Abstract
DNA damage response is crucial for maintaining genomic integrity and preventing cancer by coordinating the activation of checkpoints and the repair of damaged DNA. Central to DNA damage response are the two checkpoint kinases ATM and ATR that phosphorylate a wide range of substrates. RING finger and WD repeat domain 3 (RFWD3) was initially identified as a substrate of ATM/ATR from a proteomic screen. Subsequent studies showed that RFWD3 is an E3 ubiquitin ligase that ubiquitinates p53 in vitro and positively regulates p53 levels in response to DNA damage. We report here that RFWD3 associates with replication protein A (RPA), a single-stranded DNA-binding protein that plays essential roles in DNA replication, recombination, and repair. Binding of RPA to single-stranded DNA (ssDNA), which is generated by DNA damage and repair, is essential for the recruitment of DNA repair factors to damaged sites and the activation of checkpoint signaling. We show that RFWD3 is physically associated with RPA and rapidly localizes to sites of DNA damage in a RPA-dependent manner. In vitro experiments suggest that the C terminus of RFWD3, which encompass the coiled-coil domain and the WD40 domain, is necessary for binding to RPA. Furthermore, DNA damage-induced phosphorylation of RPA and RFWD3 is dependent upon each other. Consequently, loss of RFWD3 results in the persistent foci of DNA damage marker γH2AX and the repair protein Rad51 in damaged cells. These findings suggest that RFWD3 is recruited to sites of DNA damage and facilitates RPA-mediated DNA damage signaling and repair.
Keywords: Checkpoint Control, DNA Damage, DNA Repair, E3 Ubiquitin Ligase, Protein Phosphorylation
Introduction
The cellular response to genotoxic stress initiates multiple signal transduction pathways that include transcription regulation, cell cycle arrest, DNA damage repair, and apoptosis (1, 2). Central to DDR2 are two checkpoint kinases ATM and ATR that phosphorylate many downstream effectors to execute these functions (3, 4). Identification of key players and the characterization of their molecular functions enable a more thorough understanding of the DDR pathways, which are critical for maintaining genomic integrity. Several recent proteomic screens have drastically expanded the landscape of DDR pathways with the identification of hundreds of putative ATM/ATR substrates (5–7).
Recently, we investigated the role of a previously uncharacterized protein, RING finger and WD repeat domain 3 (RFWD3, also known as RNF201 and FLJ10520; GenBankTM number 55159) in DDR (5, 8). Originally identified from a proteomic screen for ATM/ATR substrates, RFWD3 was found phosphorylated at several conserved SQ sites in cells that were treated with ionizing radiation (IR) and replication blocking agents. Subsequent biochemical analysis revealed that RFWD3 can form a complex with MDM2 and p53 and is required for the maintenance of high levels of p53 upon DNA damage induction. The RFWD3 protein contains several well characterized functional domains as well as domains of unknown functions. The N-terminal RING finger domain is a conserved E3 ubiquitin (Ub) ligase domain. In vitro reactions demonstrate that RFWD3 exhibits robust E3 Ub ligase activity toward p53. In the presence of MDM2, a well characterized p53 E3 Ub ligase, RFWD3, appears to restrict MDM2 polyubiquitination activity, shifting the ubiquitination products to shorter ubiquitin chains, thus stabilizing p53. RFWD3 also contains two protein-protein interaction modules at its C terminus, namely a coiled-coil domain and three WD40 repeats. How RFWD3 participates in DDR, particularly in response to DNA replication arrest through these functional domains, is not clear.
The replication protein A complex (RPA) has emerged as a central player in DDR (9). RPA is a heterotrimeric complex (70-kDa RPA1, 32-kDa RPA2, and 14-kDa RPA3) that is involved in many aspects of DNA metabolism in unstressed cells as well as in cells exposed to replication block and DNA-damaging agents (9, 10). RPA is involved in several steps of DNA replication, including origin recognition, initiation, and elongation. It also plays an important role in the early stages of DNA damage signaling cascade and is directly involved in DNA repair (11, 12). Binding of RPA to single-stranded regions of DNA is critical for the recruitment of two independent checkpoint complexes, ATR/ATRIP and Rad17-RFC2–5/Rad1/Hus1/Rad9, to sites of DNA damage, where checkpoint activation leads to the phosphorylation of Chk1 (11, 13, 14). RPA is also shown to be directly involved in homology-directed repair. RPA interacts with homologous recombination (HR) repair protein Rad51, Rad52, and BRCA2 (15–23). Depletion of RPA by siRNA knockdown impairs the recruitment of Rad51 to sites of DNA repair and increases sensitivity to DNA damaging agents (18, 24). RPA function is regulated by phosphorylation both in a cell cycle-dependent manner and in response to genotoxic stress. The phosphorylation of the 32-kDa subunit of RPA2 is well characterized in these processes. At least 10 phosphorylation sites (Ser-4, Ser-8, Ser-10, Ser-11, Ser-12, Thr-21, Ser-23, Ser-29, Ser-33, and Thr-98) and 4 kinases (ATM, ATR, DNA-PKcs, Cdk1, and Cdk2) have been suggested (25–32). It is shown that DNA damage-induced RPA hyperphosphorylation is critical for Rad51 recruitment and HR-mediated repair after replication block but is not essential for IR and I-Sce-I endonuclease-stimulated HR (28). Moreover, recent studies suggest that RPA dephosphorylation is also essential for Rad51-mediated HR and for cells to reenter the cell cycle during recovery from replication block (24, 33), further highlighting the importance of regulating RPA2 phosphorylation in a coordinated manner. The RPA-mediated HR repair is also regulated by SUMOylation (34). The 70-kDa RPA1 associates with a Sentrin/SUMO-specific protease, SENP6, and is maintained in a hypoSUMOylated state during S-phase. Upon treatment with Camptothecin, an inducer of replication stress, RPA1 is modified by SUMO2/3, and this modification facilitates the recruitment of Rad51 to the damage foci to initiate DNA repair. Importantly, RPA was found as a binding partner of the annealing helicase SMARCAL1 (or HARP), a member of the SNF2 family that is mutated in Schimke immunoosseous dysplasia (35–39). SMARCAL1 is recruited to sites of DNA damage in a RPA-dependent manner and is required for the stabilization and restart of the stalled replication fork. These new findings provide further evidence that RPA-mediated DNA repair plays a crucial role in genome maintenance.
