Abstract
OBJECTIVE
Comprehensive proteomic profiling of the human adipocyte secretome identified dipeptidyl peptidase 4 (DPP4) as a novel adipokine. This study assessed the functional implications of the adipokine DPP4 and its association to the metabolic syndrome.
RESEARCH DESIGN AND METHODS
Human adipocytes and skeletal and smooth muscle cells were used to monitor DPP4 release and assess the effects of soluble DPP4 on insulin signaling. In lean and obese subjects, depot-specific expression of DPP4 and its release from adipose tissue explants were determined and correlated to parameters of the metabolic syndrome.
RESULTS
Fully differentiated adipocytes exhibit a substantially higher release of DPP4 compared with preadipocytes or macrophages. Direct addition of DPP4 to fat and skeletal and smooth muscle cells impairs insulin signaling. A fivefold higher level of DPP4 protein expression was seen in visceral compared with subcutaneous fat of obese patients, with no regional difference in lean subjects. DPP4 serum concentrations significantly correlated with adipocyte size. By using adipose tissue explants from lean and obese subjects, we observed a twofold increase in DPP4 release that strongly correlated with adipocyte volume and parameters of the metabolic syndrome and was decreased to the lean level after weight reduction. DPP4 released from adipose tissue correlated positively with an increasing risk score for the metabolic syndrome.
CONCLUSIONS
DPP4 is a novel adipokine that may impair insulin sensitivity in an autocrine and paracrine fashion. Furthermore, DPP4 release strongly correlates with adipocyte size, potentially representing an important source of DPP4 in obesity. Therefore, we suggest that DPP4 may be involved in linking adipose tissue and the metabolic syndrome.
Obesity is the hallmark of the metabolic syndrome and represents a major global health problem that frequently associates with the development of chronic diseases, including type 2 diabetes and cardiovascular disease (1). A complex interorgan cross-talk scenario between adipose tissue and other central and peripheral organs underlies the progression of these diseases, with adipose tissue on top of the cross-talk hierarchy (2). This is attributed to the huge diversity of signaling and mediator molecules released from adipose tissue, which is now considered one of the major endocrine organs (3,4). Recent data show that adipokines, which are proteins and peptides released by various adipose tissue cells, create a complex interconnected network of feedback loops (5). Enlargement of adipose tissue leads to dysregulation of adipokine secretion, representing a potential critical pathogenic link among obesity, insulin resistance (IR), and type 2 diabetes (1). Therefore, we conducted a comprehensive proteomic profiling of conditioned media derived from differentiated, primary human adipocytes. This resulted in the identification of novel adipokines, including the exoprotease dipeptidyl peptidase 4 (DPP4).
DPP4 is a ubiquitously expressed transmembrane glycoprotein that cleaves N-terminal dipeptides from a variety of substrates, including growth factors and hormones, neuropeptides, and chemokines (6). Two substrates of DPP4, glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), are released from the intestinal mucosa and responsible for ∼60% of postprandial insulin secretion, the so-called incretin effect (7). Because GLP-1 remains active under hyperglycemic conditions in type 2 diabetes, DPP4 has gained considerable interest as a therapeutic target, and a variety of DPP4-inhibitors that prolong the insulinotropic effect of GLP1 are now in clinical use as antidiabetic drugs (8). Substantial DPP4 activity is also found in plasma and other body fluids because of a soluble form of DPP4 lacking the cytoplasmic tail and the transmembrane region of this protein (9). Both the membrane abundance and the circulating activity of DPP4 have been found to be altered in a variety of neurologic and inflammatory diseases (6). However, although a fraction of soluble DPP4 most likely originates from cells of the immune system (10), the major source of circulating DPP4 and its regulation remain unknown.
Furthermore, essentially no data are currently available regarding the potential effects of soluble DPP4 on insulin target tissues, including muscle and fat. In the present investigation, we combined in vitro experiments with two independent clinical studies, aiming to validate DPP4 as a novel adipokine and to characterize the association of DPP4 to different parameters of the metabolic syndrome. We show that 1) DPP4 is a novel adipokine released from differentiated human adipocytes and that it may exert autocrine and paracrine effects leading to IR; 2) DPP4 expression is substantially elevated in visceral fat of obese subjects and that serum DPP4 correlates with adipocyte size and all parameters of the metabolic syndrome; and 3) adipose tissue explants from obese subjects release substantially more DPP4 with a prominent decrease after weight reduction. In light of the well accepted interference of DPP4 with the incretin system, we now suggest that DPP4 may play a role in linking obesity to IR and the metabolic syndrome.
RESEARCH DESIGN AND METHODS
Materials
Reagents for SDS-PAGE were supplied by GE Healthcare (Freiburg, Germany) and Sigma-Aldrich (Munich, Germany). Polyclonal antibodies for adiponectin and actin were supplied by Abcam (Cambridge, U.K.). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse IgG antibodies were supplied by Promega (Mannheim, Germany). Collagenase NB4 was obtained from Serva (Heidelberg, Germany). Troglitazone, tumor necrosis factor (TNF)-α, and BSA (fraction V, fatty acid free, low endotoxin) were obtained from Sigma-Aldrich. Adiponectin was purchased from Biovendor (Heidelberg, Germany). Complete protease inhibitor cocktail and PhosStop phosphatase inhibitor cocktail were provided by Roche (Mannheim, Germany). FCS was supplied by Gibco (Invitrogen, Carlsbad, CA). All other chemicals were of the highest analytic grade commercially available and purchased from Sigma-Aldrich. Human recombinant DPP4 was purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany), and a polyclonal antibody was purchased from Abnova (Heidelberg, Germany). The specific DPP4 inhibitor K579 was purchased from Biozol (Eching, Germany).
