Abstract
Ca2+/calmodulin (CaM)-dependent protein kinase (CaMK) kinase (CaMKK) is a member of the CaMK cascade that mediates the response to intracellular Ca2+ elevation. CaMKK phosphorylates and activates CaMKI and CaMKIV, which directly activate transcription factors. In this study, we determined the 2.4 Å crystal structure of the catalytic kinase domain of the human CaMKKβ isoform complexed with its selective inhibitor, STO-609. The structure revealed that CaMKKβ lacks the αD helix and that the equivalent region displays a hydrophobic molecular surface, which may reflect its unique substrate recognition and autoinhibition. Although CaMKKβ lacks the activation loop phosphorylation site, the activation loop is folded in an active-state conformation, which is stabilized by a number of interactions between amino acid residues conserved among the CaMKK isoforms. An in vitro analysis of the kinase activity confirmed the intrinsic activity of the CaMKKβ kinase domain. Structure and sequence analyses of the STO-609-binding site revealed amino acid replacements that may affect the inhibitor binding. Indeed, mutagenesis demonstrated that the CaMKKβ residue Pro274, which replaces the conserved acidic residue of other protein kinases, is an important determinant for the selective inhibition by STO-609. Therefore, the present structure provides a molecular basis for clarifying the known biochemical properties of CaMKKβ and for designing novel inhibitors targeting CaMKKβ and the related protein kinases.
Keywords: Apoptosis, Calcium, Calmodulin, Calcium Calmodulin-dependent Protein Kinase (CaMK), Drug Design, Multifunctional Enzymes, Protein Kinases, Protein Structure, Signal Transduction, Transcription Regulation
Introduction
Calcium is a ubiquitous second messenger that modulates diverse cellular responses. The Ca2+ receptor protein calmodulin (CaM)3 is involved in Ca2+ signaling through its effects on a variety of CaM-binding proteins, including a family of Ser/Thr protein kinases known as Ca2+/CaM-dependent protein kinases (CaMKs). The members of this family, CaMKI, CaMKIV, and CaMK kinase (CaMKK), constitute the CaMK cascade that directly activates transcription factors, such as cAMP-response element-binding protein, thereby regulating Ca2+-dependent gene expression (1–4). CaMKK phosphorylates the equivalent Thr residues of CaMKI and CaMKIV (Thr177 and Thr196, respectively) and enhances their activities (5, 6). There are two mammalian CaMKK isoforms, CaMKKα and CaMKKβ, which are localized in the cytoplasm and the nucleus, respectively (7–9). Extensive cross-talk between the CaMK cascade and other signaling pathways has been demonstrated. For example, the CaMK cascade activates the MAP kinases, such as the extracellular signal-regulated kinase (ERK) and the c-Jun N-terminal kinase (JNK) (10, 11). The CaMKK activity can be suppressed through phosphorylation by the cAMP-dependent kinase (PKA) (12). CaMKK directly phosphorylates and activates protein kinase B (PKB) and modulates apoptosis (13). CaMKK also activates the AMP-activated protein kinase (AMPK), a critical regulator of energy balance (14). Recently, a brain-specific AMPK family member, SAD-B, has been identified as a novel CaMKK target (15).
The members of the CaMK cascade share a common domain organization: the catalytic kinase domain (KD) and the C-terminal autoinhibitory domain (AID), which partially overlaps with the CaM-binding domain (CBD) (16) (see Fig. 1A). The crystal structure of the CaMKI KD-AID fragment revealed that the interaction of KD with AID prevents both substrate and ATP binding and maintains the kinase in an inactive state (17). Ca2+/CaM binding relieves the autoinhibition of the kinase, although the CaM·CBD complex structures determined previously for CaMKK and CaMKI revealed different interaction modes (18–20).
FIGURE 1.
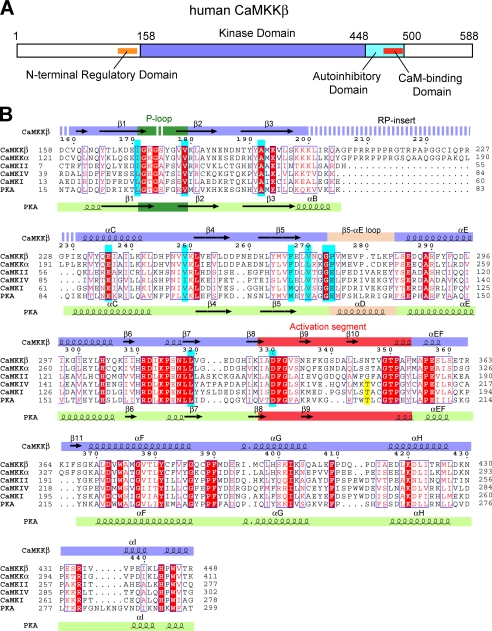
Primary structure of CaMKKβ. A, domain organization of human CaMKKβ. B, sequence alignment of the KDs of human CaMKKβ, human CaMKKα, human CaMKI, human CaMKIV, and mouse PKA. The STO-609-interacting residues are colored cyan, and the activation loop phosphorylation sites are yellow. The secondary structural elements of CaMKKβ and PKA are indicated above and below the sequences, respectively. The glycine-rich P-loop is colored green, the activation segment is red, and the β5-αE loop (CaMKKβ) and the helix αD (PKA) are wheat. Disordered regions are indicated as dashed lines. This figure was generated using the program ESPript (49).
Previous studies demonstrated the different biochemical properties between the CaMKK isoforms (21–23); the CaMKKα activity is strictly regulated by Ca2+/CaM, whereas CaMKKβ exhibits significant “autonomous” activity (60–70% of the total activity) in the absence of Ca2+/CaM. A 23-amino acid region (residues 129–151) at the N terminus of rat CaMKKβ was identified as the regulatory domain that relieves the kinase from autoinhibition, thus allowing its Ca2+/CaM-independent activity (23).
