowly than cells expressing wild type Sch9 when transferred from nitrogen deprivation conditions to a complete growth medium (results not shown). Taken together, these data indicate a tight coupling between PDK1 site phosphorylation of Sch9 and nutrient-induced growth resumption.
FIGURE 4.
Phosphorylation of PDK1 site in Sch9 is controlled by nitrogen availability. A, nitrogen deprivation of fermenting cells reduces phosphorylation, whereas transfer of the nitrogen-deprived cells to complete SC-glucose medium (lacking uracil for plasmid maintenance) results in rephosphorylation. Values represent the intensity of phospho-Thr-570 signal over HA signal with exponential phase (exp) taken as reference. B, addition of cycloheximide (CHX; 25 μg/ml; added 30 min prior to medium change) abrogates this rephosphorylation. HA3-Sch9 was immunoprecipitated from strain BY4742 transformed with pRS316-HA3-Sch9 and detected via Western blotting. exp, exponential phase; Nst, nitrogen starvation.
Mammalian PDK1 responds to phosphoinositides generated by phosphatidylinositol 3-kinase after cell stimulation by insulin or growth factors. In this way, regulatory cues from intercellular messengers are signaled to PDK1. S. cerevisiae does not produce the PtdIns molecules that activate mammalian PDK1. Instead, Pkh proteins were reported to be regulated by the long chain bases phytosphingosine (PHS) and dihydrosphingosine, precursors of sphingolipid biosynthesis (28, 37). The addition of 20 μm PHS, however, did not increase Sch9 PDK1 site phosphorylation, nor did inhibition of long chain base synthesis by 1.25 μm myriocin decrease this phosphorylation (supplemental Fig. S2). These experiments were done with exponentially growing cells and thus do not rule out an effect of these lipids under other conditions.
Pkh1 Binds to Tpk1 and Phosphorylates Its PDK1 Site in Vitro
Sch9 is not the only nutrient-regulated kinase in yeast containing a PDK1 phosphorylation site. Such a site is also present in the catalytic subunits of PKA encoded by TPK1, TPK2, and TPK3. Tpk1 contains a phosphothreonine at position 241, the putative PDK1 site (29). However, the kinase responsible for this phosphorylation has never been identified. The corresponding site in mammalian PKA is also phosphorylated, and for many years, the upstream kinase was not known with certainty (30, 32). PKA autophosphorylation and PDK1-dependent phosphorylation are the two main candidate mechanisms. Recently, increasing evidence is pointing to PDK1 as the main kinase responsible for this phosphorylation in mammalian cells and also in Schizosaccharomyces pombe (38). Both mammalian PKA and the yeast Tpks have a C-terminal FXXF motif but lack the characteristic phosphoacceptor residue of the HM.
Using an in vitro pulldown assay, we could demonstrate interaction between yeast Pkh1-HA and GST-Tpk1 (Fig. 5A). Furthermore, a considerably lower amount of Pkh1 was pulled down by a Tpk1 mutant protein in which one or both of the Phe residues in the C-terminal FXXF motif were mutated to Ala (Fig. 5B). Mutagenesis of Leu-199 to Glu in the PIF pocket of Pkh1 completely abolished Pkh1-Tpk1 interaction, underscoring the importance of this pocket (Fig. 5A). Hence, despite the absence of the phosphorylatable residue in its HM, Tpk1 displays a similar interaction with Pkh1 as Sch9. Similarly, mammalian PDK1 interacts with the C-terminal part of PKA in a yeast two-hybrid screen, and mutations in the FXXF motif of PKA reduce this interaction (34).
FIGURE 5.
