Abstract
Increasing evidence suggests that endoplasmic reticulum (ER) stress plays an important role in the pathogenesis of type 2 diabetes mellitus. SEL1L is an ER membrane protein that is highly expressed in the pancreatic islet and acinar cells. We have recently reported that a deficiency of SEL1L causes systemic ER stress and leads to embryonic lethality in mice. Here we show that mice with one functional allele of Sel1l (Sel1l+/−) are more susceptible to high fat diet (HFD)-induced hyperglycemia. Sel1l+/− mice have a markedly reduced β-cell mass as a result of decreased β-cell proliferation. Consequently, Sel1l+/− mice are severely glucose-intolerant and exhibit significantly retarded glucose-stimulated insulin secretion. Pancreatic islets from Sel1l+/− mice stimulated with a high concentration of glucose in vitro express significantly higher levels of unfolded protein response genes than those from wild-type control mice. Furthermore, dominant-negative interference of SEL1L function in insulinoma cell lines severely impairs, whereas overexpression of SEL1L efficiently improves protein secretion. Taken together, our results indicate that haploid insufficiency of SEL1L predispose mice to high fat diet-induced hyperglycemia. Our findings highlight a critical and previously unknown function for SEL1L in regulating adult β-cell function and growth.
Keywords: Diabetes, Endoplasmic Reticulum (ER), Glucose, Glucose Metabolism, Insulin, ER Homeostasis, β-Cell, Unfolded Protein
Introduction
Type 2 diabetes mellitus (T2DM)3 is a major chronic disease currently affecting over 24 million Americans with annual costs exceeding $170 billion (1). Deficit in β-cell mass and intra-islet amyloid deposition are hallmark features of the islet in T2DM (2). Obesity is a well characterized risk factor for the development of T2DM (3, 4). The molecular mechanisms underlying the β-cell failure in T2DM and by which obesity promotes β-cell failure and intracellular amyloidogenesis remain elusive.
Accumulating evidence suggests that stress in the endoplasmic reticulum (ER) plays an important causal role in the pathogenesis of T2DM (5, 6). The ER is a specialized organelle where nearly all secreted and membrane proteins undergo post-translational modifications such as glycosylation, disulfide bond formation, folding, and multimeric protein complex assembling (7, 8). Properly folded/assembled proteins travel through the secretory pathway and reach various cellular destinations, whereas nonnative or unassembled proteins are retained in the ER lumen and eventually retrotranslocated into the cytosol for degradation by the proteasomal system (9). Disequilibrium of ER protein load and folding capacity results in accumulation of unfolded/misfolded proteins in the ER lumen, leading to ER stress (10). In response to ER stress, cells activate at least three intracellular signal transduction pathways, cumulatively referred to as the unfolded protein response (11). The unfolded protein response, coordinated by three distinct ER stress signal transducers, PERK, IRE1a, and ATF6 (12), result in a general attenuation of protein translation (13) and an increased expression of ER chaperones and ER-associated degradation machinery (9, 14). Consequently, there is a reduced production of proteins that enter the ER, and there is an increase in the capacity of the ER to handle unfolded proteins. Together, these adaptive measures provide a multifaceted mechanism to maintain ER homeostasis under temporary and reversible ER stress conditions.
ER homeostasis is particularly important for the function and viability of professional secretory cells such as the pancreatic β-cell (15, 16). The unique function of the β-cell is to integrate nutrient signals into an appropriate insulin secretory rate to maintain normal glucose homeostasis. In response to serum glucose stimulus, β-cells secrete insulin from a readily available pool while activating proinsulin biosynthesis in the ER (17). Proinsulin undergoes protein folding and disulfide bond formation in the lumen of the ER (17). Properly folded proinsulin is then released to the Golgi apparatus and packaged into secretory granules, where conversion of proinsulin into mature insulin takes place (18). Because of the rapid fluctuation of serum glucose levels in animals, it is essential that β-cells control proinsulin folding in the ER with exquisite sensitivity (19). It has been suggested that conditions either evoking an overload of newly synthesized proinsulin or compromising protein folding capacity in the ER may negatively affect the homeostasis of β-cells, leading to ER stress and onset of T2DM (6). Consistent with this, markers of ER stress have been shown to be up-regulated in the islets from T2DM patients (20, 21) and from several animal models of obesity and diabetes (22–25).
Suppressor enhancer lin12 1-like (Sel1l) is highly expressed in the developing and matured pancreatic cells (26, 27). Sel1l encodes an ER membrane protein (type I) with a complex domain structure (28). Previous biochemical and molecular studies in vitro showed that SEL1L nucleates an ER membrane protein complex that is required for dislocation of unfolded or misfolded proteins from the ER lumen into the cytosol for degradation (29–34). We recently reported that mice homozygous for a gene trap mutation in Sel1l develop systemic ER stress and die during mid-gestation (35). In addition, we revealed that SEL1L deficiency impairs the growth and differentiation of pancreatic epithelial cells during early mouse embryonic development (36). These genetic data are consistent with the hypothesis that SEL1L regulates ER homeostasis in mammalian cells by facilitating ER-associated degradation of unfolded proteins (32, 33).
The physiological role of SEL1L in adult islets remains unclear. Here, we show that mice heterozygous for the gene-trap mutation in Sel1l (Sel1l+/−) are more susceptible to diet-induced hyperglycemia. Sel1l+/− mice are glucose intolerant and have a reduced β-cell mass. Cultured islets from Sel1l+/− mice show significantly higher expression of unfolded protein response marker genes than wild-type control islets. Thus, our data suggest that SEL1L is a critical regulator of β-cell function and growth in adult mice.
MATERIALS AND METHODS
Mice
Generation of Sel1l gene trap mice (Sel1l+/−) was described previously (35). For physiological studies, Sel1l+/− mice were back-crossed to C57/B6 mice for five generations and then intercrossed to generate wild-type and Sel1l+/− mice. All mice were weaned at 3 weeks of age, and genotyping was done by PCR using the following primers: F1-Sel1l, 5′-TGGGACAGAGCGGGCTTGGAAT-3′; R1-Sel1l, 5′-CACCAGGAGTCAAAGGCATCACTG-3′; R-βGeo, 5′-ATTCAGGCTGCGCAACTGTTGGG-3′.