Ser74, Thr108, and Thr458 of rat CaMKKα are the inhibitory sites phosphorylated by PKA. Phosphorylation of Thr458 within the CBD suppresses the CaM binding to CaMKK (12). Phosphorylation of Ser74 in the region N-terminal to the KD promotes the binding of protein 14-3-3, which suppresses the CaMKK activity (24). Intriguingly, the three PKA-mediated phosphorylation sites are conserved throughout the isoforms and species, suggesting the existence of a common mechanism of intramolecular regulation among the CaMKKs.
We now report the crystal structure of the human CaMKKβ KD complexed with STO-609, a selective inhibitor of CaMKK. STO-609 inhibits both the CaMKKα and the CaMKKβ isoforms, but CaMKKβ is more sensitive to STO-609 than CaMKKα (25). The CaMKKβ·STO-609 complex structure determined in this study provides a basis for understanding the selective inhibition of CaMKKβ by STO-609 as compared with CaMKKα and other protein kinases. We also report the first evidence that CaMKKβ lacks the activation loop phosphorylation site, which accounts for its high autonomous activity, and discuss the other structural properties of CaMKKβ that reflect its unique substrate recognition and autoinhibition.
EXPERIMENTAL PROCEDURES
Plasmids
The human CaMKKβ cDNA clone was obtained from the Kazusa collection (Kazusa clone ID: KIAA0787). Throughout this study, we utilize the residue numbers of Fig. 1B, according to the human CaMKKβ protein (Swiss-Prot accession code: Q96RR4) with the N terminus at Met1. The DNA encoding human CaMKKβ KD (residues 158–448) was subcloned into the expression vector pCR2.1 TOPO (Invitrogen) as a fusion with an N-terminal His tag and a tobacco etch virus protease cleavage site. The expression plasmid for the human CaMKI KD (residues 1–281) (OriGene Technologies) for use in kinase assays was similarly constructed. Point mutations were introduced into the CaMKKβ and CaMKI KDs by using a QuikChange site-directed mutagenesis kit (Stratagene).
Protein Expression and Purification
The protein was synthesized by the Escherichia coli cell-free system (26, 27). The internal solution was dialyzed in dialysis tubes (Spectra/Por 7 molecular weight cut-off, 15,000; Spectrum) against the external solution at 30 °C for 2.5 h with shaking, and then it was centrifuged at 16,000 × g at 4 °C for 20 min. The supernatant was loaded onto a HisTrap (GE Healthcare) column and eluted with a buffer containing 20 mm Tris-HCl (pH 8.0), 500 mm NaCl, 10% glycerol, and 500 mm imidazole. The eluate was incubated overnight with tobacco etch virus protease to cleave the His tag and was dialyzed against 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 10% glycerol, and 20 mm imidazole. To separate the His tag and the tobacco etch virus protease, the protein was loaded on a HisTrap column, and the flow-through fractions were collected. The protein was further purified by ion exchange on a HiTrap Q column and size-exclusion chromatography on a Superdex 200 column (GE Healthcare), in a final buffer containing 20 mm Tris-HCl (pH 7.5), 300 mm NaCl, 10% glycerol, and 2 mm DTT.
Crystallization and Data Collection
Before crystallization, the purified protein (8.0 mg/ml) was mixed with 1 mm STO-609 (Sigma-Aldrich) and 5 mm MgCl2 and was incubated at 4 °C overnight. Diffraction quality crystals of CaMKKβ complexed with STO-609 were grown in drops composed of 1 μl of protein solution, 1 μl of 0.5% agarose solution (Hampton Research), and 1 μl of reservoir solution, containing 0.1 m sodium cacodylate (pH 5.9), 0.2 m sodium acetate, and 18% PEG8000 (Hampton Research), by the hanging drop vapor diffusion method at 20 °C. Data collection was performed at 100 K, with the reservoir solution containing 29% glycerol as a cryoprotectant. The data were collected at a wavelength of 1.0 Å at BL41XU, SPring-8 (Hyogo, Japan) and were recorded on an MX225-HE CCD detector. The diffraction data were processed with the HKL2000 program (28).
Structure Determination and Refinement
The structure was solved by the molecular replacement method with the program PHASER (29, 30), using the structure of human CaMKIIδ isoform 1 (Protein Data Bank (PDB) code 2VN9) as the search model. The model was corrected iteratively using the program Coot (31), and the structure refinement was performed with the Crystallography and NMR System (CNS) (32). All refinement statistics are presented in Table 1. The quality of the model was inspected by the program PROCHECK (33). Structural similarities were calculated with the program Dali (34). The graphic figures were created using the program PyMOL (35).
TABLE 1.
Crystallographic statistics
All numbers in parentheses refer to the highest resolution shell statistics.
| Data collection | |
|---|---|
| Wavelength (Å) | 1.00 |
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (Å) | 69.4, 77.2, 84.3 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution range (Å) | 50–2.4 (2.49–2.40) |
| Redundancy | 4.5 |
| Unique reflections | 17,475 |
| Completeness (%) | 98.5 (99.9) |
| I/σ(I) | 25.9 (2.8) |
| Rsyma | 0.057 (0.607) |
| Refinement | |
| Resolution range (Å) | 37.0–2.40 (2.55–2.40) |
| No. of reflections | 16,795 |
| R-factor/Free R-factorb | 0.203/0.252 |
| No. of atoms | |
| Protein | 2066 |
| Ligand | 24 |
| Water | 53 |
| Average B-values (Å2) | |
| Protein | 50.1 |
| Ligand | 35.7 |
| Water | 40.4 |
| r.m.s.c deviations | |
| Bond lengths (Å) | 0.006 |
| Bond angles (°) | 1.3 |
| Ramachandran plot | 89.3, 9.4, 0.9, 0.4 |
a Rsym = σ |Iavg − Ii|/σIi, where Ii is the observed intensity and Iavg is the average intensity.
b Free R-factor is calculated for 5% of randomly selected reflections excluded from refinement.
c r.m.s., root mean square.
