Abstract
We previously reported that isobutylmethylxanthine (IBMX), a derivative of oxypurine, inhibits citrulline synthesis by an as yet unknown mechanism. Here, we demonstrate that IBMX and other oxypurines containing a 2,6-dione group interfere with the binding of glutamate to the active site of N-acetylglutamate synthetase (NAGS), thereby decreasing synthesis of N-acetylglutamate, the obligatory activator of carbamoyl phosphate synthase-1 (CPS1). The result is reduction of citrulline and urea synthesis. Experiments were performed with 15N-labeled substrates, purified hepatic CPS1, and recombinant mouse NAGS as well as isolated mitochondria. We also used isolated hepatocytes to examine the action of various oxypurines on ureagenesis and to assess the ameliorating affect of N-carbamylglutamate and/or l-arginine on NAGS inhibition. Among various oxypurines tested, only IBMX, xanthine, or uric acid significantly increased the apparent Km for glutamate and decreased velocity of NAGS, with little effect on CPS1. The inhibition of NAGS is time- and dose-dependent and leads to decreased formation of the CPS1-N-acetylglutamate complex and consequent inhibition of citrulline and urea synthesis. However, such inhibition was reversed by supplementation with N-carbamylglutamate. The data demonstrate that xanthine and uric acid, both physiologically occurring oxypurines, inhibit the hepatic synthesis of N-acetylglutamate. An important and novel concept emerging from this study is that xanthine and/or uric acid may have a role in the regulation of ureagenesis and, thus, nitrogen homeostasis in normal and disease states.
Keywords: Ammonia, Enzyme Inhibitors, Urea, Urea Cycle, Uric Acid, Carbamoyl Phosphate Synthase-I, N-Acetylglutamate, N-Carbamylglutamate, Citrulline, Xanthine
Introduction
Ureagenesis is the major metabolic pathway for the removal of waste nitrogen (1). The liver forms nontoxic urea from NH3 that is derived from various metabolic processes, including the metabolism of dietary protein and the breakdown of endogenous protein. An impairment of urea synthesis can cause acute or chronic hyperammonemia, a highly toxic perturbation that results in dysfunction of the central nervous system (2). The initial step in urea synthesis is the conversion of NH4+ and HCO3− into carbamoyl phosphate, catalyzed by mitochondrial carbamyl phosphate synthetase 1 (CPS1)2 (EC 6.3.4.16) (3–5), followed by the reaction of carbamoyl phosphate with ornithine to form citrulline catalyzed by ornithine transcarbamoylase (1) (Fig. 1). A major functional distinction of CPS1 is its absolute dependence on N-acetylglutamate (NAG) for catalysis through the formation of the CPS1-NAG complex. The latter is the initial and obligatory step for synthesis of carbamoyl phosphate and, thereby, citrulline (1, 6). The binding of NAG to CPS1 produces a monomeric and unstable but catalytically active protein (7–9). CPS1 is highly abundant (0.5–1.5 mm) in hepatic mitochondria (1, 10–12). Thus, the regulation of citrulline synthesis, and hence, ureagenesis may chiefly depend upon synthesis and availability of NAG. The latter is synthesized from glutamate and acetyl-CoA (ACoA) by mitochondrial N-acetylglutamate synthase (NAGS) (9). An inhibition of NAG synthesis may occur in organic acidemias and/or treatment with valproic acid (9). In these cases, organic acids form CoA esters that competitively inhibit the binding of ACoA to NAGS (13, 14).
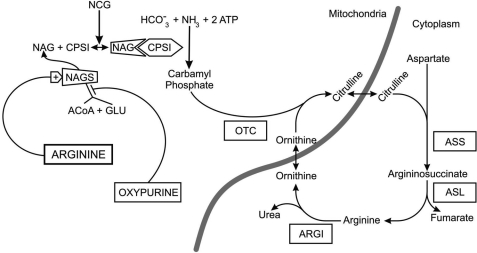
FIGURE 1.
Schematic presentation of the possible action of oxypurines on citrulline and urea synthesis. We proposed that xanthine or uric acid (oxypurines) inhibits NAGS activity and, thus, depletes mitochondrial NAG levels. The result is decreased formation of the CPS1-NAG complex, down-regulation of flux through CPS1, and an impairment of urea synthesis. However, supplementation of N-carbamylglutamate (NCG) and/or an allosteric activation of NAGS by arginine may increase generation of the CPS1-NAG complex and activate CPS1. The result is increased flux through CPS1 and urea synthesis. OTC, ornithine transcarbamylase; ARGI, arginase-I; ASS, argininosuccinate synthetase; ASL, argininosuccinate lyase; GLU, glutamate.
Here, we hypothesized that an inhibition of NAGS activity may also occur following exposure to selected oxypurines (supplemental Fig. 1S). This hypothesis is based on our recent finding that isobutylmethylxanthine (IBMX), a derivative of oxypurine and a non-selective inhibitor of phosphodiesterases, inhibits citrulline and urea synthesis in a liver perfusion system (15). However, the mechanism of the IBMX-induced inhibition of citrulline synthesis is still elusive. Therefore, in the current study, we explored the hypothesis that IBMX may inhibit citrulline synthesis via inhibition of NAGS. We also hypothesized that xanthine (XAN) and/or uric acid (UA), which contain a 2,6-dione group similar to IBMX (supplemental Fig. 1S), may inhibit NAGS and ureagenesis in a comparable manner. The current hypothesis posits that one or more oxypurines may interfere with the binding of glutamate and/or ACoA to its active site in NAGS, thereby leading to inhibition of NAGS, decreasing NAG synthesis, deactivating CPS1, and, thus, diminishing citrulline and urea synthesis (Fig. 1). Based on these hypotheses, specific questions addressed in the current work include the following. 1) Is oxypurine-induced inhibition of citrulline synthesis solely mediated via inhibition of NAGS, and/or it is combined with direct action on CPS1? 2) What is the time- and dose-dependent inhibition of NAG and/or citrulline synthesis? 3) What is the relationship between NAGS inhibition, the production of the CPS1-NAG complex, and flux through CPS1? 4) Can oxypurine-induced NAGS inhibition be reversed by an allosteric activation of NAGS? 5) Does supplementation with N-carbamylglutamate protect against oxypurine-induced inhibition of NAGS?
We used oxypurine-induced inhibition of NAG synthesis as a tool to assess the efficacy of l-arginine or N-carbamylglutamate to protect against inhibition of NAGS and, therefore, to maintain ureagenesis. N-Carbamylglutamate is a stable structural analog of NAG that is transported into mitochondria and activates CPS1 (9). Administration of N-carbamylglutamate to rats with hyperammonemia or patients deficient in NAGS increased ureagenesis and decreased the blood ammonia concentration (16–18). N-Carbamylglutamate therapy stimulates flux through CPS1 and improves urea synthesis in a variety of clinical settings, including organic acidemias and treatment with valproic acid (9, 16–18). l-Arginine is an allosteric activator of NAGS, and its supplementation increased mitochondrial NAG concentrations (19–22). Previous studies have shown that arginine is essential to several metabolic and physiological functions. Supplementation of arginine to patients with inflammatory diseases, trauma, surgery, and tumors may help maintain normal physiologic functions (23).
To test the above hypotheses and address the related questions, we used isolated mitochondria or mitochondrial lysates prepared from the liver of overnight fasted rats as a source of CPS1 and NAGS (15, 19, 22, 24). Experiments with isolated mitochondria provided insight into the action of oxypurines on the synthesis of NAG, production of the CPS1-NAG complex, and flux through CPS1 in a complex physiological system. A separate series of experiments was performed with isolated hepatocytes, a model system that possesses the entire urea cycle. To distinguish between a possible action on NAGS and/or CPS1, an additional series of experiments was performed with purified hepatic CPS1 or recombinant mouse NAGS. Experiments were performed with 15N-labeled ammonia, glutamate, or glutamine. We assayed the synthesis of 15N-labeled NAG, citrulline, or urea with liquid chromatography-mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS) as we described previously (15, 19, 25). The production of 15N-labeled NAG from [15N]glutamate was used as a measure of NAGS activity, and the production of 15N-labeled citrulline from 15NH4Cl was used as a proxy for flux through CPS1 (19, 22, 24, 26). We used the level of NAG and CPS1 to calculate the formation of the CPS1-NAG complex as described (6).
The current findings demonstrate that xanthine and uric acid significantly inhibit NAGS activity but have little effect on CPS1. The inhibition of NAG synthesis resulted in decreased formation of the CPS1-NAG complex and reduction of citrulline and urea synthesis. However, this action was reversed by supplementation of N-carbamylglutamate.
EXPERIMENTAL PROCEDURES
Materials and Animals
Male Sprague-Dawley rats (Charles River) were fed ad lib a standard rat chow diet. Chemicals, enzymes, and cofactors were obtained from Sigma-Aldrich. 15N-Labeled NH4Cl, glutamine, glutamate, or ornithine, 99 mol % excess, were from Isotec.
Experimental Design
Experiments with Mitochondrial Preparation
The initial series of experiments was performed with mitochondrial lysates in order to determine the action of oxypurines on NAG synthesis and the resulting effect on flux through CPS1. To obtain mitochondria with reduced endogenous concentration of NAG, rats were fasted overnight. Mitochondria were isolated from the liver by differential centrifugation and isolation medium consisting of 225 mm mannitol, 75 mm sucrose, 1 mm EDTA, and 5 mm Hepes, pH 7.4 (15, 19). Mitochondrial suspensions were kept on ice in the isolation medium and used either for preparation of mitochondrial lysates or incubation of intact mitochondria. Respiratory control and oxygen consumption were determined (15, 19). Experiments were carried out with mitochondria having a state 3/state 2 ratio greater than 3.
