Abstract
OBJECTIVE
Type 1 diabetes leads to impairments in growth, function, and regenerative capacity of skeletal muscle; however, the underlying mechanisms have not been clearly defined.
RESEARCH DESIGN AND METHODS
With the use of Ins2WT/C96Y mice (model of adolescent-onset type 1 diabetes), muscle regeneration was characterized in terms of muscle mass, myofiber size (cross-sectional area), and protein expression. Blood plasma was analyzed for glucose, nonesterified fatty acids, insulin, and plasminogen activator inhibitor-1 (PAI-1). PAI-039, an effective inhibitor of PAI-1, was orally administered to determine if PAI-1 was attenuating muscle regeneration in Ins2WT/C96Y mice.
RESULTS
Ins2WT/C96Y mice exposed to 1 or 8 weeks of untreated type 1 diabetes before chemically induced muscle injury display significant impairments in their regenerative capacity as demonstrated by decreased muscle mass, myofiber cross-sectional area, myogenin, and Myh3 expression. PAI-1, a physiologic inhibitor of the fibrinolytic system and primary contributor to other diabetes complications, was more than twofold increased within 2 weeks of diabetes onset and remained elevated throughout the experimental period. Consistent with increased circulating PAI-1, regenerating muscles of diabetic mice exhibited excessive collagen levels at 5 and 10 days postinjury with concomitant decreases in active urokinase plasminogen activator and matrix metalloproteinase-9. Pharmacologic inhibition of PAI-1 with orally administered PAI-039 rescued the early regenerative impairments in noninsulin-treated Ins2WT/C96Y mice.
CONCLUSIONS
Taken together, these data illustrate that the pharmacologic inhibition of elevated PAI-1 restores the early impairments in skeletal muscle repair observed in type 1 diabetes and suggests that early interventional studies targeting PAI-1 may be warranted to ensure optimal growth and repair in adolescent diabetic skeletal muscle.
With type 1 diabetes onset predominantly occurring during youth, a time of critical growth and development, two important issues related to the current study must be considered: 1) atrophic stimuli placed on young, growing muscle results in a rapid and irreversible remodeling process (1–3), and 2) populations with pediatric type 1 diabetes consistently display elevated plasminogen activator inhibitor-1 (PAI-1) levels, irrespective of HbA1c (4). Unfortunately, assessment of skeletal muscle health in type 1 diabetes has not been a consideration in the clinical setting because it is assumed that insulin therapy alone is enough to restore normal muscle health by balancing protein synthesis and degradation. However, several studies have demonstrated that insulin treatment does not restore this balance (5–8), and the information to date indicates that young patients with diabetes score significantly lower on maximal strength tests (9) and that adolescents newly diagnosed with type 1 diabetes experience reduced muscle fiber size and altered muscle morphology (10). Studies using appropriate animal models of adolescent type 1 diabetes also demonstrate significant limitations in muscle growth and contractile function (11–13).
For skeletal muscle tissue to stay healthy, it must continuously be maintained, adapt to changing needs, and be capable of repair in instances of overuse, exercise, or trauma. The repair of skeletal muscle is a complex orchestration of events including degeneration, extracellular matrix (ECM) remodeling, and repair/replacement of damaged muscle fibers (14). This regenerative process must proceed in an orderly and efficient manner if skeletal muscle is to be maintained as a healthy, functioning organ. Although it has been reported that the type 1 diabetes environment may affect muscle regeneration after injury (15–17), it has been proposed, although never demonstrated, that the lack of insulin’s anabolic action is the sole reason for the deficits observed. However, the role of insulin in skeletal muscle repair and regeneration has yet to be established. It is now becoming increasingly evident from studies conducted in various tissues that other factors, such as alterations in circulating PAI-1, may be as important in diabetes complications as hypoinsulinemia/hyperglycemia (18–20). In skeletal muscle, alterations in PAI-1 levels, an inhibitor of the fibrinolytic system, can have profound effects on ECM remodeling and ultimately delay muscle regeneration after injury (21–25).
In the current study, we sought to determine the temporal pattern of regeneration and elucidate the underlying mechanism(s) resulting in deficits in the regenerative capacity of skeletal muscle in adolescent type 1 diabetes using a genetic murine model of the disease, the Ins2WT/C96Y mouse.
RESEARCH DESIGN AND METHODS
Animal care.
Male C57BL/6-Ins2Akita/J (hereafter Ins2WT/C96Y) mice and their wild-type (WT) littermates were purchased at 3 weeks of age from Jackson Laboratory (Bar Harbor, ME). Mice (N = 16/group) were studied over a period of 8 to 13 weeks of untreated type 1 diabetes. A separate group of Ins2WT/C96Y and WT mice (N = 3/group) were used for the 1 week of type 1 diabetes regeneration study. Ins2WT/C96Y mice become spontaneously diabetic at ∼4 weeks of age because of a heterozygous mutation in the Ins2 gene (26). Exact onset of diabetes was determined by monitoring blood glucose as previously described (12). The Ins2WT/C96Y mice were chosen instead of the commonly used streptozotocin-induced diabetic rodent model because of known growth-arresting effects of streptozotocin on skeletal muscle (27).
The animal room was maintained at 21°C, 50% humidity, and 12-h/12-h light–dark cycle. All mice had access to standard breeder chow and water ad libitum.
Blood glucose and body mass were measured biweekly (fed state: 1200–1400 h) in the 8-week experimental groups. Blood samples were collected at 2, 4, and 6 weeks of diabetes for analysis of metabolites and hormones. All animal experiments were approved by the McMaster and York University Animal Care Committees in accordance with Canadian Council for Animal Care guidelines.
Skeletal muscle injury.
Skeletal muscle injury was induced with an intramuscular injection of 10 μM cardiotoxin (CTX; Latoxan, France) as previously described (28). Injuries were generated in the left tibialis anterior (TA) and quadriceps muscles of both Ins2WT/C96Y and WT mice at 1 and 8 weeks of diabetes. The 1-week group was harvested at 10 days postinjury, whereas the 8-week group was subdivided into four recovery time points: 5, 10, 21, and 35 days.
Tissue collection.
After the specified regeneration period, animals were killed and blood was collected from the thoracic cavity after heart excision. Injured and uninjured TA muscles were coated in optimum cutting temperature embedding compound and frozen in isopentane cooled by liquid nitrogen, and injured quadriceps muscles were snap-frozen and stored at −80°C.