Kinase Assays
The AMPK peptide, including the sequence surrounding the phosphorylation site of AMPK (167GEFLRTSCGSP177), was synthesized at the Support Unit for Bio-material Analysis in the RIKEN Brain Science Institute (BSI) Research Resources Center (RRC). Appropriate quantities of the purified CaMKKβ KD and full-length CaMKKβ (Carna Biosciences) were each incubated in the presence or absence of 500 μm AMPK peptide at 30 °C, in a reaction solution (20 μl) containing 50 mm HEPES (pH 7.5), 300 mm NaCl, 1 mm DTT, 10 mm MgCl2, 400 μm ATP, and 10% glycerol, with or without 0.5 μm STO-609. For the full-length CaMKKβ, 5 μm calmodulin (Sigma-Aldrich) and 1 mm CaCl2 were added to the reaction solution. ATP consumption was determined by using a Kinase-GloTM Max luminescent kinase assay (Promega) kit, which quantifies the amount of ATP in the reaction solution. Glow-type luminescence was recorded after 10 min, using a FusionTM universal microplate analyzer (Packard).
RESULTS AND DISCUSSION
Overall Structure
We crystallized human CaMKKβ KD (residues 158–448) in the presence of STO-609. The crystal belongs to the primitive orthorhombic space group P212121 with unit cell constants of a = 69.4 Å, b = 77.2 Å, c = 84.3 Å and contains one CaMKKβ·STO-609 complex in the asymmetric unit. The crystal structure of the CaMKKβ·STO-609 complex was determined by the molecular replacement method and refined to 2.4 Å resolution (Table 1). The final model includes residues 160–173, 176–199, and 231–448 of CaMKKβ, one STO-609 molecule, and 53 water molecules. A conserved Gly-rich segment (172GKGSYG177) in the phosphate-binding loop (P-loop) was partially disordered in the crystal structure. In addition, the characteristic Arg-Pro-rich insert (RP-insert) of the two CaMKK isoforms (Fig. 1B) was not identified due to lack of electron density, indicating a high degree of mobility in this region.
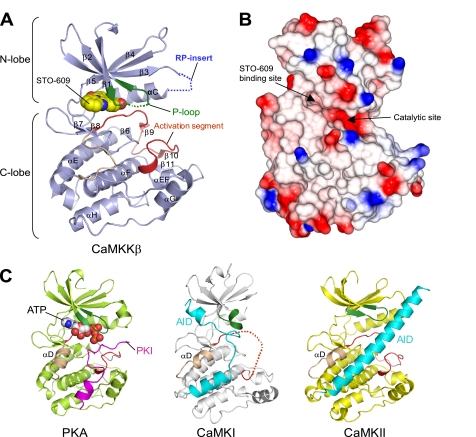
Fig. 2A shows a ribbon representation of the crystal structure of the CaMKKβ·STO-609 complex. STO-609 is bound in the ATP-binding pocket of the CaMKKβ KD, which is consistent with the previous report that the inhibition mechanism of STO-609 is ATP-competitive (25). The CaMKKβ KD adopts the canonical protein kinase fold, except that there is no counterpart to the αD helix. The equivalent region of CaMKKβ contains the conserved Pro residues among the CaMKKs (Fig. 1B) and forms a loop (the β5-αE loop) rather than a helical turn. The region surrounding the β5-αE loop displays a hydrophobic molecular surface (Fig. 2B), which may be involved in the binding of the substrate sequence (see next section for details).
FIGURE 2.
Structure of the CaMKKβ·STO-609 complex. A, ribbon representation of the CaMKKβ KD structure. The P-loop is colored green, the activation segment is red, and the β5-αE loop is wheat. The STO-609 is shown in a sphere representation. Disordered regions are indicated as dashed lines. B, the electrostatic surface of the CaMKKβ KD (red, negative charge; blue, positive charge), shown in the same orientation as in A. The locations of the STO-609-binding site and the catalytic site are indicated. This figure was generated using the program CCP4 MG (50). C, structure comparison with other protein kinases. Structures of the PKA KD complexed with PKA inhibitor (PKI) (left), the CaMKI KD-AID (middle), and the CaMKII KD-AID (right), shown in the same orientation as that of CaMKKβ (A). In these KD structures, the P-loop is colored green, the activation segment is red, and the helix αD is wheat. The PKI bound to PKA is colored magenta, and the AIDs of CaMKI and CaMKII are cyan. The ATP bound to PKA is shown in a sphere representation.
A structural comparison with other protein kinases revealed that the CaMKKβ·STO-609 complex adopts a closed conformation (36), resembling the active state of kinases such as PKA (PDB code 1ATP) (37) (Fig. 2C, left panel) (root mean square deviation 1.6 Å over 245 Cα atoms, 73% of the total 336 Cα atoms) and phosphorylase kinase (PDB code 1PHK) (38) (root mean square deviation 1.7 Å over 252 Cα atoms, 91% of the total 277 Cα atoms), with sequence identities of 36 and 31%, respectively. The CaMKKβ structure in this study is quite different from the previous structures of CaMKI (PDB code 1A06) (17) and CaMKII (PDB code 2BDW) (39) (Fig. 2C, middle and right panels), which adopt an open conformation with the N-lobe located away from the C-lobe due to the presence of the AID in the C terminus.
