Abstract
OBJECTIVE
Circulating glucose inhibits glucose production in normal rodents and humans, but this glucose effectiveness is disrupted in diabetes due partly to sustained hyperglycemia. We hypothesize that hyperglycemia in diabetes impairs hypothalamic glucose sensing to lower glucose production, and changes of glucose transporter-1 (GLUT1) in the hypothalamic glial cells are responsible for the deleterious effects of hyperglycemia in vivo.
RESEARCH DESIGN AND METHODS
We tested hypothalamic glucose effectiveness to increase hypothalamic glucose concentration and lower glucose production in rats induced with streptozotocin (STZ) uncontrolled diabetes, STZ and phlorizin, and whole-body and hypothalamic sustained hyperglycemia. We next assessed the content of glial GLUT1 in the hypothalamus, generated an adenovirus expressing GLUT1 driven by a glial fibrillary acidic protein (GFAP) promoter (Ad-GFAP-GLUT1), and injected Ad-GFAP-GLUT1 into the hypothalamus of rats induced with hyperglycemia. Pancreatic euglycemic clamp and tracer-dilution methodologies were used to assess changes in glucose kinetics in vivo.
RESULTS
Sustained hyperglycemia, as seen in the early onset of STZ-induced diabetes, disrupted hypothalamic glucose sensing to increase hypothalamic glucose concentration and lower glucose production in association with reduced GLUT1 levels in the hypothalamic glial cells of rats in vivo. Overexpression of hypothalamic glial GLUT1 in STZ-induced rats with reduced GLUT1 acutely normalized plasma glucose levels and in rats with selectively induced hypothalamic hyperglycemia restored hypothalamic glucose effectiveness.
CONCLUSIONS
Sustained hyperglycemia impairs hypothalamic glucose sensing to lower glucose production through changes in hypothalamic glial GLUT1, and these data highlight the critical role of hypothalamic glial GLUT1 in mediating glucose sensing to regulate glucose production.
Sustained hyperglycemia per se disrupts glucose homeostasis (1). When hyperglycemia is normalized in diabetic rodents (2–4) and humans (5), insulin action, β-cell insulin secretion, and hepatic glucose production regulation are restored in diabetes. With respect to glucose production regulation, an increase in circulating glucose levels inhibits glucose production in normal rodents (6) and humans (5,7,8) independent of changes in plasma insulin levels. This glucose effectiveness in lowering glucose production is completely disrupted in diabetic rodents (6) and humans (7). Importantly, normalization of plasma glucose levels in diabetic rodents with an oral inhibitor of the sodium-dependent glucose transporter (4) and in diabetic humans with a 3-day insulin treatment (5) rescues glucose effectiveness to lower glucose production.
To date, the mechanisms responsible for the deleterious effect of sustained hyperglycemia on glucose production regulation remain unclear. In light of recent discoveries that the central nervous system (CNS) (9,10), in particular hypothalamic glucose sensing (11), lowers glucose production in rodents, we here investigated whether sustained hyperglycemia impairs CNS glucose sensing to regulate glucose production and whether changes of glucose transporter-1 (GLUT1) in the hypothalamic glial cells are responsible for the deleterious metabolic effects of sustained hyperglycemia in vivo (Fig. 1A).
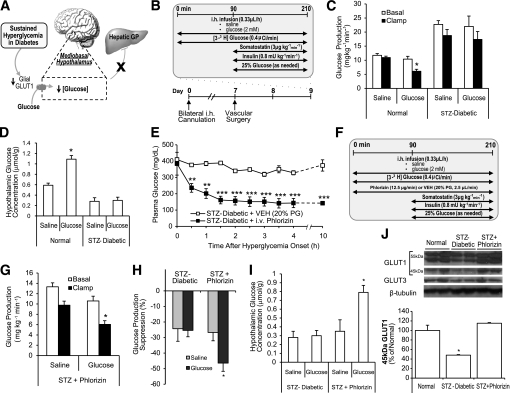
FIG. 1.
