Abstract
Angiogenesis is required for bone development, growth, and repair. It is influenced by the local bone environment that involves cross-talks between endothelial cells and adjacent bone cells. However, data regarding factors that directly contribute to angiogenesis by bone cells remain poorly understood. Here, we report that EGFL6, a member of the epidermal growth factor (EGF) repeat superfamily proteins, induces angiogenesis by a paracrine mechanism in which EGFL6 is expressed in osteoblastic-like cells but promotes migration and angiogenesis of endothelial cells. Co-immunoprecipitation assays revealed that EGFL6 is secreted in culture medium as a homodimer protein. Using scratch wound healing and transwell assays, we found that conditioned medium containing EGFL6 potentiates SVEC (a simian virus 40-transformed mouse microvascular endothelial cell line) endothelial cell migration. In addition, EGFL6 promotes the endothelial cell tube-like structure formation in Matrigel assays and angiogenesis in a chick embryo chorioallantoic membrane. Furthermore, we show that EGFL6 recombinant protein induces phosphorylation of ERK in SVEC endothelial cells. Inhibition of ERK impaired EGFL6-induced ERK activation and endothelial cell migration. Together, these results demonstrate, for the first time, that osteoblastic-like cells express EGFL6 that is capable of promoting endothelial cell migration and angiogenesis via ERK activation. Thus, the EGLF6 mediates a paracrine mechanism of cross-talk between vascular endothelial cells and osteoblasts and might offer an important new target for the potential treatment of bone diseases, including osteonecrosis, osteoporosis, and fracture healing.
Keywords: Bone, Cell Migration, Endothelium, ERK, Gene Expression, Growth Factors, Angiogenesis, EGF-like, Osteoblast
Introduction
Angiogenesis plays a pivotal role in bone formation, remodeling, and healing (1). In early osteogenesis during embryonic development, vascularization is required for the replacement of the hypertrophied cartilage core with bone marrow. During adulthood, angiogenesis is closely coupled with the process of bone remodeling (2, 3). Evidence has been presented that bone remodeling takes place in specialized vascular structures, bone remodeling compartments that contain osteoblastic-like cells and a vascular structure (4, 5). Lack of bone vascularity is associated with decreased bone formation and bone mass (1, 6). Furthermore, inhibition of angiogenesis during fracture repair in animals results in the formation of fibrous tissue and atrophic nonunions, leading to impaired bone healing (7).
It has been widely speculated that bone remodeling requires an intimate connection between blood vessels and bone cells. Vascular endothelial cells, bone building osteoblasts, and bone-resorbing osteoclasts contribute multiple regulatory proteins that interplay autocrine/paracrine modes of regulation for the recruitment, proliferation, differentiation function, and survival of these vascular and bone cells (1, 8). For instance, endothelial cells produce many factors that influence bone cells, including M-CSF, RANKL,2 and chemokines (1, 9). Conversely, both osteoclasts and osteoblasts are thought to regulate endothelial cells through parathyroid hormone and proinflammatory cytokines (1). However, data regarding factors that have a direct effect on inducing angiogenesis by bone cells remain poorly understood.
EGF-like proteins are characterized by their multiple EGF repeats. The binding of EGF-like proteins to their receptors triggers a wide range of biological functions, including proliferation, differentiation, apoptosis, adhesion, and migration (10). EGF receptors are expressed in a number of cell types, including osteoblasts, osteoclasts, and endothelial cells (11–13). Recently, EGF-like proteins such as TGF-α, Heparin-binding-EGF, amphiregulin, and betacellulin have been shown to play important roles in bone development (11, 14, 15). Interestingly, EGF-like proteins have also been linked to the regulation of angiogenesis (13, 16–21).
EGFL6, also called MAEG, was previously mapped to human Xp22 chromosome and shown to be expressed primarily in fetal tissues and during early development (22, 23). In addition, MAEG expression was detected in several tissues, including all of the dermatome derivatives as the dermis of the trunk, the hair follicles, and the mesenchyme of the cranio-facial region (24). Localization studies revealed that MAEG protein targets to the basement membrane of embryonic skin and developing hair follicles (25). More recently, EGFL6 has been proposed as a possible biomarker in ovarian cancer (26). However, the potential expression and role of EGF-like family genes on angiogenesis and bone biology have yet to be addressed.
In the present study, we show that osteoblastic-like cells express EGFL6 transcripts that encode a secreted protein. Moreover, EGFL6 is capable of promoting cell migration and angiogenesis of endothelial cells through the activation of the ERK pathway. We propose that EGFL6 mediates a paracrine mechanism of cross-talk between osteoblastic-like cells and vascular endothelial cells to regulate angiogenesis in the bone local environment.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Human fibroblast growth factor-basic (bFGF) was purchased from PeproTech, Inc. Lipofectamine 2000 transfection reagent was purchased from Invitrogen. Glutathione S-transferase (GST)-rRANKL160–318 (rRANKL) recombinant proteins were expressed and purified as described previously (27). U0126 was purchased from Promega. Arginine-glycine-aspartic acid (RGD) peptide was purchased from Sigma. Polyclonal anti-GST-EGFL6 was generated by the Polyclonal Antibody Production Facility in the School of Molecular and Biomedical Sciences, University of Adelaide. Other antibodies used were mouse monoclonal anti-c-myc (Sigma), mouse monoclonal anti-HA (Sigma), mouse monoclonal anti-p-ERK (Santa Cruz Biotechnology), rabbit polyclonal anti-ERK, rabbit polyclonal anti-p-AKT, rabbit polyclonal anti-AKT (Cell Signaling Technology), mouse monoclonal anti-β-actin (JLA20) (Developmental Studies Hybridoma Bank, University of Iowa). All antibodies were used at the concentrations recommended by the supplier.