For high fat diet feeding experiments, wild-type and heterozygous male mice at 6–8 weeks of age were put on a TD.06414 diet (Harlan, Madison, WI), which contains 23.5, 27.3, and 34.3% protein, carbohydrate, and fat, respectively. All animal experiments were performed in accordance with the Cornell Animal Care and Use Guidelines.
Physiological Studies
Fasting blood glucose was measured using an Ascensia Elite XL glucometer (Bayer). Glucose and insulin tolerance tests and glucose-stimulated insulin secretion (GSIS) were performed essentially as described (37). For the glucose tolerance test, mice were fasted for 6–8 hours and then injected intraperitoneal with 2 g of d-glucose/kg body weight. Blood glucose was measured at 0, 5, 15, 30, 60, and 120 min after glucose injection. For the insulin tolerance test, mice were fasted for 6 hours and then injected intraperitoneally with 1.0 unit of regular human insulin (Eli Lilly, Indianapolis, IN)/kg body weight dissolved in phosphate-buffered saline (PBS). Blood glucose was measured at 0, 5, 15, 30, 60, and 120 min post-insulin injection. 4-Phenylbutyric acid (4-PBA) was administered into mice by gavage as previously described (38).
Morphological Analysis of Pancreas
Pancreata were fixed in 4% paraformaldehyde overnight at 4 °C and paraffin embedded. Pancreatic sections were cut at 5-μm and mounted on glass slides. Insulin immunostaining was performed by using guinea pig anti-human insulin (Linco, 1:1000). Secondary antibodies used were either Cy2- or HRP-conjugated donkey anti-guinea pig immunoglobulin (IgG) (Jackson ImmunoResearch Laboratories, 1:500). Nuclear counterstaining was performed using 4′,6-diamidino-2-phenylindole (DAPI). β-Cell mass was determined as previously described (37). Briefly, total pancreatic and β-cell areas were measured using the AxioVision software (Version 4.1). β-Cell mass was calculated by multiplying the total pancreatic weight with the percentage of β-cell area. To determine β-cell proliferation, pancreatic sections were co-immunostained with a mouse monoclonal anti-Ki67 (Vector Laboratories, 1:500) and anti-insulin antibodies. Nuclei were counterstained with DAPI. The percentage of Ki67+ cells was derived by dividing the total number of counted β-cell nuclei with the number of Ki67+ nuclei. β-Cell apoptosis was assessed by TUNEL assay using the ApopTag In Situ Apoptosis detection kit (Chemicon, Temecula, CA) according to the manufacturer's instructions. All images were acquired using an Axiovert 40 microscope (Zeiss) equipped with an AxioCam camera.
Pancreatic Insulin Content
Pancreatic insulin content was determined as previously described (39). Briefly, pancreata were removed from mice and homogenized in 1 ml of acid ethanol (95% ethanol, 10.2 n HCl in a 50:1 ratio). The homogenized pancreata were incubated overnight at 4 °C and centrifuged. Insulin concentration in the supernatant was determined using ELISA (Crystal Chem, Downers Grove, IL) and normalized by total protein content.
Islet Isolation and in Vitro GSIS
Mouse islet isolation was performed as previously described (40). Briefly, after sacrifice of each mouse, the pancreas was perfused with 1× Hanks' balanced salt solution, pH 7.4, containing 2.0 mg/ml type V collagenase (Sigma). The inflated pancreas was removed from the body and incubated at 37 °C for 20 min. The collagenase-digested pancreas was vigorously shaken for 5 s, washed 3 times with 5–7 ml of ice-cold 1× Hanks' balanced salt solution buffer, and re-suspended in 2 ml of 28% Ficoll (VWR, West Chester, PA). The islet and acinar mixture was loaded onto the top of gradient Ficoll solutions and centrifuged at 2250 rpm for 7 min at 4 °C. Islets were collected and washed three times with ice-cold 1× Hanks' balanced salt solution before processing for further analysis.
GSIS for islets was performed as previously described with minor modifications (41). Briefly, isolated islets were washed twice with 1× KRBH buffer (118.5 mm NaCl, 2.54 mm CaCl2·2H2O, 1.19 mm KH2PO4, 4.74 mm KCl, 25 mm NaHCO3, 1.19 mm MgSO4·7H2O, 10 mm HEPES, 0.1% BSA, 5 mm glutamic acid, 5 mm fumaric acid, 5 mm pyruvic acid, pH 7.4) and equilibrated in the same buffer containing 2.8 mm glucose for 60 min at 37 °C. Islets were then incubated in 1× KRBH buffer containing either 2.8 or 16.8 mm glucose for 30 min at 37 °C. The low and high glucose-incubated islets were briefly centrifuged, and the supernatants were assayed for secreted insulin, which was normalized to islet insulin content. Islet insulin content was normalized to the total protein concentration.
RNA Extraction and Quantitative RT-PCR
RNA isolation and real-time RT-PCR were performed as previously described (35). PCR primers were designed using the PrimerSelect program of Lasergene 7.1 Sequence Analysis Software (DNAStar, Madison, WI). Relative mRNA expression was calculated by dividing the expression value in Sel1l+/− islets (set to 1) with that in wild-type islets.
Cell Culture
Min6 cells were maintained as previously described (42). For establishing stable cell lines, Min6 cells were grown to 50–60% confluency, trypsinized, washed three times in cold PBS, and resuspended in PBS. 5.6 × 106/ml was mixed with 10–20 μg of plasmid DNA, electroporated (0.24 kV and 500 microfarads) and plated into a 24-well plate. The electroporated Min6 cells were incubated in regular media for 48 h and then in neomycin resistance selection medium (G418 at 1 mg/ml) for 10 days. Stable Min6 clones were maintained in regular medium. GSIS for Min6 cells was performed essentially as previously described (43).
INS-1 cells were maintained as previously described (44). For generation of stable cell lines, INS-1 cells were seeded at a confluency of <10% and infected overnight with lentivirus expressing the reverse tet transactivator (rtTA), rtTA plus SEL1L-GFP, or rtTA plus dSEL1L-GFP. To induced expression of transgenes, doxycycline (Sigma) was added into the culture medium of Min6 and INS-1 cells (200 μg/ml).
