The prevalence of obesity continues to increase worldwide and represents one of the most pressing public health issues due to its associated morbidity, mortality, and health care costs. Novel research is demonstrating that alterations of the maternal environment can impact the intrauterine development of the fetus and influence the offspring’s risk for obesity and type 2 diabetes over the lifecourse. This article reviews the epidemiological evidence with a focus on the fetal overnutrition hypothesis. We discuss potential mechanisms and suggest future directions for research.
THE FETAL OVERNUTRITION PATHWAY TO OBESITY
While postnatal lifestyle is the most immediate cause of obesity, the influence of the maternal in utero environment is evidenced in the U- or J-shaped relationship between birth weight and adult obesity and metabolic disease demonstrating that both a nutritionally limited or excessive in utero environment can lead to postnatal obesity and related chronic diseases. Developmental biology has taught us about the role of a mismatch between a constrained prenatal and a plentiful postnatal environment in the pathogenesis of obesity, i.e., a so-called thrifty obesity pathway (1). This is likely operating in developing countries and populations undergoing rapid transition. Another developmental pathway to obesity, which is likely more important in Western societies, is the fetal overnutrition pathway. This pathway reflects the effects of hypernutrition during fetal life and creates the conditions for the later pathophysiological effects of an obesogenic environment (1). Although these developmental mechanisms are likely distinct, they are both associated with an increased risk of obesity later in life. This article focuses on the fetal overnutrition pathway.
Research in this field started with the hypothesis of fuel-mediated teratogenesis (2) and clinical studies suggesting that offspring exposed to diabetes in utero have greater birth weight and greater weight for length during childhood. Animal studies have demonstrated that the metabolic imprinting caused by the obese and diabetic intrauterine environment can be transmitted across generations. It has been suggested that a “vicious cycle” results, explaining the increases in obesity, gestational diabetes mellitus (GDM), and type 2 diabetes seen over the past several decades (3).
LONG-TERM CONSEQUENCES OF EXPOSURE TO MATERNAL DIABETES IN UTERO
Childhood growth, obesity, and the role of postnatal environment.
The role of exposure to diabetes in utero on infant and childhood growth has been examined in several studies. The offspring of Pima Indian women with preexistent type 2 diabetes and GDM were larger for gestational age at birth, and, after approximately 5 years of age, were heavier than the offspring of prediabetic or nondiabetic women (4). The Diabetes in Pregnancy study in Chicago has also reported excessive growth in multiethnic offspring of women with type 1 diabetes during pregnancy (5).
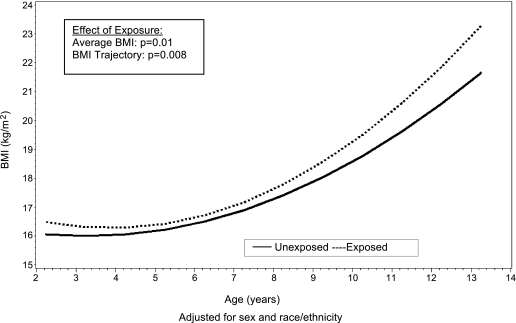
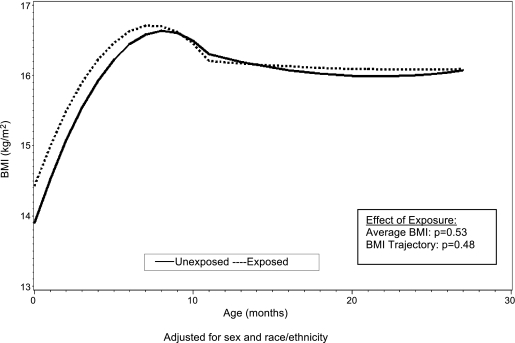
Similar, though somewhat less conclusive, findings were noted among youth of Caucasian origin in the Growing Up Today Study (6) and in the Avon Longitudinal Study of Parents and Children (ALSPAC) (7). Recently, the Exploring Perinatal Outcomes among Children (EPOCH) study found that exposure to maternal GDM was associated with higher BMI, waist circumference, both visceral and subcutaneous adipose tissue, and a more centralized fat distribution pattern in 6- to 13-year-old multiethnic youth (8). Moreover, youth exposed to maternal GDM in utero had an overall higher average BMI growth trajectory from 27 months through 13 years of age (Fig. 1) and higher BMI growth velocity starting at age 10 to 13 years (9). Importantly, no significant differences in growth trajectories between exposed and unexposed offspring were noted in infancy and early childhood (Fig. 2). These findings suggest that the long-term effects of in utero GDM exposure are not always evident in early childhood, but rather emerge during puberty, another sensitive period for the development of obesity.