Cells and Cell Culture
RAW264.7 cells were cultured in complete α-modified Eagle's medium (α-MEM) (10% (v/v) fetal bovine serum (FBS), 2 mm l-glutamine, 100u/ml penicillin, 100 μg/ml streptomycin) RAW264.7 cells were stimulated with 100 ng/ml rRANKL for 7 days for the formation of multinucleated osteoclasts. Primary mouse osteoblasts were prepared from the calvariae of neonatal C57BL/6J mice by enzymatic digestion. Calvariae were dissected from neonatal mice, washed with serum-free α-MEM, and sequentially transferred to 5 ml of cell digestion solution (1 mg/ml collagenase and 2 mg/ml dispase). Tissue was incubated at 37 °C, with constant shaking at 100 rpm, for 10 min. The tissue was then transferred into fresh digestion solution and incubated for an additional 10 min at 37 °C. Fluid was collected and calvariae repeatedly digested in fresh digestion solution for 10 min. All fluid-containing released cells were collected and cultured in complete α-MEM. For osteoblast differentiation, primary calvarial osteoblastic cells were seeded onto 12-well plates at a cell density of 4 × 104 cells/well and grown in complete α-MEM until confluent. Cell medium was then exchanged with osteogenic differentiation medium, α-MEM containing 50 μg/ml ascorbic acid and 10 mm β-glycerophosphate. The medium was replaced every 2–3 days with fresh differentiation medium up to 21 days. For the detection of EGFL6 protein expression during osteoblast differentiation, culture medium was changed to Opti-MEM reduced serum medium 24 h prior to harvesting supernatant and cell lysates. KusaO cells were maintained in complete α-MEM. COS-7 and SVEC cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) FBS, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Semiquantitative Reverse Transcription (RT)-PCR
Total cellular RNA was isolated from cultured cells using RNeasy Mini kit (Qiagen) in accordance with the manufacturer's protocol. For RT-PCR, single-stranded cDNA was reverse transcribed from 2 μg total RNA using reverse transcriptase with an oligo(dT) primer. All PCR was carried out using 1 μl of each cDNA using cycling parameters 94 °C, 40 s; 55 °C, 40 s; 72 °C, 40 s for 30 cycles with primers designed against the following mouse sequences: EGFL2 (forward, 5′-CATAGATCTATGGATTTAATCTCAACCTTG-3′; reverse, 5′-AGATCTAGATCTGTATTTAACAGAAAGCCA-3′), EGFL3 (forward, 5′-GGTGGATCCACCATGGGACCCACCTGCCTG-3′; reverse, 5′-AAGCATCTAGATGCTTCATGGCCTTGCTGC-3′), EGFL5 (forward, 5′-TTCATGAATTCATGAATGGCGAGCCGAGC-3′; reverse, 5′-CCGAATTCGGCTTTGTAGTTATGAATAGGT-3′), EGFL6 (forward, 5′-AAGCTTGGATCCGAATTCAGTATGCAGCCGCCCTGG-3′; reverse, 5′-CTCGAGTCTAGAAGATCTACCTTCTACAGATAAAAAGT-3′),EGFL7 (forward, 5′-AGTGGGGATCCCCACTTACAATGCAGAC-3′; reverse, 5′-TCTTCTAGAAGATCTTTTTTGCAGGAGCAG-3′), EGFL8 (forward, 5′-CCCATGGATCCATGGGGCTCTGGGCTGAG-3′; reverse, 5′-GTGGTCTAGACCACGCAGGCTTGGGCCCAG-3′), EGFL9 (forward, 5′-GCCACCATGGGTGCCCACTGTGAGGT-3′; reverse, 5′-GGATCCCAGCGCTGTGGTCTTACCAGG-3′), Runx2 (forward, 5′-CGCATTCCTCATCCCAGTAT-3′; reverse, 5′-TGTAGGTAAAGGTGGCTGGG-3′), osterix (forward, 5′-TTCCCTCACTCATTTCCTGG-3′; reverse, 5′-CCCTTAACCCAGCTCCCTAC-3′), osteocalcin (forward, 5′-GCGCTCTGTCTCTCGTGACCT-3′; reverse, 5′-ATAGATGCGTTTGTAGGCGG-3′), osteopontin (forward, 5′-TCTGATGAGACCGTCACTGC-3′; reverse, 5′-TCTCCTGGCTCTCTTTGGAA-3′), alkaline phosphatase (forward, 5′-AACTGCTGGCCCTTGACCCCT-3′; reverse, 5′-TCCTGCCTCCTTCCACCAGCA-3′), 36B4 (forward, 5′-TCATTGTGGGAGCAGACA-3′; reverse, 5′-TCCTCCGACTCTTCCTTT-3′), 18S (forward, 5′-ACCATAAACGATGCCGACT-3′; reverse, 5′-TGTCAATCCTGTCCGTGTC-3′), bFGF (forward, 5′-AGAGCGACCCACACGTCAAACTACA-3′; reverse, 5′-ATGGCCTTCTGTCCAGGTCCCG-3′), VEGF (forward, 5′-CCAGGCTGCACCCACGACAG-3′; reverse, 5′-CGCATCAGCGGCACACAGGA-3′). PCR samples were analyzed by DNA-agarose gel electrophoresis.
Western Blotting
Total cellular proteins were extracted from cultured cells using radioimmune precipitation assay lysis buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate) supplemented with Protease Inhibitor Mixture (Roche Applied Science). Lysates were cleared by centrifugation at 16,000× g for 20 min at 4 °C, and postnuclear supernatants were collected. For immunoblotting, equivalent amounts of extracted proteins diluted in SDS-sampling buffer were resolved by SDS-polyacrylamide (10%) gels and then electroblotted onto nitrocellulose membranes (Hybond ECL; Amersham Biosciences). Following transfer, membranes were blocked with 5% skim milk in TBS-Tween (0.05 m Tris, 0.15 m NaCl, pH 7.5, and 0.2% Tween 20) for 1 h and then probed with primary antibodies diluted in 1% (w/v) skim milk powder in TBS-Tween for 2 h. Membranes were washed and then incubated with HRP-conjugated secondary antibodies for 1 h. Immunoreactivity was visualized using the ECLTM-Plus Western Blot Detection system (Amersham Biosciences) and FujiFilm LAS-3000 Gel Documentation system (FujiFilm).
Co-immunoprecipitation
COS-7 Cells were singly or co-transfected with 2 μg each of phCMV-EGFL6-HA and pcDNA3.1-EGFL6-c-myc expression constructs using Lipofectamine 2000 (Invitrogen). The same amounts of supernatant were collected after 48-h incubation for co-immunoprecipitation. The supernatant was then incubated at 4 °C overnight with 5 μg of anti-c-myc antibody (Sigma). Antibody-bound proteins were then immobilized on GammaBind G-Sepharose beads (Amersham Biosciences) at 4 °C for 2 h and washed three times with wash buffer (20 mm HEPES-NaOH, pH 7.2, 150 mm NaCl, 1 mm MgCl2, 0.2% Triton X-100, 1 mm phenylmethylsulfonyl fluoride). Antibody-protein bead complexes were then subjected to SDS-PAGE and transfer to Western blot analysis.
Expression and Purification of GST-EGFL6
To generate GST-EGFL6 expression vector, forward primer 5′-TCTCAAGCTTGGATCCCTGTGAAGGAAATACTCACA-3′ and reverse primer 5′-CGTCGACCTGCAGTTGAATTCTAAGTCTTTGTGCTT-3′ were designed to amplify a region between the EGF domains and MAM (meprin/A5-protein/PTPmu) domain of EGFL6. The resulting nucleotide sequence was then cloned into the pGEX-3X expression vector downstream of the GST sequence. Generation of a positive clone was confirmed by restriction enzyme digestion and DNA sequencing. GST fusion proteins were expressed and purified as described previously. Briefly, pGEX-3X-GST-EGFL6 was transformed into bacterial strain BL-21 cells. The expression of GST-EGFL6 fusion proteins was induced by 0.1 mm isopropyl 1-thio-β-d-galactopyranoside. Bacteria was harvested and lysed for protein purification using glutathione-agarose beads.