For determination of flux through NAGS (NAG synthesis) and flux through CPS1 (citrulline synthesis), mitochondrial lysates were prepared from mitochondrial suspensions following three cycles of freezing in liquid nitrogen and thawing. Incubations were carried out in Erlenmeyer flasks (2-ml final volume) at 37 °C in a shaking water bath for the time indicated. The basic incubation medium consisted of 50 mm Tris, 1 mm EDTA, 5 mm KCl, 5 mm MgCl2, 15 mm KHCO3, and 5 mm KH2PO4, pH 7.4. Substrates for citrulline and NAG synthesis were added to this basic medium as indicated below and/or in the figure legends.
We first performed experiments to determine 1) the time course activity of NAGS in mitochondrial lysate, 2) the relationship between mitochondrial protein and NAGS activity, and 3) the kinetic parameters of NAGS under the current mitochondrial preparation and experimental conditions. The time course activity was determined with mitochondrial lysates and fixed 2 mg/ml mitochondrial protein. Incubations for various times between 0.5 and 7.5 min were performed with basic medium (indicated above), plus 5 mm MgATP, 5 mm ornithine, 1 mm acetyl-CoA, 10 mm [15N]glutamate, and 1 mm 15NH4Cl. For determining the relationship between mitochondrial protein and NAGS activity, incubations were carried out for 5 min with similar medium and increasing concentrations of lysate protein between 0.1 and 2 mg/ml. To determine the kinetic parameters of NAGS, lysate protein (2 mg/ml) was incubated with basic medium plus fixed 5 mm ACoA and varied [15N]glutamate concentrations between 0.2 and 40 mm or with fixed 40 mm [15N]glutamate and varied ACoA concentrations between 0.1 and 5 mm.
In addition, a series of pilot experiments was carried out to determine 1) the action of oxypurines on ornithine transcarbamoylase activity, 2) whether ornithine transcarbamoylase is limiting for the synthesis of citrulline, and 3) whether the synthesis of citrulline by mitochondrial lysates can be used as a proxy measure of flux through CPS1. To this end, mitochondrial lysates were incubated for 5 min with basic medium (as detailed above) plus [α-15N]ornithine (10 mm), carbamoyl phosphate (1 mm), MgATP (5 mm), with or without 2 mm IBMX or xanthine.
Next, experiments were carried out with various oxypurines in order to determine whether the inhibition of citrulline synthesis is unique to IBMX or shared by other oxypurines. Experiments were done with mitochondrial lysates (2 mg/ml protein) incubated for 20 min (steady-state) with a complete medium consisting of 50 mm Tris, 1 mm EDTA, 5 mm KCl, 5 mm MgCl2, 15 mm KHCO3, and 5 mm KH2PO4, pH 7.4, 10 mm ornithine, 5 mm MgATP, 10 mm 15NH4Cl, 10 mm [15N]glutamate, 0.5 mm ACoA, and either 1 mm IBMX (dissolved in DMSO) or 1 mm selected oxypurines, including, xanthine, hypoxanthine, uric acid, allopurinol, or caffeine (supplemental Fig. 1S). An equal amount of DMSO was also added to incubations without IBMX.
As demonstrated in supplemental Fig. 2S, only IBMX, xanthine, or uric acid significantly inhibited NAG and citrulline synthesis. Therefore, the rest of the experimental design was focused on the action of these compounds on NAG and citrulline synthesis. We determined the dose dependence of IBMX, xanthine, or uric acid on the flux through NAGS and CPS1. Mitochondrial lysates were incubated with complete medium (indicated above) and increasing concentrations (0–2 mm) of xanthine, uric acid, or IBMX. A separate series of experiments was performed to determine the time course of oxypurine action on NAG and/or citrulline synthesis. Incubations with complete medium with or without oxypurine were performed for various times as indicated in the legend to the figure.
The action of a given inhibitor on NAGS, CPS1, and/or generation of the CPS1-NAG complex may be affected by mitochondrial uptake. In addition, the relative efficacy and the dose of N-carbamylglutamate, a substitute of NAG, and/or arginine, an allosteric activator of NAGS, may also depend on their mitochondrial uptake. Therefore, experiments were performed with intact mitochondrial suspensions (2 mg/ml protein) and an increasing concentration of IBMX, xanthine, or uric acid or a constant concentration (2 mm) of oxypurine and increasing concentrations of N-carbamylglutamate or arginine. Incubations for 15 min were performed with medium consisting of 50 mm Tris, 1 mm EDTA, 5 mm KCl, 5 mm MgCl2, 15 mm KHCO3, and 5 mm KH2PO4, pH 7.4, plus 10 mm ornithine, 1 mm 15NH4Cl, 10 mm [15N]glutamate, 0.25 mm acetyl-CoA. ATP was replaced by 5 mm ADP and 5 mm pyruvate (respiring on pyruvate). A physiological osmolarity of 300 mosm was achieved by the addition of mannitol as indicated (27).
Next we examined whether the inhibitory action of oxypurine persisted even after its removal from the mitochondrial incubation. To this end, we used IBMX as a representative of oxypurine because mitochondria can be separated from the medium containing IBMX plus DMSO by centrifugation. Intact mitochondria were preincubated for 20 min at room temperature with medium consisting of 50 mm Tris, 1 mm EDTA, 5 mm KCl, 5 mm MgCl2, 15 mm KHCO3, and 5 mm KH2PO4, with or without IBMX (2 mm) dissolved in about 5% DMSO or only 5% DMSO without IBMX as a control. Thereafter, mitochondria pellets (control and preincubated with IBMX) were separated from incubation medium by centrifugation (6000 × g for 15 min). Mitochondrial pellets were washed with isolation medium (indicated above) containing 5% DMSO, followed by a second round of centrifugation. Control mitochondria were similarly washed with DMSO. Finally, mitochondria preincubated with IBMX plus DMSO or only DMSO (control) were reincubated for 5 min without IBMX and with basic medium plus 5 mm ornithine, 5 mm ADP, 5 mm pyruvate, 1 mm 15NH4Cl, 5 mm [15N]glutamate, 0.1 mm acetyl-CoA, with or without 1 mm arginine, as indicated in the Fig. 6 legend.
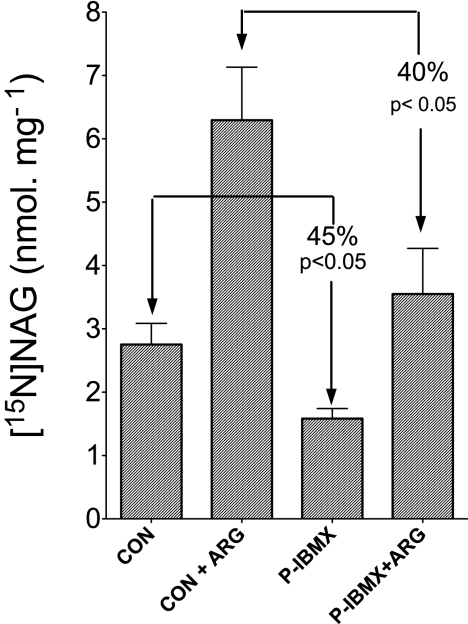
FIGURE 6.
The inhibition of NAGS activity was sustained following preincubation with IBMX. Intact mitochondria were preincubated for 20 min at room temperature with medium consisting of 50 mm Tris, 1 mm EDTA, 5 mm KCl, 5 mm MgCl2, 15 mm KHCO3, and 5 mm KH2PO4 and 2 mm IBMX dissolved in 5% DMSO (P-IBMX). Control (CON) preincubation was performed with an equal amount of DMSO without IBMX. Thereafter, mitochondrial pellets were separated from IBMX by centrifugation and then were washed with basic medium containing 5% DMSO by a second round of centrifugation (see “Experimental Procedures”). Mitochondria from control underwent similar centrifugation and were washed with DMSO. Then mitochondrial pellets obtained following preincubation with IBMX or control with only DMSO were reincubated for 5 min with medium containing 2.5 mm ADP, 5 mm pyruvate (respiring on pyruvate), 5 mm ornithine, 0.5 mm acetyl-CoA, 5 mm [15N]glutamate, 1 mm 15NH4Cl. CON, control; CON + ARG, control plus 1 mm arginine; P-IBMX, following preincubation with IBMX; P-IBMX + ARG, following preincubation with IBMX plus 1 mm arginine. Each histogram represents the mean ± S.D. (error bars) of three independent experiments. The percentage represents the differences between the indicated experimental conditions.
Experiments with Purified Hepatic CPS1 or a Recombinant Mouse NAGS
The above experiments were designed to explore the action of oxypurines on the relationship between flux through NAGS, the production of the NAG-CPS1 complex, and flux through CPS1 in a complex physiological system. However, to determine whether the action of oxypurine is at NAGS, CPS1, or both, experiments were carried out with a purified hepatic CPS1 or recombinant mouse NAGS as follows.
Rat liver CPS1 (specific activity 26–30 μmol of carbamyl phosphate/15 min/mg) was prepared essentially as described by Guthöhrlein and Knappe (28) and stored at −20 °C as an ammonium sulfate precipitate. Before use, it was dissolved in 50 mm glycylglycine and 0.1 m KCl (pH 7.4), and it was freed from ammonium sulfate by centrifugal gel filtration (29). The enzyme was assayed spectrophotometrically at 340 nm by coupling ADP production to NADH oxidation using pyruvate kinase and lactate dehydrogenase (28, 30). Assays were performed with 2.5–2.8 μg of CPS1 incubated at 37 °C for 10 min in 1 ml of a solution containing 50 mm glycylglycine, 0.1 m KCl, 35 mm NH4Cl, 5 mm ATPMg, 15 mm MgSO4, 20 mm KHCO3, 1 mm DTT, 2.5 mm phosphoenolpyruvate, 0.25 mm NADH, 40 μg/ml pyruvate kinase, 25 μg/ml lactate dehydrogenase, and 10 mm NAG (pH 7.4). Separate assays were performed with 0.2 mm NAG and 1 mm NH4Cl to approximate mitochondrial conditions. In a separate series of incubations, 5 mm xanthine, 3 mm uric acid, or 5 mm IBMX was added. IBMX was dissolved in DMSO. An equal amount of DMSO was also added to control incubations without IBMX.