Transient transfection and Gaussia luciferase (G-luc) assay in Min6 cells were performed as previously described (35). Cell growth profile was generated, and thymidine incorporation assay was performed as previously described (45).
Western Blot Analysis
Preparation of tissue/cell lysates, protein quantification, electrophoresis, and blotting were performed as previously described (35). Immunodetection was carried out using the Western blotting Luminol Reagent kit (Origene) according to the manufacturer's specifications. The primary antibodies used were: GFP (Abcam, 1:5,000), tubulin (Cell Signaling, 1:10,000), calnexin (Assay Design, 1:10,000), p-eIF2a (Cell Signaling, 1:2000), eIF2a (Cell Signaling, 1:2,000), ERP57 (Assay Design, 1:2,000), PDI (Assay Design, 1:5,000), HRD1 (Novus Biologicals, 1:1,000), ERO1L (Novus Biologicals, 1:2,000) and GRP78 (Santa Cruz, 1:1,000).
Statistical Analysis
Differences between compared groups were evaluated by performing two-tailed Student's t test, and p < 0.05 is considered significant.
RESULTS
Hemizygosity of Sel1l Predisposes Mice to HFD-induced Hyperglycemia
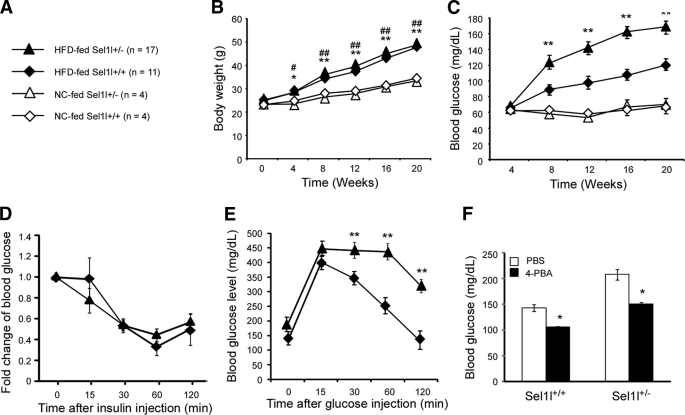
We recently reported that mice homozygous for a gene trap mutation in Sel1l (Sel1l−/−) develop systemic ER stress and die during mid-gestation (35). To determine the functional role of SEL1L in adult pancreatic β-cells, we investigated whether haploid insufficiency of SEL1L affects glucose homeostasis in mice. Wild-type and Sel1l+/− male mice at 10 weeks of age were fed with normal chow (NC) and a high fat diet (HFD) for 20 weeks (Fig. 1A). Sel1l+/− and wild-type mice showed similar body weight gain profiles in response to the two diets (Fig. 1B). Basal fasting blood glucose levels of Sel1l+/− and wild-type mice were comparable (supplemental Fig. S1A) and remained insignificantly changed in response to NC feeding (Fig. 1C). However, after 8 weeks of HFD, Sel1l+/− mice showed significantly higher blood glucose levels than the wild-type control mice (Fig. 1B). These results indicate that mice with one functional allele of Sel1l are genetically predisposed to HFD-induced hyperglycemia.
FIGURE 1.
Sel1l+/− mice are susceptible to HFD-induced hyperglycemia. A, shown is a schematic representation of mouse groups and diets. 10-Week-old male Sel1l+/− and Sel1l+/+ mice were randomly divided and fed with NC or a HFD, respectively, for 20 weeks. B, shown are body weight gains of Sel1l+/− and Sel1l+/+ mice on a HFD or NC. *, p < 0.05; **, p < 0.01, Sel1l+/− HFD versus NC; #, p < 0.05; ##, p < 0.01, Sel1l+/+ HFD versus Sel1l+/+ NC. C, shown are fasting blood glucose levels of Sel1l+/− and Sel1l+/+ mice on HFD or NC. **, p < 0.01, Sel1l+/− HFD versus Sel1l+/+ HFD. D, shown is insulin tolerance of Sel1l+/− and Sel1l+/+ mice fed with HFD for 24 weeks. E, shown is glucose tolerance of Sel1l+/− and Sel1l+/+ mice on HFD for 20 weeks. **, p < 0.01, Sel1l+/− HFD (n = 10) versus Sel1l+/+ HFD (n = 11). F, shown are fasting blood glucose levels of Sel1l+/− and Sel1l+/+ mice on HFD for 20 weeks and treated with either PBS or 4-PBA for 1 week; *, p < 0.05 4-PBA versus PBS (n = 5 per genotype). All values are expressed as the mean ± S.E.
Hyperglycemia in mice may be the result of a defective insulin production, action, or both. To determine the specific causes underlying the impaired glucose homeostasis in Sel1l+/− mice, we first assessed whether haploid insufficiency of SEL1L reduces insulin sensitivity in organs such as liver, skeletal muscle, and adipose tissue. Insulin tolerance tests showed that Sel1l+/− mice showed comparable insulin sensitivity to wild-type mice (Fig. 1D), suggesting that haploinsufficiency of SEL1L did not significantly affect peripheral insulin signaling. Next, we examined whether Sel1l+/− mice had an impaired β-cell function using glucose tolerance tests. Sel1l+/− and wild-type mice fed with NC showed similar glucose tolerance to wild-type control mice (supplemental Fig. S1B). However, Sel1l+/− mice fed with HFD exhibited significantly higher glucose intolerance than wild-type control mice (Fig. 1E). Administration of 4-PBA, a chemical chaperone previously shown to enhance insulin signaling (38), resulted in a significant reduction of serum glucose levels in both Sel1l+/− and wild-type mice fed with HFD (Fig. 1F). Together, these data suggest that in our current diet-induced obesity model, haploid insufficiency of Sel1l impairs insulin production in pancreatic β-cells and had little effect on insulin action in peripheral organs.
