Abstract
p21-activated kinase (PAK) 2, a member of the PAK family of serine/threonine protein kinases, plays an important role in physiological processes such as motility, survival, mitosis, and apoptosis. However, the role of PAK2 in resistance to chemotherapy is unclear. Here we report that PAK2 is highly expressed in human breast cancer cell lines and human breast invasive carcinoma tissue compared with a human non-tumorigenic mammary epithelial cell line and adjacent normal breast tissue, respectively. Interestingly, we found that PAK2 can bind with caspase-7 and phosphorylate caspase-7 at the Ser-30, Thr-173, and Ser-239 sites. Functionally, the phosphorylation of caspase-7 decreases its activity, thereby inhibiting cellular apoptosis. Our data indicate that highly expressed PAK2 mediates chemotherapeutic resistance in human breast invasive ductal carcinoma by negatively regulating caspase-7 activity.
Keywords: Anticancer Drug, Breast Cancer, Caspase, Drug Action, Protein Phosphorylation
Introduction
Breast cancer comprises 10.4% of all cancers diagnosed in women and is the fifth most common cause of cancer death worldwide (1). In 2009, 209,060 new cases of breast cancer were diagnosed, and 40,230 deaths occurred from breast cancer in the United States (2). One of the important problems confronted during the course of breast cancer treatment is resistance to chemotherapy (3). Accumulating evidence indicates that the expression of several growth factor receptors, including the epidermal growth factor receptor (4), human epidermal growth factor receptor type 2 (HER2) (5), and insulin-like growth factor-1 receptor (6), is often elevated in chemotherapy-resistant breast tumors. The elevation of these growth factors is associated with increased expression and/or activation of their downstream targets such as Akt (7), extracellular signal-regulated kinases (ERKs), p38 (8), and p21-activated kinase (PAK)4 1 (9). This suggests that the expression and modification of growth factor receptors and their downstream kinases might correspond with breast cancer chemotherapy resistance (10).
The PAK family of serine/threonine kinases, which were initially identified as binding partners of the Rho GTPases Cdc42 and Rac1 (11), plays an important role in physiological processes such as motility, survival, mitosis, apoptosis, and hormone signaling (12). Six PAK family members have been identified in mammalian cells. PAK1–3 belong to the group I PAK family, and PAK4–6 belong to the group II PAK family (13). PAK1 is known to promote cell survival through its phosphorylation of Bad on Ser-112 and Ser-136 (14). PAK1 also phosphorylates the estrogen receptor at Ser-305, leading to increased cyclin D1 expression and hormone independence of breast cancer cells (15, 16). Interestingly, PAK2, which has an overall 76% homology with PAK1 and 96% homology in the kinase domain, has a dual role in both cell survival and cell death pathways. Full-length PAK2 is activated by phosphoinositide 3-kinase (PI3K) and Akt to promote cell survival (12, 17–19). However, when cellular stresses occur, such as exposure to UV irradiation or chemotherapeutic drugs, PAK2 can be cleaved and activated by caspase-3, -8, and -10 (17–20) to generate a proteolytic fragment, referred to as PAK2p34, which translocates to the nucleus and leads to cellular apoptosis (17, 21, 22). Accumulating evidence indicates that PAKs, especially PAK1 and PAK4, are either up-regulated or hyperactivated in a variety of human cancers, including breast, ovarian, colorectal, thyroid, and pancreatic cancers (23). Our previous study demonstrated that PAK2 phosphorylated c-Jun at five threonine sites (Thr-2, Thr-8, Thr-89, Thr-93, and Thr-286) and played an important role in EGF-induced cell proliferation and transformation (24). However, the underlying mechanism of the role of PAK2 in resistance to chemotherapy needs to be further explored.
In the present study, we show that PAK2 is highly expressed in human breast cancer cell lines and human breast invasive ductal carcinoma tissue. PAK2 can bind with caspase-7 and phosphorylate caspase-7 at Ser-30, Thr-173, and Ser-239 residues, resulting in an inhibition of caspase-7 activity and decreased cellular apoptosis. Our results suggest that PAK2 is a novel target of chemotherapy for breast cancer.
MATERIALS AND METHODS
Reagents and Antibodies
Staurosporine, Streptomyces sp., was purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Doxorubicin HCl was bought from LKT Laboratories, Inc. (St. Paul, MN). Dulbecco's modified Eagle's medium (DMEM) and other supplements were from Invitrogen. The antibody to specifically detect the PAK2 C terminus (γPAK-C19) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies to detect phosphorylated PAK2 (Ser-141), total PAK2, total caspase-7, cleaved caspase-7 (Asp-198), cleaved PARP (Asp-214), and phosphorylated threonines were purchased from Cell Signaling Technology, Inc. (Danvers, MA). The small hairpin RNA (shRNA) construct against pak2 (number 1 sense sequence, CCCAATATTTCGGGATTTCTT; number 2 sense sequence, CCAATCACAGTTTGAAACCTT) used in this study was from the BioMedical Genomics Center at the University of Minnesota. PAK2 active kinase and the human caspase-8 active recombinant proteins were purchased from Millipore Corp. (Billerica, MA).
Cell Culture and Transfection
MCF-7 human breast cancer cells were cultivated in DMEM supplemented with 10% fetal bovine serum (FBS), 1 mm sodium pyruvate, 0.01 mg/ml bovine insulin, 100 units/ml penicillin, and 100 mg/ml streptomycin and maintained at 37 °C in a 5% CO2, humidified incubator. SK-BR-3 breast cancer cells were maintained in McCoy's 5A medium (modified) supplemented with 10% FBS. MDA-MB-468 breast cancer cells were cultured in Leibovitz's L-15 medium supplemented with 10% FBS and maintained at 37 °C in a 100% air, humidified incubator. MCF-10A cells were maintained in DMEM/F-12 medium supplemented with 5% horse serum, 20 ng/ml EGF, 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, 5 μg/ml insulin, 100 units/ml penicillin, and 100 mg/ml streptomycin and maintained at 37 °C in a 5% CO2, humidified incubator. HEK293T cells were cultured in DMEM supplemented with 10% FBS. For transfection experiments, jetPEI (Qbiogen, Inc., Carlsbad, CA) reagent was used according to the manufacturer's instructions.