Recombinant mouse NAGS was overexpressed in Escherichia coli, purified, and stored at 4 °C as described previously (25). We used recombinant NAGS to determine 1) the time course of NAGS activity in the presence or absence of xanthine, 2) the relationship between the level of enzyme protein and velocity of NAGS in the presence or absence of xanthine, 3) the action of various oxypurines on NAGS activity, and 4) the action of various concentrations of xanthine or uric acid on the kinetic parameters of NAGS. The time course of NAGS activity in the presence or absence of xanthine was determined by assays performed for various times with fixed 1 mm ACoA, 10 mm [15N]glutamate with or without fixed 2 mm xanthine. The reaction was performed at 30 °C in 250 μl of 50 mm Tris-HCl buffer, pH 8.5, containing about 2.5 μg of enzyme and 1 mm arginine. The relationship between the level of enzyme protein and velocity of NAGS in the presence or absence of xanthine was determined by assays performed for 5 min with various concentrations of enzyme protein and fixed 1 mm ACoA, 10 mm [15N]glutamate with or without fixed 2 mm xanthine. The reaction was performed at 30 °C in 250 μl of 50 mm Tris-HCl buffer, pH 8.5, containing 1 mm arginine. The action of various oxypurines on NAGS activity was determined by assays performed with fixed 1 mm ACoA and 10 mm [15N]glutamate and fixed or increasing concentrations of various oxypurines as indicated in the Fig. 8 legend. The reaction was performed at 30 °C for 5 min in 250 μl of 50 mm Tris-HCl buffer, pH 8.5, containing about 2.5 μg of enzyme and 1 mm arginine. The action of various concentrations of xanthine or uric acid on the kinetic parameters of NAGS was determined by assays performed with 1 mm arginine, varied ACoA concentration between 0.1 and 4 mm, and fixed 10 mm [15N]glutamate or fixed 1 mm ACoA and varied [15N]glutamate in the range of 1–20 mm, as indicated in the Fig. 10 legend. The reaction was performed at 30 °C for 5 min in 250 μl of 50 mm Tris-HCl buffer, pH 8.5, containing about 2.5 μg of enzyme and 1 mm arginine.
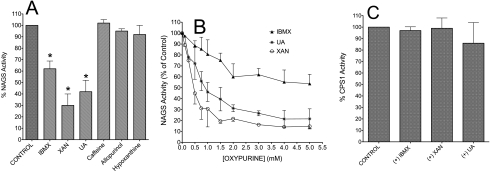
FIGURE 8.
The action of oxypurine on the activity of the recombinant NAGS or purified hepatic CPS1. A, action of a fixed concentration of oxypurines (1 mm) on percentage of NAGS activity. Assays were performed with fixed 1 mm ACoA and 10 mm [15N]glutamate. The reaction was carried out at 30 °C for 5 min in 250 μl of 50 mm Tris-HCl buffer, pH 8.5, containing about 2.5 μg of enzyme and 1 mm arginine. B, NAGS activity (percentage of control) with increasing concentrations of the indicated oxypurine. Assays were performed as indicated in A. 100% of NAGS activity (control value without oxypurine) was about 25 nmol/μg/min. C, percentage of CPS1 activity with fixed concentration (6, 5, or 3 mm) of IBMX, XAN, or UA, respectively. Results are expressed as a percentage of the enzyme activity in the absence of the indicated oxypurine. The 100% CPS1 activity was about 3.2 nmol/mg/min, as determined by formation of ADP (see “Experimental Procedures”). The CPS1 amount used was 2.5–2.8 μg/ml. Data points are mean ± S.D. (error bars) of three independent experiments.
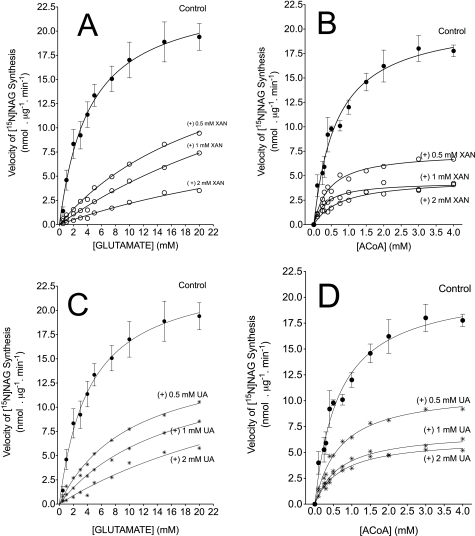
FIGURE 10.
The velocity of recombinant NAGS as a function of xanthine or uric acid concentrations. The reaction was performed at 30 °C for 5 min in 250 μl of 50 mm Tris-HCl buffer, pH 8.5, containing 2.5 mg of enzyme and 1 mm arginine. A and B, velocity of [15N]NAG synthesis in the presence of various concentrations of XAN as indicated and with either varied [[15N]glutamate] and fixed 1 mm ACoA (A) or varied [ACoA] and fixed (10 mm) [15N]glutamate (B). C and D, velocity of [15N]NAG synthesis in the presence of various concentrations of UA, as indicated, and with either varied concentrations of [15N]glutamate and fixed (1 mm) ACoA (C) or varied [ACoA] and fixed (10 mm) [15N]glutamate (D). The control parameters (without oxypurine) are mean ± S.D. (error bars) of 3–4 determinations performed with an independent preparation of recombinant NAGS. Parameters obtained with the addition of xanthine or uric acid are means of two determinations performed with an independent preparation of recombinant NAGS.
Experiments with Isolated Hepatocytes
Urea synthesis is a compartmentalized process initiated in the mitochondria and completed in the cytosol (1). Thus, to examine the effect of oxypurine with or without N-carbamylglutamate or arginine on urea synthesis, experiments were performed with isolated hepatocytes as indicated (31). Incubations were carried out for 60 min at 37 °C in a shaking water bath with Krebs buffer (pH 7.4) plus 10 mm lactate, 1.5 mm pyruvate, 5 mm [5-15N]glutamine, and 1 mm 15NH4Cl, with or without 1 mm oxypurine, a combination of 1 mm oxypurine plus 0.5 mm N-carbamylglutamate, 1 mm oxypurine plus 0.5 mm arginine, or 1 mm oxypurine plus a combination of 0.5 mm arginine and 0.5 mm N-carbamylglutamate, as indicated.
Sample Preparation and Measurements
At the end of either incubation outlined above, an aliquot was taken for protein determination, and incubation was stopped with HClO4. Metabolite measurements were done in neutralized extracts as follows.
Measurement of 15N isotopic enrichment in citrulline or NAG was performed in non-derivatized samples using the Agilent 6410 Triple Quad liquid chromatography-mass spectrometry system. 2 μl of sample was injected into a Cogent Diamond Hydride column, 100 mm × 2.1 mm (Microsolv Technology). Metabolites were separated with a gradient of Solution A, which contained 0.1% formic acid in H2O, and Solution B, containing acetonitrile plus 0.1% formic acid and 0.005% TFA. In a few experiments, measurements of 15N enrichment in citrulline or urea were carried out using gas chromatography-mass spectrometry as described (15, 19, 31). The concentration of amino acids was determined by HPLC, utilizing precolumn derivatization with o-phthalaldehyde (32) and total urea as indicated (19, 31). The concentration of NAG was determined using an isotope dilution approach (15, 19, 31).
Calculations and Statistical Analysis
Data obtained were analyzed with GraphPad Prism-5 software for linear or nonlinear curve fitting. In experiments with mitochondrial preparation, a sigmoidal dose response was used to determine the relative inhibition constant (IC50) for inhibition of the flux through CPS1 or NAGS. The time course of [δ-15N2]citrulline production from 15NH4Cl was used to calculate flux through CPS1. The flux through NAGS was determined using the formation of 15N-labeled NAG from [15N]glutamate (19). We first determined the concentration of NAG by isotope dilution and then calculated the rate of [15N]NAG synthesis by the product of the initial (before spiking the sample) 15N enrichment, mol % excess/100 × concentration (nmol/mg protein). The time course of formation of 15N-labeled NAG or citrulline was fitted to a linear regression or a one-phase exponential association (Y = Ymax*(1 − e−kt)). Depending upon the time course of production of 15N-labeled metabolite, the flux rates (nmol/mg/min) were calculated by the product of the corresponding Ymax × k or by the slope of the linear curve.
For determining the kinetic parameters of NAGS, Michaelis-Menten kinetics was used to determine the Km for glutamate or ACoA that was required achieving one-half Vmax for NAG synthesis using Prism-5 equations for enzyme kinetics. The S.E. value of the kinetic parameters was determined from the curve fitting.
Statistical analysis was carried out using Prism-5 software for the Macintosh. Either one-way analysis of variance or Student's t test was employed to determine differences between groups. A p value less than 0.05 was determined as a statistically significant difference.