The promyelocytic leukemia protein nuclear bodies (PML NBs) are proteinaceous structures that are involved in a wide range of cellular functions including DDR. Many DDR and repair proteins including p53, MDM2, ATR, RPA, and BLM are found in PML-NBs and more frequently in cells that employ alternative telomere lengthening (40). Important checkpoint proteins such as ATR and RPA that reside in PML in undamaged cells rapidly translocate to punctuate abundant nuclear foci, which represent sites of DNA damage and repair in cells that are treated with IR (41). As both RPA and ATR function upstream in the DDR, defects in this localization are often associated with impaired checkpoint and DNA repair (4).
Here we report the characterization of RFWD3 in DNA damage checkpoint and repair. RFWD3 partially colocalizes to PML NBs during S/G2 phase and translocates to sites of DNA damage in a RPA-dependent manner. Biochemical analysis demonstrates that RFWD3 physically interacts with RPA, and the loss of RFWD3 results in attenuated RPA2 phosphorylation. Consequently, the loss of RFWD3 delays Rad51 focus formation in response to replication block and correlates with persistence of Rad51 and γH2AX foci during recovery from replication block, indicating defective DNA repair.
EXPERIMENTAL PROCEDURES
Cell Culture
HeLa and 293T cells were maintained in DMEM, and U2OS cells were maintained in McCoy's 5A media, all supplemented with 10% fetal bovine serum (FBS). Cell synchronization was performed by the double thymidine block method.
Expression Vectors, Recombinant Proteins, and Antibodies
The DNA sequence corresponding to the full-length protein of human RFWD3 was cloned as described in Fu et al. (8). The FLAG-RFWD3 domain deletion mutants were generated by subcloning RFWD3 into pSG5 vector (Stratagene). SFB-RPA1- and MBP-tagged RPA1, RPA2, and RPA3 construction were generous gifts from Dr. Junjie Chen (MD Anderson Cancer Center). DNA transfections were performed using TransIT-LT1 reagent (Mirus). GST fusion proteins were purified using the glutathione-Sepharose 4B (GE Healthcare) according to the manufacturer's protocols. Antibodies used are as follows: anti-RFWD3 (BL2378, Bethyl Laboratories) or Ab1 (generated and affinity-purified in-house against a recombinant fragment of amino acids 135–286 of human RFWD3), RPA1 (Ab-1, Calbiochem), RPA2 (Ab-2, Calbiochem), RPA3 (Ab-2, Calbiochem), RPA2-Ser(P)-4/8 (Bethyl Laboratories), RPA2-Thr(P)-21 (Abcam), RPA2-pS33 (Bethyl Laboratories), CHK1-pS317 (Bethyl Laboratories), γH2AX (Millipore), Rad51 (14B4, Abcam), γ-tubulin (Sigma), and FLAG (M2, Sigma).
Immunoprecipitation, Mass Spectrometry, and in Vitro Binding Assays
HeLa nuclear extracts were prepared from cycling or hydroxyurea (HU) (1 mm, 16 h)-treated cells. Immunoprecipitation and mass spectrometry analysis were carried out as described in Malovannaya et al. (42). Recombinant GST-RFWD3 (full-length or domain-deletion mutants) was incubated with SFB (S protein, FLAG, and streptavidin-binding peptide) triple-tagged-RPA1 bound to FLAG-M2 beads at 4 °C for 1 h in NETN buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40). After washing three times with NETN, bound protein was eluted by Laemmli buffer. MBP-tagged RPA1–3 were incubated with GST-RFWD3 on beads and analyzed in the same manner.
RNA Interference
siRNAs were purchased from Dharmacon or Invitrogen. The siRNA sequences for RFWD3 and RPA2 were described in Fu et al. (8) and Wu et al. (43), respectively. siRNA transfections were carried out using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocols.
Immunofluorescence
Cells grown on poly-d-lysine-coated coverslips were washed with PBS followed by pre-extraction for 2 min in pre-extraction buffer (25 mm HEPES, 7.4, 50 mm NaCl, 1 mm EDTA, 3 mm MgCl2, 300 mm sucrose, and 0.1% Triton X-100). Cells were fixed with 4% formaldehyde (w/v) in PBS for 20 min. After three washes with PBST (PBS with 0.1% Tween 20), cells were blocked for 1 h in 5% BSA in PBST. Cells were incubated with primary antibody in 2% BSA, PBST overnight and then with Alexa 488- or 594-conjugated secondary antibodies in 2% BSA, PBST for 30 min and counterstained with 4′, 6′-diamino-2-phenylindole (DAPI).
Laser Microirradiation
GFP-RFWD3-expressing cells were cultured in CO2-independent medium (Invitrogen) with 10% FBS and were maintained at 37 °C in a temperature-controlled container in glass-bottom dishes (MatTek). Laser microirradiation was carried out with a pulsed nitrogen laser (Micropoint Ablation laser system; Photonic Instruments Inc.) coupled to a Zeiss Axiovert200M microscope and focused through a Plan-Apochromat 63×/NA 1.40 oil immersion objective (44). The output laser power was set at 75% of maximum, which creates double-stranded breaks (DSBs) (as confirmed by γH2AX staining) and induces accumulation of EGFP-tagged RFWD3 in living cells. Time-lapse images were acquired through an AxioCam HRm CCD camera.