Western Blotting Analysis
Cellular proteins were extracted using radioimmune precipitation assay lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS, 1 mm EDTA, and protease inhibitor mixture), separated by SDS-PAGE, and then transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences). The membranes were incubated with an appropriate specific primary antibody and a horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized by an enhanced chemiluminescence (ECL) reagent (Amersham Biosciences).
Tissue Array
A human breast tissue array (U.S. Biomax, Inc., Rockville, MD) was deparaffinized in 100% xylene and rehydrated in different concentrations of alcohol, and the antigens were retrieved by boiling in 10 mm sodium citrate buffer for 10 min. After cooling, the tissues were blocked with 5% normal donkey serum in 1× PBS and 0.3% Triton X-100, pH 6.0 for 1 h at room temperature and then incubated with a 1:50 dilution of a PAK2 goat antibody in 1× PBS and 0.3% Triton X-100 containing 1% donkey serum while rocking at 4 °C overnight. After washing, the tissues were incubated in the dark with a 1:200 dilution of Cy5-donkey anti-goat antibody for 1.5 h at room temperature. Images were captured by laser scanning confocal microscopy (Nikon C1si Confocal Spectral Imaging System, Nikon Instruments Co., Melville, NY) and analyzed by the ImageJ (v1.37v, National Institutes of Health) software program. The average fluorescence intensity score in each case indicated the PAK2 expression level.
Immunoprecipitation
After transfection with pc-DNA3.1-V5-pak2 and pCMV-Myc-caspase-7 for 36 h, HEK293T cellular proteins were extracted by 1% CHAPS buffer (30 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% CHAPS, and 1× protease inhibitors). Cell lysates were combined with agarose-protein A/G beads (50% slurry) by gentle rocking at 4 °C overnight. The beads were washed three times with high salt buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, and 500 mm NaCl) and low salt buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, and 150 mm NaCl) and PBS. Proteins bound to beads were boiled and resolved by 10% SDS-PAGE followed by Western blot analysis. For endogenous immunoprecipitation, MCF-7 cell lysates were immunoprecipitated with a PAK2 antibody, and the immunoprecipitated complex was probed to detect caspase-7.
Confocal Laser Scanning Fluorescence Microscopy
MCF-7 human breast cancer cells (1 × 105) were seeded in a four-chamber polystyrene vessel tissue culture glass slide (BD Biosciences) treated or not treated with 100 nm staurosporine for 6 h in a 37 °C incubator. Cells were fixed with 4% formalin for 15 min at room temperature in the dark. After gentle washing twice for 10 min each with 1× PBS containing Ca2+ and Mg2+, cells were blocked with 1× PBS, 0.02% Tween 20, and 1% BSA in a 37 °C incubator for 1 h. Cells were then incubated with a 1:50 dilution of a PAK2 goat antibody and a 1:50 dilution of a caspase-7 rabbit antibody in 1× PBS and 3% BSA by gently rocking at 4 °C overnight. After washing twice for 5 min each with 1× PBS, 0.02% Tween 20, and 1% BSA, cells were incubated with a 1:200 dilution of Cy3-donkey anti-goat and a 1:200 dilution of Cy2-donkey anti-rabbit antibodies for 1 h in the dark. Co-localization of proteins was observed by laser scanning confocal microscopy (Nikon C1si Confocal Spectral Imaging System, Nikon Instruments Co.) using a CFI Plan Fluor 40× oil objective and then analyzed using the EZ-C1 (v3.20) software program.
Plasmid Preparation and Purification of Caspase-7 Protein
The human PAK2 plasmid was purchased from Addgene (Cambridge, MA) and subcloned into pcDNA3.1. Mutants were constructed using a caspase-7 plasmid (pcDNA3.1-caspase-7) with 3 mutated sites (S30A, T173A S239A) and the QuikChange Mutagenesis kit (Stratagene, Inc., La Jolla, CA). Constructs were then subcloned into the pET-46 Ek/LIC vector and confirmed by restriction mapping and DNA sequencing. His-wild type caspase-7 (WT-caspase-7) and His-mutant caspase-7 (mut-caspase-7) proteins were induced in Escherichia coli BL21 bacteria at 25 °C for 4 h by the addition of 0.25 mm isopropyl 1-thio-β-d-galactopyranoside. WT-caspase-7 and mut-caspase-7 proteins were purified using nickel-nitrilotriacetic acid-agarose (Qiagen, Inc., Valencia, CA), eluted with 200 mm imidazole, and then purified by FPLC on a HiLoad 16/60 System using Superdex 75 gel filtration chromatography (GE Healthcare).
In Vitro Kinase Assay
Purified WT-caspase-7, mut-caspase-7, and caspase-7 peptides were incubated with active PAK2 (Upstate Biotechnology, Inc., Boston, MA) with 1 mCi of [γ-32P]ATP, 100 μm unlabeled ATP, and 1× kinase buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 1 mm EDTA, 1 mm DTT, and 0.01% Brij 35; Cell Signaling Technology, Inc.). The reactions were run at 30 °C for 30 min and stopped by adding 6× SDS sample buffer. The proteins were boiled, then resolved by SDS-PAGE, and visualized by autoradiography.
In Vitro Caspase-7 Activity Assay
To detect caspase-7 activity in vitro, WT- and mut-caspase-7 proteins (3 μg each) were individually incubated with active PAK2. Caspase-7 activity was detected using the Caspase-3 Colorimetric Assay kit (Millipore Corp.) because caspase-3 and caspase-7 have the same substrate. For detecting caspase-7 activity in cells, MCF-7 sh-pak2 and MCF-7 sh-mock cells were harvested after treatment with staurosporine (100 nm) for 6 h, and 200 μg of protein was used to detect caspase-7 activity according to the manufacturer's instructions.
Flow Cytometry Analysis
Staurosporine-induced cellular apoptosis was determined using the annexin V-FITC apoptosis detection kit (MBL International Corp., Woburn, MA) following the manufacturer's suggested protocols. After treatment with different concentrations of staurosporine, cells were harvested at 24 h, washed with phosphate-buffered saline, and then incubated for 5 min at room temperature with annexin V-FITC plus propidium iodide. Apoptosis was analyzed by a FACSCalibur flow cytometer (BD Biosciences).