RESULTS
Characterization and Kinetic Parameters of N-Acetylglutamate Synthetase in the Current Preparation of Mitochondrial Lysate
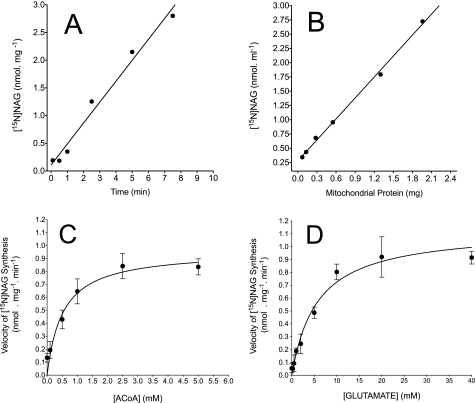
The initial series of experiments were designed to determine 1) the time course of NAGS activity in mitochondrial lysates; 2) the relationship between lysate protein and NAGS activity; and 3) the Km values for [glutamate] and [ACoA] that are required to achieve one-half Vmax for NAG synthesis under the current mitochondrial preparation and experimental conditions. Data in Fig. 2, A and B, demonstrate that the NAGS reaction was linear between 0.5 and 7.5 min of incubation, and between 0.1 and 2 mg of protein (r2 = 0.97; p < 0.0001). Fig. 2, C and D, represents the velocity of NAGS with a fixed (40 mm) concentration of [15N]glutamate and varied ACoA concentrations (0.1–5 mm) (Fig. 2C) or a fixed concentration (5 mm) of ACoA and varied [15N]glutamate concentrations (0.2–40 mm) (Fig. 2D). Fitting these data to the Michaelis-Menten equation yielded a Km of about 6 mm for glutamate and 0.6 mm for ACoA, in agreement with previous reports (9, 13, 34). In all subsequent experiments, we used ∼2 mg of mitochondrial protein (within the linear range, as shown in Fig. 2B) and the Km values of glutamate and ACoA to determine the effect of oxypurines on NAG and citrulline synthesis.
FIGURE 2.
Linearity and kinetic parameters for N-acetylglutamate synthetase in mitochondrial lysates. Experiments were carried out with mitochondrial lysates prepared from the liver of overnight fasted rats. A, activity of NAGS versus times of incubation. Mitochondrial lysates (2 mg/ml protein) were incubated for the times indicated with basic medium (see “Experimental Procedures”) plus 5 mm MgATP, 5 mm ornithine, 1 mm acetyl-CoA, 10 mm [15N]glutamate, and 1 mm 15NH4Cl. B, activity of NAGS versus increasing amounts of mitochondrial lysate protein. Incubations for 5 min were performed with various lysate protein, as indicated, and with basic medium (see “Experimental Procedures”) plus 5 mm MgATP, 5 mm ornithine, 1 mm acetyl-CoA, 10 mm [15N]glutamate, and 1 mm 15NH4Cl. C and D, velocity of [15N]NAG synthesis by the current mitochondrial lysate preparation. Incubations for 5 min were carried out with basic medium and either varied [ACoA] and fixed 40 mm [15N]glutamate (C) or varied [15N]glutamate concentrations and fixed (5 mm) ACoA (D). Data points in A and B represent the means of two experiments. Data points in C and D are means ± S.D. of 3–4 measurements performed on 3–4 independent mitochondrial lysate preparations.
Inhibition of NAG and Citrulline Synthesis by Selected Oxypurines
First, we performed a series of pilot experiments to establish the effect of oxypurines on ornithine transcarbamoylase activity and whether the synthesis of citrulline by mitochondrial lysates can be used as a proxy for flux through CPS1 under the current experimental conditions (see “Experimental Procedures”). The results demonstrated that 15N-labeled ornithine was immediately converted to 15N-labeled citrulline regardless of the presence or absence of 2 mm IBMX or xanthine (data not shown). Thus, oxypurines do not act on ornithine transcarbamoylase, and therefore, the production of 15N-labeled citrulline does reflect flux through CPS1, in accord with many previous investigations (10, 19, 22).
Next, a series of studies was designed to examine whether the inhibition of citrulline synthesis is a specific characteristic of IBMX or whether other oxypurine compounds also are inhibitory. Experiments were performed with mitochondrial lysates incubated with a complete medium (see “Experimental Procedures”) and IBMX, xanthine, uric acid, hypoxanthine, allopurinol, or caffeine (each 1 mm). These oxypurines are representative of those physiologically present (xanthine, uric acid, and hypoxanthine) as well as those derived from drugs (allopurinol) or diet (caffeine). The results demonstrate that only xanthine and uric acid significantly inhibit citrulline and NAG synthesis in a manner similar to IBMX (supplemental Fig. 2S). Therefore, the following results describe the action of IBMX, xanthine, or uric acid on NAG and citrulline synthesis and the subsequent effect on hepatic ureagenesis.
Dose Dependence and Time Course Inhibition of NAG and Citrulline Synthesis
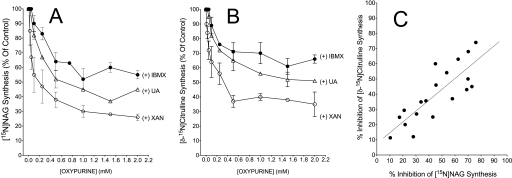
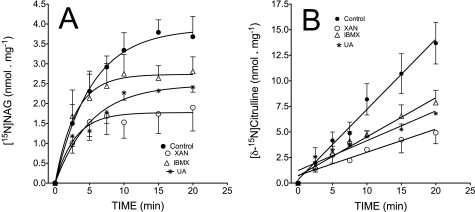
Data obtained in experiments with mitochondrial lysates demonstrate that IBMX, xanthine, or uric acid inhibits the synthesis of [15N]NAG and [δ-15N]citrulline in a dose- and time-dependent manner (Figs. 3 and 4). Fig. 3C demonstrates a linear relationship between the inhibition of NAG and citrulline synthesis. The relative IC50 values for this inhibition demonstrate that xanthine is the most potent inhibitor of both NAG and citrulline synthesis (Table 1 and supplemental Fig. 3S). Fig. 4 demonstrates a hyperbolic increase in NAG synthesis during the course of the incubation. With the addition of 1 mm xanthine or uric acid, there was ∼35–40% inhibition of 15N-labeled NAG synthesis following a 5-min incubation, and this range of inhibition was sustained to the end of a 20-min incubation. With IBMX, an inhibition of ∼20% was observed after 7.5 min and was about 25% at 20 min of incubation. The simultaneous synthesis of [δ-15N]citrulline, with or without oxypurines, linearly increased throughout the course of the incubation. However, at each time point, the synthesis of citrulline was about 3-fold lower in experiments with xanthine compared with control and 2-fold lower in experiments with uric acid. IBMX decreased flux through CPS1 by about 40%. We used the data points in Fig. 4 to calculate the rates of flux through NAGS and CPS1 in experiments with mitochondrial lysates. These calculations demonstrate about 25% decreased flux through NAGS with IBMX and about 40–50% decreased flux with xanthine or uric acid. The simultaneous calculation of flux through CPS1 demonstrates about 40% decrease with IBMX, a 2-fold decrease with uric acid, and about a 3-fold decrease with xanthine (Table 2).
FIGURE 3.
Dose dependence of oxypurine-induced inhibition of 15N-labeled NAG and citrulline synthesis. Experiments were carried out with mitochondrial lysates as a source of NAGS and CPS1. Mitochondrial lysates (∼2 mg/ml protein) were incubated for 20 min with basic medium (see “Experimental Procedures”) plus 5 mm MgATP, 5 mm ornithine, 0.5 mm acetyl-CoA, 10 mm [15N]glutamate, 1 mm 15NH4Cl, and increasing concentrations of oxypurine as indicated. A, flux through NAGS (percentage of control), as determined by synthesis of [15N]NAG. B, production of [δ-15N]citrulline synthesis (percentage of control) as a proxy for flux through CPS1. C, relationship between percentage inhibition of flux through CPS1 and flux through NAGS obtained from data in A and B. The mean control value for [15N]NAG synthesis was about 4.12 ± 0.12 nmol/mg, and the mean control value for [δ-15N]citrulline synthesis was about 25 ± 8 nmol/mg. Data points with error bars represent mean ± S.D. of 3–4 independent experiments. Data points without error bars are the mean of two independent experiments.
FIGURE 4.
Time dependence of oxypurine-induced inhibition of [15N]NAG and [δ-15N]citrulline synthesis. Mitochondrial lysates were incubated for various times with medium as outlined in the legend to Fig. 3 with or without 1 mm xanthine, uric acid, or IBMX as indicated. A, time dependence of oxypurine action on production of [15N]NAG. We first determined the concentration of NAG by isotope dilution and then calculated the rate of [15N]NAG synthesis by the product of the initial (before spiking the sample) 15N enrichment, mol % excess/100 × concentration (nmol/mg protein). B, simultaneous production of [δ-15N]citrulline represented by the product of total citrulline concentration (nmol/mg protein) × 15N enrichment, mol % excess/100. The lines present the best fit to one-phase exponential association (Y = Ymax*(1 − e−kt) of data in A or linear correlation of data in B. Data points with error bars are means ± S.D. of 3–4 independent experiments. Data points without error bars are means of two independent experiments.
TABLE 1.
The relative values of IC50 for inhibition of NAG and citrulline synthesis by IBMX, xanthine, or uric acid in experiments with mitochondrial lysate
Experiments were carried out with mitochondrial lysates as a source of NAGS and CPS1. Mitochondrial lysates (2 mg/ml protein) were incubated for 20 min with basic medium (see “Experimental Procedures”) plus MgATP (5 mm), ornithine (5 mm), acetyl-CoA (0.5 mm), [15N]glutamate (10 mm), 15NH4Cl (1 mm), and increasing concentrations of oxypurine (Fig. 3). The relative IC50 was calculated by plotting [15N]NAG and [δ-15N]citrulline synthesis (percentage of control) versus the log [oxypurine] (mm), as shown in supplemental Fig. 3S, and fitting the data to a sigmoidal dose response using GraphPad Prism-5 software.
| Experiment | IC50 |
|
|---|---|---|
| [15N]NAG synthesis | [δ-15N]citrulline synthesis | |
| mm | ||
| With IBMX | 0.36 | 0.17 |
| With xanthine | 0.17 | 0.09 |
| With uric acid | 0.24 | 0.23 |
TABLE 2.