RESULTS
RFWD3 Localizes to Sites of DNA Damage
Previously we have shown that RFWD3 is phosphorylated by ATR/ATM in response to DNA damage, suggesting that RFWD3 is a component of DDR. To further explore the function of RFWD3 in DDR, we used a pair of U2OS-derived cell lines that stably express GFP-tagged wild-type RFWD3 or an E3-inactive RFWD3 (C315A) to monitor GFP-RFWD3 cellular localization when these cells were exposed to laser microirradiations. In untreated cells, GFP-RFWD3 localizes to both nuclear and cytoplasmic compartments; upon laser microirradiation, GFP-RFWD3 rapidly accumulates to laser tracks and overlaps with DsRed-PCNA, a known component of DNA repair complex (Fig. 1A). Indirect immunofluorescence with phosphorylated H2AX (γ-H2AX), a marker for DSB sites, confirmed that RFWD3 indeed accumulates at sites of DNA damage (Fig. 1A). Time-lapse experiments suggest that GFP-RFWD3 accumulates to laser tracks within 3 min, and the signal peaks around 10 min and gradually decreases (Fig. 1B). Similar accumulation to laser tracks was also observed for an E3 ligase-inactive mutant (GFP-RFWD3-CA), suggesting that RFWD3 E3 Ub ligase activity is not required for the translocation (supplemental Fig. 1A).
FIGURE 1.
RFWD3 translocates to sites of DNA damage and colocalizes with RPA. A, U2OS cells stably expressing GFP-RFWD3 were transfected with DsRed-PCNA and treated with laser microirradiation to induce DNA-damage tracts. After 30 min, cells were fixed and immunostained with γH2AX antibodies. B, GFP-RFWD3 expressing cells were irradiated as in A and monitored with live imaging microscopy. C, U2OS cells were treated (IR+) or untreated (IR−) with 10 Gy of IR. After 6 h, cells were pre-extracted with 0.1% Triton X-100, fixed and immunostained with RFWD3 and RPA antibodies. D, U2OS cells transfected with indicated siRNAs were treated with 10 Gy of IR and immunostained with indicated antibodies.
Indirect immunofluorescence also revealed that in undamaged cells, a population of GFP-RFWD3 accumulates to a small number (2–20) of large, distinct nuclear bodies (Fig. 1C and supplemental Fig. S1B) that resemble the PML nuclear bodies. To confirm that the foci are indeed PML NBs, we co-stained GFP-RFWD3 with endogenous PML. Because RFWD3 levels appear to be elevated in the S-G2 phase (supplemental Fig. 1D), we enriched S-phase population by blocking cell cycle progression to G1/S transition with thymidine and examined RFWD3 localization at 4 h after cells were released to fresh media. To better observe the nuclear localization, we briefly pretreated the cells with detergent to extract the soluble proteins. As shown in supplemental Fig. 1B, the bright RFWD3 foci that were found in ∼20% of the cells colocalize with small fractions of PML NBs but not with γH2AX (supplemental Fig. 1C). Similar colocalization was also observed with co-transfected FLAG-tagged RFWD3 and Xpress-tagged PML in U2OS cells (supplemental Fig. 1F).
The PML nuclear bodies have been proposed as a scaffold structure for many DDR and repair proteins (43). Upon DNA damage, these proteins such as ATR and RPA undergo dynamic translocation from PML NBs to nuclear foci that represent sites of DNA damage and repair. To determine whether RFWD3 also form DNA damage-induced foci, we treated U2OS cells with DNA-damaging agents that induce DSBs (e.g. IR and doxorubicin) or replication block (hydroxyurea) and monitored the subcellular localization of endogenous RFWD3 by indirect immunofluorescence. We co-stained RFWD3 with an antibody against the 34-kDa subunit of RPA2, as it is known to translocate from PML NBs to sites of DNA damage and regulates the recruitment of many repair proteins to these sites. As shown in Fig. 1C, RFWD3 partially colocalizes with RPA during S-phase progression in undamaged cells. In cells that are exposed to IR, RFWD3 are found in abundant nuclear foci that differ in size and number from those in undamaged cells, and these foci colocalize with RPA2 foci (Fig. 1C). In contrast, PML foci generally do not colocalize with damage-induced RFWD3 foci (supplemental Fig. 1B). This is consistent with the notion that PML NBs serve as storage sites for repair factors, which are released upon DNA damage induction (45). Importantly, knockdown of RPA2 by siRNA abolished damage-induced RFWD3 focus formation, suggesting that RFWD3 localization to sites of DNA damage depends on RPA (Fig. 1D and supplemental Fig. 1G). In contrast, depleting RFWD3 by RNAi does not appreciably affect the RPA foci (Fig. 1D), indicating that RFWD3 is a downstream effector that is recruited to damage sites in a RPA-dependent manner. Similar results were also obtained in cells treated with HU that cause stalled replication forks (see below). Together these results strongly suggest that RFWD3 acts downstream of RPA in DNA damage signaling and repair.
RFWD3 Physically Interacts with RPA
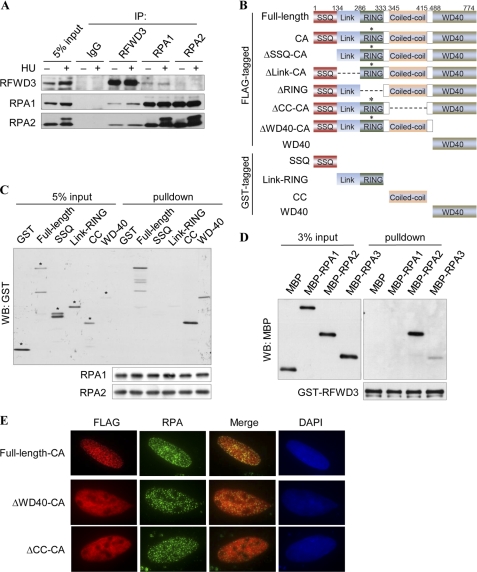
We are particularly intrigued with the colocalization of RFWD3 and RPA, as RFWD3 is phosphorylated in response to DNA damage, and RPA is a key regulator of DNA checkpoint signaling and repair. We next determined whether RFWD3 physically interacts with RPA. We isolated endogenous RFWD3 complex from cycling and HU-treated HeLa cells and identified associated proteins by mass spectrometry (MS). All three subunits of RPA are among the RFWD3-associated proteins identified by MS (supplemental Fig. 2A). Furthermore, immunoprecipitation followed by Western blotting demonstrates that RFWD3 can also be reciprocally immunoprecipitated by RPA1, and the RPA-RFWD3 association is independent of DNA damage (Fig. 2A).