Pkh1 interacts with Tpk1 and is responsible for phosphorylation of the PDK1 site in Tpk1 in vitro and in vivo. A and B, Pkh1-HA interacts with recombinant GST-Tpk1 in a GST pulldown assay (A), and this interaction is abolished by mutagenesis of the hydrophobic pocket in Pkh1-HA (A) and by mutagenesis of the hydrophobic motif in GST-Tpk1 (B). The indicated GST fusion proteins were purified from bacteria onto glutathione-Sepharose beads and incubated with cell extracts from strain BJ2168 expressing Pkh1-HA or Pkh1L199E-HA. The input fraction represents 10% of the amount added to each binding reaction. C, Pkh1-HA, but not a kinase-dead (KD) variant, phosphorylates GST-Tpk1K116M on Thr-241, its PDK1 site. A Western blot shows similar levels of both active and inactive Pkh1-HA, and Coomassie staining of the same gel as that used to obtain the autoradiogram shows equal levels of GST-Tpk1K116M and Tpk1K116M,T241A. The different small blots come from the same original autoradiogram for each experiment. D, phosphospecific antibodies directed against Thr-241 recognize immunoprecipitated HA3-Tpk1 phosphorylated on its PDK1 site but not the HA3-Tpk1T241A mutant protein. The left panel shows the result of a dot blot assay in which 100 μg of the phosphorylated (left) and unphosphorylated (right) peptide containing the PDK1 site of Tpk1 were spotted on a nitrocellulose membrane. The antibody only recognizes the phosphorylated peptide. E, in vivo phosphorylation of the PDK1 site in HA3-Tpk1. Cells of the different mutant strains (each expressing HA3-Tpk1) were grown to midexponential phase, harvested, and resuspended in medium containing 1.2 m sorbitol. Cultures were split in half: whereas control strains were incubated at 24 °C, the others were shifted to 35 °C for 30 min. A Western blot shows levels of immunoprecipitated HA3-Tpk1 from each of the strains. F, PDK1 site phosphorylation of Tpk1 occurs during or shortly after synthesis and is controlled by Pkh activity. Cells were grown to midexponential phase at 24 °C and shifted for 30 min to 35 °C before inducing expression of HA3-GFP-Tpk1 (for 2 h) by the addition of 100 μm CuSO4 (final concentration). Western blotting of immunoprecipitated Tpk1 protein shows that inactivation of the Pkh kinases drastically reduces PDK1 site phosphorylation. Values represent the intensity of phospho-Thr-241 signal over HA signal with wild type at 24 °C after induction taken as a reference.
To check whether the catalytic subunits of yeast PKA could also be substrates for Pkh, we first expressed Tpk1 as an N-terminal GST fusion protein in E. coli. Because this protein showed very strong autophosphorylation in vitro, we constructed a catalytically inactive allele of Tpk1, Tpk1K116M. Incubation with immunopurified Pkh1-HA resulted in phosphorylation of GST-Tpk1K116M as detected by incorporation of 32P in Tpk1 (Fig. 5C). This phosphorylation was significantly reduced in a Tpk1K116M,T241A mutant, indicating that Thr-241 is the main residue targeted by Pkh1 in vitro.
In Vivo, Pkh Protein Kinases Phosphorylate PDK1 Site in Newly Synthesized Tpk1
We also checked whether the PDK1 site in Tpk1 is phosphorylated in vivo using a custom-made phosphospecific antibody. This antibody was raised against a peptide encompassing the phosphorylated PDK1 site of Tpk1. Dot blot assays confirmed that the antibody is specific for the phosphorylated form of the peptide (Fig. 5D, left panel). The antibody recognized HA3-Tpk1 but not the HA3-Tpk1T241A protein, confirming that Tpk1 is phosphorylated in vivo on its PDK1 site (Fig. 5D, right panel). HA3-Tpk1 isolated from a pkh1ts pkh2Δ pkh3Δ strain incubated at 35 °C, however, still showed PDK1 site phosphorylation (Fig. 5E). Also, prolonged incubation of the temperature-sensitive strains at 35 °C did not abolish PDK1 site phosphorylation (results not shown).