Preparation of Conditioned Medium Containing EGFL6
COS-7 cells were cultured overnight in a 6-well plate at a density of 4 × 105 cells/well in complete DMEM. The following day, the culture medium was transfected with EGFL6 expression vector phCMV-EGFL6-HA or empty phCMV-HA vector, respectively, using Lipofectamine 2000 (Invitrogen). After 6 h, cells were washed twice and incubated with Opti-MEM reduced serum medium. EGFL6-enriched supernatant medium was harvested after 24 h. Supernatants were then centrifuged at 2000 rpm for 10 min to remove cell debris and snap frozen in aliquots at −80 °C. The presence of EGFL6 in the supernatant was examined by Western blotting using anti-HA and anti-EGFL6 antibodies. The concentration of EGFL6 in conditioned medium was estimated to be ∼180 ng/ml (supplemental Fig. S1).
Scratch Wound Healing Assay
SVEC cells were seeded in 24-well plates at a density of 1.6 × 104 cells/well in complete DMEM and cultured to confluence. The SVEC cell monolayer was serum starved overnight in DMEM prior to initiating of the experiment. Confluent cell monolayer were then scraped with a yellow pipette tip to generate scratch wounds and washed twice with Opti-MEM to remove cell debris. Cells were incubated at 37 °C for 24 h with the conditioned medium containing EGFL6, or vehicle control. Time lapse images were captured at 0, 12, and 24 h time points in the same position using a Nikon Eclipse TE2000-5 microscope. Four selected field of images were captured in each sample, and the wound areas are estimated by Nikon NIS-Elements computer software. For the scratch wound healing assay with MEK inhibitor and RGD peptide, SVEC monolayers were treated with MEK inhibitor U0126 (10 μm) for 1 h prior to wound generation.
Transwell Migration Assay
The transwell migration assay was performed on SVEC cells using a Fluorometric Cell Migration Assay kit with polycarbonate membrane inserts (5-μm pore size; Cell Biolabs). SVEC cells were serum-starved overnight in DMEM prior to initiation of the experiment. The lower chambers were filled with 1 ml of conditioned medium containing EGFL6 or vehicle control. SVEC cells (4 × 104) were resuspended in 200 μl of Opti-MEM and added to the upper chamber. Cells were then incubated at 37 °C for 24 h to allow cell migration through the membrane. Migratory cells were detached from the underside of the membrane and subsequently lysed and detected by CyQuant GR dye (Invitrogen). Fluorescence measurement was performed in a FluoStar Optima fluorescence plate reader with a 485/520 nm filter set.
Tube Formation Assay
GeltrexTM reduced growth factor basement membrane matrix (Invitrogen) was thawed at 4 °C overnight before use. GeltrexTM matrix was added to wells of a 24-well plate (180 μl/well) and then incubated at 37 °C for 30 min to allow polymerization. SVEC cells were starved overnight in DMEM before starting the experiment. Cells were seeded onto the layer of GeltrexTM matrix and incubated at 37 °C for 30 min with Opti-MEM. Medium was then removed and replaced with the conditioned medium containing EGFL6 or vehicle control and incubated at 37 °C for 24 h. Five random selected fields of view were captured position using Nikon Eclipse TE2000–5 microscope. Tube formation was quantified by measuring the length of tube-like structures and number of branching points by Nikon NIS-Elements computer software. Tube length was assessed by drawing lines along the tube-like structure and measuring the length of the line in pixels. For the tube formation assay with MEK inhibitor, SVEC cells were treated with MEK inhibitor U0126 (10 μm) for 1 h prior to seeding on GeltrexTM matrix.
Chick Embryo Chorioallantoic Membrane Assay
A chick embryo chorioallantoic membrane (CAM) assay was performed at the International Joint Cancer Institute, Second Military Medical University, Shanghai, China. Fertilized chicken eggs were incubated at 37 °C under conditions of constant humidity. On E3, 2–3 ml of ovalbumin was gently aspirated and removed from the egg using needle to create an air sac directly over the CAM, allowing its dissociation from the egg shell membrane. On E8, a small circular window was opened above the air sac. The opening was then sealed with tape, and the egg was incubated for 2 days. On E10, the gelatin sponge impregnated with conditioned medium containing EGFL6 or vehicle control was implanted onto the CAM, and eggs were resealed and incubated for 2 days. On E12, the images of CAM were captured and scored on a scale of 0–5 for the intensity of the angiogenic response, according to a published protocol (28).
Osteoblast Proliferation Assay
Primary mouse osteoblasts were seeded onto 96-well plate at a density of 6 × 103 cells/well and incubated overnight at 37 °C. Medium was then replaced with fresh medium containing 2% FBS, conditioned medium containing EGFL6 or vehicle control (1/2 dilution) and incubated for an additional 24, 48, or 72 h. At the end of the treatment, Cell proliferation was measured using the CellTiter 96® aqueous nonradioactive cell proliferation assay (Promega) according to the manufacturer's instructions. The measurements were performed on a microplate reader (model 680; Bio-Rad) at a wavelength of 450 nm.
Osteoblast Differentiation Assay
KusaO cells were seeded onto 24-well plates at a cell density of 5 × 103 cells/well and grown until confluent. Cells were then cultured in osteogenic differentiation medium with conditioned medium containing EGFL6 or vehicle control for 14 days. Mineral deposits were identified by alizarin red staining. For alizarin red staining, cells were washed with PBS and fixed with 4% paraformaldehyde. Cells were washed three times with 70% ethanol and stained with 1% alizarin red solution for 5 min followed by washing three times with 50% ethanol. Images were captured at 600 dpi by a scanner (Epson Perfection 3490 photo). The alizarin red-positive area was estimated using ImageJ software and is shown as a percentage compared with control.
Statistical Analysis and Data Presentation
All data shown represent one of at least three independent experiments and are expressed as mean ± S.D. Statistics were performed using Students' t test with significance taken at p < 0.05.
RESULTS
Gene Expression of EGF-like Family Members in Osteoblastic- and Osteoclastic-like Cells
In the search of novel factors involved in bone remodeling, we have uncovered several members of factors, including EGFL2, EGFL3, EGFL5, EGFL6, EGFL7, EGFL8, and EGFL9 that were differentially expressed in osteoblastic-like cells by microarray analysis, each belonging to the EGF-like family. Bioinformatic analyses indicate that EGFL2, EGFL5, and EGFL9 contain transmembrane domains, whereas EGFL3, EGFL6, EGFL7, and EGFL8 lack transmembrane domains and are predicted to be secreted proteins (Fig. 1A). By semiquantitative RT-PCR analyses, EGFL3, EGFL5, EGFL6, and EGFL9 were preferentially expressed in osteoblastic-like cells; whereas EGFL2, EGFL7, and EGFL8 were expressed in both osteoclastic- and osteoblastic-like cells (Fig. 1B).