Clinical studies of DPP-4 concentration in serum and DPP4 release from adipose tissue
For all studies, protocols were approved by local ethics committees, and all participants gave written, informed consent.
Study 1 included 20 male obese patients and 20 lean controls who were recruited at Gent University Hospital (Belgian registration number B67020084018). For all patients, anthropometric and routine blood parameters were assessed. Fasting blood samples were collected, and adipose tissue biopsies were fixed for microscopic evaluation of adipocyte surface area analysis.
Study 2 included 19 obese (BMI ≥30 kg/m2) otherwise healthy and 10 lean (BMI <25 kg/m2) healthy women who were recruited at Karolinska Institute and investigated in the morning after an overnight fast. Sixteen obese women were reinvestigated 18–24 months after gastric bypass in a weight-stable period for at least 3 months, according to self-report (reduction of BMI from 43.0 to 27.9 kg/m2). A venous blood sample was obtained for the analysis of glucose and insulin to be used as an estimation of insulin sensitivity in vivo with the homeostasis model assessment (HOMA) index as described (11). Thereafter, abdominal subcutaneous adipose tissue biopsies were obtained by needle aspiration as described previously (12). One part of the tissue was used for measurements of DPP4 release as described previously (13). Methodological experiments revealed that DPP4 release was linear with time for at least 3 h, suggesting no important cell damage (data not shown). Another part of the tissue was subjected to collagenase treatment, and mean adipocyte volume and weight were determined as described previously (14).
For calculation of the risk score for the metabolic syndrome, we used Adult Treatment Panel-III definitions as follows: 1) fasting glucose >110 mg/dL or diagnosis of type 2 diabetes, 2) blood pressure >135/85 mmHg, 3) serum triglycerides >150 mg/dL, 4) HDL-cholesterol <40 mg/dL for men and <50 mg/dL for women, and 5) abdominal obesity characterized by a waist >102 cm for men and >88 cm for women. The risk score is equal to the number of criteria fulfilled. Subjects with a risk score of ≥3 are qualified as having the metabolic syndrome.
HOMA for IR was determined in all patients, with the exception of those treated with insulin, by a mathematic transformation of fasting blood glucose and insulin measurements (HOMA = insulin [μU/mL] × glucose [mmol/L]/22.5).
Adipocyte isolation and culture
Subcutaneous adipose tissue was obtained from lean or moderately overweight women undergoing plastic surgery for mammary reduction or breast reconstruction with subcutaneous abdominal adipose tissue. The procedure was approved by the ethical committee of the Heinrich-Heine-University (Düsseldorf, Germany). All subjects were healthy and free of medication and had no evidence of diabetes according to routine laboratory test results. Preadipocytes were isolated by collagenase digestion of adipose tissue as previously described by Dietze-Schroeder et al. (15). Isolated cell pellets were resuspended in Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12) medium supplemented with 10% FCS. After overnight incubation, cultures were washed and further incubated in an adipocyte differentiation medium (DMEM/F12, 33 μmol/L biotin, 17 μmol/L d-pantothenic acid, 66 nmol/L insulin, 1 nmol/L triiodo-l-thyronine, 100 nmol/L cortisol, 10 μg/mL apotransferrin, 50 μg/μL gentamycin, 15 mmol/L HEPES, and 14 nmol/L NaHCO3, pH 7.4) for 15 days with medium change every 2–3 days and the addition of 5 μmol/L troglitazone for the first 3 days. The degree of differentiation was determined by Oil Red staining and induction of adiponectin expression. Differentiated adipocytes were used for the generation of adipocyte-conditioned media, as recently described by Dietze-Schroeder et al. (15). In brief, after in vitro differentiation, adipocytes were washed and incubated for 48 h in α-modified DMEM followed by collection of the medium. Macrophages were isolated from human adipose tissue and cultured using a method described by Curat et al. (16). For hypoxia treatment, differentiated adipocytes were incubated with a gas mixture containing 1% O2, 5% CO2, and 94% N2 in MIC-101 modular incubator chambers (Billups-Rothenburg, Del Mar, CA) at 37°C for the indicated times.
Skeletal muscle cell culture
Primary human skeletal muscle cells of healthy Caucasian donors were supplied as proliferating myoblasts (5 × 105 cells) and cultured as described previously (15). For an individual experiment, myoblasts were seeded in six-well culture dishes (9.6 cm2/well) at a density of 105 cells per well and cultured in α-modified DMEM/F12 medium containing skeletal muscle cell growth medium supplement pack up to near confluence. The cells were then differentiated and fused by culture in α-modified DMEM for 4 days and used for experiments.
Smooth muscle cell culture and proliferation
Primary human coronary artery smooth muscle cells were obtained from PromoCell (Heidelberg, Germany). Cells from four different donors were supplied as proliferating cells and kept in culture according to the manufacturer’s protocol. For all experiments, subconfluent cells of passage three were used. Cells were characterized as smooth muscle cells by morphologic criteria and by immunostaining with smooth muscle α-actin.