STZ-induced hyperglycemia reduces glial GLUT1 in the hypothalamus and inhibits CNS glucose effectiveness to elevate hypothalamic glucose concentration and lower glucose production. A: Schematic representation of the working hypothesis. Sustained hyperglycemia in diabetes disrupts hypothalamic glucose sensing to increase hypothalamic glucose concentration and lower GP through a reduction of hypothalamic glial GLUT1. B: Experimental protocol. C: GP. *P < 0.0001 vs. other clamp groups. D: Hypothalamic glucose concentration. *P < 0.001 vs. other groups. E: Continuous infusion of intravenous phlorizin rapidly normalized and maintained plasma glucose. **P < 0.01; ***P < 0.001 vs. control. F: Pancreatic clamp protocol modified to include the concurrent infusion of phlorizin or vehicle (VEH). G: GP. *P < 0.01 vs. intrahypothalamic saline-infused STZ + phlorizin rats. H: Percent suppression of glucose production from basal. *P < 0.05 vs. other groups. I: Hypothalamic glucose concentration. *P < 0.05 vs. other groups. J: Expression of GLUT1 and GLUT3 proteins in wedges of the MBH was assessed via immunoblotting and quantified using densitometry. *P < 0.05 vs. other groups. GP, glucose production; i.h., intrahypothalamic; i.v., intravenous; PG, plasma glucose.
RESEARCH DESIGN AND METHODS
Animal preparation.
SD male rats aged 8 weeks weighing 250–300 g were studied. Rats were subjected to a standard light-dark cycle and were maintained on a regular chow and had ad libitum access to distilled water. Indwelling catheters were placed in the right internal jugular vein and left carotid artery for infusion and sampling purposes. Rats were implanted with bilateral intrahypothalamic catheters placed within the mediobasal hypothalamus (MBH) as previously described (12,13). The coordinates used were 3.1 mm posterior from bregma, 0.4 mm lateral of midline, and 9.6 mm below skull surface. Recovery between surgical procedures was monitored by measuring daily food intake and body weight gain. To ensure comparable postabsorptive nutritional status, rats were limited to 20 g of food the day before experimentation. All study protocols were approved by the Institutional Animal Care and Use Committee of University Health Network.
Pancreatic (basal insulin) euglycemic clamps.
An infusion of intrahypothalamic vehicle (0.9% wt/vol NaCl) or 2 mmol/L glucose was maintained throughout the experiments. A primed-continuous infusion of [3-3H]glucose (Perkin Elmer; 40 μCi bolus; 0.4 μCi/min thereafter) was initiated at 0 min and maintained through the study to assess glucose kinetics via the use of the tracer-dilution methodology. After a basal period of 90 min, a basal pancreatic insulin clamp was performed for the final 2 h (90–210 min) of the study; a continuous coinfusion of insulin (0.8 mU/kg/min) along with somatostatin (3 μg/kg/min) was administered, and a variable infusion of 25% glucose solution was administered as needed to clamp and maintain the plasma glucose concentration at levels similar to the basal state. Plasma samples were collected every 10 min for determination of [3-3H]glucose specific activity as well as hormone levels.
Hypothalamic glucose level measurements.
MBH (10–12 mg) tissues were harvested at the end of the clamp studies and frozen in liquid nitrogen. MBH tissues were subsequently weighed and homogenized in buffer to a final volume of 15 µL (2 mmol/L Tris-HCl and 1 mmol/L EDTA, pH 7) as previously described (14). The homogenate (10 µL) was pipetted into an Analox Glucose Analyzer (Analox, London, U.K.), and glucose concentration in the homogenate was determined based on a glucose oxidase method. Readings in micromoles per liter obtained from the glucose analyzer were then converted to micromoles per gram of tissue based on the total volume of homogenate and the corresponding tissue weight (early-onset uncontrolled diabetic mode [with or without phlorizin]; whole-body and hypothalamic sustained hyperglycemic models; and construction of glial-specific adenoviruses, cell culture, and in vitro cell infection; see Supplementary Data).