Statistical Analysis
Statistically significant differences were determined using the Student's t test, and a p value less than 0.05 was considered statistically significant.
RESULTS
PAK2 Is Highly Expressed in Human Breast Cancer Tissues and Breast Cancer Cell Lines
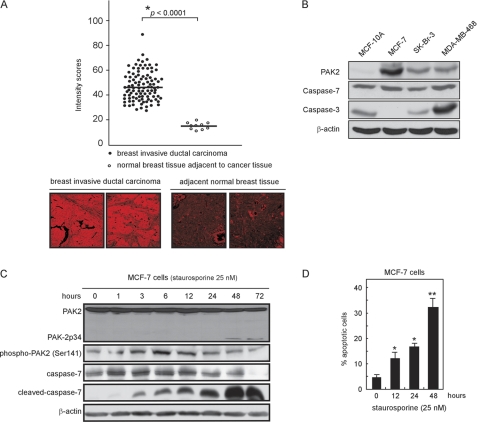
A human breast invasive ductal carcinoma tissue array was used to determine the expression level of PAK2 in human breast cancer tissue compared with normal tissue. The array slide contained duplicate core samples of 100 cases of breast invasive ductal carcinoma and 10 cases of normal breast tissues adjacent to cancer tissues. The results revealed that the PAK2 expression level was significantly higher in breast invasive ductal carcinoma tissues with a median expression level of 43.67 compared with PAK2 expression in adjacent normal tissues, which had a median score of 17.47 (Fig. 1A). Moreover, the expression levels of PAK2 in different breast cancer cell lines were determined, and the results indicated that the expression level of PAK2 was higher in the MCF-7, SK-BR-3, and MDA-MB-468 breast cancer cell lines compared with MCF-10A, a human non-tumorigenic mammary epithelial cell line (Fig. 1B). We chose MCF-7 as the major cell line in our experiments because these cells do not express the caspase-3 protein. This is important because caspase-7 has some of the same downstream target substrates as caspase-3, e.g. PARP. To avoid interference from caspase-3, we therefore chose MCF-7 as the major cell model to study the relationship of PAK2 and caspase-7. Following treatment with staurosporine (25 nm), phosphorylation of PAK2 at Ser-141 increased at 1 h, was maintained at 6 h, and decreased after 12 h. Phosphorylation of PAK2 represents its activity (25), and notably, the decreased phosphorylation of PAK2 corresponded with a marked increased expression level of cleaved caspase-7 (Fig. 1C), suggesting some kind of regulatory activity. Furthermore, staurosporine (25 nm) treatment induced apoptosis of MCF-7 cells in a time-dependent manner (e.g. 13% at 12 h and up to ∼34% at 48 h; Fig. 1D). Overall, these results indicated that PAK2 is highly expressed in breast invasive ductal carcinoma tissue and breast cancer cell lines. In addition, staurosporine can induce MCF-7 cellular apoptosis and phosphorylation of PAK2, whose activity might be directly in opposition to caspase-7 activity.
FIGURE 1.
PAK2 is overexpressed in breast invasive ductal carcinoma tissue and various breast cancer cell lines. A, immunofluorescence staining and confocal microscopy were used to detect the expression of PAK2 in 100 cases of human breast invasive ductal carcinoma tissue and in 10 cases of normal breast tissue adjacent to breast cancer tissue. PAK2 was detected by immunofluorescence staining with a PAK2 primary antibody and a Cy3-conjugated donkey anti-goat secondary antibody. The intensity score of fluorescence from each sample was determined. Data are shown as means ± S.D. from triplicate experiments; the asterisk (*) indicates a significantly (p < 0.0001) higher level (top) of PAK2 in breast cancer compared with normal tissue. Representative cases are shown (bottom). B, endogenous PAK2, caspase-3, and caspase-7 protein levels were detected in three different breast cancer cell lines compared with a human non-tumorigenic mammary epithelial cell line. Cellular proteins (40 μg) were resolved by SDS-PAGE and visualized by Western blotting. C, staurosporine (25 nm) was incubated with MCF-7 cells for various times. Cells were harvested, and total PAK2, phosphorylated PAK2, total caspase-7, and cleaved caspase-7 protein levels were detected by Western blotting using specific antibodies. D, staurosporine (25 nm) can induce apoptosis of MCF-7 cells in a time-dependent manner. Cells were harvested, and apoptosis was determined by flow cytometry. Data are shown as means ± S.D. from triplicate experiments; the asterisks indicate a significant increase in apoptosis (*, p < 0.01; **, p < 0.001) as determined by the Student's t test. For B and C, representative blots from three independent experiments are shown.
PAK2 Interacts and Co-localizes with Caspase-7
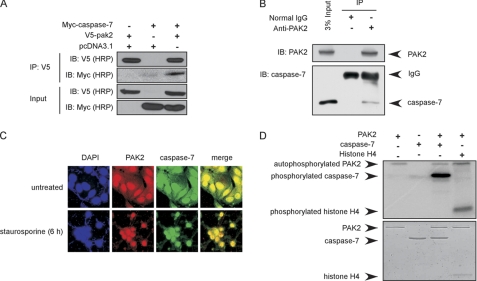
To determine whether PAK2 can directly interact with caspase-7, HEK293T cells were transiently transfected with pc-DNA3.1-V5-pak2 and pCMV-Myc-caspase-7. Anti-V5 was used to immunoprecipitate V5-PAK2, and anti-Myc was used to detect Myc-caspase-7 by Western blot. The results confirmed that PAK2 can bind with caspase-7 in vitro (Fig. 2A). To further verify that PAK2 can bind with caspase-7 in cells, MCF-7 cells were harvested and disrupted. PAK2 was immunoprecipitated with anti-PAK2, and caspase-7 was detected with anti-caspase-7. The results indicated that PAK2 can bind with caspase-7 in cells (Fig. 2B). To determine whether PAK2 and caspase-7 can co-localize in MCF-7 cells, immunocytofluorescence analysis was performed. The results showed that PAK2 and caspase-7 can co-localize in both the cytoplasm and nucleus under normal conditions. However, after stimulation with staurosporine, they co-localized in the nucleus (Fig. 2C). PAK2 is a serine/threonine protein kinase, which can phosphorylate a number of substrates, including histone H4 (26), histone H2B, and myelin basic protein (27). To determine whether PAK2 can phosphorylate caspase-7, an in vitro kinase assay was performed in the presence of [γ-32P]ATP using histone H4 as a positive control. The data indicated that PAK2 can strongly phosphorylate caspase-7 in vitro (Fig. 2D). This confirms that PAK2 can bind with and phosphorylate caspase-7 in vitro and in cells.