Immunoblotting
Adipocytes and macrophages were treated as indicated and lysed in a buffer containing 50 mmol/L HEPES, pH 7.4, 1% Triton X-100, complete protease inhibitor, and PhosStop phosphatase inhibitor cocktail. After incubation for 2 h at 4°C, the suspension was centrifuged at 10,000g for 15 min. Thereafter, 5–10 μg adipocyte lysates were separated by SDS-PAGE using 10% horizontal gels and transferred to polyvinylidene fluoride filters in a semidry blotting apparatus. Filters were blocked with Tris-buffered saline containing 0.1% Tween and 5% nonfat dry milk and subsequently incubated overnight with a 1:1,000 dilution of the appropriate antibodies. After washing, filters were incubated with secondary HRP-coupled antibody and processed for enhanced chemiluminescence detection using Immobilon HRP substrate (Millipore, Billerica, MA). Signals were visualized and evaluated on a LUMI Imager (Boehringer, Mannheim, Germany) or VersaDoc 4000 MP (Bio-Rad Laboratories, Munich, Germany) work station.
ELISA
DPP4 and heme oxygenase-1 secretion by human primary adipocytes and macrophages were determined using ELISA kits purchased from R&D Systems and Streegen Biotechnologies (Lörrach, Germany). The assays were performed in duplicates according to the manufacturer’s instructions.
Presentation of data and statistics
Data are expressed as mean ± SEM. The Shapiro–Wilcoxon test was used to test the Gaussian distribution of biological parameters. Student t test and ANOVA followed by P for linear trend post-test when appropriate were used for comparison between groups. Correlations were performed by Pearson. For adjustment (BMI, age), we applied a multiple linear regression modeling using least-squares means tests. All statistical analyses were done using JMP statistics software (SAS Institute Inc., Cary, NC) or Prism (GraphPad Software, Inc., La Jolla, CA) considering a P value <0.05 as statistically significant. Corresponding significance levels are indicated in Figs. 1 to 7.
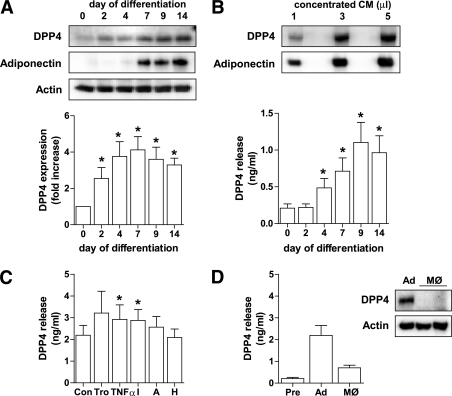
FIG. 1.
DPP4 protein level and release during adipocyte differentiation and after stimulation with different regulatory factors. A: Human primary adipocytes were differentiated as described in research design and methods, and DPP4 protein level during differentiation was analyzed by SDS-PAGE and Western blot. Adiponectin expression served as a control of differentiation. Data were normalized to the protein level of actin and are expressed relative to day 0. Data are mean values ± SEM, n ≥5, *P < 0.05 vs. preadipocytes. B: Detection of DPP4 at day 14 of differentiation using 1–5 μL of concentrated conditioned medium analyzed by SDS-PAGE and Western blot. Twenty-four–hour release of DPP4 by adipocytes determined at different time points of differentiation was analyzed by ELISA. Data are mean values ± SEM, n ≥5, *P < 0.05 vs. day 0. C: Differentiated adipocytes were treated with 5 μmol/L troglitazone, 10 ng TNF-α, 50 mmol/L insulin, 5 nmol/L adiponectin, or incubated under hypoxic conditions for 24 h. DPP4 release by differentiated adipocytes after indicated 24-h treatments as measured by ELISA. Data are mean values ± SEM, n ≥7, *P < 0.05 vs. control. D: DPP4 release by preadipocytes, differentiated adipocytes, and adipose tissue–derived and cultured human macrophages was analyzed by ELISA. Data are mean values ± SEM, n ≥3; 10 μg total lysates derived from adipocytes and macrophages were analyzed by SDS-PAGE and Western blot, and signals were detected by enhanced chemiluminescence. A, adiponectin; Ad, adipocyte; CM, conditioned medium; H, hypoxic; I, insulin; MØ, macrophage; Pre, preadipocyte; Tro, troglitazone.
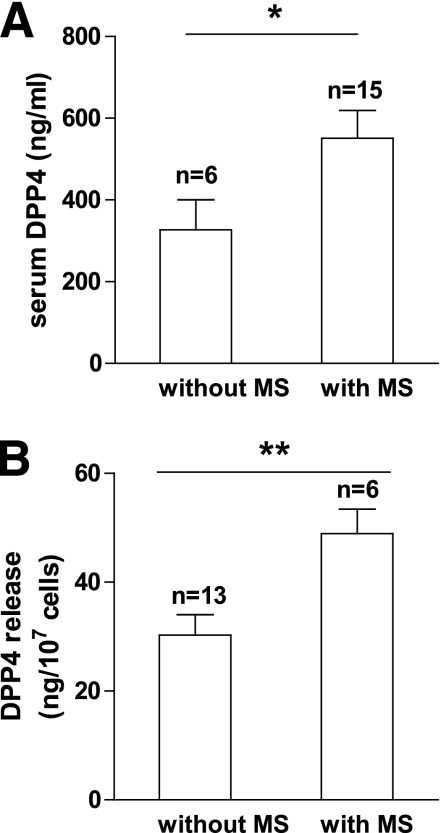
FIG. 7.
DPP4 in serum and release from adipose tissue explants in relation to a risk score for the metabolic syndrome. A risk score for the metabolic syndrome was calculated for all obese subjects in whom serum and adipose tissue explants were analyzed. Patients with a risk score of ≥3 were qualified as “with metabolic syndrome (MS).” Patients with a score of ≤2 were qualified as “without MS.” Data were analyzed using a t test. Data are mean values ± SEM. *P < 0.05, **P < 0.01.