Substrate Sequence-binding region of CaMKKβ
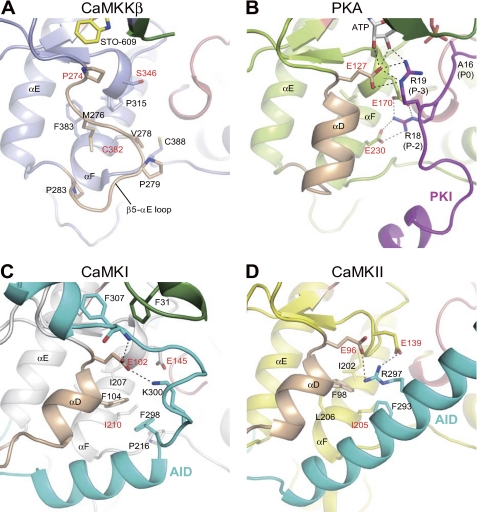
CaMKK lacks the conserved acidic residues that, in many other kinases, recognize basic residues in substrates. To identify the substrate specificity determinants of CaMKK, we compared the structure of CaMKKβ with that of PKA complexed with a pseudosubstrate, PKA inhibitor (PKI). Glu127 of PKA interacts with the Arg residue at the P-3 position of PKI, but in CaMKKβ, this acidic residue is replaced by Pro (Pro274) (Fig. 3, A and B). In addition, Glu170 and Glu230 of PKA, which interact with the Arg residue at the P-2 position of PKI, are replaced with Ser316 and Cys382, respectively, in CaMKKβ. These CaMKKβ residues are clustered with the conserved hydrophobic residues among the CaMKKs, creating a hydrophobic pocket that is suitable for the accommodation of hydrophobic residues in substrates (Fig. 3A). Consistent with these observations, an inspection of the sequences around the phosphorylation sites of various CaMKK substrates, CaMKI (172GSVLSTA178), CaMKIV (191QVLMKTV197), PKB (303GATMKTF309), AMPK (167GEFLRTS173), and SAD-B (184DSLLETS190), suggested that CaMKK prefers hydrophobic or non-polar residues, rather than basic residues, at the P-3 and P-2 positions of the substrate.
FIGURE 3.
Close-up views of the region surrounding the β5-αE loop of CaMKKβ (A) in comparison with the equivalent region of the PKA-PKI complex (B), CaMKI (C), and CaMKII (D). The coloring of the elements is the same as in Fig. 2. Hydrogen bonds are indicated as dashed lines.
The current structure of the CaMKKβ KD revealed that CaMKKβ lacks the αD helix that, in CaMKI and CaMKII, is involved in hydrophobic interactions with the AID (Fig. 3, A, C, and D). In addition, the basic residues of the CaMKI and CaMKII AIDs form hydrogen bonds with the conserved Glu residues (Glu102 and Glu96 of the CaMKI and CaMKII KDs, respectively), whereas CaMKKβ lacks the corresponding residue and instead has Pro274. Therefore, the interactive structure between the KD and AID of CaMKKβ should be quite different from those of CaMKI and CaMKII. Indeed, the length, the sequence, and the structure in the CaM-bound form (18–20) of the AID of CaMKK all differ from those of the AID of CaMK.
CaMKKβ Is an Intrinsically Active Kinase Lacking the Activation Loop Phosphorylation Site
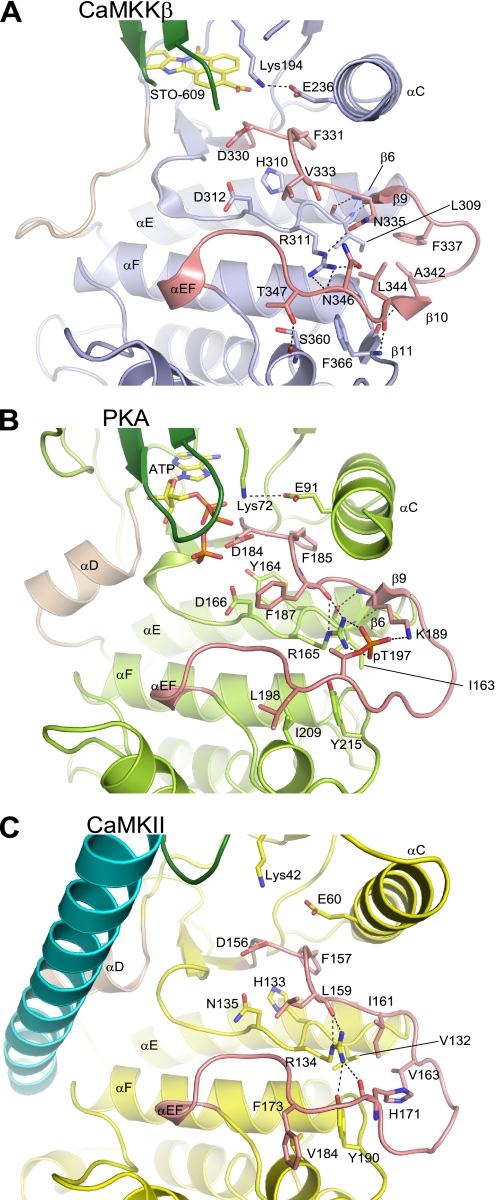
In many protein kinases, phosphorylation of the activation loop site(s) is required for the folding of the activation segment into the active-state conformation, which promotes substrate binding and catalysis (40, 41). CaMKK possesses Ser and Thr residues, which are candidates for the phosphorylation sites, within the activation loop. However, the current structure of the CaMKKβ·STO-609 complex revealed that although the activation loop Ser and Thr residues of CaMKKβ were not phosphorylated, the activation segment was folded in the active, “DFG-in” conformation. The conserved Phe residue (Phe331) within the DFG motif interacts with the αC helix, stabilizing a catalytically necessary ion pair (Glu236 and Lys194) (Fig. 4A). This result clearly indicates that CaMKKβ does not require activation loop phosphorylation to adopt the active conformation. Intriguingly, Asn346 of CaMKKβ, rather than the adjacent Ser and Thr residues (Ser345 and Thr347), is spatially equivalent to the phosphorylated Thr residue (Thr197) of PKA, in which the phosphate group of the phosphothreonine forms hydrogen bonds with the catalytic loop (Arg165) and the activation loop (Lys189) residues (Fig. 4, A and B).