IBMX, xanthine, or uric acid decreases flux through NAGS and CPS1
Experiments were carried out with mitochondrial lysates for various times as described in the legend to Fig. 4. Data were fitted to a linear regression analysis or a one-phase exponential association, Y = Ymax*(1 − e−kt). k is the rate constant. The time course production of 15N-labeled NAG (Fig. 4A) was used to calculate flux through NAGS by the product of k × Ymax. The time course production of [δ-15N]citrulline (Fig. 4B) was used to calculate flux through CPS1 by the slope of the linear production of [δ-15N]citrulline. Values are ± S.E. obtained by the curve fitting. NA, not applicable.
| Experiment | K | Flux |
|---|---|---|
| min−1 | nmol·mg protein−1·min−1 | |
| Control without oxypurine | ||
| Flux through NAGS | 0.19 ± 0.04 | 0.87 ± 0.06 |
| Flux through CPS1 | NA | 0.68 ± 0.03 |
| With 1 mm IBMX | ||
| Flux through NAGS | 0.24 ± 0.14 | 0.66 ± 0.16 |
| Flux through CPS1 | NA | 0.38 ± 0.02a |
| With 1 mm xanthine | ||
| Flux through NAGS | 0.36 ± 0.10 | 0.52 ± 0.03a |
| Flux through CPS1 | NA | 0.22 ± 0.03a |
| With 1 mm uric acid | ||
| Flux through NAGS | 0.28 ± 0.04 | 0.46 ± 0.01a |
| Flux through CPS1 | NA | 0.29 ± 0.04a |
a p < 0.05.
Because the results in Figs. 3 and 4 were obtained with mitochondrial lysates incubated with an optimal amount of MgATP and other substrates (ornithine, glutamate, acetyl-CoA, HCO3−, and NH4Cl), and because oxypurines have no effect on the ornithine transcarbamoylase activity, the current observation indicates that IBMX, xanthine, or uric acid decreases flux through NAGS. The decreased flux through CPS1 is probably mediated via inhibition of NAGS and, therefore, a diminished concentration of NAG, which is required for activation of CPS1. This observation is in accord with numerous reports indicating that a small decrease in NAG synthesis may result in a significant drop in citrulline synthesis (13, 14, 33, 34).
The Action of Oxypurines on the Synthesis of 15N-Labeled NAG and Citrulline in Intact Mitochondria
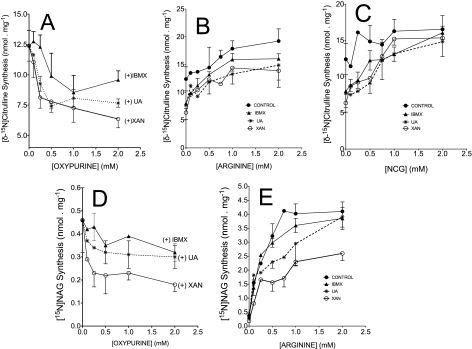
The deactivation-reactivation of NAGS and/or CPS1 may depend upon mitochondrial uptake of a given inhibitor and/or activator. Therefore, an additional series of experiments was performed with intact mitochondria. We performed incubations in a medium of physiolologic osmolarity (300 mosm) for 15 min. We selected this time period to assure maximal uptake of oxypurines into mitochondria and because the inhibitory action is time-dependent (Fig. 4A). Results in Fig. 5, A and D, demonstrate that IBMX, xanthine, or uric acid inhibits NAG and citrulline synthesis in intact mitochondria in a manner similar to that observed with mitochondrial lysate (Fig. 3).
FIGURE 5.
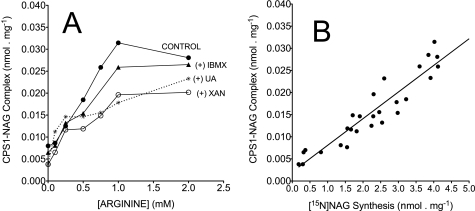
Oxypurine-induced inhibition of [15N]NAG and [δ-15N]citrulline synthesis in intact mitochondria; protection by supplementation of N-carbamylglutamate or arginine. Incubations with intact mitochondria (2 mg of protein) were carried out with iso-osmotic medium (300 mosm) for 15 min. The medium consists of basic medium (see “Experimental Procedures”) plus 5 mm ornithine, 0.1 mm acetyl-CoA, 10 mm [15N]glutamate, 1 mm 15NH4Cl, 5 mm pyruvate, and 5 mm ADP (respiring on pyruvate). A, inhibition of [δ-15N]citrulline synthesis with an increasing concentration of the indicated oxypurine without activator. B, synthesis of [δ-15N]citrulline synthesis with fixed 2 mm oxypurine and an increasing concentration of arginine as indicated. C, synthesis of [δ-15N]citrulline synthesis with fixed 2 mm oxypurine and an increasing concentration of N-carbamylglutamate (NCG). D and E, synthesis of [15N]NAG with an increasing concentration of oxypurine (D) or fixed 2 mm oxypurine and an increasing concentration of arginine (E). Data points are means ± S.D. (error bars) of three independent experiments.
If depletion of mitochondrial NAG levels is the primary mechanism for inhibition of citrulline synthesis, then supplementation of arginine, an activator of NAGS, or N-carbamylglutamate may reverse or limit this action. To test this possibility, mitochondria were incubated with IBMX, xanthine, or uric acid (each 2 mm) and increasing concentrations of N-carbamylglutamate or arginine. Fig. 5C demonstrates that the addition of N-carbamylglutamate increased the synthesis of [δ-15N]citrulline in a dose-dependent manner. With the addition of 2 mm N-carbamylglutamate, there was only little difference in citrulline synthesis in experiments with oxypurines compared with control experiments without oxypurine (Fig. 5C). This observation demonstrates that N-carbamylglutamate completely reversed the oxypurine-induced decrease in flux through CPS1 and further supports the notion that the primary action of oxypurine is an inhibition of NAGS.
The addition of increasing concentrations of arginine elevated the synthesis of both NAG (Fig. 5E) and citrulline (Fig. 5B). However, in experiments with xanthine plus 2 mm arginine, the synthesis of NAG was about 60% lower than control values. Thus, the data in Fig. 5E indicate that the addition of arginine improved flux through NAGS but did not completely abolish the inhibitory action of oxypurine, suggesting that an allosteric activation of NAGS by arginine is not sufficient to protect against the oxypurine-induced inhibition of NAGS.
Hence, data in Fig. 5E suggest that the inhibition of NAGS may be irreversible. To examine this possibility, isolated mitochondria were preincubated with IBMX as a representative oxypurine. IBMX has very limited water solubility, and its uptake into mitochondria depends upon the presence of DMSO. Intact mitochondria were preincubated for 20 min with 2 mm IBMX dissolved in about 5% DMSO or only 5% DMSO without IBMX as a control. Thereafter, mitochondria (control and preincubated with IBMX) were separated from the incubation medium by centrifugation and were washed with medium containing 5% DMSO by a second round of centrifugation as detailed under “Experimental Procedures.” Supplemental Fig. 4S demonstrates that about 97% of the 2 mm IBMX was removed by this procedure. Fig. 6 demonstrates that, following preincubation with 2 mm IBMX, about 45% inhibition of NAGS was sustained even after removal of IBMX from the incubation medium. Furthermore, when arginine was added to the incubation medium following preincubation with IBMX, although the rate of NAG synthesis was increased by about 2-fold, this rate still was about 40% lower compared with control preincubated without IBMX (Fig. 6). These observations suggest that arginine did not reverse the oxypurine-induced inhibition of NAGS, but it may have allosterically activated the portion of NAGS that was not affected by oxypurines and thus elevated NAG synthesis as indicated in Figs. 5E and 6.
The generation of the CPS1-NAG complex is the initial step in citrulline synthesis (1, 6). In the current study, we examined the relationship between the oxypurine-induced inhibition of NAGS and the generation of the CPS1-NAG complex. To this end, we used the mitochondrial levels of CPS1 and NAG concentrations obtained following incubation of intact mitochondria with oxypurine plus increasing concentrations of arginine (Fig. 5E). Based on a matrix volume of 1.2 μl/mg mitochondrial protein (25), the estimated level of CPS1 was 1.88 ± 0.3 mm, a value in good agreement with prior reports (1–1.5 mm) (1, 6, 10–12). The reaction CPS1 + NAG ↔ CPS1-NAG is at near equilibrium with a dissociation constant for NAG of 1 × 10−4 m (6, 35). Hence, the value of the [CPS1-NAG] complex at various [NAG] can be estimated by the equation, [CPS1] × [NAG]/[CPS1-NAG] = 10−4 m (35). For comparative purposes, the [CPS1-NAG] complex was expressed as nmol/mg mitochondrial protein.
Fig. 7A demonstrates that the calculated level of the CPS1-NAG complex increased with increasing concentrations of arginine. In the absence of oxypurines, the production of the CPS1-NAG complex linearly increased with increasing arginine concentrations between 0.1 and 1 mm and reached an apparent steady state above 1 mm arginine. In the presence of 1–2 mm arginine plus 2 mm xanthine or uric acid, the levels of CPS1-NAG were about 40% of control. Data in Fig. 7B demonstrate a linear relationship between the newly synthesized [15N]NAG and levels of the CPS1-NAG complex, suggesting a robust dependence between NAG synthesis and generation of the CPS1-NAG complex. These observations are in accord with previous investigations demonstrating a linear relationship between the concentrations of NAG and formation of the CPS1-NAG complex (3, 35). The current data suggest that the oxypurine-induced decreased synthesis of NAG led to decreased generation of the CPS1-NAG complex and, thus, reduction of citrulline synthesis.
FIGURE 7.