FIGURE 2.
RFWD3 interacts with RPA in vivo and in vitro. A, co-immunoprecipitation of endogenous RFWD3 and RPA was performed with nuclear extracts prepared from untreated or HU-treated HeLa cell. B, domain structure and schematics of the RFWD3 fragments are shown. C315A mutation in the RING domain abolished E3 activity. C, recombinant GST-RFWD3 (full-length or domain-deletion mutants) were incubated with SFB-RPA bound to the FLAG M2 beads. Bound proteins were eluted with Laemmli buffer and immunoblotted (WB) with the indicated antibodies. Asterisks (*) denote the intended bands. D, recombinant GST-RFWD3 bound to Glutathione-Sepharose beads was incubated with MBP-tagged RPA1, RPA2, or RPA3. Bound proteins were immunoblotted with the MBP antibody. E, U2OS cells were transfected with plasmids encoding FLAG-tagged RFWD3 or its deletion mutants and treated with HU. Cells were fixed and immunostained with FLAG and RPA antibodies.
The key functional domains of RFWD3 are the RING finger domain that possesses E3 ubiquitin ligase activity, the coiled-coil domain, and the C-terminal domain that contains three WD-40 repeats, the latter two of which are putative protein-protein interaction modules (Fig. 2B). In addition, sequence inspection reveals a cluster of SQ sites in the N terminus that is composed of three near perfect repeats of 17 amino acid residues (SSQ domain and see supplemental Fig. 2B). To map the domains of RFWD3 that mediate its interaction with RPA, we generated recombinant proteins of a series of RFWD3 domains fused with GST (Fig. 2B) and performed in vitro pulldown assays using an SFB triple tagged-RPA1 bound to FLAG-M2 beads. Domain mapping experiments show that RPA does not bind the N-terminal SSQ domain or the region encompassing the RING domain but binds strongly to the coiled-coil domain and the C-terminal WD40-containing domain (Fig. 2C). To test whether RFWD3 can directly interact with RPA, we incubated GST-RFWD3 with MBP-tagged RPA1, RPA2, or RPA3 and found that RFWD3 bound on beads is able to pull down RPA2 (Fig. 2D).
Next we test which domains are necessary for HU-induced RFWD3 focus formation using a series of FLAG-tagged RFWD3 internal deletion mutants (Fig. 2B). Because RFWD3 has strong autoubiquitination activity that leads to its own degradation, we made a cysteine 315 to alanine (C315A) mutation on all fragments that contain the RING domain to ensure similar protein expression levels. We reasoned that because the full-length RFWD3-CA mutant is able to correctly localize to DNA damage sites, the increased stability as a result of the loss of E3 activity should provide a better means for visualizing RFWD3 foci. We transfected FLAG-tagged RFWD3 deletion mutants to U2OS cells and co-stained FLAG with endogenous RPA2 after cells were treated with HU (Fig. 2E). Deletion of either SSQ, Link, or RING domain has no effect on RFWD3 focus formation and its colocalization with RPA (supplemental Fig. 2C); however, deletion of the coiled-coil domain or the C terminus that contains the WD-40 repeats abolishes RFWD3 focus formation (Fig. 2E), and WD-40 alone is able to localize to RPA foci (supplemental Fig. 2C), suggesting that binding to RPA via the C terminus of RFWD3 is necessary for RFWD3 localization to sites of DNA damage. Together these results suggest that RFWD3 functions during normal S-phase progression and translocates to sites of DNA damage.
RPA and RFWD3 Are Required for the Optimal Phosphorylation of Each Other
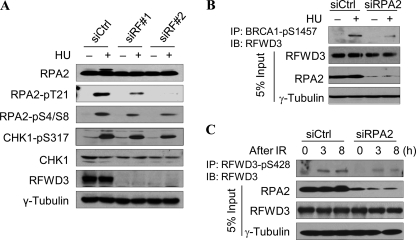
RPA is the major ssDNA-binding protein in eukaryotic cells and plays essential roles in many aspects of DNA metabolism, including replication, recombination, and repair. Binding of RPA to the ssDNA region not only protects the DNA from nuclease degradation but is also critical for ATR-mediated checkpoint activation (9). Although the N terminus of RPA2 is extensively phosphorylated in a cell cycle-dependent manner, RPA2 is hyperphosphorylated upon genotoxic stress, particularly in response to DNA replication block. This hyperphosphorylation leads to preferential interaction with DSB repair factors Rad51 and Rad52 (46) and is necessary for the recruitment of repair factors at sites of DNA damage (28). To investigate the role of RFWD3 in this response, we knocked down RFWD3 and examined the phosphorylation of checkpoint kinase CHK1 and RPA2. Cell cycle analysis shows that knockdown RFWD3 does not significantly alter cell cycle distribution (supplemental Fig. 1E). Consistent with previous reports, RPA2 is hyperphosphorylated upon HU treatment in control knockdown cells, as the slow-migrating RPA2 band in HU-treated cells is apparent (Fig. 3A). However, the hyperphosphorylated RPA2 band is absent in RFWD3 knockdown cells, indicating that RPA hyperphosphorylation is defective. In contrast, phosphorylation of CHK1 in RFWD3 knockdown cells remains largely unchanged from control knockdown cells, suggesting that RFWD3 is not essential for checkpoint activation (Fig. 3A, supplemental Fig. 3, A and B). Previous work has suggested at least nine potential phosphorylation sites within the N terminus of RPA2. To pinpoint which phosphorylation event is dependent on RFWD3, we next examined RPA2 phosphorylation by Western blotting using commercially available phospho-specific RPA2 antibodies (Ser-33, Ser-4/8, and Thr-21). As shown in Fig. 3A, phosphorylation at Thr-21 of RPA2 exhibits a marked decrease in RFWD3 knockdown cells, whereas phosphorylation at Ser-4/8 and Ser-33 are moderately defective (Fig. 3A, supplemental Fig. 3, A and B). Noticeably, the Thr-21 residue is a consensus site for phosphatidylinositol 3-kinase-like kinase family members and is shown to be phosphorylated in vitro by ATM and DNA-PKcs and in an ATM-dependent manner in response to IR (47, 48).