FIGURE 4.
Comparison of the activation segments of CaMKKβ (A), PKA (B), and CaMKII (C), showing the stabilization of the activation loop conformation. The activation segment is colored salmon. Hydrogen bonds are indicated as dashed lines.
The activation loop of CaMKKβ is stabilized by a number of intramolecular interactions (Fig. 4A). The main-chain carbonyl and imino groups of Leu309, respectively, hydrogen-bond with the main-chain imino and carbonyl groups of Asn335 and form a short anti-parallel β sheet between β6 and β9. Similarly, the main-chain carbonyl and imino groups of Leu344, respectively, hydrogen-bond with the main-chain imino and carbonyl groups of Phe366, thus forming another short anti-parallel β sheet between β10 and β11. In addition, the side chain of Asn335 hydrogen-bonds with the catalytic loop Arg residue (Arg311), which forms hydrogen bonds with the main-chain and the side-chain carbonyl groups of Asn346. On the other hand, the side chains of Phe337, Ala342, and Leu344 form hydrophobic interactions with those of Leu309 and Arg311. Furthermore, the side chain of Thr347 hydrogen-bonds with the main-chain carbonyl group of Ser360 from αEF. All of these residues are highly conserved in the CaMKKβ proteins among various species. The activation loop of CaMKKβ has some interactions with the symmetry-related molecule, but the buried surface is relatively small (∼530 Å2, corresponding to 4.1% of the total surface area), and the interacting residues are not well conserved.
CaMKII, like CaMKKβ, lacks a phosphorylation site in the activation loop. A comparison of the activation loop of CaMKKβ to that of CaMKII (Fig. 4, A and C) revealed a structural similarity in that the conserved catalytic loop Arg residue (Arg311 in CaMKKβ and Arg134 in CaMKII) hydrogen-bonds directly with the main-chain carbonyl group of the activation loop residue (Asn346 in CaMKKβ and His171 in CaMKII). Another common feature is the existence of hydrophobic interactions between the side chains of the activation loop residues, although the participating residues are not conserved between CaMKKβ and CaMKII (Phe337, Ala342, and Leu344 in CaMKKβ and Ile161, Val163, and His171 in CaMKII).
In CaMKKα, the residue corresponding to Asn346 of CaMKKβ, which is located in the equivalent position of the phosphorylated Ser/Thr residue within the activation loop, is Ser309, which is a good candidate for the regulatory phosphorylation site. However, except for Asn346, all of the CaMKKβ residues that stabilize the activation loop are conserved in CaMKKα. Thus, it is possible that CaMKKα may also adopt an active-state conformation without phosphorylation of the activation loop Ser residue (Ser309).
Characterization of the Kinase Activity
We next examined whether human CaMKKβ KD, displaying a constitutively active kinase structure, is indeed an active kinase. For this purpose, we used the human CaMKKβ KD protein purified for crystallization and a synthetic substrate peptide containing the sequence around the phosphorylation site of AMPK (167GEFLRTSCGSP177). When the purified CaMKKβ KD protein was incubated with ATP under phosphorylation conditions, significant ATP consumption was observed only in the presence of the AMPK peptide, indicating that the CaMKKβ KD has an activity to phosphorylate the AMPK peptide. The phosphorylation activity of the purified CaMKKβ KD (7.52 nmol min−1 nmol−1) was much higher than that of the full-length CaMKKβ (1.22 nmol min−1 nmol−1) (Table 2). In addition, we confirmed that the STO-609-mediated inhibition of the CaMKKβ KD was similar to that of the full-length CaMKKβ (Table 2). The result clearly indicates that the CaMKKβ KD is intrinsically active without phosphorylation of the activation loop. Considering its function at the top of the CaMK cascade, it is reasonable that CaMKK is an intrinsically active kinase that can be activated only by Ca2+/CaM.
TABLE 2.
Inhibition of CaMKKβ by STO-609
| STO-609 | Phosphorylation activity (nmol/min/nmol CaMKKβ) |
% of inhibition | |
|---|---|---|---|
| 0 μm | 0.5 μm | ||
| CaMKKβ KD | 7.52 ± 0.48 | 2.96 ± 0.40 | 60.7 ± 2.8 |
| CaMKKβ | 1.22 ± 0.17 | 0.502 ± 0.001 | 58.5 ± 5.7 |
Selective Interactions of CaMKKβ with STO-609
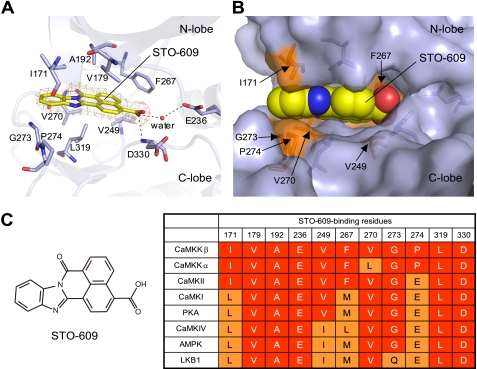
The planar molecule STO-609 is bound to the CaMKKβ KD, and its carboxylic acid moiety is slightly tilted (Fig. 5A). This conformation enables the inhibitor to fit within a narrow pocket of the CaMKKβ KD that adopts a closed conformation (Fig. 5B). The interactions between STO-609 and CaMKKβ are mostly hydrophobic, involving Ile171, Val179, Ala192, Val249, and Phe267 from the N-lobe and Gly273, Pro274, Leu319, and Asp330 from the C-lobe (Fig. 5A). In addition, STO-609 hydrogen-bonds with the backbones of Val270 and Asp330, as well as with the conserved catalytic residue Glu236, in a water-mediated manner. CaMKKβ is reportedly more sensitive to STO-609 than CaMKKα (25). As shown in Fig. 5C, among the CaMKKβ residues that interact with STO-609, only Val270 is replaced, by Leu, in CaMKKα. This result suggests that the Val to Leu replacement at this position causes the different STO-609 sensitivities between the CaMKK isoforms. Consistently, mutations of the corresponding Val residue of rat CaMKKβ (Val269) to residues with bulky side chains, including Leu, His, Met, and Phe, significantly decreased the sensitivity to STO-609 (42). Based on the structure, we suggest that this is due to steric inhibition of STO-609 binding.