Adenovirus-expressing rats with uncontrolled diabetes.
Rats received 6 μL (3 μL per side) of purified glial-specific adenovirus (GLUT1-expressing Ad-GFAP-GLUT1 or control Ad-GFAP-LacZ) via the intrahypothalamic cannula at the time of the surgery. Tissue harvesting after 4 days revealed an overexpression of the adenovirus selectively in MBH glial cells (Fig. 3). A group of rats were administered intravenous streptozotocin (STZ) (60 mg/kg) at the time of vascular catheterization 2 days after administration of intrahypothalamic Ad-GFAP-LacZ or Ad-GFAP-GLUT1. Rats received an additional dose of STZ 20 h after the initial dose to ensure rapid development of uncontrolled diabetes.
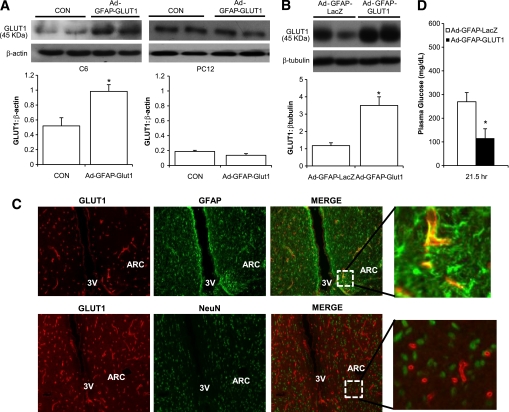
FIG. 3.
Overexpressing glial GLUT1 in the hypothalamus of STZ-induced rats acutely normalizes plasma glucose levels. A: C6 (glial) and PC12 (neuronal) cells were transduced with adenovirus expressing GLUT1 with a GFAP promoter (Ad-GFAP-GLUT1). GLUT1 expression was measured 48-h post-transduction via immunoblotting. Significant GLUT1 overexpression was detected in C6 (*P < 0.05) but not in PC12 cells relative to control cell (CON). B: Hypothalamic tissues were obtained from rats injected with adenovirus expressing LacZ with a GFAP promoter (Ad-GFAP-LacZ) or Ad-GFAP-GLUT1 for 4 days. GLUT1 expression showed a 3.5-fold increase in GLUT1 expression in rat brains injected with GLUT1 (n = 4) adenovirus vs. LacZ (n = 4) adenoviral injection. *P < 0.05. C: Rat hypothalamic frozen sections obtained 4 days after the injection of Ad-GFAP-GLUT1. Sections were stained with anti-GLUT1, anti-GFAP, and anti-NeuN antibodies. Coimmunostaining indicated that the GLUT1 signals colocalized with GFAP, but not NeuN staining, in the arcuate nucleus (ARC). D: Plasma glucose levels in STZ-injected rats harboring Ad-GFAP-LacZ or Ad-GFAP-GLUT1. *P < 0.01 vs. MBH control Ad-GFAP-LacZ–injected rats. (A high-quality color representation of this figure is available in the online issue.)
Adenovirus-expressing rats with hypothalamic hyperglycemia.
One day before the start of the 24-h infusion of 4 mmol/L glucose, Ad-GFAP-LacZ or Ad-GFAP-GLUT1 was injected 3 µL per side via the intrahypothalamic cannula. The 24-h intrahypothalamic infusion and the clamp studies were subsequently conducted as described in Supplementary Methods.
Immunofluorescence.