The relationship between the level of newly synthesized [15N]NAG and the calculated level of the CPS1-NAG complex. The level of CPS1-NAG complex was calculated using the assumption that CPS1 + NAG ↔ CPS1-NAG is at near equilibrium (35), with a dissociation constant of the CPS1 for NAG of 1 × 10−4 m (6), and, thus, [CPS1] × [NAG]/[CPS1-NAG] = 10−4 m. We estimated the concentration of CPS1 as 22% of mitochondrial matrix protein (6). Total NAG concentration (nmol/mg protein) was determined by isotope dilution (19, 31), with each arginine concentration added in experiments with intact mitochondria (CONTROL, IBMX, XAN, and UA are as indicated in Fig. 5E). A, calculated CPS1-NAG complex without oxypurine (CONTROL) or with fixed 2 mm oxypurine and increasing concentrations of arginine as indicated in Fig. 5E. B, a linear correlation between the calculated [CPS1-NAG] and the newly synthesized [15N]NAG following a 15-min incubation. The level of newly synthesized [15N]NAG was calculated by the product of the initial (before spiking the sample) 15N enrichment, mol % excess/100 × concentration of total NAG (nmol/mg protein). Data points of [15N]NAG are taken from experiments described in the legend to Fig. 5E (CON, IBMX, XAN, and UA) and are plotted against the corresponding calculated level of the CPS1-NAG complex indicated in A (r2 = 0.9 and p < 0.0001).
The Effect of IBMX, Xanthine, or Uric Acid on the Activity of Purified Hepatic CPS1 or Recombinant NAGS
The results described above provide ample evidence that the oxypurine-induced decrease of citrulline synthesis is secondary to inhibition of NAGS. However, due to the metabolic complexity inherent in experiments with isolated mitochondria, one cannot exclude the possibility that a direct disruption of CPS1 activity may occur. Therefore, to distinguish between a possible action of oxypurines on NAGS and/or CPS1, experiments were carried out with recombinant NAGS or purified CPS1 from rat liver. Data in Fig. 8A demonstrate that only IBMX, xanthine, or uric acid inhibits NAGS activity, in agreement with observations obtained with mitochondrial lysates (supplemental Fig. 2S). The inhibition of NAGS by oxypurine depends on their concentration, which decreased NAGS activity in the tested range (0.1–5 mm), with maximal inhibition achieved at about 2 mm. The relative IC50 values for this inhibition calculated by plotting percentage NAGS activity versus log [oxypurine] (supplemental Fig. 5S) were 1.64, 0.72, and 0.31 mm for IBMX, uric acid, and xanthine, respectively. An observation of special importance is that the oxypurines tested have little or no effect on CPS1 activity (Fig. 8C). Therefore, the current observations provide strong supporting evidence for the hypothesis that the decreased citrulline synthesis by IBMX (15) or other oxypurines (Figs. 3–8) is secondary to inhibition of NAG synthesis.
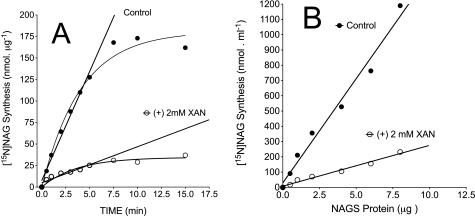
To examine the impact of xanthine or uric acid (i.e. physiologically present oxypurines) on the kinetic properties of recombinant NAGS we first determined 1) the time course of the velocity of NAGS with or without xanthine (2 mm), fixed concentrations of AcoA (1 mm), and [15N]glutamate (10 mm) and 2) the relationship between enzyme protein and NAGS velocity with or without xanthine (2 mm). Fig. 9A demonstrates that the velocity of NAGS linearly increased between 0.25 and 5 min, and the velocity reached a plateau after 5 min of incubation. The time range of the linearity and plateau was similar with or without the inclusion of xanthine. Similarly, there was a linear increase in the velocity of NAGS with an increasing concentration of NAGS protein (Fig. 9B). Therefore, to examine the action of xanthine or uric acid on the kinetic parameters of recombinant NAGS, reactions were carried out for 5 min and with ∼2.5 μg of enzyme protein in a reaction volume of 250 μl.
FIGURE 9.
Linearity of recombinant NAGS activity with regard to time and enzyme protein; determination with or without xanthine. A, time course of NAGS activity. The reaction was performed at 30 °C for the indicated times in a total reaction volume of 250 μl, containing 50 mm Tris-HCl buffer, pH 8.5, 2.5 μg of enzyme protein, 1 mm arginine, and fixed 1 mm ACoA and 10 mm [15N]glutamate. B, reactions performed with various enzyme protein, as indicated, at 30 °C in a total reaction volume of 250 μl, containing 50 mm Tris-HCl buffer, pH 8.5, 1 mm arginine, and fixed 1 mm ACoA and 10 mm [15N]glutamate. Determinations were performed without xanthine (control) or with the addition of 2 mm xanthine. Data points are means of two independent experiments.
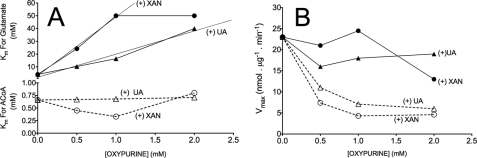
Fig. 10 represents the action of various concentrations xanthine or uric acid on the kinetic parameters of NAGS. These parameters are summarized in Table 3. We examined only the action of xanthine or uric acid because of their metabolic relevance. These oxypurines have multiple effects and mixed action on NAGS activity. Without oxypurines, the apparent Km for glutamate was 4.1 ± 0.4 mm, and that for ACoA was 0.66 ± 0.1 mm. The maximum velocity (Vmax) ranged between 21 and 23 nmol/μg/min with varied concentrations of ACoA or glutamate. In experiments with fixed (10 mm) glutamate and varied [ACoA], the addition of uric acid or xanthine to the assays slightly changed the observed Km values for ACoA and decreased the velocity of NAGS by about 2–3-fold with uric acid, by about 4-fold with 0.5 mm xanthine, and by about 5-fold with 1 or 2 mm xanthine (Table 3). In experiments with fixed 1 mm ACoA and varied [glutamate], the addition of xanthine or uric acid remarkably increased the observed Km values for glutamate in a dose-dependent manner. The increased Km values are associated with little change in the velocity of NAGS (Table 3 and Fig. 11). Hence, the data indicate that these oxypurines decrease the observed velocity of NAGS and increase the Km for glutamate with only little effect on the apparent Km for ACoA. Because 1H NMR analysis demonstrated that none of the oxypurines tested reacted with glutamate (data not shown), the current observations suggest that the inhibition of NAGS activity is probably mediated via decreased binding of glutamate to its active site.
TABLE 3.
Influence of various concentrations of xanthine or uric acid on the kinetic parameters for NAGS activity
For determination of Km and velocity values, data in Fig. 10 were analyzed with Prism-5 software using Michaelis-Menten kinetics. The kinetic parameters for ACoA were determined with varied [ACoA] and fixed (10 mm) [15N]glutamate, and parameters for glutamate were determined with varied [glutamate] and fixed (1 mm) ACoA. All analyses were carried out for 5 min with 1 mm arginine, an activator of NAGS. Enzyme concentration was 2.5 μg/250-μl assay volume. Values are ± S.E. obtained by the GraphPad curve fitting.
| Experiment | Kinetic parameters for ACoA |
Kinetic parameters for glutamate |
||
|---|---|---|---|---|
| Km(obs)a | Velocity | Km(obs) | Velocity | |
| mm | nmol·mg−1·min−1 | mm | nmol·mg−1·min−1 | |
| Control | ||||
| 0 mm oxypurine | 0.66 ± 0.1 | 21.2 ± 0.9 | 4.1 ± 0.42 | 23.5 ± 0.8 |
| With xanthine | ||||
| 0.5 mm | 0.45 ± 0.1 | 7.4 ± 0.3 | 24.2 ± 2.8 | 21.2 ± 1.6 |
| 1 mm | 0.33 ± 0.1 | 4.3 ± 0.2 | 49.51 ± 7.3 | 25.7 ± 4.6 |
| 2 mm | 0.80 ± 0.2 | 4.6 ± 0.3 | 49.9 ± 12.1 | 13.1 ± 4.4 |
| With uric acid | ||||
| 0.5 mm | 0.67 ± 0.1 | 11.1 ± 0.1 | 10.3 ± 0.8 | 15.7 ± 0.6 |
| 1 mm | 0.68 ± 0.1 | 7.1 ± 0.3 | 16.4 ± 2.1 | 15.5 ± 1.1 |
| 2 mm | 0.71 ± 0.1 | 6.3 ± 0.3 | 40.1 ± 10.1 | 18.4 ± 3.6 |
a Km(obs) is the observed concentration of substrate (ACoA or glutamate) at which one-half Vmax (velocity) for 15N-labeled NAG synthesis was achieved in the presence or absence of the indicated [oxypurine].
FIGURE 11.
The relationship between an increasing concentration of oxypurine and the Km values for glutamate or acetyl-CoA and the resulting Vmax for NAGS. A, Km values for glutamate or acetyl-CoA obtained with increasing concentration of oxypurine; B, the corresponding Vmax for NAGS. Both Km and Vmax values were taken from Table 3. Solid lines, Km values or Vmax obtained with varied concentrations of [15N]glutamate] and fixed 1 mm [ACoA]; dashed lines, Km values or Vmax obtained with varied [ACoA] and fixed (10 mm) [15N]glutamate. The straight lines in A (Km for glutamate) represent the best fit to a linear correlation for xanthine, r2 = 0.94, in the range of 0–1 mm (p = 0.04), and for uric acid, r2 = 0.99, in the range of 0–2 mm (p = 0.01). Experimental details are as indicated in the legend to Fig. 10.