RESULTS
DPP4 is a novel adipokine exhibiting regulated release from human adipocytes
Comprehensive proteomic profiling of the adipocyte secretome led to the identification of 347 proteins, with 263 proteins being predicted or annotated as secretory proteins (data to be presented in another publication). Although ∼80% of these proteins have been reported in earlier studies (17–19), our approach has identified >40 novel adipokines, including DPP4.
To validate this novel adipokine, we used in vitro differentiated human adipocytes and macrophages isolated from adipose tissue. DPP4 expression in human adipocytes is significantly increased during differentiation with a maximum reached at day 7 (fourfold over undifferentiated control) (Fig. 1A). DPP4 expression is paralleled by a marked release of this adipokine (Fig. 1B), which was significantly elevated compared with the undifferentiated control starting at day 4 and increasing up to day 9 (1.1 ng/mL released over 24 h by 3.5 × 105 cells). DPP4 in the supernatant of adipocytes was quantified by ELISA and confirmed by Western blotting (Fig. 1B). We further analyzed the release of DPP4 with prominent regulators of adipocyte secretory activity, such as troglitazone, TNF-α, insulin, and adiponectin (15,20,21). As shown in Fig. 1C, DPP4 release is significantly upregulated by TNF-α and insulin. In addition to adipocytes, adipose tissue-derived macrophages release measurable amounts of DPP4 (Fig. 1D). However, this is only one third compared with adipocytes, pointing to a major contribution of adipocytes to DPP4 output from adipose tissue.
Soluble DPP4 exerts direct effects on fat and muscle cells
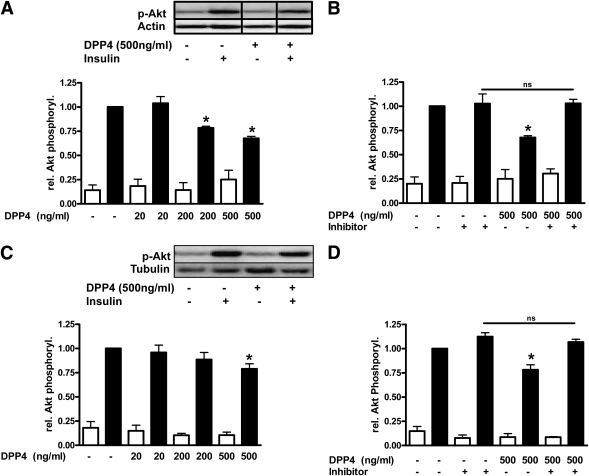
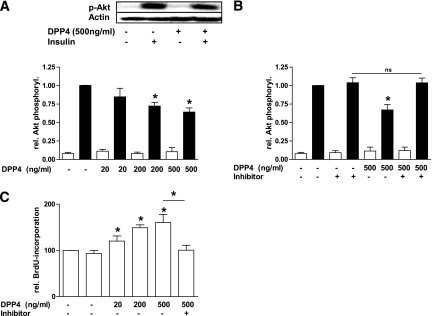
The soluble form of DPP4 may bind to the extracellular matrix (22) and affect a variety of cells, yet this has not been investigated so far. To assess potential direct effects of soluble DPP4 on peripheral cells, we studied insulin signaling in adipocytes and skeletal muscle cells. DPP4 treatment of human adipocytes results in a dose-dependent decrease in insulin-stimulated Akt phosphorylation, which reached significance using a dose of 200 ng/mL (Fig. 2A). This demonstrates an autocrine effect of DPP4 on adipocytes. It should be noted that circulating DPP4 concentrations were found in the range of 200 to 600 ng/mL in healthy patients. The effect of DPP4 on insulin-stimulated Akt phosphorylation can be completely blocked by a specific DPP4 inhibitor (Fig. 2B). Validation experiments using this compound proved inhibition of DPP4 in vitro, which remained unaltered for a period of at least 8 h (data not shown). Similar to adipocytes, DPP4 also induces IR in skeletal muscle cells at the level of Akt phosphorylation in a dose-dependent way but less prominent compared with adipocytes (Fig. 2C and D). To prove whether DPP4 has a functional impact not only on insulin signaling, we determined DPP4-stimulated proliferation and insulin signaling in primary human smooth muscle cells. In addition to the induction of IR at the level of Akt in this cell type, DPP4 induced a 1.6-fold increase in cell proliferation that can be completely blocked by the DPP4 inhibitor (Fig. 3A–C).
FIG. 2.
Effect of DPP4 on insulin-stimulated Akt phosphorylation in adipocytes and skeletal muscle cells. Differentiated human adipocytes (A and B) and skeletal muscle cells (C and D) were treated with the indicated amounts of DPP4 without and with concomitant administration of a specific DPP4 inhibitor for 24 h. After stimulation with insulin (100 nmol/L, 10 min), the cells were lysed and 5–10 μg of total lysates were resolved by SDS-PAGE and blotted to polyvinylidene fluoride membranes. Membranes were blocked with 5% milk in TBS containing 0.1% Tween 20 and incubated overnight with p-Akt antibody. After incubation with the appropriate HRP-coupled secondary antibody, the signal was detected by enhanced chemiluminescence. Signals were analyzed on a LUMI Imager Work Station (Boehringer). Data are actin normalized mean values ± SEM (n = 3–8). Representative Western blots are presented. For A, lanes were excised from a single Western blot and displayed in the presented order. Basal (white bars); insulin-stimulated (black bars). *Significantly different from insulin-stimulated control or indicated situation. ns, not significant.
FIG. 3.