FIG. 1.
Mean BMI curves for youth both exposed and unexposed to maternal diabetes in utero from 27 months of age to 13 years, adjusted for sex and race/ethnicity. From Crume et al., J Pediatrics 2011 (9), with permission.
FIG. 2.
Mean BMI curves for youth both exposed and unexposed to maternal diabetes in utero from birth to 26 months of age, adjusted for sex and race/ethnicity. From Crume et al., J Pediatrics 2011 (9), with permission.
The window for programming appears to extend into postnatal life. The early postnatal weeks of life, a time when breast-fed infants often lose weight and formula-fed infants tend to gain weight, are a crucial period for determining childhood adiposity (10). For offspring of pregnancies that carry a high risk for future obesity, early infant diet may represent an opportunity to influence long-term consequences. The EPOCH study recently demonstrated that offspring who were exposed to GDM and were breastfed for more than 6 months had lower overall and central adiposity measures at 6–13 years of age than those breastfed for <6 months (11). Since breastfeeding was also shown to protect against development of type 2 diabetes in the offspring in other reports (12), it follows that it may also attenuate the increased risk of type 2 diabetes associated with in utero exposure. These data suggest, as Plagemann and Harder (13) have recently stated, that “… we have to extend the historical concept of fuel-mediated teratogenesis beyond birth.”
Glucose abnormalities and type 2 diabetes.
In the Chicago Diabetes in Pregnancy study (5), 12-year-old offspring of mothers with type 1 diabetes and GDM had a significantly higher prevalence of impaired glucose tolerance than a nonconcurrent age- and sex-matched control group. Similarly, at every age before 20 years, offspring of Pima Indian women with type 2 diabetes and GDM had more type 2 diabetes than those of prediabetic and nondiabetic women (4). The higher rate of diabetes was partially mediated by the earlier development of obesity, but that did not account for the magnitude of the difference seen. Moreover, maternal diabetes was the strongest single risk factor for type 2 diabetes in Pima Indian youth, accounting for 40% of type 2 diabetes in that population (14). Indeed, among the Pima Indian children, the increase in the prevalence of type 2 diabetes was accounted for by the increase in the frequency of diabetic pregnancies (14), thus closing the postulated vicious cycle by which “diabetes begets diabetes.”
Data from the SEARCH Case-Control Study (15) provided novel evidence that intrauterine exposure to maternal diabetes and obesity are important determinants of type 2 diabetes in multiethnic youth. In this study, combined exposure to maternal diabetes and obesity in utero accounted for 47% of the type 2 diabetes risk in the young offspring. Similar data were recently reported among young adults from Denmark exposed to maternal type 1 diabetes in utero (16), suggesting that the long-term effects of exposure are similar regardless of diabetes type.
Despite mounting evidence that exposure to diabetes in utero is associated with type 2 diabetes in the offspring and may be responsible for the earlier onset of what was considered, until recently, a disease of adulthood, very little is known about how this exposure influences the metabolic abnormalities that are precursors of type 2 diabetes. Studies in newborns of diabetic mothers have shown an enhanced insulin secretion to a glycemic stimulus and β-cell hyperplasia (17). Impaired insulin secretion has also been proposed as a possible mechanism. Whether this is a transient phenomenon or leads to impaired glucose tolerance later in life when insulin resistance becomes important is still uncertain. Studies in humans are limited. Among Pima Indian adults, the acute insulin response to infused glucose was 40% lower in individuals whose mothers had diabetes during pregnancy than in those whose mothers developed diabetes after the birth of the subject (18). Longitudinal cohort studies are needed to explore how diabetes exposure in utero influences metabolic abnormalities that are predictors of type 2 diabetes.
Cardiovascular risk factors.
Animal studies have shown that exposure to diabetes in utero can induce cardiovascular dysfunction in the fetus and the adult offspring. However, few human studies have examined the effect of a diabetic intrauterine environment on cardiovascular risk factors in the offspring.