Initial studies on phosphorylation of the corresponding residue in mammalian PKA indicated that PKA is assembled as an active enzyme with a fully phosphorylated activation loop. One study suggested that this phosphorylation takes place during maturation of the PKA kinase. Once phosphorylated, this site is extremely stable and resistant to dephosphorylation (39, 40). In accordance, our attempts to dephosphorylate HA3-Tpk1 using commercially available phosphatases were unsuccessful (results not shown). Thus, the stability of this phosphorylation might obscure any possible effect of Pkh inactivation. To investigate the effect of Pkh inactivation on PDK1 site phosphorylation of newly synthesized Tpk1, expression of HA3-GFP-Tpk1 was induced after a 30-min incubation at 35 °C of either a WT or a pkh1ts pkh2Δ pkh3Δ strain, and the phosphorylation state of this newly synthesized Tpk1 was determined. The use of a GFP-tagged and thus larger version of Tpk1 allowed us to clearly distinguish newly synthesized Tpk1 from any endogenous PKA catalytic subunits that might co-immunoprecipitate. Fig. 5F shows that in a pkh mutant strain PDK1 site phosphorylation of Tpk1 was drastically reduced compared with a WT strain.
Next, we screened several kinase mutants, including mutants in PKA and TOR, for their possible importance for Thr-241 phosphorylation in Tpk1. None of the mutants tested affected PDK1 site phosphorylation of newly synthesized HA3-GFP-Tpk1 (supplemental Fig. S3).
We also tested whether PDK1 site phosphorylation of Tpk1 was subject to nitrogen regulation in vivo as we have found for Sch9. However, phosphorylation of this site in PKA did not drop upon nitrogen deprivation, and nitrogen resupplementation also did not cause any increase (Fig. 6). Addition of glucose to glucose-deprived cells also did not result in any change in phosphorylation of this site (supplemental Fig. S4). This seems to exclude a role for PDK1 site phosphorylation in PKA in nitrogen and glucose signaling.
FIGURE 6.
PDK1 site phosphorylation of Tpk1 is not controlled by nitrogen availability. The addition of 10 mm l-asparagine to nitrogen-deprived cells does not affect phosphorylation of Thr-241 in Tpk1. HA3-Tpk1 was immunoprecipitated from strain BY4742 and detected via Western blotting.
Importance of PDK1 Site in Tpk1 for Its Activity and Regulation
In mammalian PKA, the importance of PDK1 site phosphorylation for catalytic activity is well-established. The resolved crystal structure of active PKA (Protein Data Bank code 1U7E) shows that this phosphorylated residue engages in several hydrogen bonds and ionic interactions in the catalytic core of PKA. This results in a catalytically competent conformation of PKA and additionally creates a docking surface for the regulatory subunit (41). Therefore, mutagenesis of this residue is expected to result in a completely disordered and thus inactive kinase conformation.
Surprisingly, the yeast Tpk1T241A mutant protein was first isolated as a suppressor of the lethality of a ras1Δ ras2ts strain (42). Ras proteins are small G proteins required for adenylate cyclase activity. Deleting both RAS genes in yeast is lethal but can be rescued by additional deletion of BCY1, the PKA regulatory subunit (43). These results hinted at a role for Thr-241 in the interaction between Tpk1 and Bcy1 and, importantly, also indicated that this mutation apparently does not abolish PKA activity in yeast. To investigate in more detail the biochemical and physiological relevance of Thr-241 phosphorylation, we constructed a strain containing a PDK1 site mutant version of Tpk1 as the only source of PKA activity. Such a strain is viable (results not shown), indicating that the Tpk1T241A mutant protein has sufficient activity to sustain the essential functions of PKA.
We determined the amount of Bcy1 associated in vitro with either the wild type Tpk1 protein or the Tpk1T241A mutant protein and found, in agreement with the previous report by Levin et al. (42), that the Tpk1T241A protein did not bind Bcy1 (Fig. 7A). The authors also did not detect a significant effect of this mutation on PKA catalytic activity. To determine the effect of the T241A mutation on PKA activity in vitro, cell extracts containing the wild type Tpk1 or the mutant protein as the only source of PKA activity were used in an in vitro PKA assay with cAMP-dependent kemptide phosphorylation as a readout for PKA activity. Interestingly, whereas the wild type Tpk1 allele was stimulated by increasing cAMP levels, the Tpk1T241A mutant allele was unaffected by cAMP (Fig. 7B). The lack of binding to Bcy1 can only partially explain this result because Tpk1T241A activity corresponded to the basal level of activity observed in the absence of cAMP with the wild type Tpk1 protein (Fig. 7B). This argues for a role of Thr-241 (or its phosphorylation) in sustaining the fully active conformation of PKA.