Whole rat brains were fixed via transcardial perfusion with PBS and then 4% PFA, and brain sections containing the MBH were placed in OCT-filled disposable base molds and frozen. With the use of a cryostat (Leica CM1950), frozen sections were cut at a thickness of 10 μm. The slides were permeabilized for 10 min with 0.5% Tween, followed by 1× PBS washes. Slides underwent blocking for 1 h with 10% goat serum. Primary antibodies (overnight 4°C) used were chicken anti-β β–galactosidase (LacZ) from Abcam (1:100), mouse anti-GFAP from Sigma (1:200 for LacZ + GFAP costaining, 1:300 for GLUT1 + GFAP costaining), rabbit anti-GLUT1 from Alpha Diagnostic (1:100), and mouse anti-NeuN from Chemicon (1:400). Secondary antibodies (1 h) used were Alexa Fluor 488 anti-mouse (1:500), Alexa Fluor 546 anti-rabbit (1:500), and Alexa Fluor 546 anti-chicken (1:1,000). The slides were read with an Olympus fluorescence microscope.
Biochemical analyses.
Plasma glucose concentrations were measured via the glucose oxidase method, using an Analox Glucose Analyzer (Analox, London, U.K.). Plasma glucose tracer ([3-3H]glucose) specific activity was determined in deproteinized plasma samples with a scintillation counter (Beckman Coulter LS6500), and [3-3H]glucose measurements were used to determine the rates of glucose kinetics using steady state formulas. Radioimmunoassays (RIA) were used to determine the plasma concentrations of insulin and glucagon (kits RI-13 K and GL-32K; Linco Research, St. Charles, MO).
Calculations.
Statistical analysis was done by two-way ANOVA to compare across the groups, followed by a Tukey post hoc test to compare between groups. Data are presented as means ± SEM. The presented basal values for glucose concentration and rates of glucose fluxes are averages for times 60–90 min, whereas those for the clamp period are the averages for the final 30 min (180–210 min) of the procedure.
RESULTS
Sustained hyperglycemia in uncontrolled diabetes reduces hypothalamic glucose sensing and glial GLUT1.
In the presence of hypoinsulinemia and basal glucagon levels (Supplementary Fig. 1), basal glucose production was significantly (P < 0.001) elevated in STZ-induced diabetic rats receiving intrahypothalamic saline versus normal rats (Fig. 1B and C). During the clamps, intrahypothalamic glucose (2 mmol/L) failed to lower glucose production (Fig. 1C) and increase hypothalamic glucose concentration (Fig. 1D) in STZ-induced diabetic rats as compared with intrahypothalamic glucose in normal rats, which was effective in lowering glucose production (Fig. 1C) and increasing hypothalamic glucose concentration (Fig. 1D). Glucose uptake was comparable within groups (Supplementary Fig. 2).
We normalized glucose in diabetes and evaluated CNS glucose effectiveness. We infused intravenous phlorizin to normalize glucose in STZ-injected rats for 24 h (Fig. 1E), and during the clamps intrahypothalamic glucose lowered glucose production (Fig. 1G and H). Importantly, the ability of intrahypothalamic glucose to elevate hypothalamic glucose concentration was restored in STZ-induced diabetic rats that received phlorizin (Fig. 1I). Thus lowering of glucose levels per se in diabetes restores CNS glucose effectiveness.
MBH wedges were obtained from the STZ-induced diabetic rats, and the protein levels of glial GLUT1 (45 kDa) were significantly reduced compared with normal (Fig. 1J). A nonsignificant trend toward reduction of the neuronal GLUT3 (Fig. 1J; 27.6 ± 7.3% reduction from control; P = 0.11) and the more heavily glycosylated BBB endothelial isoforms of GLUT1 (55 kDa; Fig. 1J; 18.5 ± 10.8% reduction [doublet pooled in measurement] from control; P = 0.45) was observed. The significant reduction of hypothalamic glial GLUT1 in STZ-induced diabetic rats was reversed when hyperglycemia per se is normalized in STZ-induced rats that received intravenous phlorizin infusion (Fig. 1J). Thus hyperglycemia disrupts CNS glucose effectiveness in association with a reduction in hypothalamic glial GLUT1.