FIGURE 1.
Differentially expression of EGF-like family in osteoclasts and osteoblasts. A, schematic representations of domain structures of EGF-like family members. EGFL3, EGFL6, EGFL7, and EGFL8 are predicted as secreted protein, whereas EGFL2, EGFL5, and EGFL9 are predicted as membrane-bound protein. EGF-like family members vary in amino acid length and contain differing number of EGF domains. B, RT-PCR amplification of EGF-like family members in differentiating osteoclasts and calvaria bone. RAW264.7 cells derived osteoclasts were cultured in the presence of RANKL for up to 7 days. mRNA was extracted and subjected to RT-PCR using primers specific for EGF-like family members and 36B4. C, gene expressions of EGF-like family members up-regulated during osteoblast differentiation. RNA was extracted from primary osteoblast at 0 (mock), 7, 14, and 21 days after stimulation with β-glycerophosphate and ascorbic acid. RNA was then subjected to RT-PCR amplification using primers specific for EGF-like family members, osteoblast-specific genes, and 36B4. D, -fold change of EGFL6 gene expression during osteoblast differentiation, with its expression peaking at day 14. E, tissue expression profile of EGFL6 determined by RT-PCR analysis.
First, we examined the gene expression profile of EGF-like family members during osteoblastic differentiation. Freshly isolated mouse calvaria cells were cultured in the presence of differentiation medium for 21 days after which semiquantitative RT-PCR was performed. As shown in Fig. 1C, all of the EGF-like members were up-regulated during primary osteoblast differentiation in line with other established osteoblast markers, including Runx2, osterix, osteocalcin, osteopontin, and alkaline phosphatase. Because EGFL6 was proved highly expressed in osteoblastic-like cells, we chose to focus our studies on this isotype. EGFL6 gene expression was markedly up-regulated during osteoblast differentiation with its expression peaking at day 14 (Fig. 1D). We also examined the tissue distribution of EGFL6 from selected mouse organs by semiquantitative RT-PCR. Analysis of tissue distribution revealed that the level of EGFL6 mRNA expression was highest in the kidney, lung, and bone tissues (Fig. 1E).
EGFL6 Protein Is a Secreted Protein and Forms a Homomeric Complex
Structural analyses predicted that EGFL6 encodes a secreted protein. To validate this, we generated expression constructs phCMV3-EGFL6-HA (Fig. 2A) and carried out transient transfection in COS-7 cells. Both supernatant and cell lysates were harvested and analyzed by Western blotting with an anti-HA antibody. As shown in Fig. 2B, transient transfection of phCMV-EGFL6-HA in COS-7 cells revealed that EGFL6 was detectable in culture medium and cell lysates, consistent with being a secreted protein. It has been suggested that proteins containing EGF repeats are capable of forming a homomeric complex (29–32). To determine whether EGFL6 proteins share this feature, co-immunoprecipitation and Western blot analyses were carried out. To aid this, we generated a pcDNA3.1B-EGFL6-c-myc construct and transfected it with phCMV-EGFL6-HA. Supernatants from singly and co-transfected cells were immunoprecipitated with the anti-c-myc antibody, and the antibody-protein complexes were analyzed by immunoblotting with the anti-HA antibody. The antibody complexes were detected from the co-transfected sample but not singly transfected samples. This result demonstrated that EGFL6 existed as a homomeric complex (Fig. 2C). Taken together, these experiments indicate that EGFL6 is a secreted protein and exists as a homomeric complex.
FIGURE 2.
EGFL6 exists as a secreted homomeric complex. A, two expression constructs encoding mouse full-length EGFL6, pcDNA3.1-EGFL6-HA, and pcDNA3.1-EGFL6-c-myc were generated. pGEX-3X-GST-EGFL6 encoding EGFL6 (264–393) was generated to produce GST-EGFL6 fusion protein for antibody production. B, COS-7 cells transfected with empty vector or expression vector encoding HA-tagged EGFL6. Medium and cell lysate were collected and subjected to Western blotting using an anti-HA antibody. EGFL6 was detected in medium and cell lysates. C, COS-7 cells were singly or co-transfected with the pcDNA3.1-EGFL6-HA and pcDNA3.1-EGFL6-c-myc constructs. Two days after transfection, supernatant was collected and subjected to immunoprecipitation (IP) using an anti-c-myc antibody, followed by Western blotting (WB) using an anti-HA antibody. Immunoprecipitation results indicate that EGFL6-c-myc and EGFL6-HA proteins form a homomeric complex. D, purified GST-EGFL6 proteins shown by Coomassie Blue staining. GST-EGFL6 proteins were detected by Western blotting using an anti-GST antibody. GST alone was use as a control. E, COS-7 cells transfected with HA-tagged EGFL6 vector or an empty vector. Culture supernatants were harvested after 24 h. EGFL6 proteins in culture supernatants were detected using immunoblotting with anti-HA antibody and anti-EGFL6 antibody. F, supernatant and cell lysates harvested during primary osteoblast differentiation and subjected to Western blot analysis using the anti-EGFL6 antibody. The protein levels of EGFL6 were up-regulated during osteoblast differentiation. G, proteins extracted from limb buds (E14.5) and long bones (E18.5, week 1, and week 16) of mice and subjected to Western blot analysis. EGFL6 proteins were expressed during bone development. Cell lysate from day 21 differentiated primary osteoblast was used as a positive control. Black arrows indicate the predicted size of EGFL6 proteins.
Next, we sought to produce conditioned medium containing EGFL6 for the functional studies. To confirm the identity of the protein, we generated an antibody specific for EGFL6. Recombinant GST-EGFL6 proteins were expressed and purified (Fig. 2D) and used as antigen to generate a rabbit polyclonal antibody against EGFL6. Western blot analyses confirmed that the anti-EGFL6 antibody specifically reacted to a protein corresponding to the predicted 63 kDa in EGFL6-transfected cell supernatant but not in mock-transfected cell supernatant (Fig. 2E). Moreover, EGFL6 protein expression was examined by Western blot analyses with the anti-EGFL6 antibody. Both supernatant and cell lysates were harvested during primary osteoblast differentiation. As shown in Fig. 2F, EGFL6 protein was detected in supernatant and cell lysates and up-regulated during osteoblast differentiation. These experiments indicate that EGFL6 was produced and secreted by osteoblasts. We also examined EGFL6 protein expression during bone development. Proteins were extracted from limb buds (E14.5) and long bones (E18.5, week 1 and week 16) of mice. Western blot analyses revealed that EGFL6 protein expression was detected in developing bone (Fig. 2G).