FIGURE 2.
PAK2 interacts and co-localizes with caspase-7. A, PAK2 binds with caspase-7 in vitro. The pcDNA3.1-pak2 and pCMV-caspase-7 plasmids were transiently transfected into 293T cells, V5-PAK2 was immunoprecipitated by anti-V5, and co-immunoprecipitated Myc-caspase-7 was detected by Western blotting. B, PAK2 interacts with endogenous caspase-7. MCF-7 cell lysates were immunoprecipitated with anti-PAK2, and the immunoprecipitated complex was probed to detect caspase-7. C, PAK2 and caspase-7 can co-localize in MCF-7 cells. PAK2 and caspase-7 were detected by immunofluorescence staining with PAK2 and caspase-7 primary antibodies, a Cy3-conjugated donkey anti-goat secondary antibody for PAK2 and a Cy2-conjugated donkey anti-rabbit antibody for detection of caspase-7. D, PAK2 phosphorylates caspase-7 in vitro. Results of an in vitro kinase assay in the presence of [γ-32P]ATP were visualized by autoradiography. Histone H4 was used as a positive control. Data shown are representative of results from triplicate independent experiments. IP, immunoprecipitation; IB, immunoblot.
PAK2 Can Phosphorylate Caspase-7 at Ser-30, Thr-173, and Ser-239
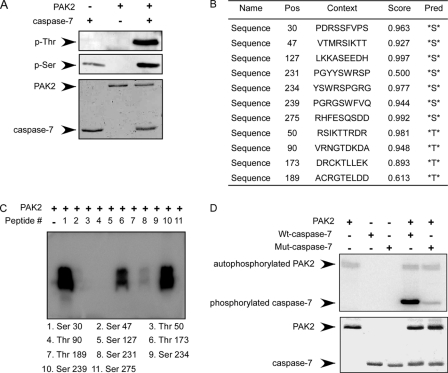
After confirming that PAK2 can phosphorylate caspase-7, the next step was to identify the site(s) of caspase-7 that can be phosphorylated by PAK2. First, we performed an in vitro kinase assay with PAK2 and caspase-7 to detect phosphorylated serines and threonines by Western blotting. The results indicated that PAK2 can phosphorylate caspase-7 at both serine and threonine sites (Fig. 3A). The potential serine and threonine phosphorylation sites were predicted by the NetPhos 2.0 program (28), and the data suggested 7 serine and 4 threonine residues as potential sites of caspase-7 that might be phosphorylated by PAK2 (Fig. 3B). Based on this analysis, 11 peptides were designed and synthesized commercially (PEPTIDE 2.0, Houston, TX). In vitro kinase assays were performed in the presence of [γ-32P]ATP using the different peptides as substrates for PAK2. The results indicated that Ser-30, Thr-173, and Ser-239 of caspase-7 are likely the most important sites to be phosphorylated by PAK2 (Fig. 3C). To further confirm the phosphorylation sites, all three of these caspase-7 sites were mutated to alanine. The His-mutant caspase-7 protein (S30A,T173A,S239A) and WT-caspase-7 protein were expressed as described under “Materials and Methods.” In vitro kinase assays using active PAK2 and WT-caspase-7 or mut-caspase-7 protein were performed in the presence of [γ-32P]ATP. The results showed that phosphorylation of the mut-caspase-7 protein by PAK2 decreased dramatically compared with WT-caspase-7, suggesting that Ser-30, Thr-173, and Ser-239 are the most important sites of caspase-7 to be phosphorylated by PAK2 (Fig. 3D).
FIGURE 3.
PAK2 phosphorylates caspase-7 at Ser-30, Thr-173, and Ser-239 sites. A, PAK2 phosphorylates caspase-7 at both serine and threonine residues as determined by Western blotting following an in vitro kinase assay. B, potential serine and threonine phosphorylation sites on caspase-7 were predicted by the NetPhos 2.0 program. Scores indicate possible sites that might be phosphorylated in cells. Pos, phosphorylation site, Pred, predicted site. C, peptide mapping of 11 synthesized peptides containing potential sites predicted to be phosphorylated by PAK2. Sites were examined using an in vitro kinase assay with PAK2 in the presence of [γ-32P]ATP and visualized by autoradiography. D, confirmation of the peptide mapping results using an in vitro kinase assay with PAK2 and His-WT-caspase-7 or His-mut-caspase-7. Results were visualized by autoradiography. For A, C, and D, representative blots from three independent experiments are shown.