PAI-039 treatment.
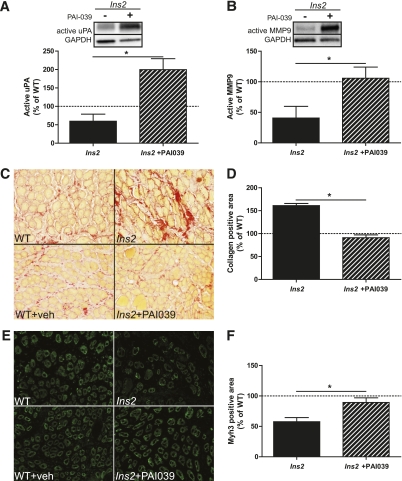
To determine if elevations in circulating PAI-1 were contributing to impaired skeletal muscle regeneration in the diabetic animals, PAI-039, an orally effective inhibitor of active PAI-1 (29), was administered throughout the regenerative process. An additional group of WT and Ins2WT/C96Y mice (N = 4) were treated via oral gavage with vehicle (2% Tween-80 and 0.5% methylcellulose in sterile H2O) or vehicle plus PAI-039 (2 mg/kg; Axon Medchem, the Netherlands), respectively. On the day of CTX injury (at 8 weeks of diabetes), mice were treated with vehicle or vehicle plus PAI-039 at 1100 h, received CTX injury to the TA at 1200 h, and received PAI-039 treatment again at 1500 h. PAI-039 treatment was continued twice daily (1100 and 1500 h) throughout the 5-day regeneration period, at which point the animals were killed and tissues were dissected and stored as described above. Those treatment time points were chosen to best attenuate the peak of PAI-1 activity because of its circadian expression pattern (30). WT mice treated with vehicle (WT + vehicle) demonstrated no significant difference from untreated WT in active urokinase plasminogen activator (uPA), active matrix metalloproteinase (MMP)-9, collagen levels, and Myh3; therefore, these two groups were pooled for comparison with Ins2WT/C96Y mice and Ins2WT/C96Y mice treated with PAI-039 (Ins2WT/C96Y+PAI-039) as illustrated in Fig. 3.
FIG. 3.
Pharmacologic treatment against PAI-1 improves fibrinolytic pathway activity, collagen degradation, and regeneration at 5 days post-CTX injury in type 1 diabetes. A: Treatment with PAI-039 caused an increase in free uPA in Ins2WT/C96Y compared with untreated Ins2WT/C96Y (t test: P = 0.004; N = 4). B: Similarly, active MMP9 was elevated in PAI-039–treated Ins2WT/C96Y (t test: P = 0.025; N = 4). These findings are characteristic of restored fibrinolytic pathway activity, which presumably led to the (C and D) reduced collagen levels (t test: P < 0.001; N = 4) and (E and F) increased Myh3-positive area (t test: P = 0.011; N = 4) observed in PAI-039–treated Ins2WT/C96Y compared with untreated Ins2WT/C96Y (labeled Ins2, C and E). A–E: Black bars represent Ins2WT/C96Y, and striped bars represent PAI-039–treated Ins2WT/C96Y. Data are presented relative to the mean of the WT and WT + vehicle pooled data. *Differences between groups identified by t test. (A high-quality digital representation of this figure is available in the online issue.)
Blood analyses.
Heparinized blood plasma was analyzed for insulin and total PAI-1 (MADPK-71 K; Millipore, Billerica, MA) at all collection time points. Plasma was also analyzed for nonesterified fatty acids with the use of a colorimetric assay (Wako Diagnostics, Richmond, VA).
Western blot analysis.
Snap-frozen quadriceps or TA samples were homogenized, analyzed for protein concentration, electrophoretically separated on acrylamide gels, and transferred to polyvinylidene fluoride membranes as previously described (28). Primary antibodies included Myh3 (Hybridoma Bank F1.652), GAPDH (Abcam 8245, loading control; Cambridge, MA), myogenin (Hybridoma Bank F5D), MMP9 (Abcam 38898), and uPA (Abcam 28230). uPA was analyzed for unbound (active) uPA and uPA bound to PAI-1 as a measure of PAI-1 activity in the muscle (31). Active uPA is found at ∼48 kDa, and inactive (PAI-1-bound) uPA is found at ∼93 kDa. Appropriate horseradish peroxidase-conjugated secondary antibodies were used and visualized with the addition of chemiluminescent reagent (Amersham, Piscataway, NJ). Images were acquired with a Fusion Fx7 imager (Vilber Lourmat, Eberhardzell, Germany) and analyzed with ImageJ.
Histochemical and immunofluorescent analyses.
Eight-micron skeletal muscle cross-sections were mounted on glass slides and stained as described below.
Hematoxylin–eosin.
Hematoxylin–eosin staining was used to determine the average fiber area of uninjured and injured TA. Three images spaced evenly throughout the TA (∼1 mm apart) were used for analysis where 25 fibers per image were analyzed for area (75 total fibers per TA). We have previously demonstrated that in this muscle, quantification of this number of fibers provides a representative analysis of fiber area (12,32).
Picrosirius red.
To stain for collagen content, sections were immersed in picrosirius red solution (0.1% w/v Direct Red 80 [Sigma 365548; St. Louis, MO] in a saturated aqueous solution of picric acid [Sigma p6744]) for 1 h. Sections were briefly rinsed in two changes of acidified dH20 (0.5% glacial acetic acid), dehydrated, cleared, and mounted.
Immunofluorescence.
Sections were fixed with ice-cold 2% paraformaldehyde, blocked with 10% normal goat serum/1.5% BSA, followed by mouse IgG Block (BMK 2202; Vector Laboratories Inc., Burlingame, CA), and incubated with 1:1 dilution of anti-Myh3 overnight at 4°C. Alexa 488 anti-mouse secondary antibody (A-11001; Invitrogen, Carlsbad, CA) was used for detection, and 4,6-diamidino-2-phenylindole was used to identify nuclei.
Image analysis.
Images obtained with a Nikon 90i-eclipse microscope (Nikon Inc., Melville, NY) were analyzed using NIS Elements software (Nikon, Inc., Melville, NY). Analysis included determination of collagen positive area and Myh3 positive area using signal threshold settings as the detection method. Fiber area was determined manually using NIS Elements software.