Whole body or hypothalamic hyperglycemia impairs hypothalamic glucose sensing.
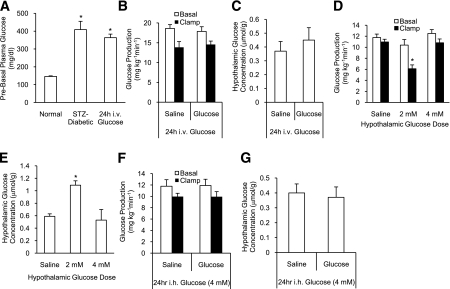
We infused intravenous glucose into normal rats and mimicked the hyperglycemic insult in uncontrolled diabetes (Fig. 2A). Before the clamps, the glucose infusion was stopped and the plasma glucose levels were normalized in 1–1.5 h. Glucose intrahypothalamic failed to lower glucose production (Fig. 2B) and increase hypothalamic glucose concentration (Fig. 2C) in 24-h intravenous glucose-infused rats. Another group of normal rats instead received intrahypothalamic glucose for 24 h to induce hypothalamic hyperglycemia. To select the glucose concentration to be used, we first performed dose-response clamps for intrahypothalamic glucose. Intrahypothalamic glucose (4 mM) for 210 min was unable to increase hypothalamic glucose concentration and lower glucose production as compared with 2 mmol/L intrahypothalamic glucose (Fig. 1B–D and Fig. 2D and E). This inability of intrahypothalamic glucose (4 mmol/L) to increase hypothalamic glucose concentration was also seen in rats with either diabetes or whole-body hyperglycemia when intrahypothalamic glucose (2 mmol/L) was administered (Fig. 1D and Fig. 2C). Consequently, we determined to infuse glucose intrahypothalamically at 4 mmol/L to induce hypothalamic hyperglycemia.
FIG. 2.
Whole-body and hypothalamic sustained hyperglycemia impair CNS glucose effectiveness to increase hypothalamic glucose concentration and lower glucose production. A: Plasma glucose levels before clamp studies after the 24-h intravenous infusion period (whole-body glucotoxicity). *P < 0.01 vs. control. B: Glucose production. C: Hypothalamic glucose concentration. D: Glucose production. *P < 0.0001 vs. other groups. E: Hypothalamic glucose concentration. *P < 0.05 vs. other groups. F: Glucose production. G: Hypothalamic glucose concentration. i.h., intrahypothalamic; i.v., intravenous.
Although the 24-h intrahypothalamic glucose (4 mmol/L)-infused rats did not display elevated plasma glucose levels, the intrahypothalamic glucose (4 mmol/L) infusion was still terminated 1.5 h before the start of the clamps to keep consistent with 24-h intravenous glucose infused rats. This 24-h hypothalamic glucose challenge negated the ability of intrahypothalamic glucose to lower glucose production (Fig. 2F) and increase hypothalamic glucose concentration (Fig. 2G). Thus an induction of whole-body or hypothalamic hyperglycemia impairs CNS glucose effectiveness.
Overexpressing hypothalamic glial GLUT1 in vitro and in vivo.