PAK2 Inhibits Caspase-7 Activity in Vitro
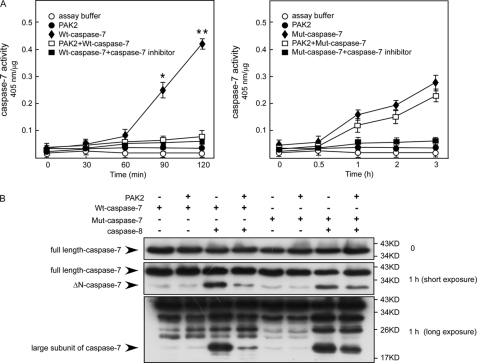
Caspase-7 is one of the important executioner caspases, which regulate mitochondrial events in the apoptotic pathway (29) and inflammatory ailments (30). Accumulating evidence suggests that cell death pathways are finely regulated by multiple signaling events, including direct phosphorylation of caspases by kinases, which can promote or inhibit caspase activity. For example, DNA-dependent protein kinase phosphorylates and activates caspase-2, whereas Akt phosphorylates and inhibits human caspase-9 activity (31). Therefore, we determined whether the phosphorylation of caspase-7 by PAK2 has an effect on caspase-7 activity. First, an in vitro kinase assay using PAK2 and caspase-7 was performed, and caspase-7 activity was analyzed using the Caspase-3 Colorimetric Assay kit as described under “Materials and Methods.” The results indicated that after phosphorylation by PAK2 WT-caspase-7 activity decreased almost 4-fold compared with WT-caspase-7 alone (Fig. 4A, left panel). In contrast, mut-caspase-7 activity was not decreased dramatically (Fig. 4A, right). Caspase-7 is proteolytically activated and cleaved by the initiator caspase-8 during death receptor- and DNA damage-induced apoptosis (30). The N-peptide of caspase-7 must be removed first to generate a ΔN-caspase-7, and then a full maturation occurs by a cut between the large and small subunits (32). Next, we determined whether phosphorylation of caspase-7 by PAK2 can inhibit caspase-7 cleavage by caspase-8. Active caspase-8 was added after an in vitro kinase assay of PAK2 with WT-caspase-7 or mut-caspase-7. The results showed that cleavage of ΔN-WT-caspase-7 and the large subunit of WT-caspase-7 by caspase-8 decreased after phosphorylation by PAK2. In contrast, the cleavage of ΔN-mut-caspase-7 and the large subunit of mut-caspase-7 by caspase-8 did not decrease dramatically (Fig. 4B). Overall, these results indicated that PAK2 can inhibit caspase-7 activity and suppress the cleavage of caspase-7 by caspase-8 in vitro.
FIGURE 4.
PAK2 inhibits caspase-7 activity in vitro. A, His-WT-caspase-7 and His-mut-caspase-7 proteins were expressed as described under “Materials and Methods.” An in vitro kinase assay was performed using PAK2 and WT-caspase-7 (3 μg) or mut-caspase-7 (3 μg) protein. Caspase-7 activity was detected at different time points using the Caspase-3 Colorimetric Assay kit. Data are shown as means ± S.D. from triplicate experiments. The asterisks indicate a significant inhibition (*, p < 0.01; **, p < 0.001). B, PAK2 suppresses the cleavage of caspase-7 by caspase-8. An in vitro kinase assay was performed using PAK2 and WT-caspase-7 or mut-caspase-7 protein. The proteins were then incubated with active caspase-8 for 1 h. The cleavage of caspase-7, ΔN-caspase-7 (caspase-7 with the N-peptide removed), and the large subunit of caspase-7 by caspase-8 was visualized by Western blotting. Data shown are representative of results from triplicate independent experiments.
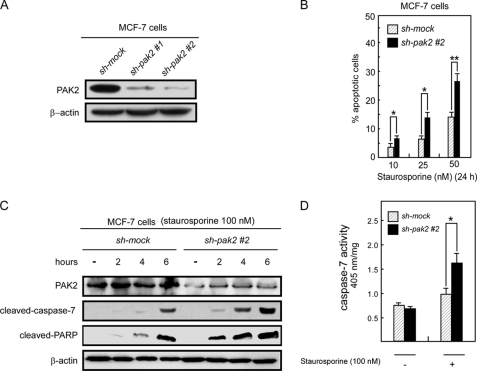
Knockdown of PAK2 Induces Increased Apoptosis in MCF-7 Breast Cancer Cells
Based on our in vitro results, PAK2 can phosphorylate caspase-7 at Ser-30, Thr-173, and Ser-239 and suppress caspase-7 activity. To determine whether PAK2 can inhibit caspase-7 activity in cells, we designed shRNA (1 and 2) against PAK2 and introduced the construct into MCF-7 cells. PAK2 knockdown stable cells were established with puromycin selection. The knockdown efficiency was assessed by Western blotting, and the results indicated that the PAK2 expression level was suppressed in MCF-7 cells expressing sh-pak2-1 or sh-pak2-2 compared with control MCF-7 cells that expressed GFP-shRNA (sh-mock; Fig. 5A). Using these cells, we investigated staurosporine-induced apoptosis. After treatment with staurosporine (50 nm) for 24 h, 27% of MCF-7 sh-pak2-2 cells exhibited apoptosis compared with 14% of control cells (Fig. 5B). MCF-7 sh-pak2-1 cells (supplemental Fig. 1A) exhibited similar effects (supplemental Fig. 1B). Thus, knockdown of PAK2 causes a greater degree of apoptosis in MCF-7 cells. Furthermore, to determine whether knockdown of PAK2 can induce more caspase-7 cleavage by caspase-8 in MCF-7 cells, we assessed the level of cleaved caspase-7 as well as the level of cleaved PARP, which is a downstream target of caspase-7 (33). The Western blotting results showed that knockdown of PAK2 induced more cleaved caspase-7 and cleaved PARP than that seen in control MCF-7 cells (Fig. 5C). Moreover, to investigate whether knockdown of PAK2 can affect caspase-7 activity, MCF-7 sh-pak2 and MCF-7 sh-mock cells were treated with staurosporine and harvested after 4 h, and cell lysates were used to detect caspase-7 activity. Data indicated that staurosporine-induced caspase-7 activity in MCF-7 sh-pak2 cells was much higher than that observed in MCF-7 sh-mock cells (Fig. 5D). Overall, these results indicated that knockdown of PAK2 can enhance caspase-7 activity and induce more apoptosis in MCF-7 breast cancer cells expressing PAK2.
FIGURE 5.