Statistical analysis.
For all experiments, the appropriate t test or two-way ANOVA with Bonferroni post hoc analysis was performed between Ins2WT/C96Y and WT groups. Two-way ANOVA was run on datasets with dependent variables measured over time, and one-tailed t tests were carried out on data with only single comparisons. One-tailed t tests were justified for these comparisons because differences in a specific direction were hypothesized a priori on the basis of our data and previous reports (21–25). Data are presented as mean ± SEM with P < 0.05 considered significant. Asterisks denote significant differences identified by t test or Bonferroni post hoc test in pairwise comparisons, and significant main effect of diabetes or significant interaction between diabetes and time is listed in Figs. 1 to 3.
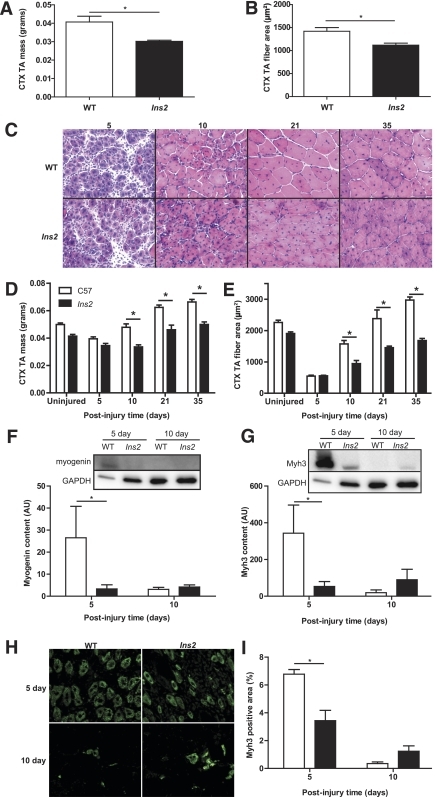
FIG. 1.
Type 1 diabetes impairs regeneration of skeletal muscle after CTX injury. Ten days after injury, TA muscle of the 1-week diabetic Ins2WT/C96Y mice demonstrates a significant loss of (A) mass (t test: P = 0.015; N = 3) and (B) myofiber cross-sectional area (t test: P = 0.014; N = 3) compared with WT, indicating impaired regeneration after injury at only 7 days of type 1 diabetes. C: Hematoxylin–eosin staining of TA injured at 8 weeks of type 1 diabetes demonstrates loss of (D) muscle mass (N = 16) and (E) myofiber area (N = 16) compared with WT beginning at 10 days postinjury. The uninjured time point in both panels is the contralateral TA to the 5-day post-CTX muscle and is included to illustrate the decrease in muscle mass and myofiber area associated with the impaired growth of skeletal muscle in the type 1 diabetic state. The values for the uninjured time point are not included in the statistical analysis. A main effect of diabetes (main effect: P < 0.001) is observed in both mass and fiber area with the asterisk (*) denoting specific differences between WT and Ins2WT/C96Y as defined by post hoc analysis. Note that the type 1 diabetic muscle does not return to WT mass/fiber size at later time points, but continues to lag in regeneration. Because early expression of myogenic proteins is critical to the early stages of regeneration, (F) myogenin (main effect: P = 0.062, interaction: P = 0.017; N = 8) and (G) embryonic myosin heavy chain (Myh3) expression (main effect: P = 0.117, interaction: P = 0.009; N = 8) were determined in quadriceps muscle and demonstrate significantly increased expression at 5 days postinjury in WT but not Ins2WT/C96Y (labeled Ins2, F and G). H: Immunofluorescent staining of injured TA with anti-Myh3 confirms (I) the lack of Myh3 positive fibers in Ins2WT/C96Y compared with WT (main effect: P = 0.013; interaction: P < 0.001; N = 8) at 5 days postinjury. *Differences between groups at specific time points identified by Bonferroni post hoc analysis after 2-way ANOVA (D–G, I). A–I: White bars represent WT, and black bars represent Ins2WT/C96Y. (A high-quality digital representation of this figure is available in the online issue.)
RESULTS
Ins2WT/C96Y mice spontaneously developed type 1 diabetes (hypoinsulinemia/hyperglycemia) at ∼4 weeks of age, which was maintained throughout the study period (Tables 1 and 2) compared with WT littermates. Relative to WT mice, Ins2WT/C96Y mice also displayed decreased body mass gain and developed hyperlipidemia by 6 weeks of untreated diabetes (Tables 1 and 2), consistent with previous findings (33).
TABLE 1.
Characteristics of WT and diabetic mice at 8 weeks of diabetes (∼13 weeks old)
| Weeks of diabetes |
Diabetes main effect and interaction with time |
||||||
|---|---|---|---|---|---|---|---|
| Measure | Group | 2 | 4 | 6 | 8 | P value | |
| Body mass (g) | 8 weeks + variable time post-CTX | WT | 20.1 ± 0.3 | 23.1 ± 0.4 | 24.9 ± 0.4 | 26.2 ± 0.4 | DME = 0.0004 |
| Ins2 | 19.1 ± 0.3 | 21.5 ± 0.3* | 22.9 ± 0.4* | 23.9 ± 0.4* | Int = 0.0035 | ||
| Insulin (pg/mL) | WT | 789 ± 86 | 778 ± 175 | 814 ± 106 | DME <0.0001 | ||
| Ins2 | 225 ± 24* | 224 ± 35* | 222 ± 56* | Int = NS | |||
| Blood glucose (mmol/L) | WT | 11.3 ± 0.6 | 9.1 ± 0.3 | 9.1 ± 0.3 | 8.2 ± 0.3 | DME <0.0001 | |
| Ins2 | 29.1 ± 1.0* | 32.2 ± 0.7* | 32.4 ± 0.7* | 33.1 ± 0.5* | Int <0.0001 | ||
| Nonesterified fatty acids (mmol/L) | WT | 0.63 ± 0.06 | 0.77 ± 0.08 | 1.10 ± 0.06 | DME <0.0001 | ||
| Ins2 | 1.05 ± 0.10 | 0.85 ± 0.07 | 2.14 ± 0.12* | Int = 0.0003 | |||
Biweekly data for the long-term (8 weeks) type 1 diabetic and WT groups (N = 16). Ins2WT/C96Y mice are labeled Ins2. Two-factor ANOVA was run to determine the main effects of diabetes, time, and interaction; P values for diabetes main effect and interaction are listed next to the respective data. Absence of a significant main effect or interaction is indicated as NS.