GLUT1 in the MBH glial cells regulates glucose uptake from the extracellular to the intracellular compartment. If glucotoxicity disrupts CNS glucose sensing via a reduction in hypothalamic glial GLUT1 and consequently hypothalamic intracellular glucose concentration, then rescuing the reduction of hypothalamic glial GLUT1 in rats with sustained hyperglycemia could restore CNS glucose effectiveness. We generated an adenovirus expressing LacZ driven by a glial fibrillary acidic protein (GFAP) promoter (Ad-GFAP-LacZ). Transduction of Ad-GFAP-LacZ in C6 glial cells, but not PC12 neuronal cells, revealed strong β-galactosidase staining (Supplementary Fig. 3A). Direct injection of Ad-GFAP-LacZ into the MBH expressed LacZ in GFAP-expression cells in the arcuate nucleus in vivo (Supplementary Fig. 3B). GFAP was colocalized with 98.8 + 1.2% of LacZ (n = 4). We then constructed an adenovirus expressing GLUT1 driven by GFAP (Ad-GFAP-GLUT1). Transduction of Ad-GFAP-GLUT1 in C6 significantly increased glial isoforms of GLUT1 (Fig. 3A), whereas Ad-GFAP-GLUT1 had no effects in PC12 (Fig. 3A). Injection of Ad-GFAP-GLUT1 into the MBH increased glial GLUT1 (Fig. 3B), which were colocalized with GFAP (95.9 + 1.1% of GLUT1 [n = 5]), but not NeuN (Fig. 3C). We developed a molecular approach to increase GLUT1 in the hypothalamic glial cells in vivo.
Overexpressing hypothalamic glial GLUT1 in rats induced with uncontrolled diabetes and hypothalamic hyperglycemia.
To evaluate whether overexpressing hypothalamic glial GLUT1 in STZ-induced diabetic rats with reduced hypothalamic glial GLUT1 (Fig. 1J) could rescue MBH glucose sensing, we injected MBH Ad-GFAP-GLUT1 or Ad-GFAP-LacZ into STZ-injected rats. Plasma glucose levels were elevated to ∼280 mg/dL in rats that received Ad-GFAP-LacZ and a 21.5 h-prior STZ injection (Fig. 3D). In contrast, plasma glucose levels were normalized in rats with Ad-GFAP-GLUT1 who underwent the same STZ injection protocol (Fig. 3D).
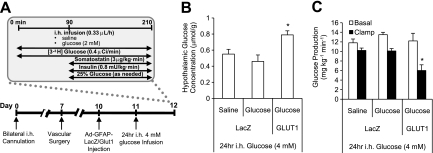
Next, we injected Ad-GFAP-GLUT1 or LacZ into MBH of rats with hypothalamic hyperglycemia (Fig. 4A and Fig. 2F and G). The ability of intrahypothalamic glucose to increase hypothalamic glucose concentration was restored in hypothalamic hyperglycemic rats when injected with hypothalamic Ad-GFAP-GLUT1 (Fig. 4B), suggesting that overexpressing MBH glial GLUT1 resuscitates the elevation of hypothalamic glucose concentration in the intracellular compartment. This restoration of elevated hypothalamic glucose concentration rescued the ability of intrahypothalamic glucose (MBH glucose sensing) to lower glucose production in hypothalamic hyperglycemic rats (Fig. 4C). Of note, hypothalamic glucose concentration (0.34 +/− 0.07 µmol/g) and glucose production during the clamps (9.2 +/− 0.6 mg ⋅ kg−1 ⋅ min−1) were not altered in hypothalamic hypergylycemic rats injected with MBH Ad-GFAP-GLUT1 and that underwent intrahypothalamic saline clamp studies (n = 4). This suggests that changes in MBH glial GLUT1 mediate MBH glucose sensing (but not under saline-infused condition) to regulate hypothalamic glucose concentration and glucose production. Together, overexpressing hypothalamic glial GLUT1 in two independent hyperglycemic models restores CNS glucose effectiveness.
FIG. 4.
Overexpressing hypothalamic glial GLUT1 in the hypothalamic hyperglycemic rats rescues CNS glucose sensing. A: Experimental protocol. B: Hypothalamic glucose concentration. *P < 0.05 vs. other groups. C: Glucose production. *P < 0.05 vs. other groups. i.h., intrahypothalamic.
DISCUSSION
The underlying mechanisms responsible for the deleterious effects of sustained hyperglycemia on glucose homeostasis remain unclear in both rodents and humans. The current set of data demonstrated sustained hyperglycemia as seen in uncontrolled diabetes impairs CNS glucose effectiveness to increase hypothalamic glucose concentration and lower glucose production through changes in hypothalamic glial GLUT1.