Haploid Insufficiency of SEL1L Impairs Glucose-stimulated Insulin Secretion and Adaptive Proliferation of β-Cells
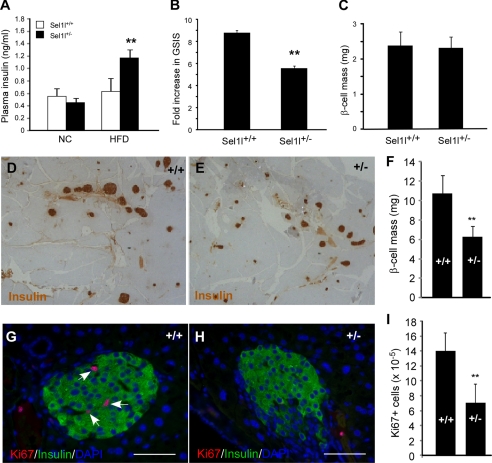
We next assessed the serum insulin levels in Sel1l+/− mice fed with NC and HFD using insulin ELISA. No significant difference in serum insulin level was observed between Sel1l+/− and wild-type mice fed with NC. However, Sel1l+/− mice fed with HFD showed significantly higher serum insulin levels than wild-type control mice (Fig. 2A). The pancreatic insulin contents of wild-type and Sel1l+/− mice fed with HFD were comparable (supplemental Fig. S2A). We next carried out glucose-stimulated insulin secretion studies in Sel1l+/− mice. Although Sel1l+/− mice fed with NC showed no difference in GSIS to wild-type control mice (Fig. 2C), Sel1l+/− mice fed with HFD showed a significantly lower GSIS as compared with wild-type mice (Fig. 2B). These results together with the finding that Sel1l+/− mice were glucose-intolerant (Fig. 1E) indicate that haploid insufficiency of SEL1L also causes impairment in glucose-stimulated insulin secretion.
FIGURE 2.
Sel1l+/− mice fed with a high fat diet exhibit impaired GSIS and reduced β-cell mass. A, shown are plasma insulin levels of Sel1l+/− and Sel1l+/+ mice on NC or HFD for 20 weeks. **, p < 0.01 HFD-fed Sel1l+/− versus Sel1l+/+ mice (n = 7). B, shown is fold change of GSIS of Sel1l+/− and Sel1l+/+ mice. **, p < 0.01, Sel1l+/− versus Sel1l+/+ mice (n = 5 per genotype). C, shown are β-cell masses of Sel1l+/− and Sel1l+/+ mice on HFD for 8 weeks (n = 7 per genotype). D–E, shown are representative images of insulin immunostained pancreatic sections from Sel1l+/+ (D) and Sel1l+/− (E) mice on HFD for 20 weeks. F, shown is quantification of β-cell mass in Sel1l+/+ and Sel1l+/− mice on HFD for 20 weeks. **, p < 0.01 Sel1l+/+ versus Sel1l+/− mice (n = 5 per genotype). β-Cell mass was determined by timing the pancreatic weight with percentage of the β-cell area. G and H, representative images are shown of Ki67 immunofluorescence of pancreatic sections from Sel1l+/+ (G) and Sel1l+/− (H) mice on HFD for 20 weeks. Nuclei were countered stained with DAPI. I, shown is quantification of Ki67-positive cells in Sel1l+/+ and Sel1l+/− mice on HFD for 20 weeks. The rate of β-cell proliferation was calculated by dividing the number of Ki67-positive cells with the total number of nuclei counted. **, p < 0.01 Sel1l+/+ versus Sel1l+/− mice (n = 4 per genotype). All values are expressed as the mean ± S.E.
The functional β-cell mass is a critical factor in determining how much insulin is released into the blood in response to glucose stimulation. As Sel1l+/− mice showed an impaired pancreatic function, we speculated that Sel1l+/− mice fed with HFD might have a reduced β-cell mass. To test this, we carried out morphological analysis of the endocrine pancreas in Sel1l+/− fed with HFD. Wild-type and Sel1l+/− mice on HFD for 8 weeks showed no difference in β-cell masses (Fig. 2C). However, after 20 weeks of HFD-feeding, Sel1l+/− mice exhibited a markedly reduced β-cell mass as compared with that of wild-type mice (Fig. 2, D–F). We then investigated whether the reduction of β-cell mass in Sel1l+/− mice was due to an increased apoptosis or a decreased β-cell proliferation. In situ cell apoptosis assays showed that there was no significant difference in β-cell apoptosis between Sel1l+/− and wild-type mice (supplemental Fig. S2B). However, Sel1l+/− islets contained a significantly lower number of Ki67-positive β-cells than wild-type islets (Fig. 2, G–I). Together, these results indicate that Sel1l+/− mice fed with HFD for 20 weeks had a reduced β-cell mass, and the reduction of β-cell mass in Sel1l+/− mice was due at least in part to an impaired compensatory β-cell growth.
Haploinsufficiency of SEL1L Predisposes Liver and Pancreatic Islets to Obesity-induced Endoplasmic Reticulum Stress
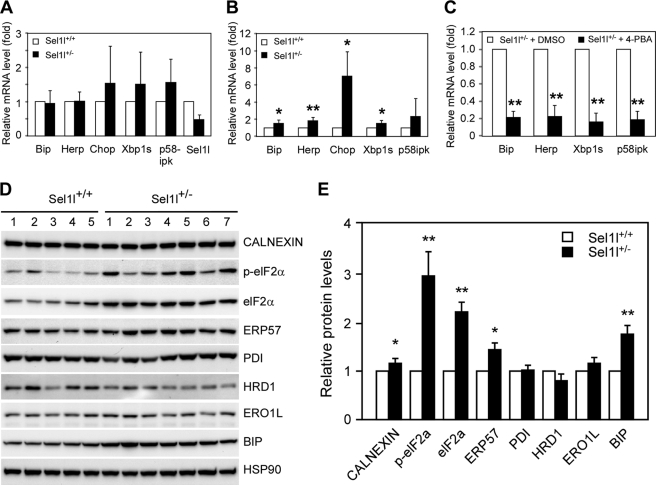
SEL1L is a critical component of the ER-associated protein degradation machinery, and SEL1L deficiency results in systemic ER stress in mouse embryonic cells (35). We, therefore, next investigated possible alterations of the UPR pathway in liver and pancreatic islets of Sel1l+/− mice fed with HFD. First, we cultured islets isolated from wild-type and Sel1l+/− mice in the presence of low (10 mm) and high (30 mm) glucose and performed quantitative RT-PCR analysis of UPR marker genes. Wild-type and Sel1l+/− islets treated with low glucose showed comparable expression of Bip, Herp, Chop, Xbp-1s, and p58IPK at the mRNA level (Fig. 3A). However, Bip, Herp, Chop, and Xbp-1s were significantly up-regulated in high glucose-treated Sel1l+/− islets as opposed to wild-type control islets (Fig. 3B). Treatment of Sel1l+/− pancreatic islets with 30 mm glucose in the presence of the chemical chaperon 4-PBA markedly reduced the transcription of Bip, Herp, Xbp-1s, and p58IPK (Fig. 3C).