Effect of DPP4 on insulin-stimulated Akt phosphorylation and proliferation in smooth muscle cells. A and B: Smooth muscle cells were treated with the indicated amounts of DPP4 without and with concomitant administration of a specific DPP4 inhibitor for 24 h. After stimulation with insulin (100 nmol/L, 10 min) the cells were lysed and Western blots performed as indicated in Fig. 2. Data are actin normalized mean values ± SEM (n = 3–6). Basal (white bars); insulin-stimulated (black bars). C: Proliferation of smooth muscle cells was determined by measuring the incorporation of BrdU into DNA. Data are expressed relative to the basal control value, taken as 100%. Data are mean values ± SEM (n = 3–8). ns, not significant. *Significantly different from control or indicated situation.
DPP4 is elevated in serum of obese patients and correlates with various anthropometric and clinical parameters (clinical study 1)
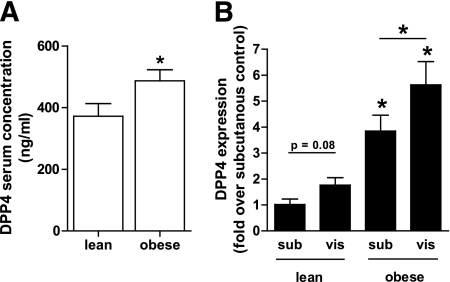
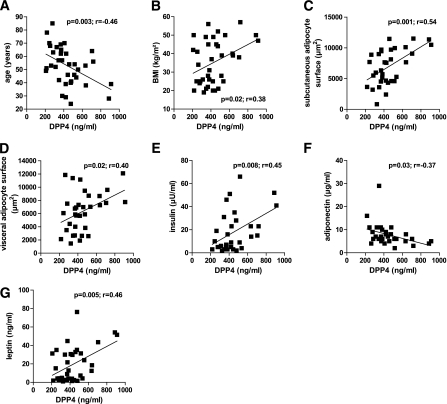
Measuring DPP4 in serum from age-matched lean and morbidly obese subjects (patient characteristics in Supplementary Table 1) revealed that obese subjects are characterized by significantly increased DPP4 concentrations (Fig. 4A). DPP4 expression in adipose tissue biopsies from the same patients revealed that DPP4 protein expression is regulated by both the fatness of the individual and the adipose tissue depot (Fig. 4B). Although there is only a trend for higher DPP4 expression in visceral fat of lean subjects, obese patients are characterized by significantly higher DPP4 in visceral adipose tissue compared with subcutaneous adipose tissue. Furthermore, expression of DPP4 in both depots is significantly higher in obese subjects compared with lean subjects. DPP4 levels positively correlate with BMI, the size of subcutaneous and visceral adipocytes, insulin, and leptin, whereas a negative correlation with age and adiponectin could be found (Fig. 5A–G). Adjusting DPP4 for age has no impact on these correlations. However, when adjusted for BMI, DPP4 serum concentrations significantly correlate only with the size of subcutaneous adipocytes (P = 0.04, r = 0.32), pointing to a close relation between the size of adipocytes and the release of this adipokine.
FIG. 4.
DPP4 serum concentration and expression in adipose tissue from lean compared with obese patients (clinical study 1). A: Sera from lean (n = 20) and morbidly obese (n = 20) men were analyzed for their DPP4 concentration by ELISA. Data are mean values ± SEM, *P < 0.05 vs. lean group. B: DPP4 protein level in adipose tissue biopsies was analyzed by SDS-PAGE and Western blot. Data were normalized to the protein level of actin and are expressed relative to subcutaneous adipose tissue from lean subjects. Data are mean values ± SEM, n = 8 for lean and n = 14 for obese patients, *P < 0.05 respective subcutaneous or designated group.
FIG. 5.
DPP4 serum concentrations correlate with various clinical and biochemical parameters (clinical study 1). Sera from lean (n = 20) and morbidly obese (n = 20) men were analyzed for their DPP4 concentration by ELISA. Linear regression analysis of DPP4 serum concentration and patient characteristics such as age (A), BMI (B), size of subcutaneous (C) and visceral (D) adipocytes, insulin concentration (E), adiponectin concentration (F), and leptin concentration (G). Statistical evaluation is indicated in each graph. vis, visceral.
DPP4 is released from subcutaneous adipose tissue in vitro (clinical study 2)
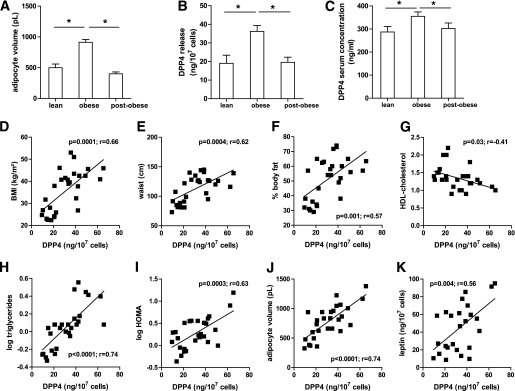
We investigated lean and obese subjects after weight reduction and analyzed the release of DPP4 from whole adipose tissue. Adipocytes from lean subjects were significantly smaller than those from obese patients (Fig. 6A). Surgery-induced weight loss reduced the average size of adipocytes below the size from lean subjects. DPP4 release is significantly increased from adipose tissue of obese subjects compared with lean subjects (Fig. 6B), whereas weight reduction by bariatric surgery normalized the DPP4 release to the lean level. This was paralleled by a significant reduction in the circulating DPP4 level, supporting the notion that adipose tissue is an important source of serum DPP4 (Fig. 6C). In the group of lean and obese subjects, DPP4 release from adipose tissue significantly correlates with BMI, waist circumference, percent body fat, triglycerides, HOMA, adipocyte volume, and leptin, whereas the correlation is negative with HDL-cholesterol (Fig. 6D–K). All of these factors are denominators of the metabolic syndrome. It is noteworthy that leptin shows similar correlations with the above mentioned parameters, with the exception of triglycerides, for which no correlation could be found. Notably, the release of DPP4 from adipose tissue correlates with many parameters that correlate with circulating DPP4 concentrations. There is also a strong correlation between adipose secretion of leptin and DPP4 (Fig. 6K).