FIGURE 3.
RFWD3 and RPA are required for optimal checkpoint activation after replication block. A, HeLa cells were transfected with 2 different RFWD3 siRNA sequences, then untreated or treated with 2 mm HU for 2 h before being analyzed for the effect on RPA and CHK1 phosphorylation. B, HeLa cells were transfected with RPA2 siRNA and treated or untreated with HU. Phosphorylation of RFWD3 was immunoprecipitated (IP) with BRCA1-phospho-Ser-1457 antibody and detected by Western blotting (IB) with an RFWD3 antibody. C, HeLa cells were transfected with RPA2 siRNA and untreated or treated with 10 Gy of IR. Phosphorylated RFWD3 was immunoprecipitated with anti-RFWD3-phospho-S428 antibody and detected by Western blotting with an RFWD3 antibody.
RFWD3 is phosphorylated by both ATM and ATR kinases (5, 6, 8). Whereas ATM phosphorylates RFWD3 at early times upon DNA damage, ATR is the major kinase that phosphorylates RFWD3 at later times (8). To determine whether RPA regulates RFWD3 phosphorylation, we knocked down RPA2 by siRNA transfection and examined RFWD3 phosphorylation by immunoprecipitation with anti-BRCA1-phospho-Ser-1457, which also recognizes phosphorylated RFWD3 (5), and with anti-RFWD3 Ser(P)-428-specific antibody. Western blotting with an RFWD3 antibody shows that loss of RPA2 results in a reduction of RFWD3 phosphorylation in IR- and HU-treated HeLa cells (Fig. 3, B and C, and supplemental Fig. 3C), suggesting that phosphorylation of RFWD3 at the sites of DNA damage is regulated in part by RPA, presumably through the activation of PI-3 kinases.
Loss of RFWD3 Leads to Delayed Recruitment of Rad51, Persistent Rad51, and γH2AX Foci and Increased DNA Damage Sensitivity
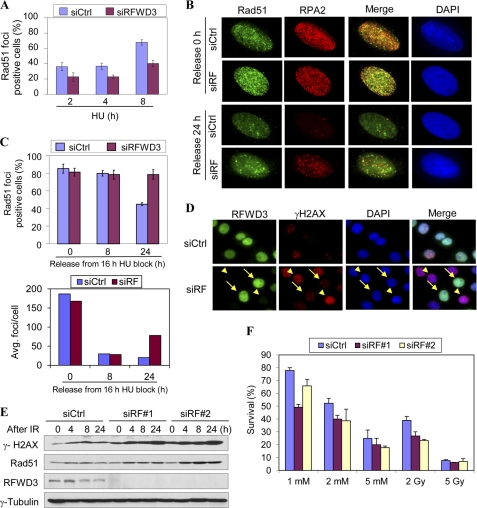
A consequence of the accumulation of ssDNA at stalled replication forks is the generation of DSBs, the most detrimental form of DNA damage that, if left unrepaired, leads to genomic instability. A key enzyme in HR is Rad51, which forms a presynaptic complex on ssDNA and mediates strand exchange reactions (17, 19, 20). Importantly, it was shown that both Rad51 and Rad52 preferentially associate with hyperphosphorylated RPA2 (46). Therefore, we tested the hypothesis that defective RPA phosphorylation in RFWD3 knockdown cells may affect HU-induced recruitment of Rad51 to DNA damage foci. Consistent with this notion, we found that attenuated RPA2 phosphorylation in RFWD3-depleted cells correlates with delayed formation of HU-induced Rad51 foci compared with that in control knockdown cells (Fig. 4A). However, a comparable number of Rad51 foci-containing cells are found after prolonged exposure to HU in both control and RFWD3 knockdown cells (Fig. 4, B and C). This suggests that loss of RFWD3 delays but not completely abrogates Rad51 recruitment. Next, we treated the cells with HU for 16 h, then removed the HU and monitored the dispersal of Rad51 foci as an indication of completion of DNA repair. We found that the number of Rad51 foci-containing cells decreased to ∼50% in the control knockdown cells 24 h after HU removal. In contrast, the number of Rad51 foci-positive cells in RFWD3-depleted cells remained essentially the same after HU removal (Fig. 4, B and C). Furthermore, the average number of Rad51 foci in RFWD3-depleted cells is more than two-fold higher than that in control knockdown cells (Fig. 4C), indicating the accumulation of unrepaired DNA.
FIGURE 4.
Loss of RFWD3 leads to delayed recruitment of Rad51, persistent Rad51, and γH2AX foci and increased DNA damage sensitivity. A, U2OS cells transfected with RFWD3 or control siRNA were treated with 2 mm HU and fixed at the indicated time to perform Rad51 immunostaining. More than 200 cells were counted at each time point. Cell with more than 10 bright nuclear foci were counted as Rad51 foci-positive cells. B and C, U2OS cells transfected with RFWD3 or control siRNA were treated with 1 mm HU for 16 h before the HU was removed. Cells were allowed to recover for indicated times and fixed for Rad51 immunostaining. Representative images are shown in B, and the statistical analysis is shown in C. The average number of Rad51 foci/cell was calculated among foci-positive cells. D, HeLa cells transfected with RFWD3 or control siRNA were treated with 10 Gy of IR and were allowed to recover for up to 24 h. The γH2AX as a marker for DSB was analyzed by immunostaining at 24 h. The arrows in siRFWD3-transfected cells point to cells that still express RFWD3, whereas arrowheads point to cells that have lost RFWD3 expression. E, cells were transfected and treated as in D but were analyzed by Western blotting. F, DNA damage sensitivity was measured by the colony formation assay in HeLa cells transfected with the indicated siRNAs. Two hundred cells were plated in triplicate and treated with 1 mm HU for 24 h or 2 Gy IR. Colonies were counted after recovering for 1 week.