Knockdown of PAK2 increases staurosporine-induced apoptosis of MCF-7 cells. A, MCF-7 sh-pak2-1, sh-pak2-2, and sh-mock cells were generated by stable infection of sh-pak2-1, sh-pak2-2, or sh-mock plasmids into MCF-7 cells. Lysates (40 μg) prepared from MCF-7 sh-pak2-1, sh-pak2-2, and sh-mock cells were used to detect PAK2 by Western blotting. B, staurosporine induced more apoptosis in MCF-7 sh-pak2-2 cells compared with sh-mock cells. MCF-7 sh-pak2-1 had the same effect (supplemental Fig. 1C). Using different concentrations of staurosporine to stimulate MCF-7 sh-pak2-2 and sh-mock cells, apoptosis was determined by flow cytometry. Data are shown as means ± S.D. from triplicate experiments. The asterisks indicate a significant increase in apoptosis (*, p < 0.01; **, p < 0.001) as determined by the Student's t test. C, staurosporine induced more cleaved caspase-7 and cleaved PARP in MCF-7 sh-pak2-2 cells compared with sh-mock cells. MCF-7 sh-pak2-1 had the same effect (supplemental Fig. 1B). Staurosporine (100 nm) was used to simulate MCF-7 sh-pak2-2 and sh-mock cells. Cells were harvested at various time points, and PAK2, cleaved caspase-7, and cleaved PARP were detected by Western blotting using specific antibodies. Data shown are representative of results from triplicate independent experiments. D, caspase-7 activity is higher in MCF-7 sh-pak2-2 cells compared with sh-mock cells. Staurosporine (100 nm) was used to stimulate MCF-7 sh-pak2-2 and sh-mock cells. After 4 h, cells were harvested, and caspase-7 activity was detected as described under “Materials and Methods.” Data are shown as means ± S.D. from triplicate experiments. The asterisk indicates a significant increase in apoptosis (*, p < 0.01) as determined by the Student's t test.
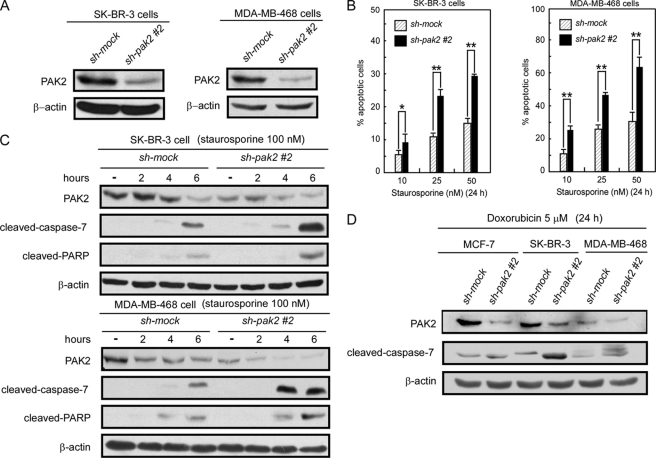
Knockdown of PAK2 Induces Increased Apoptosis in SK-BR-3 and MDA-MB-468 Breast Cancer Cells
Based on previous data, knockdown of PAK2 can restore caspase-7 activity and induce increased apoptosis in MCF-7 cells, which do not express caspase-3. We next determined whether knockdown of PAK2 has a similar effect in SK-BR-3 and MDA-MB-468 breast cancer cells, which express both caspase-3 and caspase-7 proteins. The shRNA (1 or 2) construct was introduced into SK-BR-3 and MDA-MB-468 cells. Knockdown PAK2 stable cells were established with puromycin selection. The knockdown efficiency was assessed by Western blotting, and the results indicated that knockdown of PAK2 suppressed the endogenous PAK2 protein expression level compared with control cells (Fig. 6A). Staurosporine could induce much more apoptosis in sh-pak2 cells compared with control cells (Fig. 6B) and also caused more cleavage of caspase-7 and PARP in sh-pak2 cells compared with control cells (Fig. 6C). In addition to staurosporine, doxorubicin HCl, a chemotherapeutic drug in clinical use for treatment of breast cancer, had similar effects on MCF-7, SK-BR-3, and MDA-MB-468 cells (Fig. 6D and supplemental Fig. 2). These data indicated that knockdown of PAK2 expression can enhance caspase-7 activity and induce more apoptosis in breast cancer cells with or without caspase-3.
FIGURE 6.
Knockdown of PAK2 increases apoptosis in SK-BR-3 and MDA-MB-468 cells. A, SK-BR-3 and MDA-MB-468 sh-pak2 (1 and 2) and sh-mock cells were generated by stable infection with sh-pak2 (1 and 2) or sh-mock plasmids into SK-BR-3 and MDA-MB-468 cells, respectively. Lysates (40 μg) prepared from SK-BR-3 and MDA-MB-468 sh-pak2 and sh-mock cells were used to detect PAK2 expression by Western blotting. B, staurosporine induces more apoptosis in SK-BR-3 and MDA-MB-468 sh-pak2-2 cells compared with sh-mock cells. SK-BR-3 and MDA-MB-468 sh-pak2-2 and sh-mock cells were treated with different concentrations of staurosporine, and apoptosis was determined by flow cytometry. Data are shown as means ± S.D. from triplicate experiments, and the asterisks indicate a significant increase in apoptosis (*, p < 0.01; **, p < 0.001) as determined by the Student's t test. C, staurosporine induces more cleaved caspase-7 and cleaved PARP in SK-BR-3 and MDA-MB-468 sh-pak2-2 cells compared with sh-mock cells. Staurosporine (100 nm) was used to treat SK-BR-3 and MDA-MB-468 sh-pak2-2 and sh-mock cells, and cells were harvested at various time points. PAK2, cleaved caspase-7, and cleaved PARP were detected by Western blotting using specific antibodies. Data shown are representative of results from triplicate independent experiments. D, doxorubicin HCl induces more cleaved caspase-7 and cleaved PARP in MCF-7, SK-BR-3, and MDA-MB-468 sh-pak2-2 cells compared with sh-mock cells. MCF-7, SK-BR-3, and MDA-MB-468 sh-pak2-1 had the same effect (supplemental Fig. 2). Doxorubicin HCl (5 μm) was used to treat MCF-7, SK-BR-3, and MDA-MB-468 sh-pak2-2 and sh-mock cells. Cells were harvested at 24 h, and cleaved caspase-7 and PAK2 were detected by Western blotting using specific antibodies. Data shown are representative of results from triplicate experiments.