FIGURE 3.
Endoplasmic reticulum stress in pancreatic islets and liver of Sel1l+/− mice fed with a high fat diet. A and B, shown is a comparison of UPR gene expression in Sel1l+/+ and Sel1l+/− pancreatic islets. Islets were isolated from 30-week-old Sel1l+/+ and Sel1l+/− mice and cultured overnight in media containing 10 mm (A) or 30 mm (B) glucose. The mRNA expression of five UPR genes, including Bip, Herp, Chop, Xbp1s, and p58IPK, were analyzed by real-time RT-PCR. C, real-time PCR analysis of UPR gene expression in Sel1l+/− islets treated with and without the chemical chaperon, 4-PBA is shown. For each gene, the expression in the vehicle (DMSO) treated islets was set to 1 and used to calculate a relative expression of the gene in 4-PBA treated islets. D, shown is UPR gene expression at the protein level in livers of Sel1l+/− and Sel1l+/+ mice on HFD for 20 weeks. Liver lysates from 5 Sel1l+/+ and 7 Sel1l+/− mice (30 μg per sample) were immunoblotted and probed with antibodies against the indicated gene products. HSP90 was used as a loading control. E, shown is quantification of protein expression in Sel1l+/+ and Sel1l+/− mice indicated in D. For each protein the expression in Sel1l+/+ liver was set to 1 and used to calculate a relative expression in Sel1l+/− liver. *, p < 0.05, **, p < 0.01 Sel1l+/− mice (n = 7) versus Sel1l+/+ (n = 5). All values are expressed as the mean ± S.E.
ER stress has previously been mechanistically linked to obesity-induced insulin resistance in mice (25). Because Sel1l+/− mice were modestly insulin-intolerant (Fig. 1D), we speculated that this might be due to low levels of ER stress in peripheral organs such as liver and adipose tissue. To test this, we prepared lysates from livers of Sel1l+/− and wild-type control mice fed with HFD and examined the expression of a number of UPR genes through Western blot analyses (Fig. 3D). The liver of Sel1l+/− mice showed significantly elevated expression of CALNEXIN, elF2a, ERP57, and BIP as compared with the liver of wild-type mice fed with the same diet (Fig. 3E). Quantitative RT-PCR also revealed significantly elevated expression of Chop and Ero1l mRNA (supplemental Fig. S3). These results indicate that haploinsufficiency of SEL1L predisposes liver and pancreatic islets to ER stress induced by obesity.
Interference of SEL1L Function in Vitro Markedly Inhibits Glucose-stimulated Insulin Secretion and β-Cell Proliferation
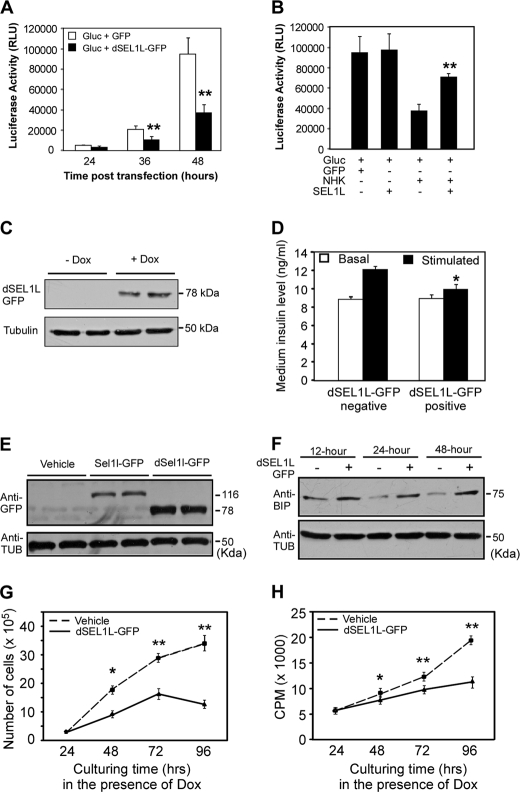
To further understand the role of SEL1L in regulating β-cell function and growth, we performed functional studies of SEL1L in insulinoma cell lines, Min6 and INS-1. First, we assessed the effect of ectopic expression of dSEL1L-GFP, a GFP-tagged SEL1L deletion mutant, on the secretion of G-luc, a naturally secreted luciferase reporter (46). G-luc and dSEL1L-GFP were transiently expressed in Min6 cells. Conditioned media of Min6 cells were collected at various time points after transfection and analyzed by luciferase assays. G-luc activities in the conditioned media of dSEL1L-GFP-expressing cells were significantly lower than those in the conditioned medium of GFP-expressing control cells (Fig. 4A). We then asked whether overexpression of SEL1L facilitates or enhances the secretion of G-luc. For this study we co-expressed SEL1L-GFP with a NHK-GFP, GFP-tagged null-Hong-Kong variant mutant human α1-antitrypsin in Min6 cells. NHK-GFP, an inherent folding-incompetent protein, was previously shown to induce ER stress in mammalian cells (47). Co-expression of SEL1L-GFP with NHK-GFP significantly increased the G-luc activity of conditioned media (Fig. 4B).
FIGURE 4.