Phosphorylation of histone variant H2AX at serine 139 is one of the early events after DNA damage and is thought to mark the damage sites where repair factors assemble (49, 50). Phosphorylated H2AX (also known as γH2AX) then progressively diminishes with kinetics that correlate with completion of DNA repair (51, 52). To investigate whether delayed recruitment of Rad51 affects DNA repair in RFWD3 knockdown cells, we examined the γH2AX foci at later times after DNA damage was induced. Compared with a control knockdown, γH2AX foci persist in cells that have lost RFWD3, indicating that loss of RFWD3 impairs DNA damage repair, although it does not affect checkpoint (Fig. 4D and Ref. 5). Consistent with this, Western blotting also showed higher levels of γH2AX protein at various times after IR (Fig. 4E and supplemental Fig. 4). Interestingly, levels of γH2AX were elevated in siRFWD3 knockdown cells before DNA damage induction, indicating that loss of RFWD3 may result in spontaneous DNA damage during normal cell cycle progression.
If RFWD3 is important for DNA damage repair, it is expected that cells lacking RFWD3 should be more sensitive to DNA damage. We tested this hypothesis by colony formation assays. As shown in Fig. 4F, RFWD3-depleted cells indeed display increased sensitivity when exposed to replication blocking agent HU and IR. Taken together, our data suggest that impaired recruitment of DNA repair factors to sites of DNA damage as a result of loss of RFWD3 correlates with persistent DNA damage and increased sensitivity.
DISCUSSION
The RPA complex has emerged as a key player in orchestrating DNA damage checkpoint signaling, stabilization of the replication fork, and DNA repair. Binding of RPA to ssDNA not only protects resected DNA ends from degradation by nucleases but is also necessary for checkpoint activation. In the ssDNA region, RPA has been shown to recruit two independent checkpoint complexes, ATRIP/ATR and Rad1/Rad9/Hus1, for initiation of checkpoint signaling, leading to optimal CHK1 phosphorylation. Recently, RPA has been shown to recruit the annealing helicase SMARCAL1to blocked replication forks and their stabilization. In this manuscript we show that RPA is required to recruit an E3 Ub ligase RFWD3 to DNA damage foci by physical interaction. This is consistent with the notion that RPA serves as an assembly platform for the repair factors, including HR proteins Rad51, Rad52, BLM, and WRN helicases, ERCC1-XPF and XPG endonucleases, and core NER factors TFIIH and XPA, through either direct recruitment or interaction at the sites of damage.
We have demonstrated previously that RFWD3 displays robust E3 Ub ligase activity toward p53 in vitro and positively regulates p53 levels in vivo. However, because p53 is generally considered as an effector in the hierarchy of the DNA damage pathway, whether RFWD3 also functions in checkpoint signaling and is directly involved in the repair remains unknown. We show here that, RFWD3, like RPA, is enriched in the PML-NBs during the S and G2 phases in normal growing cells but rapidly translocates to sites of DNA damage in a RPA-dependent manner. Upon treatment with DNA-damaging agents, RFWD3 is necessary for optimal RPA phosphorylation and recruitment of Rad51 to sites of repair. Consequently, depletion of RFWD3 leads to persistent Rad51 and γH2AX foci, presumably correlated with defective DNA repair. Interestingly, these phenotypes resemble to a large extent those observed in SMARCL1-depleted cells. SMARCAL1, or HARP (HepA-related protein), is a distant member of SNF2 family of DNA-dependent ATPases that has annealing helicase activity. SMARCAL, like RFWD3, is phosphorylated by ATM/ATR kinase and recruited to sites of DNA damage in a RPA-dependent manner. SMARCAL1-depleted cells have impaired DNA repair and hypersensitivity to DNA damage. Thus, our data add RFWD3 to the increasing list of repair enzymes that are recruited by RPA and indicate that RFWD3-mediated ubiquitination may also contribute to repair.
Dynamic Translocation of RFWD3 Links It to RPA and ATR-mediated Pathways
Using immunofluorescence and stably expressed GFP-RFWD3, we show that RFWD3 localizes to both the cytoplasm and nucleus. Although the function of cytoplasmic RFWD3 is unclear, the localization of nuclear RFWD3 to PML NBs in unstressed cells, and its translocation to sites of DNA damage strongly suggest that nuclear RFWD3 plays a role in DDR. The PML NBs, named after the tumor suppressor PML that is essential for PML nuclear body formation, are distinct nuclear structures whose components regulate almost every aspect of cellular functions, including transcription, DNA repair, and apoptosis. Among the several mechanisms proposed to explain the involvement of PML in such diverse functions, one suggests PML NBs act as protein storage sites that, when necessary, release the repair factors to sites of repair. Consistent with this notion, we found that after exposure to DNA-damaging agents, RFWD3 rapidly translocates to discrete, abundant foci that also contain RPA but not PML. Importantly, phosphorylation of RFWD3 was weakened in RPA knockdown cells, and phosphorylation of RPA at Thr-21, a conserved site for ATR kinase, was weakened in RFWD3 knockdown cells. This suggests that both RFWD3 and RPA are important for optimal activation of ATR. Given that focus formation in RPA hyperphosphorylation mutant is normal, together these observations suggest that phosphorylation occurs after recruitment to damage sites.