Induction of Cell Migration and Angiogenesis of Endothelial Cells by EGFL6
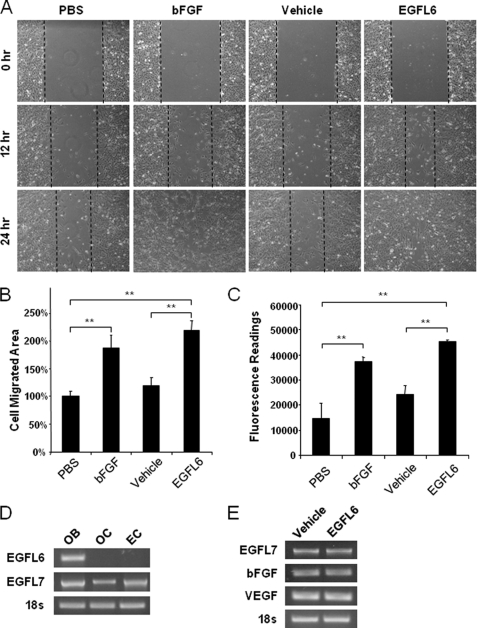
EGF-like proteins have previously been implicated in endothelial cell migration and angiogenesis (13, 16–21). Therefore, we hypothesized that EGFL6 might also participate in angiogenesis. As a first step toward investigating this possibility, we examined the effect of EGFL6 on endothelial cell migration. To this end, we performed scratch wound healing assays using SVEC endothelial cells. As shown in Fig. 3A–B, EGFL6 conditioned medium was found to increase endothelial cell migration by up to ∼40% at 12 h compared with the vehicle control. bFGF was included as a positive control. The effect of EGFL6 on endothelial cell migration was also assessed using transwell migration assays. As shown in Fig. 3C, EGFL6 significantly increased cell migration compared with vehicle-treated cells. Interestingly, endothelial cells did not express EGFL6 (Fig. 3D), indicating that EGLF6 mediates a paracrine mechanism of cross-talk between vascular endothelial cells and osteoblastic-like cells. To determine whether the cell migration effect was induced directly by EGFL6 or other angiogenic factors in response to the stimulation of EGFL6 semiquantitative RT-PCR was performed. The SVEC endothelial cells were stimulated with conditioned medium containing the recombinant EGFL6 proteins or vehicle control for 24 h. Semiquantitative RT-PCR analyses revealed that EGFL6 did not alter the mRNA expression level of angiogenic factors, including bFGF, VEGF, and EGFL7 (Fig. 3E). An important aspect of angiogenesis involves the organization of endothelial cells into a three-dimensional tube-like structures. To determine whether EGFL6 promotes angiogenesis, we next performed tube formation assays. Geltrex is a reduced growth factor basement membrane matrix that allows endothelial cells to form tube-like structures. The SVEC endothelial cells were cultured on Geltrex and treated with conditioned medium containing the recombinant EGFL6 proteins or vehicle control. In this instance, bFGF and PBS served as positive and negative controls, respectively. As shown in Fig. 4A, tube-like structures were evident on the Matrigel after 24 h of culture. Treatment of EGFL6 condition medium significantly increased the total tube length compared with vehicle treatment and PBS control (Fig. 4B).
FIGURE 3.
EGFL6 promotes endothelial cell migration. A, scratch wound healing assays performed in SVEC cells treated with conditioned medium containing EGFL6 or vehicle control for 24 h. Representative microscopic views at 0 h, 12 h, and 24 h are shown. B, quantitative analysis of cell migration area after 12 h. C, transwell migration assay. SVEC cells were seeded in the upper chamber, conditioned medium containing EGFL6 or vehicle control was placed at the bottom side. After 24 h, migrated cells were quantified by fluorescence dye. PBS and bFGF were used as a negative and positive control, respectively. D, RT-PCR amplification of EGFL6 and EGFL7 in calvaria bone (OB), RAW264.7 cell-derived osteoclasts (OC), and SVEC endothelial cells (EC). E, RT-PCR amplification of angiogenic genes in endothelial cells treated with conditioned medium containing EGFL6 or vehicle control for 24 h. **, p < 0.01. Error bars, S.D.
FIGURE 4.
EGFL6 promotes tube formation on Matrigel and induces angiogenesis in a CAM assay. A, SVEC cells previously starved overnight seeded on GeltrexTM matrix. Cells were cultured in the conditioned medium containing EGFL6 or vehicle control for 24 h. PBS and bFGF were used as a negative and positive control, respectively. Representative photographs of each sample are shown. B, quantitative analysis of tube length. C, photographs of the chick CAMs after 48 h containing the gelatin sponge carrying EGFL6 proteins and vehicle control. Representative images are shown. D, average scores of the angiogenesis response intensity. **, p < 0.01. Error bars, S.D.
EGFL6 Induces Angiogenesis in a Chick Embryo CAM
The previous in vitro experiments suggested that EGFL6 may have an important role in angiogenesis. However, studies have shown that proteins affecting cell proliferation, migration, or differentiation in vitro may not necessarily regulate endothelial cell activity in vivo (33). To explore this possibility, we performed a CAM assay which is an in vivo angiogenesis assay allowing blood vessel formed on chorioallantoic membrane. As illustrated in Fig. 4C, EGFL6 significantly induced angiogenesis in a CAM assay. After a 48-h incubation, the gelatin sponge treated with EGFL6 was surrounded by allantoic vessels that developed radially toward the implant in a “spoked wheel ” pattern. Normal vascularized CAMs were observed with the gelatin sponge treated with vehicle. VEGF was used as a positive control (data not shown). As shown in Fig. 4D, the average score of the angiogenic response intensities measured in EGFL6 samples was significantly higher than the vehicle control. Taken together, these studies showed that EGFL6 is involved in various aspects of angiogenesis: promotion endothelial cell migration by a scratch wound healing assay and a transwell migration assay, enhancement of tube-like structure by a tube formation assay, formation of new blood vessels in a CAM assay. Thus, these data document the first evidence of the proangiogenic effect of EGFL6.
Activation of ERK Signaling Pathway by EGFL6
In an effort to address the mechanism of action of EGFL6 in angiogenesis, we searched for key signaling pathways of EGFL6. We first tested the effect of EGFL6 on the activation of potential signaling pathways related to angiogenesis using specific phosphorylated antibodies to Akt and MAPK. As shown in Fig. 5A, the ERK pathway is strongly activated by EGFL6 whereas Akt remains constant in SEVC cells. To confirm further that ERK is involved in EGFL6-mediated angiogenesis and cell migration, we also examined the effect of the MEK inhibitor on the EGFL6-induced activation of ERK and cell migration in endothelial cells. Cells were pretreated with a well known MEK inhibitor (U0126) for 1 h followed by stimulation with conditioned medium of EGFL6. As shown in Fig. 5B, the activation of ERK by EGFL6 was blocked in the presence of the MEK inhibitor. In parallel, EGFL6-induced cell migration of endothelial cells was also inhibited, suggesting a possible role of ERK in this process (Fig. 5, C and D). To assess the role of ERK in EGFL6-induced tube-like structure formation, a tube formation assay was carried out in the absence and presence of U0126. EGFL6-induced tube-like structure formation was significantly impaired by U0126 compared with the controls (Fig. 5E).