GLUT1 facilitates glucose uptake into the intracellular compartment for subsequent metabolism (15,16). Consistent with the working hypothesis that a reduction of hypothalamic glial GLUT1 is responsible for the metabolic impairments induced by hyperglycemia, hyperglycemia suppresses BBB glucose transport in STZ-induced rodents (17,18), although glucose uptake into the glial cells was not determined. In addition, hyperglycemia in maternal diabetes suppresses GLUT1 and glucose transport in preimplantation embryos (19) and may lead to diabetic embryopathy (20). In parallel, Alzheimer disease and neurodegeneration are caused by impaired glucose uptake and metabolism as well as hyperphosphorylation of τ in the brain (21,22). A reduction in brain GLUT1 is associated with these defects in a human brain with Alzheimer disease (23). Together, the ability of GLUT1 to facilitate intracellular glucose uptake and regulate glucose metabolism plays an important role in certain disease progression, and changes in hypothalamic glial GLUT1, as suggested by the current study, alter peripheral glucose regulation in diabetes.
A surprising aspect of our findings is that in all three hyperglycemic models, hypothalamic glucose concentration was not elevated after either MBH saline or 2 mmol/L glucose (glucose-sensing) clamps. These data indicate a potential MBH intracellular adaptive response to hyperglycemia. In light of the fact that MBH glial GLUT1 was reduced in uncontrolled diabetes (Fig. 1J) and that hyperglycemia suppresses GLUT1 (supported by the current phlorizin study as well as the studies described above), it is reasonable to postulate that the lack of increase in MBH glucose concentration as observed in the hyperglycemic models represents a lack of elevation of intracellular MBH glucose concentration. In fact, when the hypothalamic hyperglycemic rats were injected with MBH Ad-GFAP-GLUT1, the ability of MBH glucose sensing to elevate MBH glucose concentration was restored. Future experiments aimed to clarify the ability of hyperglycemia to alter GLUT1 protein expression and glucose uptake in a glial cell culture model will strengthen this working hypothesis.
Another important unanswered question in our study is how restoration of hypothalamic glucose elevation induced by CNS glucose sensing lowers glucose production in hyperglycemic models. We put forward a working hypothesis that in diabetes overexpressing hypothalamic glial GLUT1 enhances glucose uptake into glial cells and elevates intracellular glucose and lactate concentration in response to CNS glucose sensing. Lactate will be shuttled into neurons to trigger downstream biochemical/signaling pathways leading to the inhibition of glucose production. Consistent with this hypothesis, MBH lactate infusion lowers glucose production in uncontrolled diabetes (24). Since glucose sensing in POMC neurons regulates peripheral glucose homeostasis in rodents (25), it would be necessary also to evaluate whether the restoration of hypothalamic glucose elevation (or indirectly via lactate) triggers signaling events within MBH POMC neurons to lower glucose production in our current hyperglycemic models.
In summary, our data highlight the critical role of hypothalamic glial GLUT1 in mediating hypothalamic glucose sensing to regulate glucose production in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a research grant to T.K.T.L. from the Canadian Institute of Health Research (CIHR; MOP-86554). M.C. was supported by the Ontario Graduate Scholarship and a graduate scholarship from the Banting and Best Diabetes Centre at the University of Toronto (BBDC). C.S.Y. is supported by graduate scholarships from the CIHR and BBDC. C.K.L.L., A.K., and G.W.C.C. were supported by the CIHR graduate scholarship. T.K.T.L. holds the Canada Research Chair in Obesity and the John Kitson McIvor Endowed Chair in Diabetes Research at the Toronto General Research Institute and University of Toronto.
No potential conflicts of interest relevant to this article were reported.