By 10–14 years of age, offspring of diabetic pregnancies enrolled in the Chicago study had higher blood pressure than offspring of nondiabetic pregnancies (19). Similar results were noted among 7- to 11-year-old Pima Indian offspring, independent of adiposity (20). Higher concentrations of markers of endothelial dysfunction (intracellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin), as well as increased cholesterol-to-HDL ratio were also reported among offspring of mothers with type 1 diabetes compared with offspring of nondiabetic pregnancies, independent of current BMI (21). Similarly, EPOCH offspring exposed to maternal GDM had significantly increased E-selectin, vascular cell adhesion molecule-1, and systolic blood pressure, and decreased adiponectin levels compared with nonexposed children (22). Together, these data suggest that in utero exposure to diabetes confers risks for the development of cardiovascular disease later in life that are independent of adiposity and may be in addition to genetic predisposition to diabetes. Studies are needed to explore the mechanisms through which exposure to diabetes in utero influences cardiovascular risk in the offspring.
IS MATERNAL OBESITY IMPORTANT IN THE VICIOUS CYCLE OF OBESITY?
There is increasing interest in the hypothesis that maternal obesity is associated with lifelong obesity and related metabolic consequences in offspring because “the obesity epidemic could accelerate through successive generations independent of further genetic or environmental factors” (23).
Epidemiological data suggest a direct association between maternal prepregnancy BMI and fetal growth, offspring BMI, or obesity in later life (24). Children exposed to maternal obesity are also at increased risk of developing type 2 diabetes (15). Moreover, studies have found that higher gestational weight gain (GWG) is associated with higher weight for height in infancy, childhood, and adolescence (25), with some suggestion that this association is stronger in obese mothers. These data suggest that increasing trends of maternal weight and GWG may generate an intergenerational vicious cycle of obesity and diabetes because heavier mothers give birth to heavier daughters, who are at increased risk to be obese themselves during their reproductive years, thus perpetuating the cycle. It remains to be determined whether improved weight gain patterns can be achieved throughout pregnancy that would prevent the short- and long-term consequences on the offspring described here.
WHAT IS THE ROLE OF MATERNAL DIET?
The role of maternal diet on fetal overnutrition has been less studied than its relationship with fetal undernutrition. However, several studies suggest that high energy intakes resulting in maternal obesity, and diets high in fat, cholesterol, and carbohydrates may influence fetal growth and development and have programming consequences. Offspring of rats on a high-fat diet had increased pancreatic β-cell mass, replication, and neogenesis, leading to hyperglycemia and type 2 diabetes in the adult life (26). Other experimental data in animals have shown that the consumption of a diet rich in saturated fat starting before conception and continuing through weaning leads to increased hyperinsulinemia, adiposity, hypertension, and endothelial dysfunction in offspring at 6 months of age (27). AMP-activated protein kinase signaling pathways were shown to be downregulated, and skeletal muscle development was impaired in fetuses of obese, overnourished sheep (fed 150% of National Research Council recommendations) (28).
In humans, limited evidence suggests that maternal nutritional intake is associated with maternal insulin resistance and fetal weight. Clapp et al. (29) demonstrated that infants of mothers on high-glycemic index diets had higher birth weight and skinfold thickness than those exposed to a low-glycemic diet. Additional research is needed to understand whether maternal diet and specific nutrient intakes during pregnancy have effects on neonatal growth, body composition, and metabolism that can be specifically targeted by future interventions.
EVIDENCE FOR SPECIFIC INTRAUTERINE EFFECTS
Several mechanisms that are not mutually exclusive may explain the associations between exposure to maternal diabetes/obesity in utero and long-term metabolic outcomes in the offspring. These include shared genes, shared familial socioeconomic and lifestyle factors, as well as specific intrauterine effects.
Type 2 diabetes is a disease with familial clustering and clearly has a genetic component. In particular, early-onset (<35 years of age) type 2 diabetes is associated with a more severe insulin secretion defect than older-onset diabetes, suggesting that there may be genes associated with both early-onset diabetes and more pronounced reduction in glucose-stimulated insulin secretion. Similarly, a strong body of literature suggests that BMI variability within a population is largely due to heritable genetic differences.