RFWD3 May Facilitate DNA Repair through Regulation of RPA Phosphorylation
The 32-kDa subunit RPA2 undergoes extensive phosphorylation in cells treated with replication blocking agents. However, the functional consequence of these modifications remains unclear. Earlier studies suggest that RPA phosphorylation may not be essential for its function, as deletion of the N terminus of RPA where the phosphorylation sites are located does not affect its ssDNA binding activity, heterotrimeric complex formation, or the ability to support DNA replication in vitro (53). More recently, several studies suggest that hyperphosphorylated RPA plays an important role in homologous recombination-mediated repair at stalled replication fork (28, 46). In vitro studies suggest that hyperphosphorylation of RPA may cause conformational change (54) and enhance its interaction with Rad51 (46). Consistent with this model, it was shown that recruitment of Rad51 to stalled replication fork is markedly reduced in cells expressing a phosphorylation-defective mutant of RPA (28). In this work we show that depletion of RFWD3 resulted in attenuation of RPA phosphorylation and delayed recruitment of Rad51. These findings are consistent with a model that RFWD3 is directly involved in DNA repair through its interaction with RPA and the regulation of RPA phosphorylation.
RFWD3 was originally identified as a substrate of ATM/ATR kinase from a proteomic screen. Our previous work showed that RFWD3 is capable of ubquitinating p53 in vitro and positively regulates p53 levels through its interaction with MDM2. Here, we provide additional evidence that RFWD3 may play a more direct role in DNA damage repair through its interaction with RPA, suggesting RFWD3 acts as a potential tumor suppressor. Our data also raise several interesting questions, the most intriguing being the identity of the substrate(s) of RFWD3 E3 Ub ligase. As DDR-induced ubiquitination is an integral part of the DDR, at least four E3 Ub ligases have been suggested to be involved in the process (2). For example, RNF8, which is recruited by MDC1 through its FHA domain, activates the DDR-induced ubiquitination reaction by adding Lys-63-linked Ub chains to histones, which recruits another E3 Ub ligase RNF168 to potentiate the ubiquitination reactions. Moreover, the BRCA1/BARD1 E3 Ub ligase as a part of the BRCA1-A complex is then recruited to the sites of repair through binding of ubiquitinated histone by Rap80. Notably, although many E3s involved have been identified, few substrates are known, and they are mainly histones that are known to be involved in this process. Thus, identification of ubiquitin acceptors in DDR remains a great challenge for better understanding of DDR. Another interesting question is how RFWD3 affects RPA phosphorylation. Using commercially available antibodies, we show that Thr-21 is a residue that is regulated by RFWD3. It is likely that additional sites are also RFWD3-dependent. In addition to kinases, two phosphatases, PP2A and PP4, have been shown to be required for RPA dephosphorylation (24, 33). Loss of these proteins is correlated with impaired recovery from replication block. Given that RFWD3 is an E3 Ub ligase, an alternative mechanism for RFWD3-dependent regulation would be fine-tuning the RPA phosphorylation through degradation of the phosphatase, thereby maintaining the proper phosphorylation levels. This hypothesis will be tested in future studies.
Supplementary Material
Acknowledgments
We thank Dr. Junjie Chen for providing reagents and sharing unpublished results, Dr. Jun Qin for mass spectrometry analysis, and members of Qin laboratory for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant GM080703 (to Y. W.). This work was also supported by McLean Foundation at Baylor College of Medicine (to Y. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- DDR
- DNA damage response
- HR
- homologous recombination
- RFWD3
- RING finger and WD repeat domain 3
- SFB
- S protein, FLAG, and streptavidin-binding peptide
- DSB
- double-strand break
- PML
- promyeloytic leukemia protein
- NB
- nuclear body
- IR
- ionizing radiation
- HU
- hydroxyurea
- SMARCAL1
- SWI/SNF-related, matrix associated, actin-dependent regulator of chromatin, subfamily a-like 1
- Ub
- ubiquitin
- Gy
- gray
- RPA
- replication protein A
- MBP
- maltose binding protein
- BLM
- Bloom Syndrome, RecQ helicose-like
- DNA-PKcs
- DNA-dependent protein kinase, catalytic subunit.
REFERENCES
- 1. Harper J. W., Elledge S. J. (2007) Mol. Cell 28, 739–745 [DOI] [PubMed] [Google Scholar]
- 2. Ciccia A., Elledge S. J. (2010) Mol. Cell 40, 179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shiloh Y. (2003) Nat. Rev. Cancer 3, 155–168 [DOI] [PubMed] [Google Scholar]
- 4. Cimprich K. A., Cortez D. (2008) Nat. Rev. Mol. Cell Biol. 9, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mu J. J., Wang Y., Luo H., Leng M., Zhang J., Yang T., Besusso D., Jung S. Y., Qin J. (2007) J. Biol. Chem. 282, 17330–17334 [DOI] [PubMed] [Google Scholar]
- 6. Matsuoka S., Ballif B. A., Smogorzewska A., McDonald E. R., 3rd, Hurov K. E., Luo J., Bakalarski C. E., Zhao Z., Solimini N., Lerenthal Y., Shiloh Y., Gygi S. P., Elledge S. J. (2007) Science 316, 1160–1166 [DOI] [PubMed] [Google Scholar]
- 7. Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu X., Yucer N., Liu S., Li M., Yi P., Mu J. J., Yang T., Chu J., Jung S. Y., O'Malley B. W., Gu W., Qin J., Wang Y. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 4579–4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richard D. J., Bolderson E., Khanna K. K. (2009) Crit. Rev. Biochem. Mol. Biol. 44, 98–116 [DOI] [PubMed] [Google Scholar]
- 10. Wold M. S. (1997) Annu. Rev. Biochem. 66, 61–92 [DOI] [PubMed] [Google Scholar]