FIGURE 7.
PDK1 site phosphorylation in HA3-Tpk1 is important for interaction with Bcy1 and for catalytic activity. A, mutagenesis of the PDK1 site in HA3-Tpk1 abolishes Bcy1 binding. HA3-Tpk1 or HA3-Tpk1T241A was immunoprecipitated from strains KV28 (tpk1,2,3Δ pRS313-Tpk1) and KV29 (tpk1,2,3Δ pRS313-Tpk1T241A), each expressing HA3-Tpk1. The amount of Bcy1 associated with each of these proteins was determined with anti-Bcy1 antibody. B, PDK1 site mutation renders Tpk1 insensitive to stimulation by cAMP. Crude cell extracts from strains KV28 (Tpk1 only; ●) and KV29 (Tpk1T241A only; ○) were used, and kemptide phosphorylation is expressed as a function of increasing cAMP concentration. spec. PKA act., specific PKA activity.
DISCUSSION
Pkh1 Interacts with Sch9 and Tpk1 in Manner Characteristic of PDK1-Substrate Binding
Interaction of mammalian PDK1 with a significant number of its substrates is mediated by the binding of a C-terminal phosphorylated hydrophobic motif in the substrate to a hydrophobic pocket in the kinase domain of PDK1. The yeast Pkh proteins show high sequence similarity to mammalian PDK1 in their catalytic domain, including the region comprising the hydrophobic pocket. Using an in vitro GST pulldown assay, we could demonstrate interaction of Pkh1, with Sch9, and Tpk1. Mutagenesis of the hydrophobic pocket in Pkh1 abolished the interaction with Sch9 and Tpk1. Mutagenesis of the Phe residues located in the hydrophobic motifs of Sch9 and Tpk1 reduced the interaction with Pkh1 but did not completely disrupt it.
PDK1 Site Phosphorylation of Sch9 by Pkh1–3 Is Sensitive to Nitrogen Status of Medium
Cell integrity signaling by Pkh1–2 is mediated by PDK1 site phosphorylation of the kinases Ypk1, Ypk2, and Pkc1. Our results now show that the Pkh kinases also phosphorylate the PDK1 site in Sch9. We demonstrated in vivo loss of this phosphorylation during nitrogen deprivation and rapid rephosphorylation when nitrogen was resupplemented.
At present, we cannot exclude the possibility that nitrogen availability (also) regulates the activity of a protein phosphatase targeting the PDK1 site in Sch9. Interestingly, the nitrogen-sensitive TORC1 complex regulates the activity of the type 2A and type 2A-related protein phosphatases by controlling their interaction with the Tap42 protein. Phosphoproteomics analyses, however, indicate that Tap42 and Sch9 represent two distinct effectors of TORC1 signaling, functioning parallel to each other (22). Indeed, treatment of exponentially growing cells with rapamycin (inhibiting TORC1 and thus activating the aforementioned phosphatases) does not reduce PDK1 site phosphorylation of Sch9 (27).
Phosphorylation of Sch9 by both TORC1 and Pkh is required to obtain a fully active kinase, but these phosphorylation sites are differentially regulated. PDK1 site phosphorylation of Sch9 was reduced during nitrogen deprivation but not when cells were subjected to heat stress (results not shown) or treated with rapamycin (27). The latter two conditions do reduce TORC1-dependent phosphorylation of Sch9 (27). This underscores the divergent regulation of different phosphorylation sites in Sch9 and also points to different factors that control Pkh and TORC1 activity.