DME, diabetes main effect; Int, interaction with time.
*Significant difference at that time point by Bonferroni post hoc comparison.
TABLE 2.
Characteristics of WT and diabetic mice during skeletal muscle regeneration after a period of untreated type 1 diabetes
| Days post-CTX injury |
Diabetes main effect and interaction with time |
||||||
|---|---|---|---|---|---|---|---|
| Measure | Group | 5 | 10 | 21 | 35 | P value | |
| Body mass (g) | 8 weeks + variable time post-CTX | WT | 26.8 ± 1.2 | 26.8 ± 0.9 | 28.5 ± 0.7 | 29.9 ± 0.9 | DME <0.0001 |
| Ins2 | 25.0 ± 0.6 | 23.0 ± 0.6* | 24.6 ± 0.5* | 25.5 ± 0.4* | Int = NS | ||
| Insulin (pg/mL) | WT | 1,294 ± 286 | 1,242 ± 268 | 1,818 ± 461 | 2,413 ± 349 | DME <0.0001 | |
| Ins2 | 235 ± 23* | 235 ± 41* | 194 ± 115* | 157 ± 53* | Int = NS | ||
| Blood glucose (mmol/L) | WT | 8.2 ± 0.2 | 9.2 ± 0.4 | 8.7 ± 0.3 | 8.6 ± 0.4 | DME <0.0001 | |
| Ins2 | 31.4 ± 0.7* | 33.2 ± 0.7* | 33.2 ± 0.9* | 32.1 ± 0.6* | Int = NS | ||
| Nonesterified fatty acids (mmol/L) | WT | 0.69 ± 0.09 | 0.56 ± 0.06 | 0.53 ± 0.08 | 0.73 ± 0.04 | DME <0.0001 | |
| Ins2 | 1.27 ± 0.13* | 1.54 ± 0.06* | 1.26 ± 0.19* | 1.97 ± 0.25* | Int = NS | ||
| Uninjured TA mass (g) | WT | 0.047 ± 0.001 | 0.049 ± 0.002 | 0.050 ± 0.001 | 0.054 ± 0.004 | DME <0.0001 | |
| Ins2 | 0.039 ± 0.002 | 0.038 ± 0.001* | 0.044 ± 0.002 | 0.045 ± 0.003* | Int = NS | ||
| Uninjured TA fiber area (µm2) | WT | 2,168 ± 135 | 2,349 ± 121 | 2,182 ± 89 | 2,338 ± 229 | DME = 0.0007 | |
| Ins2 | 1,905 ± 103 | 1,813 ± 32* | 2,074 ± 158 | 1,834 ± 36* | Int = NS | ||
| Uninjured TA fiber area (µm2) | 1 week + time post-CTX | WT | 1,832 ± 96 | ||||
| Ins2 | 1,768 ± 61 | ||||||
Data for the long-term (8 weeks + variable time post-CTX; N = 16 [4 per time point]) and short-term (1 week + 10 days post-CTX; N = 3) type 1 diabetic and WT groups. Ins2WT/C96Y mice are labeled Ins2. Two-factor ANOVA was run to determine the main effects of diabetes, time, and interaction; P values for diabetes main effect and interaction are listed next to the respective data. Absence of a significant main effect or interaction is indicated as NS.
DME, diabetes main effect; Int, interaction with time.
*Significant difference at that time point by Bonferroni post hoc comparison. For single comparison of short-term groups, t test revealed no significant difference.
Histologic assessment of the uninjured TA served as an index for the effects of type 1 diabetes on skeletal muscle growth. We found that uninjured Ins2WT/C96Y muscles displayed no impairment in myofiber cross-sectional area within the first 17 days of type 1 diabetes (Table 1), whereas a significant reduction (12%) in myofiber cross-sectional area accrual occurred by 8 weeks of type 1 diabetes that did not significantly worsen with increasing disease duration (up to 13 weeks, Table 2). This suggests that impaired growth, rather than progressive atrophy, is responsible for the reduced myofiber area observed in Ins2WT/C96Y, at least until such time as significant neuropathic complications develop (34).
In response to muscle damage, deficits in muscle health became considerably more apparent. Ins2WT/C96Y muscles exposed to the type 1 diabetes environment for 1 week before injury demonstrated a 21% decrement in myofiber cross-sectional area at the 10-day regeneration mark (Fig. 1B). This novel finding suggests that even short-term exposure to type 1 diabetes has profound effects on skeletal muscle’s ability to repair after damage.
The regenerative capacity was also impaired at 8 weeks of disease progression because muscle masses and myofiber cross-sectional areas of regenerating Ins2WT/C96Y muscles were significantly less than WT muscles from 10 days of regeneration onward (Fig. 1C–E). Loss of mass and myofiber area was significant even when expressed as a percentage of either the uninjured, contralateral TA or body mass (Supplementary Fig. 1), confirming that the poor regeneration in type 1 diabetes extends beyond the reduced growth rate. These changes in overall mass and myofiber area were preceded by alterations in the protein expression of markers of the regenerative process, myogenin and embryonic myosin heavy chain (Myh3). Myogenin is a myogenic regulatory factor that is expressed during early time points in regeneration and is important for cell-cycle exit of myoblasts and consequent terminal differentiation (35,36). Western blot analysis showed suppressed expression of myogenin in Ins2WT/C96Y muscle compared with WT muscle at 5 days postinjury (Fig. 1F). Myh3, a developmental myosin isoform, is expressed transiently during skeletal muscle regeneration (37) and used as a reference point to assess the process of differentiation (38). Similar to myogenin, the protein expression of Myh3 was reduced in Ins2WT/C96Y muscle at 5 days of regeneration. Both Western blot and immunofluorescent staining of regenerating muscles demonstrated this expression pattern (Fig. 1G–I). Neither group displayed expression of Myh3 at 21 or 35 days after regeneration, suggesting that the impairments in the regenerative process are within the early phases after injury with diabetes development (≤10 days), and after this time, maturation proceeds, albeit delayed.