M.C. and C.S.Y. conducted and designed the experiments, performed data analyses, and wrote the manuscript. C.K.L.L., K.L., P.M., A.K., and G.W.C.C. assisted in in vivo experiments. T.Y.Y.L. and P.Y.T.W. assisted in in vitro experiments and constructed the adenoviruses. T.K.T.L. supervised the project, designed the experiments, and edited the manuscript.
The authors are extremely grateful to Dr. Amira Klip from The Hospital for Sick Children and the University of Toronto for advice on Western blotting for GLUT1.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0120/-/DC1.
REFERENCES
- 1.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care 1990;13:610–630 [DOI] [PubMed] [Google Scholar]
- 2.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto Y, Torres TP, Donahue EP, Shiota M. Glucose toxicity is responsible for the development of impaired regulation of endogenous glucose production and hepatic glucokinase in Zucker diabetic fatty rats. Diabetes 2006;55:2479–2490 [DOI] [PubMed] [Google Scholar]
- 5.Hawkins M, Gabriely I, Wozniak R, Reddy K, Rossetti L, Shamoon H. Glycemic control determines hepatic and peripheral glucose effectiveness in type 2 diabetic subjects. Diabetes 2002;51:2179–2189 [DOI] [PubMed] [Google Scholar]
- 6.Rossetti L, Giaccari A, Barzilai N, Howard K, Sebel G, Hu M. Mechanism by which hyperglycemia inhibits hepatic glucose production in conscious rats. Implications for the pathophysiology of fasting hyperglycemia in diabetes. J Clin Invest 1993;92:1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mevorach M, Giacca A, Aharon Y, Hawkins M, Shamoon H, Rossetti L. Regulation of endogenous glucose production by glucose per se is impaired in type 2 diabetes mellitus. J Clin Invest 1998;102:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacca L, Hendler R, Sherwin RS. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab 1978;47:1160–1163 [DOI] [PubMed] [Google Scholar]
- 9.German J, Kim F, Schwartz GJ, et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med 2010;16:392–395 [DOI] [PubMed] [Google Scholar]
- 11.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005;309:943–947 [DOI] [PubMed] [Google Scholar]
- 12.Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes 2011;60:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang CS, Lam CK, Chari M, et al. Hypothalamic AMP-activated protein kinase regulates glucose production. Diabetes 2010;59:2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam TK, Gutierrez-Juarez R, Pocai A, et al. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med 2007;13:171–180 [DOI] [PubMed] [Google Scholar]
- 15.Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J 1994;8:1003–1011 [DOI] [PubMed] [Google Scholar]
- 16.Mueckler M, Caruso C, Baldwin SA, et al. Sequence and structure of a human glucose transporter. Science 1985;229:941–945 [DOI] [PubMed] [Google Scholar]
- 17.Matthaei S, Horuk R, Olefsky JM. Blood-brain glucose transfer in diabetes mellitus. Decreased number of glucose transporters at blood-brain barrier. Diabetes 1986;35:1181–1184 [DOI] [PubMed] [Google Scholar]
- 18.McCall AL, Millington WR, Wurtman RJ. Metabolic fuel and amino acid transport into the brain in experimental diabetes mellitus. Proc Natl Acad Sci USA 1982;79:5406–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol 1998;275:E38–E47 [DOI] [PubMed] [Google Scholar]
- 20.Heilig CW, Saunders T, Brosius FC, 3rd, et al. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci USA 2003;100:15613–15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avila J. Tau phosphorylation and aggregation in Alzheimer’s disease pathology. FEBS Lett 2006;580:2922–2927 [DOI] [PubMed] [Google Scholar]
- 22.Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol 2004;541:135–152 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett 2008;582:359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chari M, Lam CK, Wang PY, Lam TK. Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes 2008;57:836–840 [DOI] [PubMed] [Google Scholar]
- 25.Parton LE, Ye CP, Coppari R, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007;449:228–232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.