However, work from the Pima Indian population indicates that the effect of maternal diabetes on offspring long-term outcomes is not fully explained by genetic factors. Dabelea et al. (30) compared outcomes in siblings discordant for intrauterine exposures. Among 21 of the 28 Pima sib pairs discordant both for type 2 diabetes and diabetes exposure in utero, the diabetic sibling was born after the mother’s diagnosis of diabetes, and in only 7 of the 28 pairs, the diabetic sibling was born before (odds ratio 3.0, P < 0.01) (30). Moreover, in this study the mean BMI in Pima Indian siblings born after the onset of the mother’s diabetes was significantly higher (by 2.6 kg/m2) than among age-matched siblings who were not exposed to diabetes in utero (30). In another study, the prevalence of obesity among 2- to 18-year-old siblings born after maternal bariatric surgery was 52% lower than among age-matched siblings born when their mother was obese (31). Because siblings discordant for intrauterine exposures carry a similar risk of inheriting the same susceptibility genes and share a similar postnatal environment, such studies provide strong evidence that the excess risk of childhood obesity and type 2 diabetes associated with fetal overnutrition reflects a specific intrauterine effect.
WHAT ARE THE SPECIFIC INTRAUTERINE EFFECTS?
The mechanisms responsible for these effects are not clearly understood but represent an area of intense research, spanning from animal models to human epidemiological and mechanistic studies and, more recently, epigenetic research. Although considerable progress has been made toward a better understanding of how fetal overnutrition operates, there are still numerous unanswered questions and opportunities for future research.
What is the role of maternal fuels?
Hyperglycemia.
The fuel-mediated teratogenesis hypothesis was first proposed to explain the association between maternal hyperglycemia in pregnancy and excessive growth in the developing fetus. While maternal glucose freely crosses the placenta to the fetus, maternal insulin does not. The developing fetal pancreas responds to the glucose load by producing additional insulin, which in turn acts as a fetal growth hormone promoting growth and adiposity.
Maternal hyperglycemia, extreme enough to be recognized as GDM, is a clear risk factor for macrosomia. However, most macrosomic infants are not born to mothers diagnosed with GDM. Several studies suggest that the relation between glycemia during pregnancy and infant body size is linear. In a study of 6,854 consecutive pregnant women screened for GDM, increasing glucose concentration at screening was associated with higher prevalence of macrosomia (32). In a large community-based study in Mysore, South India, maternal fasting glucose at 30 weeks of gestation was positively associated with infant birth weight, ponderal index, and head circumference (33). In the multinational Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study, there was a linear association between maternal glucose levels during pregnancy and macrosomia, serum C-peptide, and neonatal adiposity, without evidence of a specific cut point (34), indicating the need to reconsider current GDM diagnostic criteria.
Although the immediate effects of maternal glucose on fetal growth are well described, the long-term consequences on the offspring are less clear. A recent analysis of the HAPO study found no relationship between maternal glucose levels and child obesity at age 2 years (35). However, Hillier et al. (36) reported a dose-response relationship between levels of maternal hyperglycemia in pregnancy and offspring obesity at age 5–7 years in a retrospective cohort study of 9,439 Kaiser Permanente mother-child pairs, even after controlling for GWG. Chandler-Laney et al. (37) also found a dose-response relationship between maternal glucose concentrations during pregnancy and prepubertal offspring lean and fat mass (P < 0.001). However, in a recent follow-up study (38) of a randomized clinical trial in Australia, intensive treatment of mild GDM during pregnancy did not result in BMI differences in offspring at 4–5 years of age, although it substantially reduced macrosomia, raising doubts about the effectiveness of a pregnancy intervention based on tight glycemic control in preventing childhood obesity. There are several alternative explanations for this negative result. As suggested by EPOCH, it is possible that the effects of exposure to diabetes in pregnancy on offspring BMI only become apparent during early adolescence when exposed offspring transition through puberty (9). It is also possible that the detrimental effects of in utero exposures are affecting other markers of fatness than BMI, especially early in life. Finally, it is likely that future pregnancy interventions need to control for the effects of other fuels, in addition to glucose, which may be altered in obese and diabetic pregnancies and may have programming consequences.
Other fuels.
Descriptive evidence suggests that alterations in maternal lipid metabolism may also contribute to the fuel-mediated teratogenesis. Free fatty acids (FFAs) and triglycerides have been shown to be increased in mothers of obese neonates and because of the limited de novo lipogenic capacity of the fetus, most precursors for fetal fat accretion are supplied by transplacental transfer of maternal substrates derived from lipids. Placental lipoprotein lipase hydrolyzes triglycerides to FFA that can then be transferred to the fetus. Lipids play a role in adipocyte differentiation, therefore increased FFA transfer could affect fetal adiposity by influencing the number, size, or lipoprotein lipase activity of fetal fat cells. Limited studies in humans suggest that, in well-controlled GDM pregnancies, maternal lipids are strong predictors for fetal lipids and fetal growth (39). Epidemiological, clinical, and mechanistic studies are needed to test the hypothesis that programming of adiposity is, at least in part, mediated through an abnormal maternal lipid supply to the fetus.