Long chain bases, the only known regulators of Pkh proteins to date, do not appear to regulate Thr-570 phosphorylation. Data presented by Liu et al. (28) showed an increase in overall Sch9 phosphorylation after PHS addition to an in vitro kinase reaction containing both Sch9 and Pkh1. However, an increase was also seen when Pkh1 was replaced by a kinase-dead variant, indicating that PHS could exert (part of) its effect through stimulation of Sch9 autophosphorylation (28). Recent work also indicates that changes in the level of intracellular PHS do not alter PDK1 phosphorylation of the well-characterized Pkh target Ypk1 (44).
It remains to be investigated how the nitrogen signal is transduced to Pkh and Sch9. It is possible that the nitrogen-responsive transceptors Gap1 and/or Mep2 (45–47) are involved in Pkh-dependent control of Sch9 phosphorylation. However, whereas rapid signaling by these transceptors occurs independently of protein synthesis, treatment of cells with cycloheximide prevented nitrogen-induced PDK1 site phosphorylation of Sch9. Moreover, when only asparagine or ammonium was added to nitrogen-deprived cells, rephosphorylation was significantly impaired and did not reach the level observed in exponentially growing cells. Transferring cells to complete growth medium did result in complete rephosphorylation of this site. Complete growth medium consists of a mixture of all amino acids, ammonium sulfate, vitamins, and minerals required for growth. The latter two components are also present in medium containing only l-Asn or NH4+, ruling out the possibility that these components (and not the nitrogen sources) are actually affecting the phosphorylation. Because of the auxotrophies of the yeast strain used in these experiments, addition of a single nitrogen source, such as asparagine, was unable to sustain growth. Hence, PDK1 site phosphorylation of Sch9 may be connected to nitrogen-induced growth.
On the other hand, reduced phosphorylation of this site does not appear to be a simple consequence of growth arrest because not all conditions that arrest growth abolish PDK1 site phosphorylation like nitrogen deprivation does. For instance, treatment of cells with the drugs myriocin (this work) or rapamycin (27) induced growth arrest but did not reduce PDK1 site phosphorylation of Sch9. This indicates that disappearance of PDK1 site phosphorylation of Sch9 during nitrogen deprivation is not simply due to growth arrest but could be a specific response to the absence of an adequate nitrogen supply to sustain growth. It is also interesting to note that in nature yeast is unlikely to encounter only a single type of nitrogen source. When cells are confronted with new conditions, this usually involves a mixture of nutrients, including nitrogen sources. Hence, transfer of nitrogen-deprived cells to a complete growth medium might actually resemble natural conditions better than mere addition of a pure nitrogen source.
In Vivo Phosphorylation of Tpk1 by Pkh Protein Kinases Occurs during (or Shortly after) Protein Synthesis and Is Highly Stable
PKA catalytic subunits also contain a putative PDK1 phosphorylation site, and we could demonstrate for the first time that Pkh1 is able to phosphorylate this site (Thr-241) in vitro. For mammalian PKA, it is not clear whether PDK1 site phosphorylation is a regulated event or whether this phosphorylation is constitutive. Studies indicate that during its synthesis mammalian PKA is assembled as an active kinase with a phosphorylated activation loop. It has been suggested that PDK1 is responsible for this phosphorylation during maturation of the PKA catalytic subunits (30), although phosphorylation of this site by PKA itself also has been suggested (48). Our results now show for the first time that in yeast Pkh activity is required for PDK1 site phosphorylation of newly synthesized Tpk1 protein. This phosphorylation appears to be extremely stable and not nitrogen-regulated. The resolved crystal structure of active mammalian PKA gives insight into the highly stable nature of this phosphorylation and helps to formulate a model for PDK1 site phosphorylation of Tpk1. Phosphorylation on newly synthesized Tpk1 protein creates a phosphogroup that can engage in several structurally important interactions within the catalytic core of Tpk1, thus creating an active kinase conformation. The resulting conformational changes also prevent access of phosphatases to this phosphogroup, explaining its stability. Importantly, PKA autophosphorylation does not seem to be involved in regulating phosphorylation on this site (supplemental Fig. S3A).