Experiments with Isolated Hepatocytes
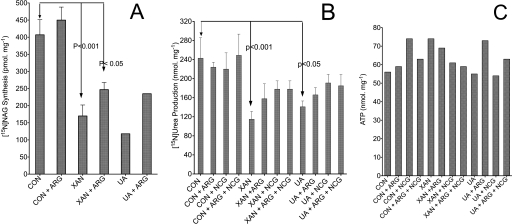
Experiments with isolated hepatocytes were performed in order to determine 1) the relationship between the inhibition of NAGS and ureagenesis by physiologically present oxypurines (i.e. xanthine or uric acid) and 2) the efficacy of N-carbamylglutamate or arginine as a protective agent against oxypurine-induced inhibition of NAGS and, thus, urea synthesis. Data in Fig. 12 demonstrate that xanthine or uric acid significantly inhibits NAG synthesis (Fig. 12A) and [15N]urea synthesis (Fig. 12B) in isolated hepatocytes in a pattern that is similar to their inhibition of [δ-15N]citrulline synthesis in isolated mitochondria (Fig. 5, A and D). However, the inhibition of ureagenesis was attenuated by the addition of arginine and/or 0.5 mm N-carbamylglutamate (Fig. 12B). This observation is consistent with the restoration of citrulline synthesis in experiments with isolated mitochondria (Fig. 5, B and C). Of interest is the finding that these oxypurines have only little effect on ATP levels (Fig. 12C), indicating that the inhibition of ureagenesis is not related to a change of energy status. Therefore, experiments with isolated hepatocytes further support the notion that the inhibition of NAGS activity is the primary mechanism by which xanthine or uric acid inhibits urea synthesis. This conclusion again agrees with the concept that the synthesis of citrulline and urea is determined in large measure by NAG synthesis (13, 14, 34).
FIGURE 12.
Xanthine or uric acid inhibits 15N-labeled NAG and urea synthesis in isolated hepatocytes; the action of N-carbamylglutamate (NCG) or arginine supplementation. Isolated hepatocytes were incubated for 60 min in Krebs medium plus 5 mm [5-15N]glutamine, 1 mm 15NH4Cl, 10 mm lactate, 1.5 mm pyruvate and with or without 1 mm oxypurine or oxypurine plus 0.5 mm activator, as indicated. A, amount of newly synthesized 15N-labeled NAG following a 60-min incubation. 15N-Labeled NAG was formed from 15N-labeled glutamate. The latter was generated from 15NH3 via reductive amination of α-ketoglutarate in the mitochondrial compartment, as indicated (36). The amount of [15N]NAG was calculated as follows. We first determined the concentration of NAG by isotope dilution (19, 31) and then calculated the level of [15N]NAG by the product of the initial (before spiking the sample) 15N enrichment, mol % excess/100 × concentration (nmol/mg protein). B, production of [15N]urea, which is the sum of Um+1 and Um + 2 isotopomers (urea contains one 15N or two 15N) obtained by the product of total urea (nmol/mg protein) times 15N enrichment (atom % excess/100) as indicated (31). C, levels of ATP in hepatocytes at the end of the incubation. p values were obtained using Prism-5 software and one-way analysis of variance comparing the indicated (by arrows) experimental conditions with control without oxypurine (CON). Error bars, S.D.
An important observation involves 15N enrichment in NAG. Hepatocyte incubations were done with 15NH3 and [5-15N]glutamine, but enrichment in NAG (35–50 mol % excess) reflects labeling of mitochondrial [15N]glutamate. The latter must be formed from reductive amination of α-ketoglutarate through the action of mitochondrial glutamate dehydrogenase (36). Hence, the label in NAG may be used as a surrogate for enrichment in mitochondrial [15N]glutamate.
DISCUSSION
We previously reported that IBMX inhibits hepatic citrulline and urea synthesis (15) by a mechanism that had been elusive. Because IBMX is an oxypurine derivative, we hypothesized that IBMX and, possibly, other oxypurines (supplemental Fig. 1S) may interfere with the binding of glutamate or ACoA to its active site on NAGS, thereby decreasing the velocity of NAGS, diminishing NAG synthesis, leading to a failure of CPS1 activation, and resulting in inhibition of citrulline and urea synthesis (Fig. 1). This hypothesis implies that one or more oxypurines that have a chemical structure similar to IBMX, perhaps by including a 2,6-dione group (supplemental Fig. 1S), may disrupt the binding of glutamate or ACoA to its active site at NAGS and thereby decrease the velocity of NAGS. The current findings suggest that the 2,6-dione group of oxypurines disrupts the binding of glutamate to its binding site at NAGS and thereby decreases synthesis of NAG. The result is less citrulline and urea synthesis. This conclusion is strongly supported by the following observations. 1) IBMX, xanthine, or uric acid, all of which contain a 2,6-dione group (supplemental Fig. 1S), significantly inhibits NAGS activity (Figs. 3, 4, 5, and 8) with little or no effect on CPS1 activity (Fig. 8C). However, other oxypurines that lack the 2,6-dione group have only little or no effect on NAGS activity (supplemental Fig. 2S and Fig. 8A). 2) Xanthine or uric acid remarkably increased the observed Km values for glutamate with little effect on Km values for ACoA. The increased Km for glutamate was accompanied by decreased velocity of NAGS (Table 3 and Fig. 11), suggesting that the inhibition of NAGS activity is mediated via decreased affinity of glutamate with its active site at NAGS. This conclusion is further supported by 1H NMR analysis, which demonstrates that neither oxypurine directly reacted with glutamate (data not shown). 3) The generation of the CPS1-NAG complex, the initial step in citrulline synthesis (1, 6), is linearly correlated with the inhibition of NAG synthesis (Fig. 7B), suggesting a robust coupling between inhibition of NAGS, decreased generation of the CPS1-NAG complex, and the observed inhibition of citrulline and urea synthesis (Figs. 4–6 and 12). 4) The supplementation of N-carbamylglutamate, an artificial substitute for NAG, completely reversed the oxypurine-induced inhibition of citrulline synthesis (Fig. 5C) and ureagenesis (Fig. 12B).
Supplemental Fig. 1S illustrates a structural similarity between the adenosine group of ACoA and the oxypurines tested in the current study. Thus, one might expect that the inhibition of NAGS may be mediated by disruption of ACoA binding to its active site at NAGS. However, the current findings indicate that the inhibition of NAGS activity is mediated via decreased affinity of glutamate to its active site on the enzyme. Because NAGS is mainly inhibited by oxypurines containing a 2,6-dione group (supplemental Fig. 1S), it appears that this structure plays a key role in disrupting the binding of glutamate to its active site. An apparent exception to this notion is caffeine. Although caffeine contains a 2,6-dione group, about 20% inhibition in NAGS activity was observed only with [caffeine] above 5 mm (data not shown). The presence of methyl groups in position 7 and/or positions 1 and 3 in the caffeine molecule may decrease its potency.
A fundamental question arising from the current findings and conclusion is how the 2,6-dione group decreases glutamate affinity to its binding site at NAGS. A prior study of the crystal structure of NAGS in microorganisms indicated that mammalian NAGS and its bacterial homolog have similar affinities for ACoA and glutamate (37) as well as for their allosteric regulator arginine (38). Therefore, we may assume that the binding of substrates in rats and bacterial NAGS is similar. In Neisseria gonorrhoeae, NAGS consists of two independently folded domains, an N-terminal amino acid kinase and a C-terminal N-acetyltransferase domain (38). The C-terminal N-acetyltransferase domain has a central anti-parallel β sheet, which is divided into two arms that resemble a “V” shape, with ACoA and glutamate binding between them (34, 38). Glutamate binds to the active site by anchoring its two carboxyl groups using the polar side chains of Arg316, Arg425, and Ser427 and the main chain nitrogen atoms of Cys356 and Leu314 in the C-terminal N-acetyltransferase domain of N. gonorrhoeae (38). The anchoring of glutamate to an Arg residue in NAGS may resemble the anchoring of xanthine, uric acid, or hypoxanthine to an Arg residue in mammalian xanthine oxidoreductase (39). Thus, it is possible that the 2,6-dione group in oxypurines may disrupt the anchoring of one or both carboxyl groups of glutamate to NAGS. This possibility is supported by the data in Fig. 11, which demonstrate a linear relationship between increasing levels of xanthine or uric acid in the reaction assays and increased Km values for glutamate, which was accompanied by decreased Vmax for NAGS with little effect on the Km for ACoA. Thus, data in Figs. 10 and 11 demonstrate distinct differences between the action of oxypurine on the binding of glutamate or ACoA to their active site at NAGS and support the notion that the 2,6-dione group in oxypurines may disrupt the anchoring of one or both carboxyl groups of glutamate to NAGS. Further crystallography/structural studies should determine the validity of this model.
The proposed mechanism of oxypurine inhibition of NAGS rules out a putative action on the arginine allosteric site for these reasons: 1) arginine binds to the C-terminal end of the N-terminal amino acid kinase domain that is distant from the catalytic sites (40, 41); 2) our current preliminary data without oxypurines (data not shown) and previously published data (30) indicate that in the absence of arginine there was decreased NAGS velocity with only little change in the apparent Km values for both glutamate and ACoA. However, the kinetic parameters in Table 3, which were obtained in the presence of 1 mm arginine, demonstrate little change in the observed Km for ACoA but a remarkable increase in the observed Km for glutamate, a change that was accompanied by decreased NAGS velocity (Table 3). Together, the data suggest that the action of oxypurine is not at the site of allosteric activation of NAGS.
Xanthine and uric acid are cytoplasmic metabolites, which are formed and metabolized via oxidoreductases. Data in Figs. 5 and 12 (A and B) indicate that both xanthine and uric acid were taken up by mitochondria and inhibited NAGS. This observation is in line with a previous study demonstrating mitochondrial uptake of allopurinol, an oxypurine analog (42). Because the synthesis of NAG provides the natural allosteric activator of ureagenesis and because xanthine or uric acid inhibits NAG synthesis, an important concept emerging from the current work is that xanthine and/or uric acid may have a functional role in the regulation of ureagenesis and ammonia detoxification in normal and disease states. Many cases of idiopathic hyperammonemia, in a variety of clinical settings, are associated with elevated plasma levels of xanthine and/or uric acid, including prolonged exercise (43), the metabolic syndrome (44), and hyperammonemia following chemotherapy (45). The current findings may shed new light on these cases of idiopathic hyperammonemia and suggest a plausible mechanism for induction of hyperammonemia (i.e. an inhibition of NAG synthesis). The current study suggests that administration of N- carbamylglutamate might be therapeutically efficacious in clinical settings characterized by hyperammonemia secondary to an increase of the level of oxypurines within hepatic mitochondria. NAG would not be effective because it is swiftly hydrolyzed via amino acid acylase, but N-carbamylglutamate has been successfully utilized (17, 18). This study also suggests that the combination of arginine and N-carbamylglutamate may be even more effective to augment flux through NAGS (Fig. 12B).