FIGURE 5.
The STO-609-binding site. A, the binding site structure with the STO-609 modeled in the 2Fo − Fc electron density map. The STO-609 and the residues of CaMKKβ that coordinate STO-609 are represented as stick models. Hydrogen bonds are indicated as dashed lines. B, the inhibitor-binding residues, shown with a superposition of their molecular surfaces. The STO-609 is shown in a sphere representation. C, chemical diagram of STO-609 and sequence comparison of the STO-609-binding residues of CaMKKβ with other protein kinases.
We next compared the amino acid sequence of the STO-609-binding site of CaMKKβ with those of other protein kinases, such as CaMKI, CaMKII, CaMKIV, PKA, AMPK, and LKB1, which are much less sensitive to STO-609 than CaMKKα and CaMKKβ. The inhibitor potencies of STO-609 against these protein kinases are different, and the IC50 values were previously ranked as CaMKKβ < CaMKKα < CaMKII < CaMKI = PKA < CaMKIV (25) and as CaMKKβ < AMPK < LKB1 (43). As shown in Fig. 5C, the CaMKKβ residues that interact with STO-609, such as Ile171, Val249, Phe267, Gly273, and Pro274, are replaced with various residues in these protein kinases, and the degrees of the amino acid replacements correlate well with the different inhibitor potencies displayed by STO-609. In particular, Pro274 of CaMKKβ is replaced by Glu in all of these protein kinases except for CaMKKα, suggesting that Pro at this position is the most important determinant for the selective binding of STO-609 in CaMKK as compared with the other protein kinases.
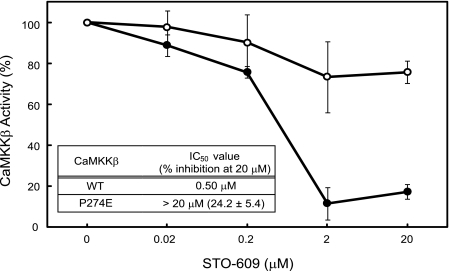
This finding led us to examine the effect of the CaMKKβ P274E mutation on the inhibition by STO-609. As shown in Fig. 6, the P274E mutation significantly reduced the inhibitory effect of STO-609 on the CaMKKβ KD. Although the kinase activity of the wild-type CaMKKβ KD was almost completely inhibited in the presence of 20 μm STO-609, the P274E mutant was only slightly affected (24.2% inhibition) by the same concentration of STO-609. The IC50 value of STO-609 for the P274E mutant was estimated to be more than 20 μm, which was much higher than that of the wild-type protein (IC50 = 0.50 μm). This mutagenesis result indicated that the Pro side chain at this position contributes to the selective inhibition of CaMKKβ by STO-609.
FIGURE 6.
Effect of STO-609 on the activities of the wild-type and P274E mutant CaMKKβ KDs. The wild-type (WT) (closed circles) and P274E mutant (open circles) CaMKKβ KDs were assayed to measure the inhibitory effects of STO-609 (at the indicated concentrations) on their activities to phosphorylate the AMPK peptide. The experiments were performed in duplicate for each condition, as described under “Experimental Procedures.” The amounts of ATP consumption in the absence of STO-609 were set to 100. Error bars indicate S.D.
In the current structure of the CaMKKβ·STO-609 complex, Pro274 is located at the gate of the STO-609-binding pocket, and the pyrrolidine ring forms hydrophobic contacts with the inhibitor (Fig. 5B). It is therefore likely that the presence of an acidic residue, which in the other protein kinases recognizes a basic residue at the P-3 position of the substrate (Fig. 3, B–D), in place of the Pro in CaMKKα and CaMKKβ, would reduce the binding of STO-609 due to the loss of hydrophobic contacts.
Implications for Selective Substrate Protein Recognition by the RP-insert
It was previously demonstrated that PKB is a relatively poor substrate for CaMKK as compared with the downstream kinases (CaMKI and CaMKIV) (44). In addition, the intact CaMKI and CaMKIV proteins are much better substrates for CaMKK as compared with synthetic peptides containing the sequence around the phosphorylation sites of CaMKI and CaMKIV (45). The RP-insert of CaMKK is required for the selective high affinity interactions with CaMKI and CaMKIV, although it is dispensable for its autophosphorylation, CaMKIV peptide phosphorylation, and PKB activation (46). Because the Arg to Glu mutations within the RP-insert of rat CaMKKα drastically reduced its ability to activate CaMKIV (46), it is likely that the positively charged Arg residues within the CaMKK RP-insert are required for the recognition of the negatively charged residues in CaMKI and CaMKIV.