Whereas the activity of most other AGC kinases, such as Sch9, appears to be regulated by phosphorylation of the PDK1 site in their activation loop, PKA may be controlled largely by the classical cAMP-dependent dissociation of the catalytic and regulatory subunits. On the other hand, several results indicate the existence of additional controls on PKA besides cAMP regulation (49–52). It has been reported that glucose affects the overall phosphorylation state of Tpk1. Addition of glucose to cells growing on the respiratory carbon source glycerol leads to an increase in the phosphorylation of Tpk1 and a concomitant increase in PKA activity (53). Conversely, upon glucose exhaustion, phosphorylation decreases. The precise site(s) phosphorylated under these conditions has not been identified. Our results now exclude the most obvious candidate, phosphorylation of Thr-241 in the activation loop, from involvement because this phosphorylation does not respond to nutrient stimuli, such as glucose and nitrogen.
Taken together, our results identify the yeast PDK1 orthologs as important regulators of Sch9 and PKA. Both kinases are phosphorylated on their PDK1 site by the yeast Pkh protein kinases both in vitro and in vivo. Although this phosphorylation is regulated post-translationally by nitrogen availability for Sch9, it occurs during synthesis of the protein for PKA. In both cases, PDK1 site phosphorylation is important for kinase activity. Our results further extend the similarity between these yeast and mammalian signaling modules, which play a crucial role in many cellular regulatory processes.
Supplementary Material
Acknowledgments
We thank Dr. R. M. Biondi for the suggestion of evaluating PDK1 site phosphorylation during synthesis of Tpk1. We thank M. De Jonge, T. Adriany, and R. Wicik for excellent technical assistance; N. Vangoethem for help with preparation of the figures; and D. Castermans for critically reading the manuscript. We are grateful to M. Deak, P. Herman, M. Hall, K. Irie, J. Thorner, and M. Tyers for the kind gift of strains and plasmids.
This work was supported by a Ph.D. fellowship from the Fund for Scientific Research-Flanders (FWO) (to K. V. and M. K.), a fellowship from the Agency for Innovation by Science and Technology (IWT-Flanders) (to S. H.), a return grant from the Belgian Federal Science Policy Office (to M. V.), and grants from the Fund for Scientific Research-Flanders, Interuniversity Attraction Poles Network P5/30 and P6/14 and the Research Fund of the KULeuven (Concerted Research Actions) (to J. M. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Materials and Methods, Figs. S1–S4, and Tables 1 and 2.
- PKA
- protein kinase A
- PDK1
- 3-phosphoinositide-dependent protein kinase-1
- HM
- hydrophobic motif
- PIF
- PDK1-interacting fragment
- ts
- temperature-sensitive
- PKB
- protein kinase B
- PKG
- protein kinase G
- PtdIns
- phosphatidylinositol
- (m)TOR
- (mammalian) target of rapamycin
- SC
- synthetic complete
- PHS
- phytosphingosine
- SD
- synthetic defined
- PI3K
- phosphatidylinositol 3-kinase.