What is the role of the adipoinsular axis?
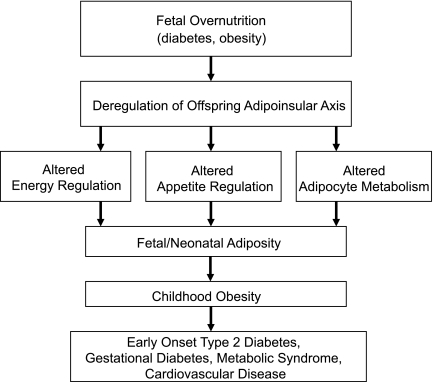
The adipoinsular axis is an endocrine feedback loop that connects the endocrine pancreas with adipose tissue and the brain to regulate hunger and fat storage through the hormones insulin and leptin. Insulin promotes development of fat mass and leptin production, while leptin acts to reduce energy intake and also suppresses insulin secretion via leptin receptors on pancreatic β-cells. Abnormal functioning of this feedback loop may therefore lead to excess adiposity, hyperphagia, and dysregulation of the energy balance regulating systems (40) (Fig. 3).
FIG. 3.
Potential pathways linking fetal overnutrition to long-term consequences in the offspring. Modified from McMillen, Reproduction 2006 (40), with permission.
Hyperinsulinemia has been reported in fetuses exposed to diabetes in utero. High amniotic fluid insulin levels are associated with increased obesity and impaired glucose tolerance in children exposed to a diabetic intrauterine environment (5). Fetal hyperinsulinemia is adipogenic in late fetal and early infant life and is hypothesized to increase both fat cell number and size. Animal studies have also suggested that fetal hyperinsulinemia may lead to permanent morphological changes within central nervous system nuclei regulating metabolism and body weight. Permanent dysplasia and pronounced neuronal hypotrophy have been shown to occur within the ventromedial hypothalamic nucleus as a result of exposure to increased insulin concentrations during fetal development (41). Permanent disruption of hypothalamic structures has been demonstrated in animals treated with insulin peripherally or intrahypothalamically (42). No studies in humans have specifically explored these mechanisms.
In addition to the alterations described in fetal insulin secretion, there is evidence that fetal leptin concentrations are affected by maternal diabetes during pregnancy. Fetal leptin is produced by adipocytes and by the placenta, and cord blood concentrations positively correlate with birth weight and adiposity. Elevated cord blood leptin concentrations were found in both infants of type 1 diabetic and GDM mothers as compared with control subjects (43), even after controlling for differences in birth weight, suggesting a direct influence of maternal hyperglycemia on neonatal fat mass and leptin levels. Exposure to GDM was associated with both hyperleptinemia and hyperinsulinemia in the newborn (44). It is possible that exposure to GDM may lead to increased insulin secretion and adiposity in the infant despite the rising plasma leptin concentrations, which are unable to control the release of insulin and weight gain (44). Leptin resistance or altered leptin signaling in the postnatal life may therefore manifest as an inability of rising plasma leptin concentrations to control the release of insulin and/or to reduce body size and fat mass. No human studies have linked insulin and leptin levels at birth and postnatally to longitudinal measures of obesity among offspring exposed to overnutrition in utero in order to test these hypotheses.
What is the role of epigenetics?
Epigenetic processes control genetic function without changing DNA sequence. DNA methylation, genomic imprinting, and chromatin changes involving modifications in histones H3 and H4 are types of posttranslational DNA modifications that result in differential levels of gene expression without altering DNA sequence. The epigenome is particularly susceptible to alterations during gestation because the DNA synthesis rate is high and the DNA methylation patterning required for normal tissue development is established during this period.
Epigenetic modifications in response to overnutrition may lead to metabolic imprinting or permanent alterations in genes involved in the regulation of energy homeostasis. Several such genes have been shown to be regulated by DNA methylation and histone modifications, including genes for leptin (45), SOCS3 (46), and glucose transporter (47). Animal studies have characterized an obese phenotype in cloned mice that do not transmit obesity to their progeny through natural mating, indicating that an epigenetic modification rather than a genetic change may be responsible for producing the obese phenotype. Recently, Bouchard et al. (48) found maternal hyperglycemia to be correlated with placental leptin gene DNA methylation levels. These findings suggest that that the intrauterine environment of diabetic and obese women may impact the epigenome of the offspring and lend support to further studies in this area.