FIGURE 5.
EGFL6 promotes endothelial cell migration through activation of ERK. A, cellular phosphorylation levels of ERK1/2 determined by Western blot analysis after SVEC cells were stimulated with conditioned medium containing EGFL6 or vehicle control for the indicated times. B, EGFL6-induced ERK phosphorylation inhibited by MEK inhibitor, U0126. C, effect of U0126 on SVEC cell migration. A scratch wound healing assay was performed with the conditioned medium containing EGFL6 or vehicle control in the presence or absence of U0126 for 24 h. D, quantitative analysis of the scratch wound healing assay with U0126 after 12 h. E, tube formation assay performed with the conditioned medium containing EGFL6 or vehicle control in the presence or absence of U0126 for 24 h. PBS and bFGF were used as a negative and positive control, respectively. *, p < 0.05; **, p < 0.01. Error bars, S.D.
RGD Peptide Impaired EGFL6-induced Endothelial Cell Migration
EGFL6 was reported to contain an RGD region that binds to integrins (22, 23). To determine whether EGFL6 potentially interacts with integrins, we examined the effect of RGD peptide on the EGFL6-induced endothelial cell migration. Cells were pretreated with RGD peptides for 1 h prior to performing scratch wound healing assays. As shown in Fig. 6, EGFL6-induced endothelial cell migration was significantly blocked in the presence of RGD peptide. These results suggest the possibility that EGFL6 interacts with integrins and regulates angiogenic activities.
FIGURE 6.
EGFL6-induced endothelial cell migration was blocked by RGD peptides. A, scratch wound healing assays performed with the conditioned medium containing EGFL6 or vehicle control in the presence or absence of RGD peptides for 12 h. B, quantitative analysis of cell migration area after 12 h. **, p < 0.01. Error bars, S.D.
EGFL6 Does Not Induce Osteoblast Proliferation and Mineralization
Because we identified EGFL6 originally in osteoblasts, we next sought to determine whether EGFL6 also regulate the activities of these cells. To this end, we first examined the effect of EGFL6 on osteoblast proliferation. Freshly isolated mouse calvaria cells were cultured in the presence of 2% FBS for 72 h after which CellTiter 96® aqueous nonradioactive cell proliferation assay was performed. As shown in Fig. 7A, EGFL6 conditioned medium had no effect on osteoblast proliferation compared with a vehicle control. 10% FBS was used as a positive control. We next investigated the effect of EGFL6 on bone mineralization in KusaO cells using alizarin red staining assay. KusaO cell is a mouse preosteoblast cell line established for studying osteoblast differentiation (34, 35). As shown in Fig. 7B, mineralization area assessments by alizarin red staining were performed after 14 days of culture with the conditioned medium containing EGFL6 or vehicle control under differentiation condition. Culture without differentiation medium represented a negative control. Bone morphogenetic protein-2 (BMP-2) was included as a positive control. We found that EGFL6 had no effect on osteoblast mineralization (Fig. 7C).
FIGURE 7.
EGFL6 does not induce proliferation and mineralization of osteoblastic cells. A, freshly isolated mouse calvaria cells were cultured in the presence of 2% FBS with conditioned medium containing EGFL6 or vehicle control. Cell viability was measured at 24 h, 48 h, and 72 h using the CellTiter 96® aqueous nonradioactive cell proliferation assay. 2% FBS and 10% FBS were used as a negative and positive control, respectively. B, KusaO cells were cultured in differentiation medium with conditioned medium containing EGFL6 or vehicle control for 14 days and stained with alizarin red. KusaO cells cultured without differentiation medium were used as a negative control. BMP-2 was used as a positive control. (C) The mineralization areas were quantified and presented in percentages comparing to control. *, p < 0.05; **, p < 0.01. Error bars, S.D.
DISCUSSION
The regulation of endothelial cells and angiogenesis is highly complex and orchestrated by multiple intercellular signaling networks, and knowledge of these networks will be useful for finding angiogenic inhibitors or activators with therapeutic potential. In this study, we have shown that EGFL6, although highly expressed by osteoblastic-like cells, directly regulates migration and angiogenesis of endothelial cells via activation of the ERK signaling pathway. This finding represents a novel paracrine mechanism in which EGFL6 regulates a potential cross-talk between osteoblastic-like cells and endothelial cells in the bone local environment. A close association between vascularization and bone cells has long been recognized to play an important role in bone remodeling and fracture repair (1, 3, 6, 7, 36). Thus, the identification of EGFL6 as an angiogenic factor might provide a potential mechanistic insight into the intercellular communication between bone cells and endothelial cells. Although a number of factors that are expressed by bone cells can regulate angiogenesis via an indirect mechanism on endothelial cells, based on these studies, EGFL6 appears to have a direct effect on endothelial cells.
EGFL6, as a member of the EGF repeat superfamily proteins, consists of the EGF domain. However, the biological functions of EGFL6 have not been previously examined. Using RNA in situ hybridization, EGFL6/MAEG expression was detected in several sites, including all of the dermatome derivatives as the dermis of the trunk, the hair follicles, and the mesenchyme of the cranio-facial region. It was suggested that EGFL6/MAEG may play a role as a mediator regulating epithelial-mesenchymal interaction during hair follicle development (25). In the present study, we have shown that EGFL6 is highly expressed in osteoblastic-like cells. Our observation is consistent with the in situ detection of EGFL6 on the mesenchyme of the cranio-facial region which potentially contains cells of osteoblastic lineage (24). Because the osteoblastic-like cells used in the present study were freshly isolated from calvaria of young age mice, we cannot exclude the possibility that, in addition to osteoblasts, it might also contain mesenchymal or more differentiated stromal-like cells. Interestingly, we have found that the EGFL6 transcripts are induced in cultured cell with osteogenic medium, indicating that EGFL6 expression lies, at least in part, within the osteoblastic/stromal cell linage.