DISCUSSION
Caspases, a family of cysteine proteases, are central components of cellular apoptosis (33, 34). Based on their different functions and structures, caspases are classified into two groups. Caspase-1, -2, -8, -9, and -10 belong to the first group, called initiator caspases, which can autocleave, and activate the second group of caspases, referred to as executioner caspases, which include caspase-3, -6, and -7 (34). Accumulating evidence reveals that inhibition of the apoptosis cascades plays an important role in tumor therapy resistance. For example, up-regulation of caspase-8 inhibitors like Flice-like inhibitory protein or inhibition of caspase-8 by Bcl-2 can induce tumor resistance to chemotherapy drugs by decreasing cellular apoptosis (35). Suppressing the activation of caspase-9 downstream can cause chemotherapy resistance in diffuse large B-cell lymphoma (36). Overexpression of the inhibitor of caspase-3 can activate deoxyribonuclease in human renal carcinoma cells, therefore enhancing their resistance to cytotoxic chemotherapy (37). These studies strongly suggest that regulating the activity of caspases might be beneficial in tumor chemotherapy.
Recently, research results suggest that caspases can be regulated by kinases through phosphorylation. Conversely, kinases can be regulated by caspase cleavage, which provides a balance of cell survival and death through cross-talk between the two enzyme families (38). For instance, caspase-8 can be phosphorylated at Tyr-465 by Lyn, which inhibits caspase-8 activity (39). Caspase-3 can be phosphorylated by protein kinase C (PKC) to enhance caspase-3 activity, whereas PKC can be downstream of caspase-3 and activated by caspase-3 cleavage, providing a positive feedback to amplify caspase-3 activity and enhance apoptotic signals (40). However, the modification or regulation of caspase-7, the other executioner caspase, remains unclear. Caspase-7 was long assumed to be redundant with caspase-3 function because of their similar structure and common protein substrates (30). Recent studies indicate that caspase-7 has some distinct functions in apoptosis and inflammation. For example, caspase-7−/− murine embryonic fibroblasts are more resistant to ultraviolet light-induced apoptosis compared with caspase-3−/− murine embryonic fibroblasts. In addition, activation of caspase-7, but not caspase-3, requires caspase-1 complexes during cell inflammation (29). Furthermore, caspase-7 knock-out, but not caspase-3-deficient, mice are also resistant to lethality triggered by intraperitoneal injections of lipopolysaccharide (41). These distinct roles of caspase-7 suggest that regulating caspase-7 activity might be beneficial in preventing cell inflammation and suppressing tumorigenesis.
Here we found that PAK2 phosphorylated caspase-7 (Fig. 2C), resulting in decreased caspase-7 activity (Fig. 4). PAK2 also suppressed the effect of chemotherapeutic drugs on apoptosis of MCF-7, SK-BR-3, and MDA-MB468 breast cancer cells. Moreover, knockdown of PAK2 in MCF-7 cells restored caspase-7 activity and increased apoptosis of MCF-7 cells (Fig. 5). Knockdown of PAK2 expression in SK-BR-3 and MDA-MB468 cells also enhanced the level of cleaved caspase-7 and increased the ability of chemotherapeutic drugs to induce apoptosis. This suggests that highly expressed PAK2 can inhibit cellular apoptosis by phosphorylating caspase-7. PAK2 has both pro- and antiapoptosis functions, making it unique in the PAK family of proteins. Full-length PAK2 can induce cell survival, and caspase-mediated cleaved PAK2p34 can induce apoptosis. A recent study indicates that full-length PAK2 can negatively regulate caspase-3-activated proteolytic PAK2p34, activation of caspase-3, and apoptotic histone H2B phosphorylation (42). This suggests that full-length PAK2 can negatively regulate proteolytic PAK2p34 to promote cell proliferation.
Accumulating evidence indicates that PAK2 is among a panel of “invasiveness-associated” genes that correspond with cancer proliferation and survival (43). Our data revealed that PAK2 is highly expressed in breast cancer cell lines and breast invasive ductal carcinoma tissue (Fig. 1). These results suggest that highly expressed PAK2 might promote breast cancer progression and restrain the cell death response induced by chemotherapeutic drugs. In conclusion, our results suggest a mechanism by which breast chemotherapy resistance might be associated with a high expression of functional PAK2, which results in decreased cellular apoptosis mediated by low caspase-7 activity. Overall, our data suggest that PAK2 might be a novel target for breast cancer chemotherapy.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA077646, CA120388, CAR37CA081064, and ES016548. This work was also supported by The Hormel Foundation and Minnesota Partnership for Biotechnology and Medical Genomics Grant L9046001101.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- PAK
- p21-activated kinase
- PARP
- poly(ADP-ribose) polymerase
- mut
- mutant.
REFERENCES
- 1. (2003) Cent. Eur. J. Public Health 11, 177–179 [PubMed] [Google Scholar]
- 2. Puglisi F., Andreetta C., Fasola G. (2006) Expert Opin. Pharmacother. 7, 2309–2318 [DOI] [PubMed] [Google Scholar]
- 3. Haagenson K. K., Wu G. S. (2010) Cancer Metastasis Rev. 29, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowsett M., Johnston S., Martin L. A., Salter J., Hills M., Detre S., Gutierrez M. C., Mohsin S. K., Shou J., Allred D. C., Schiff R., Osborne C. K., Smith I. (2005) Endocr. Relat. Cancer 12, Suppl. 1, S113–S117 [DOI] [PubMed] [Google Scholar]