Perturbation of SEL1L function in insulinoma cell lines impairs glucose-stimulated insulin secretion and β-cell proliferation. A, G-luc secretion in Min6 cells transiently expressing a dominant-negative form of SEL1L is shown. Mins6 cells were transiently co-transfected with the indicated expression plasmids. Conditioned media were sampled at 24, 36, and 48 h post-transfection and analyzed by luciferase assay. Expression of dSel1l-GFP markedly suppresses G-luc secretion in Min6 cells. B, G-luc secretion in Min6 cells overexpressing SEL1L. Min6 cells were transiently co-transfected with the indicated expression plasmids. Conditioned media were sampled at 24 h post-transfection and analyzed by luciferase assay. Expression of SEL1L-GFP restores protein secretion inhibited by NHK-GFP. **, p < 0.01. NHK-GFP + SEL1L-GFP versus NHK-GFP- expressing cells. C, shown is an immunoblotting analysis of dSEL1L-GFP fusion protein expression in Min6 cells stably integrated with a Tet-inducible Sel1l-GFP transgene. dSEL1L-GFP was detected using an anti-GFP antibody. Dox, doxycycline. D, GSIS of Min6 cells stably expressing dSEL1L-GFP is shown. *, p < 0.05 dSEL1L-GFP-negative versus dSEL1L-GFP-positive cells. Expression of dSEL1L-GFP markedly inhibits glucose-stimulated insulin secretion in Min6 cells. E, shown is an immunoblotting analysis of SEL1L-GFP and dSEL1L-GFP expression in INS-1 cells stably integrated with Tet-inducible Sel1l-GFP and dSel1l-GFP transgenes. F, shown is an immunoblotting analysis of GRP78/BIP expression in INS-1 cells stably expressing dSEL1L-GFP. Ectopic expression of dSEL1L-GFP in INS-1 cells results in up-regulation of GRP78/BIP. Tub, tubulin G and H, shown are growth profiles of INS-1 cells stably expressing dSEL1L-GFP. Cell proliferation was analyzed through cell counting (G) and by thymidine incorporation assay (H). The data were derived from three independent experiments. *, p < 0.05; **, p < 0.01, vehicle versus dSEL1L-GFP expressing cells. All values are expressed as the mean ± S.E.
Although the luciferase data described above indicate that SEL1L function is critically required for general protein secretion in Min6 cells, we do not know if it is required for glucose-stimulated insulin secretion. Thus, we next examined whether ectopic expression of dSEL1L-GFP suppresses glucose-stimulated insulin secretion in Min6 cells. For this study we generated stable Min6 cell lines containing a doxycycline-inducible dSel1l-GFP transgene. Doxycycline-dependent dSEL1L-GFP expression was verified by Western blot analysis (Fig. 4C). An insulin ELISA assay indicated that ectopic expression of dSEL1L-GFP in Min6 cells significantly reduced glucose-stimulated insulin secretion in Min6 cells (Fig. 4D).
Last, using the dominant-negative functional interference strategy detailed above, we assessed whether SEL1L function is critical for β-cell proliferation in vitro. For this study we generated INS-1 cell lines stably expressing the rtTA regulatory proteins (vehicle), rtTA + dSEL1L-GFP, and rtTA + SEL1L-GFP (Fig. 4E). Immunoblotting analysis showed that a significant up-regulation of Grp78/BIP expression was detectable at ∼12 h after the activation of the dSel1l-GFP transgene in INS-1 cells (Fig. 4F). INS-1 lines stably expressing dSEL1L-GFP exhibited a significantly reduced cell proliferation as compared with control lines, as revealed by both cell number counting (Fig. 4G) and thymidine incorporation assays (Fig. 4H). Together, these results indicate that interference of SEL1L function in β-cells impairs both function (insulin secretion) and proliferation of β-cells.
DISCUSSION
The molecular mechanisms underlying the dysfunction and/or failure of β-cells in type 2 diabetes are the subject of many studies in recent years. Genetic studies in mice have implicated several ER proteins, including PERK, elF2a, p58IPK, and WSF, in regulation of pancreatic β-cell growth and function. PERK-deficient mice became progressively diabetic during the postnatal period (48). p58IPK-deficient mice also gradually developed diabetes mellitus with increasing apoptosis of pancreatic islet cells (49). Mice homozygous null for Wfs1 developed overt diabetes (50–52). In this study we provide genetic evidence that SEL1L is critically required for both β-cell function and proliferation. We show that mice heterozygous for a gene trap mutation in Sel1l (Sel1l+/−) maintain normal glycemia under regular diet. However, when fed with a high fat diet, Sel1l+/− mice exhibit a significantly elevated level of serum glucose. Sel1l+/− mice are glucose intolerant as compared with wild-type littermate controls. In addition, Sel1l+/− mice exhibit a markedly reduced β-cell mass due to a decreased rate of β-cell proliferation. Pancreatic islets from Sel1l+/− mice, when incubated in vitro in the presence of high glucose, exhibit an elevated expression of UPR genes. Furthermore, mouse and rat insulinoma cells stably expressing a dominant-negative form of SEL1L exhibit impaired protein secretion and cell growth. Together, these data strongly suggest that haploid insufficiency of SEL1L predisposes mice to high fat-induced hyperglycemia. Our findings thus support the hypothesis that SEL1L is a key regulator of glucose homeostasis in mice.
The pancreatic defects in Sel1l+/− mice are likely to be multifaceted and develop progressively during high fat diet feeding. Sel1l+/− mice exhibited significantly higher blood glucose levels than their wild-type littermates just after 8 weeks of HFD feeding (Fig. 1C). At this stage, however, no significant difference in β-cell mass was detected between Sel1l+/− and wild-type mice (Fig. 2C). These results argue that, at least initially, impaired glucose-stimulated insulin secretion was responsible for the observed dysregulated blood glucose homeostasis in Sel1l+/− mice on HFD. During further HFD feeding, the proliferation of pancreatic β-cells was inhibited (Fig. 2, G–I), leading to a significantly reduced β-cell mass (Fig. 2, D–F). Thus, the pancreatic defects at later phases of the HFD feeding are likely to be a combination of impaired insulin secretion and reduced β-cell mass.