EGF-like proteins are structurally unrelated to VEGF, and the role of EGF-like family in angiogenesis and bone biology is not yet well defined. One of most well characterized members of EGF-like family is EGFL7, which encodes two EGF-like domains and a leucine and valine-rich C-terminal region. Intriguingly, EGFL7 is a secreted protein and shares no significant amino acid sequence homology with EGFL6. EGFL7 is expressed at high levels in the vasculature itself (37), and loss of EGFL7 function in zebrafish embryos results in loss of vascular tubulogenesis (16). Moreover, delayed development of vasculature in organs was found in EGFL7 knock-out mice (17), suggesting that it regulates angiogenesis by an autocrine mechanism involving vasculature alone. Interestingly, in contract to EGFL7, EGFL6 is not expressed in endothelial cells but by osteoblastic-like cells, which suggests that EGFL6 regulates angiogenesis in local bone environment by a paracrine mechanism. These studies hint that angiogenesis and vascular development form a complex but orderly process that is specifically controlled by different factors, including various EGF-like family proteins. Several members of EGF-like family termed EGFL2, EGFL3, EGFL5, EGFL6, EGFL7, EGFL8, and EGFL9 are comparable in EGF-like domain but distinct in their amino acid sequence. Notably, they are abundantly expressed in osteoblastic-like cells, raising the possibility that these EGF-like family proteins might play an important role in angiogenesis and bone biology. In this study, we present evidence that EGFL6 is produced by osteoblastic-like cells and plays a role in endothelial cells and angiogenesis.
During angiogenesis, there is evidence that several signaling pathways are potentially activated. These include integrins/FAK-mediated pathway, MAPK pathway, and the PIK3/Akt pathways (13, 18, 38). It is noteworthy to mention that the signaling pathway of EGFL6 has not been previously reported. In the light of the knowledge that EGFL6 promotes angiogenic endothelial cells, we tested the effect of EGFL6 on the activation of these potential signaling pathways using specific phosphorylated antibodies to Akt and MAPK. We found that ERK is activated by EGFL6 in a time-dependent manner. Furthermore, inhibition of ERK signaling pathway blocks EGFL6-induced ERK activation and endothelial cell migration. These results further attest to its role in the induction of cell migration and angiogenesis of endothelial cells via a specific signaling pathway of ERK.
It has been well established that EGF domain binds with high affinity to specific cell-surface receptors and induces their dimerization, which is essential for activating the tyrosine kinase in the receptor cytoplasmic domain, then initiating signal transduction that results in DNA synthesis, cell proliferation, and developmental processes (10, 39). However, the EGF-like family proteins might have distinct member of receptors given their sequence diversity and different expression patterns (40–42). The receptor of EGFL6 is yet to be identified. A recent report found that EGFL6 contains an RGD region that binds to α8β1 integrin (25). Mice with loss of function of α8β1 integrin because of a mutant in the α8 gene exhibited profound deficits in kidney morphogenesis (43, 44). Interestingly, β1 integrin plays an essential role in vascular development (45, 46). Our results also demonstrated that EGFL6 might interact with integrins and regulate angiogenic activities. Taken together, these raise questions whether the activation of ERK is mediated by α8β1 integrin. To our knowledge, data regarding the association of α8β1 integrin with ERK are presently lacking. It remains to be defined whether EGFL6 uses an RGD motif to bind to α8β1 integrin as a receptor or use other critical region for binding to a more specific receptor. The identification of EGFL6 cognate receptor in endothelial cells will be the focus of our future study.
In summary, we have shown that EGFL6 has a specific role in the induction of endothelial cell migration and angiogenesis through the activation of ERK pathway. Our data also suggest a potential paracrine action between endothelial cells and bone cells. Understanding the mechanisms by which EGFL6 regulates the intercellular communication in bone local environment should aid in advancing our knowledge of bone remodeling and angiogenesis and may facilitate us to design novel therapeutic strategies for the treatment of disease associated with pathological angiogenesis such as tumor and bone fracture healing.
Supplementary Material
This work was supported in part by the National Health and Medical Research Council of Australia and the Sir Charles Gairdner Hospital Research Fund.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- RANKL
- receptor activator of nuclear factor κB ligand
- bFGF
- fibroblast growth factor-basic
- CAM
- chorioallantoic membrane
- α-MEM
- complete α-modified Eagle's medium
- RGD
- arginine-glycine-aspartic acid
- SVEC
- a simian virus 40-transformed mouse microvascular endothelial cell line
- BMP-2
- bone morphogenetic protein-2.
REFERENCES
- 1. Brandi M. L., Collin-Osdoby P. (2006) J. Bone Miner. Res. 21, 183–192 [DOI] [PubMed] [Google Scholar]
- 2. Gerber H. P., Ferrara N. (2000) Trends Cardiovasc. Med. 10, 223–228 [DOI] [PubMed] [Google Scholar]
- 3. Carlevaro M. F., Cermelli S., Cancedda R., Descalzi Cancedda F. (2000) J. Cell Sci. 113, 59–69 [DOI] [PubMed] [Google Scholar]
- 4. Hauge E. M., Qvesel D., Eriksen E. F., Mosekilde L., Melsen F. (2001) J. Bone Miner. Res. 16, 1575–1582 [DOI] [PubMed] [Google Scholar]
- 5. Parfitt A. M. (2001) J. Bone Miner. Res. 16, 1583–1585 [DOI] [PubMed] [Google Scholar]
- 6. Street J., Bao M., deGuzman L., Bunting S., Peale F. V., Jr., Ferrara N., Steinmetz H., Hoeffel J., Cleland J. L., Daugherty A., van Bruggen N., Redmond H. P., Carano R. A., Filvaroff E. H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9656–966112118119 [Google Scholar]