Arginine-induced stimulation of ureagenesis (Fig. 12B) may reflect conformational changes and/or altered protein dynamics (40, 41). In addition, arginine is metabolized to agmatine in the mitochondria (46). Agmatine stimulates NAG synthesis subsequent to the stimulation of β-oxidation, thereby providing more acetyl-CoA for NAG synthesis (46). However, because acetyl-CoA was added to the incubation medium, the metabolism of arginine to agmatine or the addition of exogenous agmatine had little protective effect against the oxypurine-induced inhibition of NAGS (data not shown). Although arginine did not reverse the xanthine- or uric acid-induced inhibition of NAGS, it may allosterically activate the portion of NAGS that was not affected by oxypurines and, thus, enhance NAG and citrulline synthesis, as indicated in Figs. 5 (B and E) and 6.
In summary, the current findings demonstrate that xanthine and uric acid significantly inhibit NAGS activity but have no or little effect on CPS1. The data suggest that the 2,6-dione group in these oxypurines disrupts the binding of glutamate to NAGS. The result is decreased affinity of glutamate for NAGS and decreased enzyme velocity. The inhibition of NAGS led to diminished citrulline and urea synthesis. However, this effect was curtailed by supplementation of N-carbamylglutamate. A novel notion arising from this study is that xanthine and/or uric acid may have an important role in the regulation of ureagenesis and, thus, ammonia detoxification in normal and disease states. In addition, the current findings may contribute to better understanding of the pathophysiology of idiopathic hyperammonemia and, thus, lead to better treatment.
Supplementary Material
Acknowledgment
We are indebted to Himani Datta Majumdar (Children's National Medical Center, George Washington University) for the preparation of recombinant NAGS.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-053761 and DK-R56-053761 (to Itzhak Nissim) and Grants R01DK064913, R01DK047870, PO1HD26979, U54RR019453, and HD058567. This work was also supported in part by Spanish Ministry for Science Grant SAF2010-17993.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1S–5S.
- CPS1
- carbamoyl phosphate synthase-I
- NAG
- N-acetylglutamate
- ACoA
- acetyl-CoA
- NAGS
- N-acetylglutamate synthase
- UA
- uric acid
- XAN
- xanthine
- IBMX
- isobutylmethylxanthine.
REFERENCES
- 1. Meijer A. J., Lamers W. H., Chamuleau R. A. (1990) Physiol. Rev. 70, 701–748 [DOI] [PubMed] [Google Scholar]
- 2. Gropman A. L., Summar M., Leonard J. V. (2007) J. Inherit. Metab. Dis. 30, 865–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahuja V., Powers-Lee S. G. (2008) J. Inherit. Metab. Dis. 31, 481–491 [DOI] [PubMed] [Google Scholar]
- 4. Holden H. M., Thoden J. B., Raushel F. M. (1999) Cell Mol. Life Sci. 56, 507–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCudden C. R., Powers-Lee S. G. I. (1996) J. Biol. Chem. 271, 18285–18294 [DOI] [PubMed] [Google Scholar]
- 6. Raijman L., Jones M. E. (1976) Arch. Biochem. Biophys. 175, 270–278 [DOI] [PubMed] [Google Scholar]
- 7. Britton H. G., Rubio V. (1988) Eur. J. Biochem. 171, 615–622 [DOI] [PubMed] [Google Scholar]
- 8. Rubio V., Britton H. G., Grisolia S. (1983) Eur. J. Biochem. 134, 337–343 [DOI] [PubMed] [Google Scholar]
- 9. Caldovic L., Tuchman M. (2003) Biochem. J. 372, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen N. S., Cheung C. W., Sijuwade E., Raijman L. (1992) Biochem. J. 282, 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lusty C. J. (1978) Eur. J. Biochem. 85, 373–383 [DOI] [PubMed] [Google Scholar]
- 12. Clarke S. A. (1976) J. Biol. Chem. 251, 950–961 [PubMed] [Google Scholar]
- 13. Coude F. X., Sweetman L., Nyhan W. L. (1979) J. Clin. Invest. 64, 1544–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coude F. X., Grimber G., Parvy P., Rabier D., Petit F. (1983) Biochem. J. 216, 233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nissim I., Horyn O., Nissim I., Daikhin Y., Wehrli S. L., Yudkoff M. (2008) J. Biol. Chem. 283, 15063–15071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S., Paik W. K., Cohen P. P. (1972) Proc. Natl. Acad. Sci. U.S.A. 69, 3530–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuchman M., Caldovic L., Daikhin Y., Horyn O., Nissim I., Nissim I., Korson M., Burton B., Yudkoff M. (2008) Pediatr. Res. 64, 213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ah Mew N., Payan I., Daikhin Y., Nissim I., Nissim I., Tuchman M., Yudkoff M. (2009) Mol. Genet. Metab. 98, 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nissim I., Luhovyy B., Horyn O., Daikhin Y., Nissim I., Yudkoff M. (2005) J. Biol. Chem. 280, 17715–17724 [DOI] [PubMed] [Google Scholar]
- 20. Kawamoto S., Sonoda T., Ohtake A., Tatibana M. (1985) Biochem. J. 232, 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morimoto B. H., Brady J. F., Atkinson D. E. (1990) Biochem. J. 272, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGivan J. D., Bradford N. M., Mendes-Mourão J. (1976) Biochem. J. 154, 415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coman D., Yaplito-Lee J., Boneh A. (2008) Clin. Nutr. 27, 489–496 [DOI] [PubMed] [Google Scholar]
- 24. Lund P., Wiggins D. (1987) Biochem. J., 243, 273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caldovic L, Lopez G. Y., Haskins N., Panglao M., Shi D., Morizono H., Tuchman M. (2006) Mol. Genet. Metab. 87, 226–232 [DOI] [PubMed] [Google Scholar]
- 26. Cohen N. S., Cheung C. W., Kyan F. S., Jones E. E., Raijman L. (1982) J. Biol. Chem. 257, 6898–6907 [PubMed] [Google Scholar]
- 27. Cohen N. S., Cheung C. W. (1984) Arch. Biochem. Biophys. 234, 31–44 [DOI] [PubMed] [Google Scholar]
- 28. Guthöhrlein G., Knappe J. (1968) Eur. J. Biochem. 7, 119–127 [DOI] [PubMed] [Google Scholar]
- 29. Penefsky H. S. (1977) J. Biol. Chem. 252, 2891–2899 [PubMed] [Google Scholar]
- 30. Alonso E., Cervera J., García-España A., Bendala E., Rubio V. (1992) J. Biol. Chem. 267, 4524–4532 [PubMed] [Google Scholar]
- 31. Nissim I., Yudkoff M., Brosnan J. T. (1996) J. Biol. Chem. 271, 31234–31242 [DOI] [PubMed] [Google Scholar]
- 32. Jones B. N., Gilligan J. P. (1983) J. Chromatogr. 266, 471–482 [DOI] [PubMed] [Google Scholar]
- 33. Aoyagi K., Mori M., Tatibana M. (1979) Biochim. Biophys. Acta 587, 515–521 [DOI] [PubMed] [Google Scholar]
- 34. Caldovic L., Ah Mew N., Shi D., Morizono H., Yudkoff M., Tuchman M. (2010) Mol. Genet. Metab. 100, S13-S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alonso E., Rubio V. (1983) Eur. J. Biochem. 135, 331–337 [DOI] [PubMed] [Google Scholar]
- 36. Treberg J. R., Brosnan M. E., Watford M., Brosnan J. T. (2010) Adv. Enzyme Regul. 50, 34–43 [DOI] [PubMed] [Google Scholar]
- 37. Qu Q., Morizono H., Shi D., Tuchman M., Caldovic L. (2007) BMC Biochemistry 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi D., Sagar V., Jin Z., Yu X., Caldovic L., Morizono H., Allewell N. M., Tuchman M. (2008) J. Biol. Chem. 283, 7176–7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishino T., Okamoto K., Eger B. T., Pai E. F., Nishino T. (2008) FEBS J. 275, 3278–3289 [DOI] [PubMed] [Google Scholar]
- 40. Min L., Jin Z., Caldovic L., Morizono H., Allewell N. M., Tuchman M., Shi D. (2009) J. Biol. Chem. 284, 4873–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sancho-Vaello E., Fernández-Murga M. L., Rubio V. (2009) FEBS Lett. 583, 202–206 [DOI] [PubMed] [Google Scholar]
- 42. Rus D. A, Sastre J., Viña J., Pallardó F. V. (2007) Front. Biosci. 12, 1184–1189 [DOI] [PubMed] [Google Scholar]
- 43. Nybo L., Secher N. H. (2004) Prog. Neurobiol. 72, 223–261 [DOI] [PubMed] [Google Scholar]
- 44. Tsouli S. G., Liberopoulos E. N., Mikhailidis D. P., Athyros V. G., Elisaf M. S. (2006) Metabolism 55, 1293–1301 [DOI] [PubMed] [Google Scholar]
- 45. Jaing T. H., Lin J. L., Lin Y. P., Yang S. H., Lin J. J., Hsia S. H. (2009) J. Pediatr. Hematol. Oncol. 31, 955–956 [DOI] [PubMed] [Google Scholar]
- 46. Nissim I., Daikhin Y., Nissim I., Luhovyy B., Horyn O., Wehrli S. L., Yudkoff M. (2006) J. Biol. Chem. 281, 8486–8496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.