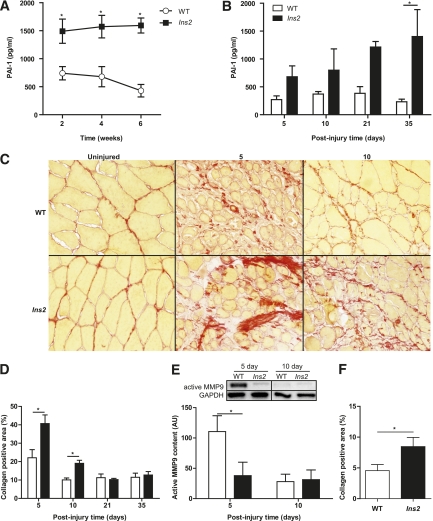
Skeletal muscle regeneration is a complex process that is heavily dependent, particularly during the early phase, on the optimal functioning of the fibrinolytic system (21–25). We speculated that impairments in type 1 diabetes muscle regeneration may be due, at least in part, to elevated PAI-1 preventing the activation of uPA and its downstream effectors, the MMPs (e.g., MMP9). Consequently, suppression of the fibrinolytic process would result in attenuation of ECM remodeling, thus creating barriers for infiltration of immune cells and efficient activation and invasion of the myogenic stem cells that are responsible for the formation of new myofibers (14). We observed total PAI-1 levels to be more than twofold higher in Ins2WT/C96Y mice than in WT mice within 2 weeks of hyperglycemia in the former group, with values remaining elevated with diabetes throughout the experimental period (Fig. 2A and B). Moreover, regenerating Ins2WT/C96Y muscle displayed elevations in collagen content at 5 and 10 days of regeneration compared with WT muscle (Fig. 2C and D). Active uPA levels at 5 days of regeneration were decreased by ∼35% in Ins2WT/C96Y muscle compared with WT muscle (WT: 5978 ± 1362 vs. Ins2WT/C96Y: 3873 ± 1241; P = 0.15). Although this decrease in active uPA was not statistically significant, active MMP9, the MMP associated with ECM remodeling in skeletal muscle (39,40), was significantly elevated at 5 days of regeneration in WT but not Ins2WT/C96Y muscle, with levels between the two groups similar by 10 days postinjury (Fig. 2E).
FIG. 2.
Type 1 diabetes causes elevated PAI-1, suppresses MMP9 activation, and increases collagen content during early regeneration time points. Significantly elevated PAI-1 levels in Ins2WT/C96Y mice compared with WT mice were found in blood plasma collected (A) throughout type 1 diabetes progression (main effect: P < 0.001; N = 16) and (B) after CTX injury (main effect: P < 0.001; N = 16). This led to the hypothesis that collagen would be elevated in the Ins2WT/C96Y mice because of suppression of the fibrinolytic pathway. C: Picrosirius red staining of injured TA sections revealed increased collagen (red color), which was statistically significant (D, main effect: P = 0.001, interaction: P = 0.004; N = 16). E: MMP9, an important protease in skeletal muscle collagen cleavage, was also found to be significantly repressed in Ins2WT/C96Y mice (labeled Ins2, E) at the 5-day time point (N = 8). F: The short-term Ins2WT/C96Y mice also exhibited increased collagen at 10 days postinjury (t test: P = 0.047, N = 3). *Differences between groups at specific time points identified by Bonferroni post hoc analysis after 2-way ANOVA (A, B, D, E). A–E: White bars/circles represent WT, and black bars/squares represent Ins2WT/C96Y. (A high-quality digital representation of this figure is available in the online issue.)
With PAI-1 elevated within ∼2 weeks of type 1 diabetes onset (Fig. 2A), we hypothesized that the deficit in regeneration observed in Ins2WT/C96Y mice diabetic for 1 week before CTX injury would also display defective ECM remodeling. As hypothesized, collagen content in regenerating Ins2WT/C96Y mice, diabetic for a total of 17 days, was significantly elevated (Fig. 2F), consistent with a role of PAI-1 in the impaired regeneration.
By having identified that increased PAI-1 levels in Ins2WT/C96Y mice are associated with early impairments in muscle regeneration, we then determined if these deficits could be restored with pharmacologic inhibition of PAI-1, even in the absence of insulin therapy. Twice-daily oral dosing of PAI-039 (tiplaxtinin), a pharmacologic inhibitor of PAI-1 (29), effectively increased the amount of active (free) uPA in the injured muscle of 8-week diabetic Ins2WT/C96Y mice compared with untreated Ins2WT/C96Y mice (Fig. 3A) and increased the ratio of active to inactive uPA (uPA/PAI-1-uPA) (Ins2WT/C96Y: 1.22 ± 0.13 AU vs. Ins2WT/C96Y+PAI-039: 1.74 ± 0.14 AU; P = 0.02). WT mice treated with vehicle alone demonstrated no significant change in active uPA levels (WT: 5978 ± 1362 AU vs. WT + vehicle: 7007 ± 778 AU; P = 0.27) or uPA/PAI-1-uPA (WT: 1.40 ± 0.18 AU vs. WT + vehicle: 1.28 ± 0.09 AU; P = 0.29). The downstream effect of elevated active uPA levels, resultant from PAI-039 treatment, was an increase in active MMP9 (Fig. 3B) and a normalization of collagen content in the regenerating Ins2WT/C96 muscles to the levels observed in WT mice (Fig. 3C and D). The recovery of the fibrinolytic pathway with PAI-039 in Ins2WT/C96 mice restored not only normal ECM remodeling but also Myh3 expression to levels similar to WT regenerating muscles (Fig. 3E and F). Injured TA fiber area demonstrated no significant difference between groups (WT + vehicle: 461 ± 29 µm2 vs. Ins2WT/C96Y+PAI-039: 396 ± 32 µm2; P = 0.19), whereas TA mass exhibited a small but significant difference (WT + vehicle: 0.039 ± 0.002 g vs. Ins2WT/C96Y+PAI-039: 0.031 ± 0.002 g; P < 0.05). Similarly, no difference was noted in fiber area at 5 days postinjury between WT and Ins2WT/C96Y mice (Fig. 1E), with a small decrease in muscle mass at that time point (Fig. 1D). The reasons underlying the apparent discrepancy between fiber area and muscle mass in the diabetic mice is unknown; however, it could be speculated that differences in fibrosis, inflammatory response, or lipid content within the muscles of the various groups could contribute to these observations.