FIG. 6.
DPP4 release of explants obtained from adipose tissue of lean controls and obese patients before and after bariatric surgery, and linear correlation with various clinical and biochemical parameters (clinical study 2). A and B: Samples of adipose tissue were obtained from lean controls (n = 10) and obese patients before (n = 19) and after (n = 16) bariatric surgery, and used to generate explants as described in research design and methods. The size of adipocytes for each subject was measured (A). DPP4 release was analyzed by ELISA and related to the quantity of adipocytes (B). C: DPP4 serum concentration was measured in lean and obese patients before and after bariatric surgery. D–K: Linear regression analysis of DPP4 release per 107 cells and patient characteristics such as BMI (D), waist circumference (E), percent of body fat (F), HDL-cholesterol concentration (G), triglycerides concentration (H), HOMA (I), adipocyte volume (J), and leptin (K). A–C: Data are mean values ± SEM. *P < 0.05 between respective groups.
DPP4 serum concentrations and release from adipose tissue are significantly related to the metabolic syndrome
In both obese patient groups providing data for circulating DPP4 levels and DPP4 release from adipose tissue explants, the respective concentrations of DPP4 are significantly increased in subjects with a risk score for the metabolic syndrome of ≥3, as calculated according to the Adult Treatment Panel-III guidelines (Fig. 7A and B). By performing the same analysis for the circulating levels of leptin, monocyte chemotactic protein-1, RANTES (regulated upon activation, normal T cell expressed and secreted), plasminogen activator inhibitor-1, chemerin, and high-sensitivity C-reactive protein, we did not find any such relationship with the metabolic syndrome (data not shown). In contrast, adiponectin serum levels were significantly decreased in patients with the metabolic syndrome (data not shown). Including the lean subjects in this type of analysis does not change the outcome of this analysis, and it should be noted that the relationship of DPP4 with the risk score for the metabolic syndrome in the obese subjects is independent from BMI.
DISCUSSION
Our proteomics approach identified DPP4 as a novel adipokine released by fully differentiated human adipocytes. This was confirmed by Western blot, ELISA, and determination of enzymatic activity. DPP4 release increased substantially on fat cell differentiation, and comparison with preadipocytes and adipose tissue macrophages showed that adipocytes most likely represent the major source of DPP4 released from the intact organ to the circulation. DPP4 is a multifunctional, type II integral membrane glycoprotein exhibiting ubiquitous expression, including adipose tissue (23), being highly abundant in the kidney, on T lymphocytes and endothelial cells (22). DPP4 is certainly different from many other adipokines in that 1) the protein is not secreted but released from the plasma membrane as soluble DPP4 subsequent to proteolytic cleavage (24), 2) DPP4 exerts dual functions both as a regulatory protease and a binding protein, and 3) this protein is already an established target for treatment of type 2 diabetes (8), supporting our notion that DPP4 may potentially link adipose tissue to type 2 diabetes and the metabolic syndrome. Regulators of DPP4 release are presently unknown, but we show that both insulin and TNF-α augment the shedding of soluble DPP4 by ∼50% despite an unaltered expression. Thus, factors related to IR and adipose tissue inflammation enhance the release of this novel adipokine from the fat cell. In addition to the endocrine effects of DPP4 released to the circulation, both cell surface resident and soluble DPP4 may have multiple autocrine and paracrine functional implications for adipose tissue physiology. First, DPP4 recruits adenosine deaminase to the cell surface (25), which may modulate the well established antilipolytic effects of adenosine. Second, DPP4 is a strong inhibitor of the antilipolytic activity of neuropeptide Y (23), which is one of the best peptide substrates of the enzyme (26). Therefore, enhanced abundance of both resident and soluble DPP4 within adipose tissue of obese subjects may substantially augment the lipolytic activity of enlarged adipocytes. Finally, DPP4 inactivates or alters the specificity of many chemokines, including RANTES, eotaxin, macrophage-derived chemokine, stromal-derived factor-1, and many others (22), making it likely that DPP4 plays a yet undefined functional role in the intraorgan cross-talk among macrophages, adipocytes, and other components of the stroma-vascular fraction.
So far, the direct effects of soluble DPP4 on isolated cells have not been investigated, although it binds to the extracellular matrix and may exert signaling functions (22). We demonstrate for the first time that DPP4 consistently impairs insulin signaling at the level of Akt in three different primary cell types, namely, adipocytes, skeletal muscle, and smooth muscle cells. Enzymatic activity of DPP4 seems to be involved in this process, but DPP4 inhibitors may also affect the binding properties of sDPP4 to a putative receptor. This issue is currently under investigation in our laboratory. It may be speculated that DPP4 exerts an autocrine action on adipocytes, which may be of particular interest for perivascular fat, where DPP4 may also act in a paracrine/endocrine fashion on the vascular wall. DPP4 induces proliferation of human vascular cells in parallel to an impairment of insulin signaling, suggesting a potential role in obesity-associated vascular complications. In this study, we used DPP4 concentrations that match circulating levels that were measured in both lean and obese subjects. Because obese patients are characterized by significantly increased circulating DPP4, it may be speculated that DPP4 may interfere with insulin sensitivity not only in adipose tissue but also in other insulin-sensitive peripheral organs. This would substantially extend the current view of DPP4 as a target for treatment of type 2 diabetes. Future work will be needed to address the mechanism and the functional role of these effects in the pathogenesis of IR and obesity-associated complications.