REFERENCES
- 1. Johnson L. N., Noble M. E., Owen D. J. (1996) Cell 85, 149–158 [DOI] [PubMed] [Google Scholar]
- 2. Pearce L. R., Komander D., Alessi D. R. (2010) Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 3. Vanhaesebroeck B., Leevers S. J., Panayotou G., Waterfield M. D. (1997) Trends Biochem. Sci. 22, 267–272 [DOI] [PubMed] [Google Scholar]
- 4. Andjelković M., Alessi D. R., Meier R., Fernandez A., Lamb N. J., Frech M., Cron P., Cohen P., Lucocq J. M., Hemmings B. A. (1997) J. Biol. Chem. 272, 31515–31524 [DOI] [PubMed] [Google Scholar]
- 5. Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G. F., Holmes A. B., Gaffney P. R., Reese C. B., McCormick F., Tempst P., Coadwell J., Hawkins P. T. (1998) Science 279, 710–714 [DOI] [PubMed] [Google Scholar]
- 6. Currie R. A., Walker K. S., Gray A., Deak M., Casamayor A., Downes C. P., Cohen P., Alessi D. R., Lucocq J. (1999) Biochem. J. 337, 575–583 [PMC free article] [PubMed] [Google Scholar]
- 7. Milburn C. C., Deak M., Kelly S. M., Price N. C., Alessi D. R., Van Aalten D. M. (2003) Biochem. J. 375, 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frödin M., Antal T. L., Dümmler B. A., Jensen C. J., Deak M., Gammeltoft S., Biondi R. M. (2002) EMBO J. 21, 5396–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frödin M., Jensen C. J., Merienne K., Gammeltoft S. (2000) EMBO J. 19, 2924–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biondi R. M., Kieloch A., Currie R. A., Deak M., Alessi D. R. (2001) EMBO J. 20, 4380–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biondi R. M., Komander D., Thomas C. C., Lizcano J. M., Deak M., Alessi D. R., van Aalten D. M. (2002) EMBO J. 21, 4219–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biondi R. M. (2004) Trends Biochem. Sci. 29, 136–142 [DOI] [PubMed] [Google Scholar]
- 13. Jacinto E., Lorberg A. (2008) Biochem. J. 410, 19–37 [DOI] [PubMed] [Google Scholar]
- 14. Casamayor A., Torrance P. D., Kobayashi T., Thorner J., Alessi D. R. (1999) Curr. Biol. 9, 186–197 [DOI] [PubMed] [Google Scholar]
- 15. Inagaki M., Schmelzle T., Yamaguchi K., Irie K., Hall M. N., Matsumoto K. (1999) Mol. Cell. Biol. 19, 8344–8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. deHart A. K., Schnell J. D., Allen D. A., Hicke L. (2002) J. Cell Biol. 156, 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roelants F. M., Torrance P. D., Bezman N., Thorner J. (2002) Mol. Biol. Cell 13, 3005–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toda T., Cameron S., Sass P., Wigler M. (1988) Genes Dev. 2, 517–527 [DOI] [PubMed] [Google Scholar]
- 19. Crauwels M., Donaton M. C., Pernambuco M. B., Winderickx J., de Winde J. H., Thevelein J. M. (1997) Microbiology 143, 2627–2637 [DOI] [PubMed] [Google Scholar]
- 20. Jorgensen P., Nishikawa J. L., Breitkreutz B. J., Tyers M. (2002) Science 297, 395–400 [DOI] [PubMed] [Google Scholar]
- 21. Jorgensen P., Rupes I., Sharom J. R., Schneper L., Broach J. R., Tyers M. (2004) Genes Dev. 18, 2491–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber A., Bodenmiller B., Uotila A., Stahl M., Wanka S., Gerrits B., Aebersold R., Loewith R. (2009) Genes Dev. 23, 1929–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trott A., Shaner L., Morano K. A. (2005) Genetics 170, 1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., Longo V. D. (2001) Science 292, 288–290 [DOI] [PubMed] [Google Scholar]
- 25. Wanke V., Cameroni E., Uotila A., Piccolis M., Urban J., Loewith R., De Virgilio C. (2008) Mol. Microbiol. 69, 277–285 [DOI] [PubMed] [Google Scholar]
- 26. Geyskens I., Kumara S. H. M. C., Donaton M. C. V., Bergsma J. C. T., Thevelein J. M., Wera S. (2000) in Molecular Mechanisms of Signal Transduction (Bos J. L. ed) pp. 117–126, IOS Press, Fairfax, VA [Google Scholar]