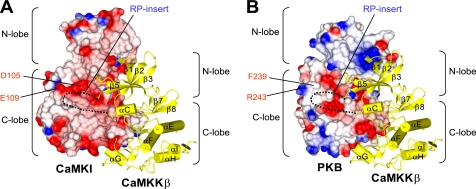
To gain insight of how CaMKKβ recognizes its substrate kinases, we generated docking models for the CaMKKβ·CaMKI and CaMKKβ·PKB complexes (Fig. 7) by superposing the crystal structures of CaMKKβ (this study), CaMKI (PDB code 1A06) (17), and PKB (PDB code 1GZN) (47) onto the structure of p38 MAPK·MAPK-activated kinase 2 complex (PDB code 2OZA) (48). These structural models suggested that the negatively charged residues in the αD helix of CaMKI (Asp105 and Glu109 in rat sequence), which are conserved in CaMKIV but not in PKB (Phe239 and Arg243), may interact with the RP-insert of CaMKKβ. Intriguingly, the mutation of the corresponding Asp residue (Asp108) to Arg in the human CaMKI KD reduced its ability to be phosphorylated by the CaMKKβ KD (supplemental Fig. 1). Therefore, it is possible that the Asp residue in the αD helix of CaMKI may be involved in the interaction with CaMKKβ. Although the residues of CaMKKβ that interact with this Asp residue of CaMKI have not been identified, based on the sequence conservation between CaMKKs (46), it is conceivable that the Arg residues within the RP-insert are involved in this interaction. The determination of the structure of the CaMKKβ·CaMKI complex will clarify the specific features of binding between these proteins.
FIGURE 7.
Hypothetical models of the heterodimeric CaMKKβ·CaMKI (A) and CaMKKβ·PKB (B) complexes. The CaMKKβ KD (yellow ribbons) is docked onto the electrostatic surfaces of the CaMKI KD and the PKB KD (red, negative charge; blue, positive charge). The disordered RP-insert region of the CaMKKβ KD is indicated as dashed lines. Selected residues in the CaMKI KD and the PKB KD are indicated.
In conclusion, this study has demonstrated the unique structural properties of the CaMKKβ KD, which provide a molecular basis for understanding the known biochemical properties of CaMKKβ and the distinct STO-609 sensitivity between the CaMKKα and CaMKKβ isoforms. Our structure of the CaMKKβ·STO-609 complex also provides a structural basis for designing novel inhibitors to specifically block CaMKKβ and related protein kinases.
Supplementary Material
Acknowledgments
We thank Hideaki Niwa, Takashi Umehara, Mitsutoshi Toyama, Mio Inoue, Mari Aoki, Mio Goto, Ken Ishii, Naomi Ohbayashi, Tomomi Uchikubo-Kamo, Ryogo Akasaka, and Kazushige Katsura for technical assistance. We are grateful to Dr. Osamu Ohara (Kazusa DNA Research Institute, Japan) for the CaMKKβ clone (KIAA0787). We thank the members of the Support Unit for Bio-material Analysis, RIKEN BSI Research Resource Center, and especially Reiko Ito, Junko Ishikawa, and Aya Abe, for AMPK peptide synthesis.
This work was supported by grants from the RIKEN Structural Genomics/Proteomics Initiative (RSGI), the National Project on Protein Structural and Functional Analyses, and the Targeted Proteins Research Program (TPRP), the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The atomic coordinates and structure factors (code 2ZV2) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- CaM
- calmodulin
- CaMK
- CaM-dependent protein kinase
- CaMKK
- CaMK kinase
- AMPK
- AMP-activated protein kinase
- KD
- kinase domain
- AID
- autoinhibitory domain
- CBD
- CaM-binding domain
- RP-insert
- Arg-Pro-rich insert.
REFERENCES
- 1. Soderling T. R. (1999) Trends Biochem. Sci. 24, 232–236 [DOI] [PubMed] [Google Scholar]
- 2. Soderling T. R., Stull J. T. (2001) Chem. Rev. 101, 2341–2352 [DOI] [PubMed] [Google Scholar]
- 3. Corcoran E. E., Means A. R. (2001) J. Biol. Chem. 276, 2975–2978 [DOI] [PubMed] [Google Scholar]
- 4. Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., Soderling T. R. (2008) Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haribabu B., Hook S. S., Selbert M. A., Goldstein E. G., Tomhave E. D., Edelman A. M., Snyderman R., Means A. R. (1995) EMBO J. 14, 3679–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selbert M. A., Anderson K. A., Huang Q. H., Goldstein E. G., Means A. R., Edelman A. M. (1995) J. Biol. Chem. 270, 17616–17621 [DOI] [PubMed] [Google Scholar]
- 7. Tokumitsu H., Enslen H., Soderling T. R. (1995) J. Biol. Chem. 270, 19320–19324 [DOI] [PubMed] [Google Scholar]
- 8. Kitani T., Okuno S., Fujisawa H. (1997) J. Biochem. 122, 243–250 [DOI] [PubMed] [Google Scholar]
- 9. Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. (1998) J. Biol. Chem. 273, 31880–31889 [DOI] [PubMed] [Google Scholar]
- 10. Enslen H., Tokumitsu H., Stork P. J., Davis R. J., Soderling T. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10803–10808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmitt J. M., Wayman G. A., Nozaki N., Soderling T. R. (2004) J. Biol. Chem. 279, 24064–24072 [DOI] [PubMed] [Google Scholar]
- 12. Wayman G. A., Tokumitsu H., Soderling T. R. (1997) J. Biol. Chem. 272, 16073–16076 [DOI] [PubMed] [Google Scholar]