Sel1l encodes an ER membrane protein that is highly expressed in the developing and mature pancreas (27). We previously demonstrated that SEL1L deficiency disrupts ER homeostasis, which leads to ER stress and activation of the unfolded protein response pathway (35). Several lines of evidence from the present study suggest that ER stress is likely to be responsible for the impaired insulin secretion and reduced β-cell proliferation in Sel1l+/− mice fed with HFD. First, we showed that Sel1l+/− islets treated with high glucose expresses significantly higher levels of UPR markers (Fig. 3B). Second, treatment of Sel1l+/− islets with the chemical chaperone, 4-PBA, markedly reduces the expression UPR genes. Third, INS-1 cells expressing a dominant-negative form of SEL1L exhibit significantly elevated expression of the ER stress sensors BIP. Further studies are required to elucidate how ER stress mechanistically affects β-cell growth and insulin secretion in adult mice.
The insulin signaling pathway in Sel1l+/− mice fed with HFD may also be impaired, as Sel1l+/− mice showed a significantly elevated plasma insulin level at the starting period of HFD feeding (Fig. 2A). The molecular mechanisms underlining the impaired insulin signaling in Sel1l+/− mice, however, remain unclear from the current investigation. SEL1L was previously shown to be a critical factor involved in regulating ER homeostasis (32, 33, 35). We show here that several factors of the unfolded protein response pathway are up-regulated in the liver of HFD-fed Sel1l+/− mice. It is thus conceivable that ER stress may be a contributing factor to the impaired insulin action in Sel1l+/− mice. This hypothesis is supported by the observation that treatment of HFD-fed Sel1l+/− mice with 4-PBA markedly lowers blood glucose levels (Fig. 1F). Proof of this requires further biochemical and molecular analyses of the liver and adipose tissues of Sel1l+/− mice on HFD.
The islet in type 2 diabetes is characterized by islet amyloid derived from islet amyloid polypeptide (53). Transgenic expression of human islet amyloid polypeptide in rodents recapitulates this islet pathology and leads to diabetes (23, 54). Collectively, these findings suggest that islet amyloid plays an important role in mediating β-cell dysfunction and death. How amyloidogenesis occurs in islets of type 2 diabetes remains to be elucidated. However, given the role of SEL1L in quality control of ER-sorted proteins (32), it is tempting to speculate that dysregulation of SEL1L may be an underlying etiology for islet amyloidogenesis.
In conclusion, we show here that mice haploinsufficient for SEL1L are susceptible to HFD diet-induced hyperglycemia. These mice have a significantly reduced β-cell mass and an impaired glucose-stimulated insulin secretion. Our findings highlight a critical and previously unknown role for SEL1L in regulating β-cell function and growth. The implication of these findings is that SEL1L may have potential as a therapeutic target to develop new drugs for the prevention and treatment of type 2 diabetes mellitus.
Supplementary Material
Acknowledgments
We thank Dr. Donald F. Steiner (University of Chicago) for providing Min6 cells and Drs. Masakazu Shiota (Vanderbilt University) and Bruce Currie and Yves Boisclair (Cornell University) for stimulating discussions and critical comments during the preparation of this manuscript.
This work was supported by the College of Agriculture and Life Sciences and the Vertebrate Functional Genomics Center, Cornell University (to Q. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- T2DM
- type 2 diabetes mellitus
- ER
- endoplasmic reticulum
- HFD
- high fat diet
- Sel1l
- suppressor enhancer Lin12 1-like
- GSIS
- glucose-stimulated insulin secretion
- rtTA
- reverse tet transactivator
- 4-PBA
- phenylbutyric acid
- NC
- normal chow
- UPR
- unfolded protein response.
REFERENCES
- 1. American Diabetes Association (2008) Diabetes Care 31, 596–615 [DOI] [PubMed] [Google Scholar]
- 2. Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 3. Carey D. G., Jenkins A. B., Campbell L. V., Freund J., Chisholm D. J. (1996) Diabetes 45, 633–638 [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo R. A., Bonadonna R. C., Ferrannini E. (1992) Diabetes Care 15, 318–368 [DOI] [PubMed] [Google Scholar]
- 5. Eizirik D. L., Cardozo A. K., Cnop M. (2008) Endocr. Rev. 29, 42–61 [DOI] [PubMed] [Google Scholar]
- 6. Scheuner D., Kaufman R. J. (2008) Endocr. Rev. 29, 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voeltz G. K., Rolls M. M., Rapoport T. A. (2002) EMBO Rep. 3, 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. English A. R., Zurek N., Voeltz G. K. (2009) Curr. Opin. Cell Biol. 21, 596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hampton R. Y. (2002) Curr. Opin. Cell Biol. 14, 476–482 [DOI] [PubMed] [Google Scholar]
- 10. Ma Y., Hendershot L. M. (2001) Cell 107, 827–830 [DOI] [PubMed] [Google Scholar]
- 11. Sundar Rajan S., Srinivasan V., Balasubramanyam M., Tatu U. (2007) Indian J. Med. Res. 125, 411–424 [PubMed] [Google Scholar]
- 12. Ron D., Walter P. (2007) Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 13. Harding H. P., Novoa I., Bertolotti A., Zeng H., Zhang Y., Urano F., Jousse C., Ron D. (2001) Cold Spring Harbor Symp. Quant. Biol. 66, 499–508 [DOI] [PubMed] [Google Scholar]
- 14. Brodsky J. L. (2007) Biochem. J. 404, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ortsäter H., Sjöholm A. (2007) Mol. Cell. Endocrinol. 277, 1–5 [DOI] [PubMed] [Google Scholar]
- 16. van Anken E., Braakman I. (2005) Crit. Rev. Biochem. Mol. Biol. 40, 269–283 [DOI] [PubMed] [Google Scholar]