Serum DPP4 is altered in many pathophysiologic conditions, such as different types of cancer, allergic asthma, or hepatitis C (10). To the best of our knowledge, this is the first study to analyze circulating DPP4 in the context of obesity and adipose tissue. Morbidly obese men are characterized by elevated DPP4 levels compared with lean controls. DPP4 serum concentrations are significantly correlated with the BMI, the size of adipocytes in subcutaneous and visceral fat, and the adipocyte hormones adiponectin (negatively) and leptin, showing that DPP4 is related to not only increased body weight but also other important parameters of adipose tissue in particular. DPP4 is negatively associated with age, but all of the above mentioned parameters are still significantly correlated with DPP4, even after adjustment for age. In a different manner, BMI adjustment causes the disappearance of most of these correlations, with the exception of the size of subcutaneous adipocytes. In addition to circulating DPP4, the protein expression of this adipokine is significantly different not only between lean and obese subjects but also between their fat depots. Former studies report contradicting data, describing both decreased and increased mRNA expression of DPP4 in adipose tissue of obese men (23,27). We now clearly demonstrate at the protein level that obesity leads to a prominent induction of DPP4 abundance in both subcutaneous and visceral adipose tissue and that the visceral fat exhibits the highest DPP4 level in obese subjects. Therefore, we conclude that enlargement of visceral adipocytes in obesity may substantially contribute to the augmented level of circulating DPP4 in obese patients. It is noteworthy that we measured DPP4 serum concentration and not its activity. However, in additional experiments, other samples from the same patients were used to determine DPP4 activity that is significantly correlated with circulating DPP4 levels (data not shown). Thus, DPP4 activity is also significantly increased in obese compared with lean subjects.
DPP4 expression in adipose tissue is increased in obese compared with lean individuals, a fact that is reflected by an increased release of DPP4 from adipose tissue explants of obese patients compared with lean controls. Similar to circulating DPP4, its release from adipose tissue correlates with various classic markers for the metabolic syndrome, namely, BMI, waist circumference and plasma triglycerides, and HOMA as an index of IR, as well as with fat cell volume and the adipokine leptin. In addition, DPP4 release can be reversed to normal levels by surgery-induced weight loss, which is also reflected by DPP4 being significantly reduced in serum of these patients. With the exception of one study reporting on DPP4 levels in obese children before and after weight loss (28), this is the first description of significantly decreased DPP4 levels after weight loss induced by obesity surgery in adults. Thus, in obesity, both circulating levels of DPP4 and DPP4 release by adipose tissue are increased but can be reduced to control levels by substantial weight loss.
Both circulating DPP4 and DPP4 release by adipose tissue correlate strongly with the metabolic syndrome. Thus, DPP4 may be of relevance as a novel biomarker of the metabolic syndrome and for detection of obese subjects at high risk for obesity-associated complications. Future studies are needed to address this important issue and to define the molecular pathways that link adipose DPP4 to the metabolic syndrome and type 2 diabetes. An adipose-specific knockout of DPP4 would be required to prove a causal role of this protein, and this mouse model is currently under development in our laboratory. However, several lines of evidence support our notion that the novel adipokine links obesity to the metabolic syndrome. First, DPP4 impairs the function of the incretin system, which is of key importance for glucose homeostasis (29). Incretin-based therapies are known to preserve β-cell function and to exert salutary effects on blood pressure and lipid profile (30). Second, DPP4 inhibitors are well known to improve glucose tolerance in animal models of obesity (31). More important, chronic DPP4 inhibition in ZDF rats was shown to delay the onset of type 2 diabetes (31). Finally, preclinical data suggest that GLP-1 is cardioprotective (32), and DPP4 inhibition was shown to improve cardiovascular outcomes in rodents (33). Our data strongly support the current view (20) that adipocytes and specifically adipose tissue play a major, most likely causative role in the pathogenesis of metabolic diseases.
In summary, we showed that DPP4 is a novel adipokine that is substantially overexpressed in visceral fat from obese subjects and exhibits an augmented release in obesity. Soluble DPP4 exerts autocrine and paracrine effects and impairs insulin signaling. We further observe a tight correlation of DPP4 release to adipocyte cell size, and plasma levels of DPP4 strongly correlate with the risk of having the metabolic syndrome. Therefore, we suggest that DPP4 is a novel biomarker and a potential link between obesity and the metabolic syndrome.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Bundesministerium für Gesundheit, the Ministerium für Innovation, Wissenschaft und Forschung NRW, Deutsche Forschungsgemeinschaft (SE 1922/2-1), Commission of the European Communities (Collaborative Project ADAPT, contract number HEALTH-F2-2008-201100, Integrated Project HEPADIP, contract number LSHM-CT-2005-018734), EU COST Action BM0602, the Swedish Research Council (K2008-54X-01034-42-4 and 2007-2489), the Swedish Diabetes Association, the Swedish Heart and Lung Association, the Diabetes Program at Karolinska Institutet, and the Novo Nordisk Foundation. No other potential conflicts of interest relevant to this article were reported.