- 13. Yano S., Tokumitsu H., Soderling T. R. (1998) Nature 396, 584–587 [DOI] [PubMed] [Google Scholar]
- 14. Witczak C. A., Sharoff C. G., Goodyear L. J. (2008) Cell. Mol. Life Sci. 65, 3737–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujimoto T., Yurimoto S., Hatano N., Nozaki N., Sueyoshi N., Kameshita I., Mizutani A., Mikoshiba K., Kobayashi R., Tokumitsu H. (2008) Biochemistry 47, 4151–4159 [DOI] [PubMed] [Google Scholar]
- 16. Tokumitsu H., Wayman G. A., Muramatsu M., Soderling T. R. (1997) Biochemistry 36, 12823–12827 [DOI] [PubMed] [Google Scholar]
- 17. Goldberg J., Nairn A. C., Kuriyan J. (1996) Cell 84, 875–887 [DOI] [PubMed] [Google Scholar]
- 18. Osawa M., Tokumitsu H., Swindells M. B., Kurihara H., Orita M., Shibanuma T., Furuya T., Ikura M. (1999) Nat. Struct. Biol. 6, 819–824 [DOI] [PubMed] [Google Scholar]
- 19. Kurokawa H., Osawa M., Kurihara H., Katayama N., Tokumitsu H., Swindells M. B., Kainosho M., Ikura M. (2001) J. Mol. Biol. 312, 59–68 [DOI] [PubMed] [Google Scholar]
- 20. Clapperton J. A., Martin S. R., Smerdon S. J., Gamblin S. J., Bayley P. M. (2002) Biochemistry 41, 14669–14679 [DOI] [PubMed] [Google Scholar]
- 21. Tokumitsu H., Soderling T. R. (1996) J. Biol. Chem. 271, 5617–5622 [DOI] [PubMed] [Google Scholar]
- 22. Tokumitsu H., Muramatsu M., Ikura M., Kobayashi R. (2000) J. Biol. Chem. 275, 20090–20095 [DOI] [PubMed] [Google Scholar]
- 23. Tokumitsu H., Iwabu M., Ishikawa Y., Kobayashi R. (2001) Biochemistry 40, 13925–13932 [DOI] [PubMed] [Google Scholar]
- 24. Davare M. A., Saneyoshi T., Guire E. S., Nygaard S. C., Soderling T. R. (2004) J. Biol. Chem. 279, 52191–52199 [DOI] [PubMed] [Google Scholar]
- 25. Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., Kobayashi R. (2002) J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 26. Kigawa T., Yabuki T., Matsuda N., Matsuda T., Nakajima R., Tanaka A., Yokoyama S. (2004) J. Struct. Funct. Genomics 5, 63–68 [DOI] [PubMed] [Google Scholar]
- 27. Kigawa T., Matsuda T., Yabuki T., Yokoyama S. (2007) Cell-free Protein Synthesis (Spirin A. S., Swartz J. R. eds) pp. 83–97, Wiley-VCH, Weinheim, Germany [Google Scholar]
- 28. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 29. Read R. J. (2001) Acta Crystallogr. D Biol. Crystallogr. 57, 1373–1382 [DOI] [PubMed] [Google Scholar]
- 30. Collaborative Computational Project, Number 4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–76315299374 [Google Scholar]
- 31. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 32. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 33. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 34. Holm L., Sander C. (1993) J. Mol. Biol. 233, 123–138 [DOI] [PubMed] [Google Scholar]
- 35. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schrödinger, LLC, New York [Google Scholar]
- 36. Cox S., Radzio-Andzelm E., Taylor S. S. (1994) Curr. Opin. Struct. Biol. 4, 893–901 [DOI] [PubMed] [Google Scholar]
- 37. Zheng J., Trafny E. A., Knighton D. R., Xuong N. H., Taylor S. S., Ten Eyck L. F., Sowadski J. M. (1993) Acta Crystallogr. D Biol. Crystallogr. 49, 362–365 [DOI] [PubMed] [Google Scholar]
- 38. Owen D. J., Noble M. E., Garman E. F., Papageorgiou A. C., Johnson L. N. (1995) Structure 3, 467–482 [DOI] [PubMed] [Google Scholar]
- 39. Rosenberg O. S., Deindl S., Sung R. J., Nairn A. C., Kuriyan J. (2005) Cell 123, 849–860 [DOI] [PubMed] [Google Scholar]
- 40. Johnson L. N., Noble M. E., Owen D. J. (1996) Cell 85, 149–158 [DOI] [PubMed] [Google Scholar]
- 41. Nolen B., Taylor S., Ghosh G. (2004) Mol. Cell 15, 661–675 [DOI] [PubMed] [Google Scholar]
- 42. Tokumitsu H., Inuzuka H., Ishikawa Y., Kobayashi R. (2003) J. Biol. Chem. 278, 10908–10913 [DOI] [PubMed] [Google Scholar]
- 43. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Cell Metabolism 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 44. Okuno S., Kitani T., Matsuzaki H., Konishi H., Kikkawa U., Fujisawa H. (2000) J. Biochem. 127, 965–970 [DOI] [PubMed] [Google Scholar]
- 45. Kitani T., Ishida A., Okuno S., Takeuchi M., Kameshita I., Fujisawa H. (1999) J. Biochem. 125, 1022–1028 [DOI] [PubMed] [Google Scholar]
- 46. Tokumitsu H., Takahashi N., Eto K., Yano S., Soderling T. R., Muramatsu M. (1999) J. Biol. Chem. 274, 15803–15810 [DOI] [PubMed] [Google Scholar]
- 47. Yang J., Cron P., Thompson V., Good V. M., Hess D., Hemmings B. A., Barford D. (2002) Mol. Cell 9, 1227–1240 [DOI] [PubMed] [Google Scholar]
- 48. White A., Pargellis C. A., Studts J. M., Werneburg B. G., Farmer B. T., 2nd (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6353–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gouet P., Courcelle E., Stuart D. I., Métoz F. (1999) Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 50. Potterton L., McNicholas S., Krissinel E., Gruber J., Cowtan K., Emsley P., Murshudov G. N., Cohen S., Perrakis A., Noble M. (2004) Acta Crystallogr. D 60, 2288–2294 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.