To rule out the possibility that PAI-039 treatment improves glycemic or insulinemic levels, thus improving the diabetic environment in ways other than affecting PAI-1 activity, whole-blood glucose and plasma insulin levels were measured. Blood glucose concentrations remained severely elevated in the Ins2WT/C96Y mice treated with PAI-039 (WT + vehicle: 8.9 ± 0.7 mmol/L vs. Ins2WT/C96Y+PAI-039: 35.0 ± 0.0 mmol/L; P < 0.05), whereas insulin levels remained low (WT + vehicle: 916 ± 74 pg/mL vs. Ins2WT/C96Y+PAI-039: 146 ± 29 pg/mL; P < 0.05).
DISCUSSION
Our results indicate that the type 1 diabetic environment negatively affects the health of skeletal muscle, as defined by impaired growth and poor regenerative capacity. The deficits in regenerative capacity occur rapidly with exposure to type 1 diabetes (within ∼2 weeks) and, as we demonstrated, are consistent with elevated PAI-1 and ineffective ECM remodeling.
Maintaining a healthy muscle mass in the type 1 diabetic population has not typically been addressed in the clinical setting. Unfortunately, many studies demonstrate impairments in skeletal muscle health (e.g., impaired morphology, decreased strength, and metabolic capacity) observed early in patients with type 1 diabetes who are receiving insulin therapy, changes that may precede other diabetes complications (13). The results presented support these previous findings as we demonstrate that repair from muscle damage is significantly blunted in the diabetic state with as little as 7 days of uncontrolled type 1 diabetes before muscle injury. Furthermore, we also demonstrate that PAI-1 is significantly elevated within the first 2 weeks of type 1 diabetes onset and that inhibition of this hormone restores the regenerative capacity of type 1 diabetic mice, irrespective of the hypoinsulinemia. Although skeletal muscle is capable of maintaining basic function in the face of extreme stressors, this does not equate to a healthy muscle mass that is functioning optimally. We and others have demonstrated that although basic indices of muscle function may not be significantly impaired, dramatic changes are occurring within the muscle demonstrating compromised health (11,12,41). If we heed lessons from other metabolic disease states (e.g., obesity), as muscle health diminishes, disease severity increases. For example, the muscle wasting that occurs with obesity (sarcopenic obesity) is a serious complication resulting in the expedition of complications within other tissues (42). Given the importance of skeletal muscle to whole-body fuel metabolism, ensuring that skeletal muscle health is maintained in metabolic disease states is obviously of critical importance.
Type 1 diabetes onset most often occurs during childhood/adolescence, and previous studies have shown that atrophic stimuli (e.g., hindlimb casting) placed on young, growing muscle result in a rapid and irreversible remodeling process, ultimately leading to a failure to achieve its full potential of adult muscle mass (1–3). We were interested to determine if type 1 diabetes may prove to be one of these “atrophic environments.” The present findings illustrate that growing skeletal muscle exposed to type 1 diabetes will display a failure to accrue muscle mass/fiber area, and these findings are not the product of progressive atrophy resultant from prolonged type 1 diabetes exposure or neuropathic complications, because no change in muscle mass or fiber area was observed once into adulthood (a further 5 weeks of uncontrolled type 1 diabetes). Although we investigated the uncontrolled diabetic state in a rodent model of diabetes, it is worth considering that there are two situations in which pediatric type 1 diabetic populations may be under this stress: 1) before diagnosis (which may last a period of months) and 2) after diagnosis when glycemic control is difficult and suboptimal (43). Consistent with our results, findings in newly diagnosed juvenile type 1 diabetic humans demonstrate a reduced myofiber area compared with healthy age-matched control subjects (10).
Repair from muscle damage consists of multiple, overlapping stages (14). After the initial injury, an inflammatory phase ensues to remove damaged cells and debris. It is during this early phase that remodeling of the ECM begins, with a dramatic increase in ECM proteins, particularly collagen, which is needed as the structural integrity of the muscle is compromised while damaged muscle fibers undergo phagocytosis. As repair continues, so does ECM remodeling, with the excess of ECM proteins undergoing degradation as nascent myofibers form and mature. Activity of the fibrinolytic system (PAI-1, uPA, MMPs) is critical during this ECM remodeling period (21–25). Newly formed myofibers will initially express immature myosin heavy chain isoforms, such as Myh3 (embryonic myosin heavy chain). As maturation continues, the muscle fibers will increase in size, returning to a preinjury state while replacing the immature contractile proteins with mature isoforms. We show in this study that in response to muscle damage, ECM remodeling is impaired in the type 1 diabetic state, and this is a direct result of elevated PAI-1. The increase in PAI-1 observed in regenerating muscle of type 1 diabetic mice decreased active uPA and MMP9 levels, thereby attenuating ECM turnover (as noted by elevated intramuscular collagen) during the first 10 days postinjury. Restoration of the fibrinolytic system in type 1 diabetic mice via pharmacologic inhibition of PAI-1 restored active MMP9 expression, returned collagen levels to normative values, and ultimately allowed for nascent muscle fiber growth to occur. Consistent with these findings, mice deficient in uPA exhibit impaired muscle regeneration, whereas PAI-1–deficient mice exhibit augmented muscle repair (22,44). It is worth noting that, in the current study, PAI-039 treatment in Ins2WT/C96Y mice resulted in active uPA levels that significantly exceeded that measured in WT mice muscles. Although this may be considered “supraphysiologic,” tissues were collected 1 h after PAI-039 administration on the day of harvest, a time when the drug effects were at their peak. With a PAI-039 half-life of 4.1 h (29), it can be speculated that more time was spent below this elevated level than within it.
Although the evidence to date suggests that insulin administration does not restore function of the fibrinolytic pathway (because insulin therapy does not reduce PAI-1 levels in human pediatric populations) (4), and thus would not improve collagen degradation and de novo myofiber formation, insulin might facilitate the rate of myofiber growth (later stages of regeneration) by modestly improving protein turnover (5–8). Future studies are needed to clearly define if PAI-039 treatment, in combination with standard insulin therapy, provides a more optimal regeneration environment. Furthermore, we administered PAI-039 just before the injury; however, future studies should consider the clinically important issue of treatment after injury, in terms of both effectiveness and timeframe. A final clinical consideration of PAI-039 treatment is the long-term effects of its administration if it were to be given prophylactically or for prolonged periods of time. To date, there is limited information on this topic. One study administered PAI-039 for 42 days through addition to the rodent chow and found an acute protection against radiation-induced intestinal injury but noted no adverse effects of drug treatment (45), whereas a second study provided a 2-month administration of PAI-039 to AngII/salt–treated mice (46). In the latter study, PAI-039 was effective in decreasing aortic remodeling with no effect of PAI-039 alone on serum amyloid A levels (an index of systemic inflammation).