- 11. Zou L., Liu D., Elledge S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13827–13832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zou Y., Liu Y., Wu X., Shell S. M. (2006) J. Cell. Physiol. 208, 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou L., Elledge S. J. (2003) Science 300, 1542–1548 [DOI] [PubMed] [Google Scholar]
- 14. Ball H. L., Myers J. S., Cortez D. (2005) Mol. Biol. Cell 16, 2372–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bochkarev A., Bochkareva E. (2004) Curr. Opin. Struct. Biol. 14, 36–42 [DOI] [PubMed] [Google Scholar]
- 16. Jackson D., Dhar K., Wahl J. K., Wold M. S., Borgstahl G. E. O. (2002) J. Mol. Biol. 321, 133–148 [DOI] [PubMed] [Google Scholar]
- 17. McIlwraith M. J., Van Dyck E., Masson J. Y., Stasiak A. Z., Stasiak A., West S. C. (2000) J. Mol. Biol. 304, 151–164 [DOI] [PubMed] [Google Scholar]
- 18. Sleeth K. M., Sørensen C. S., Issaeva N., Dziegielewski J., Bartek J., Helleday T. (2007) J. Mol. Biol. 373, 38–47 [DOI] [PubMed] [Google Scholar]
- 19. Song B., Sung P. (2000) J. Biol. Chem. 275, 15895–15904 [DOI] [PubMed] [Google Scholar]
- 20. Stauffer M. E., Chazin W. J. (2004) J. Biol. Chem. 279, 25638–25645 [DOI] [PubMed] [Google Scholar]
- 21. Sugiyama T., Kowalczykowski S. C. (2002) J. Biol. Chem. 277, 31663–31672 [DOI] [PubMed] [Google Scholar]
- 22. Wong J. M., Ionescu D., Ingles C. J. (2003) Oncogene 22, 28–33 [DOI] [PubMed] [Google Scholar]
- 23. Yuan S. S., Lee S. Y., Chen G., Song M., Tomlinson G. E., Lee E. Y. (1999) Cancer Res. 59, 3547–3551 [PubMed] [Google Scholar]
- 24. Lee D. H., Pan Y., Kanner S., Sung P., Borowiec J. A., Chowdhury D. (2010) Nat. Struct. Mol. Biol. 17, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niu H., Erdjument-Bromage H., Pan Z. Q., Lee S. H., Tempst P., Hurwitz J. (1997) J. Biol. Chem. 272, 12634–12641 [DOI] [PubMed] [Google Scholar]
- 26. Pan Z. Q., Amin A. A., Gibbs E., Niu H., Hurwitz J. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8343–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olson E., Nievera C. J., Klimovich V., Fanning E., Wu X. (2006) J. Biol. Chem. 281, 39517–39533 [DOI] [PubMed] [Google Scholar]
- 28. Shi W., Feng Z., Zhang J., Gonzalez-Suarez I., Vanderwaal R. P., Wu X., Powell S. N., Roti Roti J. L., Gonzalo S., Zhang J. (2010) Carcinogenesis 31, 994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nuss J. E., Patrick S. M., Oakley G. G., Alter G. M., Robison J. G., Dixon K., Turchi J. J. (2005) Biochemistry 44, 8428–8437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vassin V. M., Wold M. S., Borowiec J. A. (2004) Mol. Cell. Biol. 24, 1930–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vassin V. M., Anantha R. W., Sokolova E., Kanner S., Borowiec J. A. (2009) J. Cell Sci. 122, 4070–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stephan H., Concannon C., Kremmer E., Carty M. P., Nasheuer H. P. (2009) Nucleic Acids Res. 37, 6028–6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng J., Wakeman T., Yong S., Wu X., Kornbluth S., Wang X. F. (2009) Mol. Cell. Biol. 29, 5696–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dou H., Huang C., Singh M., Carpenter P. B., Yeh E. T. (2010) Mol. Cell 39, 333–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bansbach C. E., Bétous R., Lovejoy C. A., Glick G. G., Cortez D. (2009) Genes Dev. 23, 2405–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Postow L., Woo E. M., Chait B. T., Funabiki H. (2009) J. Biol. Chem. 284, 35951–35961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ciccia A., Bredemeyer A. L., Sowa M. E., Terret M. E., Jallepalli P. V., Harper J. W., Elledge S. J. (2009) Genes Dev. 23, 2415–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yuan J., Ghosal G., Chen J. (2009) Genes Dev. 23, 2394–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yusufzai T., Kong X., Yokomori K., Kadonaga J. T. (2009) Genes Dev. 23, 2400–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dellaire G., Bazett-Jones D. P. (2004) Bioessays 26, 963–977 [DOI] [PubMed] [Google Scholar]
- 41. Barr S. M., Leung C. G., Chang E. E., Cimprich K. A. (2003) Curr. Biol. 13, 1047–1051 [DOI] [PubMed] [Google Scholar]
- 42. Malovannaya A., Li Y., Bulynko Y., Jung S. Y., Wang Y., Lanz R. B., O'Malley B. W., Qin J. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 2431–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu X., Shell S. M., Zou Y. (2005) Oncogene 24, 4728–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uematsu N., Weterings E., Yano K., Morotomi-Yano K., Jakob B., Taucher-Scholz G., Mari P. O., van Gent D. C., Chen B. P., Chen D. J. (2007) J. Cell Biol. 177, 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernardi R., Pandolfi P. P. (2007) Nat. Rev. Mol. Cell Biol. 8, 1006–1016 [DOI] [PubMed] [Google Scholar]
- 46. Wu X., Yang Z., Liu Y., Zou Y. (2005) Biochem. J. 391, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zernik-Kobak M., Vasunia K., Connelly M., Anderson C. W., Dixon K. (1997) J. Biol. Chem. 272, 23896–23904 [DOI] [PubMed] [Google Scholar]
- 48. Block W. D., Yu Y., Lees-Miller S. P. (2004) Nucleic Acids Res. 32, 997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 50. Mah L. J., El-Osta A., Karagiannis T. C. (2010) Leukemia 24, 679–686 [DOI] [PubMed] [Google Scholar]
- 51. Sedelnikova O. A., Rogakou E. P., Panyutin I. G., Bonner W. M. (2002) Radiat. Res. 158, 486–492 [DOI] [PubMed] [Google Scholar]
- 52. Bouquet F., Muller C., Salles B. (2006) Cell Cycle. 5, 1116–1122 [DOI] [PubMed] [Google Scholar]
- 53. Henricksen L. A., Wold M. S. (1994) J. Biol. Chem. 269, 24203–24208 [PubMed] [Google Scholar]
- 54. Binz S. K., Lao Y., Lowry D. F., Wold M. S. (2003) J. Biol. Chem. 278, 35584–35591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.