- 5. Arpino G., Green S. J., Allred D. C., Lew D., Martino S., Osborne C. K., Elledge R. M. (2004) Clin. Cancer Res. 10, 5670–5676 [DOI] [PubMed] [Google Scholar]
- 6. Gee J. M., Robertson J. F., Gutteridge E., Ellis I. O., Pinder S. E., Rubini M., Nicholson R. I. (2005) Endocr. Relat. Cancer 12, Suppl. 1, S99–S111 [DOI] [PubMed] [Google Scholar]
- 7. Kirkegaard T., Witton C. J., McGlynn L. M., Tovey S. M., Dunne B., Lyon A., Bartlett J. M. (2005) J. Pathol. 207, 139–146 [DOI] [PubMed] [Google Scholar]
- 8. Gutierrez M. C., Detre S., Johnston S., Mohsin S. K., Shou J., Allred D. C., Schiff R., Osborne C. K., Dowsett M. (2005) J. Clin. Oncol. 23, 2469–2476 [DOI] [PubMed] [Google Scholar]
- 9. Holm C., Rayala S., Jirström K., Stål O., Kumar R., Landberg G. (2006) J. Natl. Cancer Inst. 98, 671–680 [DOI] [PubMed] [Google Scholar]
- 10. Riggins R. B., Schrecengost R. S., Guerrero M. S., Bouton A. H. (2007) Cancer Lett. 256, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. (1994) Nature 367, 40–46 [DOI] [PubMed] [Google Scholar]
- 12. Molli P. R., Li D. Q., Murray B. W., Rayala S. K., Kumar R. (2009) Oncogene 28, 2545–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hofmann C., Shepelev M., Chernoff J. (2004) J. Cell Sci. 117, 4343–4354 [DOI] [PubMed] [Google Scholar]
- 14. Schürmann A., Mooney A. F., Sanders L. C., Sells M. A., Wang H. G., Reed J. C., Bokoch G. M. (2000) Mol. Cell. Biol. 20, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nheu T., He H., Hirokawa Y., Walker F., Wood J., Maruta H. (2004) Cell Cycle 3, 71–74 [PubMed] [Google Scholar]
- 16. Rayala S. K., Talukder A. H., Balasenthil S., Tharakan R., Barnes C. J., Wang R. A., Aldaz C. M., Khan S., Kumar R. (2006) Cancer Res. 66, 1694–1701 [DOI] [PubMed] [Google Scholar]
- 17. Roig J., Traugh J. A. (1999) J. Biol. Chem. 274, 31119–31122 [DOI] [PubMed] [Google Scholar]
- 18. Walter B. N., Huang Z., Jakobi R., Tuazon P. T., Alnemri E. S., Litwack G., Traugh J. A. (1998) J. Biol. Chem. 273, 28733–28739 [DOI] [PubMed] [Google Scholar]
- 19. Jakobi R., Moertl E., Koeppel M. A. (2001) J. Biol. Chem. 276, 16624–16634 [DOI] [PubMed] [Google Scholar]
- 20. Fischer U., Stroh C., Schulze-Osthoff K. (2006) Oncogene 25, 152–159 [DOI] [PubMed] [Google Scholar]
- 21. Jakobi R., McCarthy C. C., Koeppel M. A., Stringer D. K. (2003) J. Biol. Chem. 278, 38675–38685 [DOI] [PubMed] [Google Scholar]
- 22. Ling J., Morley S. J., Traugh J. A. (2005) EMBO J. 24, 4094–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar R., Gururaj A. E., Barnes C. J. (2006) Nat. Rev. 6, 459–471 [DOI] [PubMed] [Google Scholar]
- 24. Li T., Zhang J., Zhu F., Wen W., Zykova T., Li X., Liu K., Peng C., Ma W., Shi G., Dong Z., Bode A. M., Dong Z. (2011) Carcinogenesis 32, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu Y. H., Johnson D. A., Traugh J. A. (2008) J. Biol. Chem. 283, 36397–36405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuazon P. T., Lorenson M. Y., Walker A. M., Traugh J. A. (2002) FEBS Lett. 515, 84–88 [DOI] [PubMed] [Google Scholar]
- 27. Jakobi R., Huang Z., Walter B. N., Tuazon P. T., Traugh J. A. (2000) Eur. J. Biochem. 267, 4414–4421 [DOI] [PubMed] [Google Scholar]
- 28. Blom N., Gammeltoft S., Brunak S. (1999) J. Mol. Biol. 294, 1351–1362 [DOI] [PubMed] [Google Scholar]
- 29. Lakhani S. A., Masud A., Kuida K., Porter G. A., Jr., Booth C. J., Mehal W. Z., Inayat I., Flavell R. A. (2006) Science 311, 847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamkanfi M., Kanneganti T. D. (2010) Int. J. Biochem. Cell Biol. 42, 21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cardone M. H., Salvesen G. S., Widmann C., Johnson G., Frisch S. M. (1997) Cell 90, 315–323 [DOI] [PubMed] [Google Scholar]
- 32. Yang X., Stennicke H. R., Wang B., Green D. R., Jänicke R. U., Srinivasan A., Seth P., Salvesen G. S., Froelich C. J. (1998) J. Biol. Chem. 273, 34278–34283 [DOI] [PubMed] [Google Scholar]
- 33. Kuribayashi K., Mayes P. A., El-Deiry W. S. (2006) Cancer Biol. Ther. 5, 763–765 [DOI] [PubMed] [Google Scholar]
- 34. Hajra K. M., Liu J. R. (2004) Apoptosis 9, 691–704 [DOI] [PubMed] [Google Scholar]
- 35. Kim P. K., Mahidhara R., Seol D. W. (2001) Drug Resist. Updat. 4, 293–296 [DOI] [PubMed] [Google Scholar]
- 36. Cillessen S. A., Hess C. J., Hooijberg E., Castricum K. C., Kortman P., Denkers F., Vos W., van de Wiel M. A., Schuurhuis G. J., Ossenkoppele G. J., Meijer C. J., Oudejans J. J. (2007) Clin. Cancer Res. 13, 7012–7021 [DOI] [PubMed] [Google Scholar]
- 37. Hara S., Miyake H., Arakawa S., Kamidono S., Hara I. (2001) J. Urol. 166, 2491–2494 [PubMed] [Google Scholar]
- 38. Kurokawa M., Kornbluth S. (2009) Cell 138, 838–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jia S. H., Parodo J., Kapus A., Rotstein O. D., Marshall J. C. (2008) J. Biol. Chem. 283, 5402–5413 [DOI] [PubMed] [Google Scholar]
- 40. Voss O. H., Kim S., Wewers M. D., Doseff A. I. (2005) J. Biol. Chem. 280, 17371–17379 [DOI] [PubMed] [Google Scholar]
- 41. Lamkanfi M., Moreira L. O., Makena P., Spierings D. C., Boyd K., Murray P. J., Green D. R., Kanneganti T. D. (2009) Blood 113, 2742–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marlin J. W., Eaton A., Montano G. T., Chang Y. W., Jakobi R. (2009) Neoplasia 11, 286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu R., Wang X., Chen G. Y., Dalerba P., Gurney A., Hoey T., Sherlock G., Lewicki J., Shedden K., Clarke M. F. (2007) N. Engl. J. Med. 356, 217–226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.