Although elevated PAI-1 has been linked to other complications usually associated with diabetes, such as coronary artery disease and nephropathy (18–20), it is somewhat surprising that this is the first time that a clinically relevant inhibition of PAI-1 (through pharmacologic means) has been used to treat a diabetes complication. In fact, to the best of our knowledge, only three other studies have investigated mitigating diabetes complications by altering PAI-1 levels. These studies used streptozotocin-induced diabetes in PAI-1–deficient rodents to investigate the role of PAI-1 in mediating the effects of type 1 diabetes on renal morphology and function (18,47,48). Collectively, these authors found that elevated PAI-1 was contributing to diabetic nephropathy (increased glomerular ECM, decreased glomerular filtration rate) and that amelioration of these symptoms occurred in the PAI-1–deficient background. Given the demonstrated (present study) (18,47,48) and proposed (19,20) linkage of PAI-1 with diabetes complications and the fact that PAI-1 is elevated in populations with pediatric type 1 diabetes regardless of the level of glycemic control (4,49,50), we propose that aggressive therapeutic approaches, including intensive insulin and PAI-1 inhibitor strategies, warrant further investigation for the treatment of young type 1 diabetic patients. This will not only ensure optimal accrual and maintenance of a healthy skeletal muscle mass but also will reduce the onset and progression of other diabetes complications. Clearly, future studies are needed to definitively demonstrate the causative role of PAI-1 in the impaired muscle regeneration of patients with diabetes, and these studies may also prove valuable in developing therapeutic strategies to ensure the most effective management of other diabetes complications.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Natural Science and Engineering Research Council of Canada (T.J.H.) and the Ontario Graduate Scholarship (M.P.K.).
No potential conflicts of interest relevant to this article were reported.
M.P.K. designed the study; interpreted the results; performed animal care, sample collection, and assays; performed all data analysis; and wrote the initial manuscript draft. J.M. designed the study, interpreted the results, and performed animal care, sample collection, and assays. A.A.N. performed animal care, sample collection, and assays. M.C.R. designed the study and interpreted the results. T.J.H. designed the study, interpreted the results, and performed animal care, sample collection, and assays. All authors contributed to the final version of the manuscript.
The authors thank April Scott and Janis MacDonald of the McMaster University CAF for technical assistance. The Myh3 F1.652 antibody developed by H.M. Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology (Iowa City, IA).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0007/-/DC1.
REFERENCES
- 1.Mozdziak PE, Pulvermacher PM, Schultz E. Unloading of juvenile muscle results in a reduced muscle size 9 wk after reloading. J Appl Physiol 2000;88:158–164 [DOI] [PubMed] [Google Scholar]
- 2.Wanek LJ, Snow MH. Activity-induced fiber regeneration in rat soleus muscle. Anat Rec 2000;258:176–185 [DOI] [PubMed] [Google Scholar]
- 3.Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol 1989;67:1827–1834 [DOI] [PubMed] [Google Scholar]
- 4.Zeitler P, Thiede A, Müller HL. Prospective study on plasma clotting parameters in diabetic children—no evidence for specific changes in coagulation system. Exp Clin Endocrinol Diabetes 2001;109:146–150 [DOI] [PubMed] [Google Scholar]
- 5.Bennet WM, Connacher AA, Smith K, Jung RT, Rennie MJ. Inability to stimulate skeletal muscle or whole body protein synthesis in type 1 (insulin-dependent) diabetic patients by insulin-plus-glucose during amino acid infusion: studies of incorporation and turnover of tracer L-[1-13C]leucine. Diabetologia 1990;33:43–51 [DOI] [PubMed] [Google Scholar]
- 6.Charlton MR, Balagopal P, Nair KS. Skeletal muscle myosin heavy chain synthesis in type 1 diabetes. Diabetes 1997;46:1336–1340 [DOI] [PubMed] [Google Scholar]
- 7.Godil MA, Wilson TA, Garlick PJ, McNurlan MA. Effect of insulin with concurrent amino acid infusion on protein metabolism in rapidly growing pubertal children with type 1 diabetes. Pediatr Res 2005;58:229–234 [DOI] [PubMed] [Google Scholar]
- 8.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 1995;95:2926–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fricke O, Seewi O, Semler O, Tutlewski B, Stabrey A, Schoenau E. The influence of auxology and long-term glycemic control on muscle function in children and adolescents with type 1 diabetes mellitus. J Musculoskelet Neuronal Interact 2008;8:188–195 [PubMed] [Google Scholar]
- 10.Jakobsen J, Reske-Nielsen E. Diffuse muscle fiber atrophy in newly diagnosed diabetes. Clin Neuropathol 1986;5:73–77 [PubMed] [Google Scholar]
- 11.Gordon CS, Serino AS, Krause MP, et al. Impaired growth and force production in skeletal muscles of young partially pancreatectomized rats: a model of adolescent type 1 diabetic myopathy? PLoS ONE 2010;5:e14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause MP, Riddell MC, Gordon CS, Imam SA, Cafarelli E, Hawke TJ. Diabetic myopathy differs between Ins2Akita+/- and streptozotocin-induced type 1 diabetic models. J Appl Physiol 2009;106:1650–1659 [DOI] [PubMed] [Google Scholar]
- 13.Krause MP, Riddell MC, Hawke TJ. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr Diabetes. 22 September 2010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 2001;91:534–551 [DOI] [PubMed] [Google Scholar]
- 15.Gulati AK, Swamy MS. Regeneration of skeletal muscle in streptozotocin-induced diabetic rats. Anat Rec 1991;229:298–304 [DOI] [PubMed] [Google Scholar]
- 16.Jerković R, Bosnar A, Jurisić-Erzen D, et al. The effects of long-term experimental diabetes mellitus type I on skeletal muscle regeneration capacity. Coll Antropol 2009;33:1115–1119 [PubMed] [Google Scholar]