FUTURE DIRECTIONS FOR RESEARCH
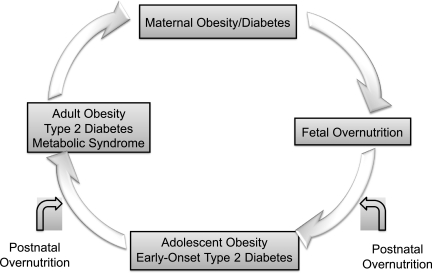
The effects of overnutrition in utero over the lifecourse may be viewed as a vicious cycle (Fig. 4) (3,23). Children who were exposed to maternal diabetes and/or obesity during pregnancy are at increased risk of becoming obese and developing type 2 diabetes at young ages. Many of these female offspring are already obese and have diabetes or abnormal glucose tolerance by the time they reach their childbearing years, thereby perpetuating the cycle. Across generations, this cycle is likely increasing the risk and/or accelerating the onset of obesity and type 2 diabetes.
FIG. 4.
The vicious cycle of diabetes and obesity.
However, the specific mediators of these long-term effects and the likely mechanisms through which they operate remain unclear. Studies on relevant animal models with rapid translation by human mechanistic studies are necessary to understand the effects of maternal fuels and the contributions of maternal diet and body status on fetal target tissues. With the recent development of high-throughput methylation profiling assays, analysis of DNA methylation can now provide unique insight into the role of epigenetics as a mechanistic link between early life overnutrition and subsequent pathophysiology. The future of epigenetic therapy holds tremendous potential not only for individualized health care but also for population-wide screening, diagnostic, and prevention strategies.
The role of the postnatal environment and the interaction between fetal overnutrition and other postnatal obesogenic environments and/or developmental periods also requires further study. Longitudinal cohort studies starting during gestation and spanning childhood and adolescence, such as ALSPAC (7), Project Viva (49), EPOCH (11), a newly established prebirth cohort in Colorado (Healthy Start), and, mostly notably, the National Children’s Study (50) are needed to rapidly translate the knowledge provided by basic research and to provide the necessary platform for addressing some of these crucial questions.
Finally, randomized trials are needed to determine whether improved weight gain patterns can be achieved throughout pregnancy that would prevent the consequences on the offspring described in this article. If this is achievable, it will in turn probably reduce the prevalence of obesity and type 2 diabetes in the next generation and, therefore, be beneficial for future generations as well as for the immediate offspring.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
D.D. wrote the manuscript, reviewed and edited the manuscript, and contributed to discussion. T.C. researched the data, reviewed and edited the manuscript, and contributed to discussion.
REFERENCES
- 1.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 2008;32(Suppl. 7):S62–S71 [DOI] [PubMed] [Google Scholar]
- 2.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [review] [DOI] [PubMed] [Google Scholar]
- 3.Pettitt DJ, Knowler WC. Diabetes and obesity in the Pima Indians: a crossgenerational vicious cycle. J Obesity Weight Regul 1988;7:61–65 [Google Scholar]
- 4.Pettitt DJ, Nelson RG, Saad MF, Bennett PH, Knowler WC. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care 1993;16:310–314 [DOI] [PubMed] [Google Scholar]
- 5.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers: relationship to fetal hyperinsulinism. Diabetes Care 1995;18:611–617 [DOI] [PubMed] [Google Scholar]
- 6.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111:e221–e226 [DOI] [PubMed] [Google Scholar]
- 7.Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010;53:89–97 [DOI] [PubMed] [Google Scholar]
- 8.Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 2011;54:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 14 January 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 10.Stettler N, Stallings VA, Troxel AB, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation 2005;111:1897–1903 [DOI] [PubMed] [Google Scholar]
- 11.Crume TL, Ogden L, Maligie M, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care 2011;34:641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer-Davis EJ, Dabelea D, Lamichhane AP, et al. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCH for Diabetes in Youth Case-Control Study. Diabetes Care 2008;31:470–475 [DOI] [PubMed] [Google Scholar]
- 13.Plagemann A, Harder T. Fuel-mediated teratogenesis and breastfeeding. Diabetes Care 2011;34:779–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of type II diabetes in American Indian children. Diabetologia 1998;41:904–910 [DOI] [PubMed] [Google Scholar]