- 27. Urban J., Soulard A., Huber A., Lippman S., Mukhopadhyay D., Deloche O., Wanke V., Anrather D., Ammerer G., Riezman H., Broach J. R., De Virgilio C., Hall M. N., Loewith R. (2007) Mol. Cell 26, 663–674 [DOI] [PubMed] [Google Scholar]
- 28. Liu K., Zhang X., Lester R. L., Dickson R. C. (2005) J. Biol. Chem. 280, 22679–22687 [DOI] [PubMed] [Google Scholar]
- 29. Levin L. R., Zoller M. J. (1990) Mol. Cell. Biol. 10, 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore M. J., Kanter J. R., Jones K. C., Taylor S. S. (2002) J. Biol. Chem. 277, 47878–47884 [DOI] [PubMed] [Google Scholar]
- 31. Nirula A., Ho M., Phee H., Roose J., Weiss A. (2006) J. Exp. Med. 203, 1733–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng X., Ma Y., Moore M., Hemmings B. A., Taylor S. S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9849–9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deminoff S. J., Howard S. C., Hester A., Warner S., Herman P. K. (2006) Genetics 173, 1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biondi R. M., Cheung P. C., Casamayor A., Deak M., Currie R. A., Alessi D. R. (2000) EMBO J. 19, 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 36. Alessi D. R., Kozlowski M. T., Weng Q. P., Morrice N., Avruch J. (1998) Curr. Biol. 8, 69–81 [DOI] [PubMed] [Google Scholar]
- 37. Sun Y., Taniguchi R., Tanoue D., Yamaji T., Takematsu H., Mori K., Fujita T., Kawasaki T., Kozutsumi Y. (2000) Mol. Cell. Biol. 20, 4411–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang Y., McLeod M. (2004) Genetics 168, 1843–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toner-Webb J., van Patten S. M., Walsh D. A., Taylor S. S. (1992) J. Biol. Chem. 267, 25174–25180 [PubMed] [Google Scholar]
- 40. Shoji S., Titani K., Demaille J. G., Fischer E. H. (1979) J. Biol. Chem. 254, 6211–6214 [PubMed] [Google Scholar]
- 41. Kim C., Xuong N. H., Taylor S. S. (2005) Science 307, 690–696 [DOI] [PubMed] [Google Scholar]
- 42. Levin L. R., Kuret J., Johnson K. E., Powers S., Cameron S., Michaeli T., Wigler M., Zoller M. J. (1988) Science 240, 68–70 [DOI] [PubMed] [Google Scholar]
- 43. Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. (1985) Cell 40, 27–36 [DOI] [PubMed] [Google Scholar]
- 44. Roelants F. M., Baltz A. G., Trott A. E., Fereres S., Thorner J. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Donaton M. C., Holsbeeks I., Lagatie O., Van Zeebroeck G., Crauwels M., Winderickx J., Thevelein J. M. (2003) Mol. Microbiol. 50, 911–929 [DOI] [PubMed] [Google Scholar]
- 46. Van Nuland A., Vandormael P., Donaton M., Alenquer M., Lourenço A., Quintino E., Versele M., Thevelein J. M. (2006) Mol. Microbiol. 59, 1485–1505 [DOI] [PubMed] [Google Scholar]
- 47. Van Zeebroeck G., Bonini B. M., Versele M., Thevelein J. M. (2009) Nat. Chem. Biol. 5, 45–52 [DOI] [PubMed] [Google Scholar]
- 48. Yonemoto W., Garrod S. M., Bell S. M., Taylor S. S. (1993) J. Biol. Chem. 268, 18626–18632 [PubMed] [Google Scholar]
- 49. Cameron S., Levin L., Zoller M., Wigler M. (1988) Cell 53, 555–566 [DOI] [PubMed] [Google Scholar]
- 50. Durnez P., Pernambuco M. B., Oris E., Argüelles J. C., Mergelsberg H., Thevelein J. M. (1994) Yeast 10, 1049–1064 [DOI] [PubMed] [Google Scholar]
- 51. Hirimburegama K., Durnez P., Keleman J., Oris E., Vergauwen R., Mergelsberg H., Thevelein J. M. (1992) J. Gen. Microbiol. 138, 2035–2043 [DOI] [PubMed] [Google Scholar]
- 52. Peeters T., Louwet W., Geladé R., Nauwelaers D., Thevelein J. M., Versele M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13034–13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Portela P., Moreno S. (2006) Cell. Signal. 18, 1072–1086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.