- 17.Vignaud A, Ramond F, Hourdé C, Keller A, Butler-Browne G, Ferry A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology 2007;74:291–300 [DOI] [PubMed] [Google Scholar]
- 18.Nicholas SB, Aguiniga E, Ren Y, et al. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int 2005;67:1297–1307 [DOI] [PubMed] [Google Scholar]
- 19.Lyon CJ, Hsueh WA. Effect of plasminogen activator inhibitor-1 in diabetes mellitus and cardiovascular disease. Am J Med 2003;115(Suppl. 8A):62S–68S [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 2009;94:3171–3182 [DOI] [PubMed] [Google Scholar]
- 21.Fibbi G, D’Alessio S, Pucci M, Cerletti M, Del Rosso M. Growth factor-dependent proliferation and invasion of muscle satellite cells require the cell-associated fibrinolytic system. Biol Chem 2002;383:127–136 [DOI] [PubMed] [Google Scholar]
- 22.Koh TJ, Bryer SC, Pucci AM, Sisson TH. Mice deficient in plasminogen activator inhibitor-1 have improved skeletal muscle regeneration. Am J Physiol Cell Physiol 2005;289:C217–C223 [DOI] [PubMed] [Google Scholar]
- 23.Naderi J, Bernreuther C, Grabinski N, et al. Plasminogen activator inhibitor type 1 up-regulation is associated with skeletal muscle atrophy and associated fibrosis. Am J Pathol 2009;175:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suelves M, Vidal B, Ruiz V, et al. The plasminogen activation system in skeletal muscle regeneration: antagonistic roles of urokinase-type plasminogen activator (uPA) and its inhibitor (PAI-1). Front Biosci 2005;10:2978–2985 [DOI] [PubMed] [Google Scholar]
- 25.Sisson TH, Nguyen MH, Yu B, Novak ML, Simon RH, Koh TJ. Urokinase-type plasminogen activator increases hepatocyte growth factor activity required for skeletal muscle regeneration. Blood 2009;114:5052–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46:887–894 [DOI] [PubMed] [Google Scholar]
- 27.Johnston AP, Campbell JE, Found JG, Riddell MC, Hawke TJ. Streptozotocin induces G2 arrest in skeletal muscle myoblasts and impairs muscle growth in vivo. Am J Physiol Cell Physiol 2007;292:C1033–C1040 [DOI] [PubMed] [Google Scholar]
- 28.Hawke TJ, Atkinson DJ, Kanatous SB, Van der Ven PF, Goetsch SC, Garry DJ. Xin, an actin binding protein, is expressed within muscle satellite cells and newly regenerated skeletal muscle fibers. Am J Physiol Cell Physiol 2007;293:C1636–C1644 [DOI] [PubMed] [Google Scholar]
- 29.Elokdah H, Abou-Gharbia M, Hennan JK, et al. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. J Med Chem 2004;47:3491–3494 [DOI] [PubMed] [Google Scholar]
- 30.Oishi K, Ohkura N, Kasamatsu M, et al. Tissue-specific augmentation of circadian PAI-1 expression in mice with streptozotocin-induced diabetes. Thromb Res 2004;114:129–135 [DOI] [PubMed] [Google Scholar]
- 31.Crandall DL, Elokdah H, Di L, Hennan JK, Gorlatova NV, Lawrence DA. Characterization and comparative evaluation of a structurally unique PAI-1 inhibitor exhibiting oral in-vivo efficacy. J Thromb Haemost 2004;2:1422–1428 [DOI] [PubMed] [Google Scholar]
- 32.Krause MP, Liu Y, Vu V, et al. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol 2008;295:C203–C212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong EG, Jung DY, Ko HJ, et al. Nonobese, insulin-deficient Ins2Akita mice develop type 2 diabetes phenotypes including insulin resistance and cardiac remodeling. Am J Physiol Endocrinol Metab 2007;293:E1687–E1696 [DOI] [PubMed] [Google Scholar]
- 34.Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles—a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia 2009;52:1182–1191 [DOI] [PubMed] [Google Scholar]
- 35.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 2007;19:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith CK, 2nd, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol 1994;159:379–385 [DOI] [PubMed] [Google Scholar]
- 37.d’Albis A, Couteaux R, Janmot C, Roulet A, Mira JC. Regeneration after cardiotoxin injury of innervated and denervated slow and fast muscles of mammals. Myosin isoform analysis. Eur J Biochem 1988;174:103–110 [DOI] [PubMed] [Google Scholar]
- 38.Schiaffino S, Gorza L, Sartore S, Saggin L, Carli M. Embryonic myosin heavy chain as a differentiation marker of developing human skeletal muscle and rhabdomyosarcoma. A monoclonal antibody study. Exp Cell Res 1986;163:211–220 [DOI] [PubMed] [Google Scholar]
- 39.Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol 2008;52:307–314 [DOI] [PubMed] [Google Scholar]
- 40.Kherif S, Lafuma C, Dehaupas M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 1999;205:158–170 [DOI] [PubMed] [Google Scholar]
- 41.Shortreed KE, Krause MP, Huang JH, et al. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS ONE 2009;4:e7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010;5:e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 44.Lluís F, Roma J, Suelves M, et al. Urokinase-dependent plasminogen activation is required for efficient skeletal muscle regeneration in vivo. Blood 2001;97:1703–1711 [DOI] [PubMed] [Google Scholar]
- 45.Abderrahmani R, François A, Buard V, et al. Effects of pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 in radiation-induced intestinal injury. Int J Radiat Oncol Biol Phys 2009;74:942–948 [DOI] [PubMed] [Google Scholar]
- 46.Weisberg AD, Albornoz F, Griffin JP, et al. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol 2005;25:365–371 [DOI] [PubMed] [Google Scholar]
- 47.Collins SJ, Alexander SL, Lopez-Guisa JM, et al. Plasminogen activator inhibitor-1 deficiency has renal benefits but some adverse systemic consequences in diabetic mice. Nephron Exp Nephrol 2006;104:e23–e34 [DOI] [PubMed] [Google Scholar]
- 48.Lassila M, Fukami K, Jandeleit-Dahm K, et al. Plasminogen activator inhibitor-1 production is pathogenetic in experimental murine diabetic renal disease. Diabetologia 2007;50:1315–1326 [DOI] [PubMed] [Google Scholar]
- 49.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010;33:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Small M, Kluft C, MacCuish AC, Lowe GD. Tissue plasminogen activator inhibition in diabetes mellitus. Diabetes Care 1989;12:655–658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.