- 15.Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 2008;31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 17.Heding LG, Persson B, Stangenberg M. B-cell function in newborn infants of diabetic mothers. Diabetologia 1980;19:427–432 [DOI] [PubMed] [Google Scholar]
- 18.Gautier JF, Wilson C, Weyer C, et al. Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 2001;50:1828–1833 [DOI] [PubMed] [Google Scholar]
- 19.Silverman BL, Rizzo T, Green OC, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 1991;40(Suppl. 2):121–125 [DOI] [PubMed] [Google Scholar]
- 20.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in Pima Indian children. J Clin Endocrinol Metab 2005;90:3225–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetol 2002;45:991-996 [DOI] [PubMed]
- 22.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011;54:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab 2003;88:3505–3506 [DOI] [PubMed] [Google Scholar]
- 24.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics 2005;116:1329–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 2008;112:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gniuli D, Calcagno A, Caristo ME, et al. Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. J Lipid Res 2008;49:1936–1945 [DOI] [PubMed]
- 27.Khan IY, Dekou V, Douglas G, et al. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol 2005;288:R127–R133 [DOI] [PubMed] [Google Scholar]
- 28.Zhu MJ, Han B, Tong J, et al. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 2008;586:2651–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapp JF, 3rd, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol 2002;186:142–147 [DOI] [PubMed] [Google Scholar]
- 30.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 31.Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 2006;118:e1644-e1649 [DOI] [PubMed]
- 32.Yogev Y, Langer O, Xenakis EM, Rosenn B. The association between glucose challenge test, obesity and pregnancy outcome in 6390 non-diabetic women. J Matern Fetal Neonatal Med 2005;17:29–34 [DOI] [PubMed] [Google Scholar]
- 33.Hill JC, Krishnaveni GV, Annamma I, Leary SD, Fall CH. Glucose tolerance in pregnancy in South India: relationships to neonatal anthropometry. Acta Obstet Gynecol Scand 2005;84:159–165 [DOI] [PubMed] [Google Scholar]
- 34.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 35.Pettitt DJ, McKenna S, McLaughlin C, Patterson CC, Hadden DR, McCance DR. Maternal glucose at 28 weeks of gestation is not associated with obesity in 2-year-old offspring: the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) family study. Diabetes Care 2010;33:1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–2292 [DOI] [PubMed] [Google Scholar]
- 37.Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care 2011;34:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction 2006;131:415–427 [DOI] [PubMed] [Google Scholar]
- 41.Dörner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res 1994;26:213–221 [DOI] [PubMed] [Google Scholar]
- 42.Plagemann A, Heidrich I, Götz F, Rohde W, Dörner G. Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp Clin Endocrinol 1992;99:91–95 [DOI] [PubMed] [Google Scholar]
- 43.Persson B, Westgren M, Celsi G, Nord E, Ortqvist E. Leptin concentrations in cord blood in normal newborn infants and offspring of diabetic mothers. Horm Metab Res 1999;31:467–471 [DOI] [PubMed] [Google Scholar]
- 44.Simmons D, Breier BH. Fetal overnutrition in polynesian pregnancies and in gestational diabetes may lead to dysregulation of the adipoinsular axis in offspring. Diabetes Care 2002;25:1539–1544 [DOI] [PubMed] [Google Scholar]
- 45.Melzner I, Scott V, Dorsch K, et al. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. J Biol Chem 2002;277:45420–45427 [DOI] [PubMed] [Google Scholar]
- 46.Campión J, Milagro FI, Martínez JA. Individuality and epigenetics in obesity. Obes Rev 2009;10:383–392 [DOI] [PubMed] [Google Scholar]
- 47.Yokomori N, Tawata M, Onaya T. DNA demethylation during the differentiation of 3T3-L1 cells affects the expression of the mouse GLUT4 gene. Diabetes 1999;48:685–690 [DOI] [PubMed] [Google Scholar]
- 48.Bouchard L, Thibault S, Guay SP, et al. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care 2010;33:2436–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196:322–328, e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landrigan PJ, Trasande L, Thorpe LE, et al. The National Children’s Study: a 21-year prospective study of 100,000 American children. Pediatrics 2006;118:2173–2186 [DOI] [PubMed] [Google Scholar]