- 17. Straub S. G., Sharp G. W. (2002) Diabetes Metab. Res. Rev 18, 451–463 [DOI] [PubMed] [Google Scholar]
- 18. Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J. D., Anderson R. G. (1986) J. Cell Biol. 103, 2273–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipson K. L., Fonseca S. G., Urano F. (2006) Curr. Mol. Med. 6, 71–77 [DOI] [PubMed] [Google Scholar]
- 20. Boden G., Duan X., Homko C., Molina E. J., Song W., Perez O., Cheung P., Merali S. (2008) Diabetes 57, 2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang C. J., Lin C. Y., Haataja L., Gurlo T., Butler A. E., Rizza R. A., Butler P. C. (2007) Diabetes 56, 2016–2027 [DOI] [PubMed] [Google Scholar]
- 22. Laybutt D. R., Preston A. M., Akerfeldt M. C., Kench J. G., Busch A. K., Biankin A. V., Biden T. J. (2007) Diabetologia 50, 752–763 [DOI] [PubMed] [Google Scholar]
- 23. Matveyenko A. V., Gurlo T., Daval M., Butler A. E., Butler P. C. (2009) Diabetes 58, 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oyadomari S., Koizumi A., Takeda K., Gotoh T., Akira S., Araki E., Mori M. (2002) J. Clin. Invest. 109, 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 26. Donoviel D. B., Donoviel M. S., Fan E., Hadjantonakis A., Bernstein A. (1998) Mech. Dev. 78, 203–207 [DOI] [PubMed] [Google Scholar]
- 27. Biunno I., Appierto V., Cattaneo M., Leone B. E., Balzano G., Socci C., Saccone S., Letizia A., Della Valle G., Sgaramella V. (1997) Genomics 46, 284–286 [DOI] [PubMed] [Google Scholar]
- 28. Biunno I., Cattaneo M., Orlandi R., Canton C., Biagiotti L., Ferrero S., Barberis M., Pupa S. M., Scarpa A., Ménard S. (2006) J. Cell. Physiol. 208, 23–38 [DOI] [PubMed] [Google Scholar]
- 29. Cattaneo M., Otsu M., Fagioli C., Martino S., Lotti L. V., Sitia R., Biunno I. (2008) J. Cell. Physiol. 215, 794–802 [DOI] [PubMed] [Google Scholar]
- 30. Cormier J. H., Tamura T., Sunryd J. C., Hebert D. N. (2009) Mol. Cell 34, 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosokawa N., Wada I., Nagasawa K., Moriyama T., Okawa K., Nagata K. (2008) J. Biol. Chem. 283, 20914–20924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller B., Klemm E. J., Spooner E., Claessen J. H., Ploegh H. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12325–12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller B., Lilley B. N., Ploegh H. L. (2006) J. Cell Biol. 175, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oresic K., Mueller B., Tortorella D. (2009) Biosci. Rep. 29, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Francisco A. B., Singh R., Li S., Vani A. K., Yang L., Munroe R. J., Diaferia G., Cardano M., Biunno I., Qi L., Schimenti J. C., Long Q. (2010) J. Biol. Chem. 285, 13694–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li S., Francisco A. B., Munroe R. J., Schimenti J. C., Long Q. (2010) BMC Dev. Biol. 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sachdeva M. M., Claiborn K. C., Khoo C., Yang J., Groff D. N., Mirmira R. G., Stoffers D. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Görgün C. Z., Hotamisligil G. S. (2006) Science 313, 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta S., McGrath B., Cavener D. R. (2010) Diabetes 59, 1937–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scharp D. W., Kemp C. B., Knight M. J., Ballinger W. F., Lacy P. E. (1973) Transplantation 16, 686–689 [DOI] [PubMed] [Google Scholar]
- 41. Zhang W., Feng D., Li Y., Iida K., McGrath B., Cavener D. R. (2006) Cell Metab. 4, 491–497 [DOI] [PubMed] [Google Scholar]
- 42. Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. (1990) Endocrinology 127, 126–132 [DOI] [PubMed] [Google Scholar]
- 43. Dowling P., O'Driscoll L., O'Sullivan F., Dowd A., Henry M., Jeppesen P. B., Meleady P., Clynes M. (2006) Proteomics 6, 6578–6587 [DOI] [PubMed] [Google Scholar]
- 44. Merglen A., Theander S., Rubi B., Chaffard G., Wollheim C. B., Maechler P. (2004) Endocrinology 145, 667–678 [DOI] [PubMed] [Google Scholar]
- 45. Li S., Francisco A. B., Han C., Pattabiraman S., Foote M. R., Giesy S. L., Wang C., Schimenti J. C., Boisclair Y. R., Long Q. (2010) FEBS Lett. 584, 4121–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Badr C. E., Hewett J. W., Breakefield X. O., Tannous B. A. (2007) PLoS One 2, e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. (1988) J. Biol. Chem. 263, 7330–7335 [PubMed] [Google Scholar]
- 48. Harding H. P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., Sabatini D. D., Ron D. (2001) Mol. Cell 7, 1153–1163 [DOI] [PubMed] [Google Scholar]
- 49. Ladiges W. C., Knoblaugh S. E., Morton J. F., Korth M. J., Sopher B. L., Baskin C. R., MacAuley A., Goodman A. G., LeBoeuf R. C., Katze M. G. (2005) Diabetes 54, 1074–1081 [DOI] [PubMed] [Google Scholar]
- 50. Akiyama M., Hatanaka M., Ohta Y., Ueda K., Yanai A., Uehara Y., Tanabe K., Tsuru M., Miyazaki M., Saeki S., Saito T., Shinoda K., Oka Y., Tanizawa Y. (2009) Diabetologia 52, 653–663 [DOI] [PubMed] [Google Scholar]
- 51. Ishihara H., Takeda S., Tamura A., Takahashi R., Yamaguchi S., Takei D., Yamada T., Inoue H., Soga H., Katagiri H., Tanizawa Y., Oka Y. (2004) Hum. Mol. Genet. 13, 1159–1170 [DOI] [PubMed] [Google Scholar]
- 52. Riggs A. C., Bernal-Mizrachi E., Ohsugi M., Wasson J., Fatrai S., Welling C., Murray J., Schmidt R. E., Herrera P. L., Permutt M. A. (2005) Diabetologia 48, 2313–2321 [DOI] [PubMed] [Google Scholar]
- 53. Butler A. E., Janson J., Soeller W. C., Butler P. C. (2003) Diabetes 52, 2304–2314 [DOI] [PubMed] [Google Scholar]
- 54. Janson J., Soeller W. C., Roche P. C., Nelson R. T., Torchia A. J., Kreutter D. K., Butler P. C. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 7283–7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.