- 7. Fang T. D., Salim A., Xia W., Nacamuli R. P., Guccione S., Song H. M., Carano R. A., Filvaroff E. H., Bednarski M. D., Giaccia A. J., Longaker M. T. (2005) J. Bone Miner. Res. 20, 1114–1124 [DOI] [PubMed] [Google Scholar]
- 8. Eriksen E. F., Eghbali-Fatourechi G. Z., Khosla S. (2007) J. Bone Miner. Res. 22, 1–6 [DOI] [PubMed] [Google Scholar]
- 9. Collin-Osdoby P., Rothe L., Anderson F., Nelson M., Maloney W., Osdoby P. (2001) J. Biol. Chem. 276, 20659–20672 [DOI] [PubMed] [Google Scholar]
- 10. Singh A. B., Harris R. C. (2005) Cell. Signal. 17, 1183–1193 [DOI] [PubMed] [Google Scholar]
- 11. Schneider M. R., Mayer-Roenne B., Dahlhoff M., Proell V., Weber K., Wolf E., Erben R. G. (2009) J. Bone Miner. Res. [DOI] [PubMed] [Google Scholar]
- 12. Wang K., Yamamoto H., Chin J. R., Werb Z., Vu T. H. (2004) J. Biol. Chem. 279, 53848–53856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta V. B., Besner G. E. (2007) Growth Factors 25, 253–263 [DOI] [PubMed] [Google Scholar]
- 14. Zhu J., Jia X., Xiao G., Kang Y., Partridge N. C., Qin L. (2007) J. Biol. Chem. 282, 26656–26664 [DOI] [PubMed] [Google Scholar]
- 15. Qin L., Tamasi J., Raggatt L., Li X., Feyen J. H., Lee D. C., Dicicco-Bloom E., Partridge N. C. (2005) J. Biol. Chem. 280, 3974–3981 [DOI] [PubMed] [Google Scholar]
- 16. Parker L. H., Schmidt M., Jin S. W., Gray A. M., Beis D., Pham T., Frantz G., Palmieri S., Hillan K., Stainier D. Y., De Sauvage F. J., Ye W. (2004) Nature 428, 754–758 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M., Paes K., De Mazière A., Smyczek T., Yang S., Gray A., French D., Kasman I., Klumperman J., Rice D. S., Ye W. (2007) Development 134, 2913–2923 [DOI] [PubMed] [Google Scholar]
- 18. Kim H. S., Shin H. S., Kwak H. J., Cho C. H., Lee C. O., Koh G. Y. (2003) FASEB J. 17, 318–320 [DOI] [PubMed] [Google Scholar]
- 19. Penta K., Varner J. A., Liaw L., Hidai C., Schatzman R., Quertermous T. (1999) J. Biol. Chem. 274, 11101–11109 [DOI] [PubMed] [Google Scholar]
- 20. Campagnolo L., Leahy A., Chitnis S., Koschnick S., Fitch M. J., Fallon J. T., Loskutoff D., Taubman M. B., Stuhlmann H. (2005) Am. J. Pathol. 167, 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leker R. R., Toth Z. E., Shahar T., Cassiani-Ingoni R., Szalayova I., Key S., Bratincsák A., Mezey E. (2009) Neuroscience 163, 233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeung G., Mulero J. J., Berntsen R. P., Loeb D. B., Drmanac R., Ford J. E. (1999) Genomics 62, 304–307 [DOI] [PubMed] [Google Scholar]
- 23. Buchner G., Orfanelli U., Quaderi N., Bassi M. T., Andolfi G., Ballabio A., Franco B. (2000) Genomics 65, 16–23 [DOI] [PubMed] [Google Scholar]
- 24. Buchner G., Broccoli V., Bulfone A., Orfanelli U., Gattuso C., Ballabio A., Franco B. (2000) Mech. Dev. 98, 179–182 [DOI] [PubMed] [Google Scholar]
- 25. Osada A., Kiyozumi D., Tsutsui K., Ono Y., Weber C. N., Sugimoto N., Imai T., Okada A., Sekiguchi K. (2005) Exp. Cell Res. 303, 148–159 [DOI] [PubMed] [Google Scholar]
- 26. Buckanovich R. J., Sasaroli D., O'Brien-Jenkins A., Botbyl J., Hammond R., Katsaros D., Sandaltzopoulos R., Liotta L. A., Gimotty P. A., Coukos G. (2007) J. Clin. Oncol. 25, 852–861 [DOI] [PubMed] [Google Scholar]
- 27. Xu J., Tan J. W., Huang L., Gao X. H., Laird R., Liu D., Wysocki S., Zheng M. H. (2000) J. Bone Miner. Res. 15, 2178–2186 [DOI] [PubMed] [Google Scholar]
- 28. Ribatti D., Nico B., Vacca A., Presta M. (2006) Nat. Protoc. 1, 85–91 [DOI] [PubMed] [Google Scholar]
- 29. Lu H. S., Chai J. J., Li M., Huang B. R., He C. H., Bi R. C. (2001) J. Biol. Chem. 276, 34913–34917 [DOI] [PubMed] [Google Scholar]
- 30. Tu C. F., Yan Y. T., Wu S. Y., Djoko B., Tsai M. T., Cheng C. J., Yang R. B. (2008) J. Biol. Chem. 283, 12478–12488 [DOI] [PubMed] [Google Scholar]
- 31. Wu B. T., Su Y. H., Tsai M. T., Wasserman S. M., Topper J. N., Yang R. B. (2004) J. Biol. Chem. 279, 37485–37490 [DOI] [PubMed] [Google Scholar]
- 32. Yang R. B., Ng C. K., Wasserman S. M., Colman S. D., Shenoy S., Mehraban F., Komuves L. G., Tomlinson J. E., Topper J. N. (2002) J. Biol. Chem. 277, 46364–46373 [DOI] [PubMed] [Google Scholar]
- 33. Liekens S., De Clercq E., Neyts J. (2001) Biochem. Pharmacol. 61, 253–270 [DOI] [PubMed] [Google Scholar]
- 34. Allan E. H., Ho P. W., Umezawa A., Hata J., Makishima F., Gillespie M. T., Martin T. J. (2003) J. Cell. Biochem. 90, 158–169 [DOI] [PubMed] [Google Scholar]
- 35. Nakamura A., Ly C., Cipetiæ M., Sims N. A., Vieusseux J., Kartsogiannis V., Bouralexis S., Saleh H., Zhou H., Price J. T., Martin T. J., Ng K. W., Gillespie M. T., Quinn J. M. (2007) Bone 40, 305–315 [DOI] [PubMed] [Google Scholar]
- 36. Carano R. A., Filvaroff E. H. (2003) Drug Discov. Today 8, 980–989 [DOI] [PubMed] [Google Scholar]
- 37. Fitch M. J., Campagnolo L., Kuhnert F., Stuhlmann H. (2004) Dev. Dyn. 230, 316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lamalice L., Le Boeuf F., Huot J. (2007) Circ. Res. 100, 782–794 [DOI] [PubMed] [Google Scholar]
- 39. Sanderson M. P., Dempsey P. J., Dunbar A. J. (2006) Growth Factors 24, 121–136 [DOI] [PubMed] [Google Scholar]
- 40. Schmidt M. H., Bicker F., Nikolic I., Meister J., Babuke T., Picuric S., Müller-Esterl W., Plate K. H., Dikic I. (2009) Nat. Cell Biol. 11, 873–880 [DOI] [PubMed] [Google Scholar]
- 41. Linton J. M., Martin G. R., Reichardt L. F. (2007) Development 134, 2501–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y., Zhao L., Smas C., Sul H. S. (2010) Mol. Cell. Biol. 30, 3480–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brandenberger R., Schmidt A., Linton J., Wang D., Backus C., Denda S., Müller U., Reichardt L. F. (2001) J. Cell Biol. 154, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Müller U., Wang D., Denda S., Meneses J. J., Pedersen R. A., Reichardt L. F. (1997) Cell 88, 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmidt A., Brixius K., Bloch W. (2007) Circ. Res. 101, 125–136 [DOI] [PubMed] [Google Scholar]
- 46. Bloch W., Forsberg E., Lentini S., Brakebusch C., Martin K., Krell H. W., Weidle U. H., Addicks K., Fässler R. (1997) J. Cell Biol. 139, 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.