D.L., S.F., N.W., and S.H. researched data; S.L. contributed to discussion and reviewed and edited the manuscript; D.M.O. contributed to discussion and reviewed and edited the manuscript; K.E. reviewed and edited the manuscript; J.M.K., M.R., S.M., and F.-G.H. researched data; J.R. researched data and reviewed and edited the manuscript; P.A. researched data and contributed to discussion; H.S. researched data and wrote the manuscript; and J.E. wrote the manuscript.
The authors thank Prof. Jutta Liebau and her team, Department of Plastic Surgery, Florence-Nightingale-Hospital Düsseldorf, for support in obtaining adipose tissue samples. The technical assistance of Andrea Cramer, Angelika Horrighs, Birgit Knobloch, and Kerstin Wåhlén and the secretarial assistance of Birgit Hurow (Paul-Langerhans-Group, German Diabetes Center) are acknowledged.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1707/-/DC1.
REFERENCES
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sell H, Dietze-Schroeder D, Eckel J. The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol Metab 2006;17:416–422 [DOI] [PubMed] [Google Scholar]
- 3.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab 2003;14:137–145 [DOI] [PubMed] [Google Scholar]
- 4.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537–1545 [DOI] [PubMed] [Google Scholar]
- 5.Breitling R. Robust signaling networks of the adipose secretome. Trends Endocrinol Metab 2009;20:1–7 [DOI] [PubMed] [Google Scholar]
- 6.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009;30:600–607 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 8.Ahrén B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care 2007;30:1344–1350 [DOI] [PubMed] [Google Scholar]
- 9.Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 2003;40:209–294 [DOI] [PubMed] [Google Scholar]
- 10.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother 2009;58:1723–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23:57–63 [DOI] [PubMed] [Google Scholar]
- 12.Kolaczynski JW, Morales LM, Moore JH, Jr, et al. A new technique for biopsy of human abdominal fat under local anaesthesia with Lidocaine. Int J Obes Relat Metab Disord 1994;18:161–166 [PubMed] [Google Scholar]
- 13.Lönnqvist F, Nordfors L, Jansson M, Thörne A, Schalling M, Arner P. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J Clin Invest 1997;99:2398–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Löfgren P, Hoffstedt J, Näslund E, Wirén M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia 2005;48:2334–2342 [DOI] [PubMed] [Google Scholar]
- 15.Dietze-Schroeder D, Sell H, Uhlig M, Koenen M, Eckel J. Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes 2005;54:2003–2011 [DOI] [PubMed] [Google Scholar]
- 16.Curat CA, Miranville A, Sengenès C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 2004;53:1285–1292 [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Choi YS, Lim S, et al. Comparative analysis of the secretory proteome of human adipose stromal vascular fraction cells during adipogenesis. Proteomics 2010;10:394–405 [DOI] [PubMed] [Google Scholar]
- 18.Rosenow A, Arrey TN, Bouwman FG, et al. Identification of novel human adipocyte secreted proteins by using SGBS cells. J Proteome Res 2010;9:5389–5401 [DOI] [PubMed]
- 19.Zhong J, Krawczyk SA, Chaerkady R, et al. Temporal profiling of the secretome during adipogenesis in humans. J Proteome Res 2010;9:5228–5238 [DOI] [PMC free article] [PubMed]
- 20.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci 2010;1212:E1–E19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sell H, Dietze-Schroeder D, Eckardt K, Eckel J. Cytokine secretion by human adipocytes is differentially regulated by adiponectin, AICAR, and troglitazone. Biochem Biophys Res Commun 2006;343:700–706 [DOI] [PubMed] [Google Scholar]
- 22.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond) 2005;108:277–292 [DOI] [PubMed] [Google Scholar]
- 23.Kos K, Baker AR, Jernas M, et al. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab 2009;11:285–292 [DOI] [PubMed] [Google Scholar]
- 24.Iwaki-Egawa S, Watanabe Y, Kikuya Y, Fujimoto Y. Dipeptidyl peptidase IV from human serum: purification, characterization, and N-terminal amino acid sequence. J Biochem 1998;124:428–433 [DOI] [PubMed] [Google Scholar]
- 25.Schrader WP, West CA, Miczek AD, Norton EK. Characterization of the adenosine deaminase-adenosine deaminase complexing protein binding reaction. J Biol Chem 1990;265:19312–19318 [PubMed] [Google Scholar]
- 26.Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept 1999;85:9–24 [DOI] [PubMed] [Google Scholar]
- 27.Bouchard L, Tchernof A, Deshaies Y, et al. ZFP36: a promising candidate gene for obesity-related metabolic complications identified by converging genomics. Obes Surg 2007;17:372–382 [DOI] [PubMed] [Google Scholar]
- 28.Reinehr T, Roth CL, Enriori PJ, Masur K. Changes of dipeptidyl peptidase IV (DPP-IV) in obese children with weight loss: relationships to peptide YY, pancreatic peptide, and insulin sensitivity. J Pediatr Endocrinol Metab 2010;23:101–108 [DOI] [PubMed] [Google Scholar]
- 29.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 [DOI] [PubMed] [Google Scholar]
- 30.Rizzo M, Rizvi AA, Spinas GA, Rini GB, Berneis K. Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin Investig Drugs 2009;18:1495–1503 [DOI] [PubMed] [Google Scholar]
- 31.Sudre B, Broqua P, White RB, et al. Chronic inhibition of circulating dipeptidyl peptidase IV by FE 999011 delays the occurrence of diabetes in male zucker diabetic fatty rats. Diabetes 2002;51:1461–1469 [DOI] [PubMed] [Google Scholar]
- 32.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 2005;54:146–151 [DOI] [PubMed] [Google Scholar]
- 33.Sauvé M, Ban K, Momen MA, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010;59:1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.