Abstract
OBJECTIVE
Overactivity of the Forkhead transcription factor FoxO1 promotes diabetic hyperglycemia, dyslipidemia, and acute-phase response, whereas suppression of FoxO1 activity by insulin may alleviate diabetes. The reported efficacy of long-chain fatty acyl (LCFA) analogs of the MEDICA series in activating AMP-activated protein kinase (AMPK) and in treating animal models of diabesity may indicate suppression of FoxO1 activity.
RESEARCH DESIGN AND METHODS
The insulin-sensitizing and anti-inflammatory efficacy of a MEDICA analog has been verified in guinea pig and in human C-reactive protein (hCRP) transgenic mice, respectively. Suppression of FoxO1 transcriptional activity has been verified in the context of FoxO1- and STAT3-responsive genes and compared with suppression of FoxO1 activity by insulin and metformin.
RESULTS
Treatment with MEDICA analog resulted in total body sensitization to insulin, suppression of lipopolysaccharide-induced hCRP and interleukin-6–induced acute phase reactants and robust decrease in FoxO1 transcriptional activity and in coactivation of STAT3. Suppression of FoxO1 activity was accounted for by its nuclear export by MEDICA-activated AMPK, complemented by inhibition of nuclear FoxO1 transcriptional activity by MEDICA-induced C/EBPβ isoforms. Similarly, insulin treatment resulted in nuclear exclusion of FoxO1 and further suppression of its nuclear activity by insulin-induced C/EBPβ isoforms. In contrast, FoxO1 suppression by metformin was essentially accounted for by its nuclear export by metformin-activated AMPK.
CONCLUSIONS
Suppression of FoxO1 activity by MEDICA analogs may partly account for their antidiabetic anti-inflammatory efficacy. FoxO1 suppression by LCFA analogs may provide a molecular rational for the beneficial efficacy of carbohydrate-restricted ketogenic diets in treating diabetes.
Overactivity of the Forkhead transcription factor FoxO1 promotes diabetic hyperglycemia and dyslipidemia, combined with pancreatic insufficiency (recently reviewed in [1]). Specifically, FoxO proteins promote hepatic glucose production because of transcriptional transactivation of genes coding for rate-limiting gluconeogenic enzymes (2,3), accompanied by FoxO1-induced switch from muscle carbohydrate to fatty acid metabolism with concomitant decrease in glucose utilization (4). Further to diabetic hyperglycemia, FoxO1 may promote diabetic dyslipidemia as a result of increased lipoprotein production complemented by inhibition of plasma lipoprotein clearance, as a result of transcriptional activation of the microsomal triglyceride transfer protein (5) and of apolipoprotein CIII (6), respectively. Suppression of pancreatic duodenal homeobox-1 expression by FoxO1 (7) may further limit the expansion of β-cell mass in response to peripheral insulin resistance. Moreover, FoxO1 may enhance the acute phase/inflammatory response to cytokines (e.g., interleukin [IL]-6, tumor necrosis factor [TNF]-α) and growth factors (e.g., epidermal growth factor [EGF], IGF) as a result of coactivation of STAT3 (8,9), thus promoting the macrovascular disease of diabetes (10). STAT3-induced SOCS3 also may result in its binding and inhibition of insulin receptor signaling, complemented by proteasomal degradation of insulin receptor substrate [IRS]1/2 (11,12). Insulin activity in maintaining balanced carbolipid metabolism and in restraining cytokine-induced inflammation is partly accounted for by phosphorylation of FoxO1 (T24, S256, S319) by activated protein kinase B (PKB)/Akt, resulting in its nuclear exclusion, poly-ubiquitination, and proteasomal degradation (reviewed in [1]).
Carbohydrate-restricted ketogenic diets were used as sole treatment for diabetes before the insulin era beginning in 1922 and are reported to outweigh the performance of isocaloric high-carbohydrate fat-restricting diets in alleviating glycemic control, dyslipidemia, and insulin resistance in type 2 diabetes (13–15). The efficacy of ketogenic diets is surprising in view of their inherent lipotoxic potential (16). This apparent paradox may be resolved by proposing that the lipotoxicity of high-fat diets may be because of downstream fatty acyl metabolites (e.g., diglycerides, triglycerides, ceramide) derived under conditions of carbohydrate and insulin excess, whereas the free long-chain fatty acid (LCFA) precursors (or their respective LCFA-CoA thioesters) may account for the surprising efficacy of carbohydrate-restricted ketogenic diets, if not allowed to be further metabolized into downstream lipotoxic products. Alternatively, synthetic LCFA that are neither esterified into lipids nor β-oxidized may mimic the proposed intrinsic efficacy of free LCFA/LCFA-CoA in the diabetes context.
MEDICA analogs (17–22) consist of long-chain, α,ω-dicarboxylic acids, tetramethyl-substituted in the αα′ (Mαα) or ββ′ carbons [HOOC-C(α′)-C(β′)-(CH2)n-C(β)-C(α)-COOH]. MEDICA analogs may be thioesterified to their respective CoA-thioesters, but these are not esterified into lipids, nor converted into ceramides, whereas the methyl-substitutions at the αα′ or ββ′ positions block their β-oxidation. Treatment with MEDICA analogs results in the formation of endogenous MEDICA-CoA thioesters, but not in depletion of intracellular CoA (23). MEDICA analogs are mostly excreted in bile as respective glucuronides (J.B.-T., unpublished data). MEDICA analogs have been previously reported to suppress hepatic glucose and lipoprotein production, with a concomitant increase in total body glucose uptake and plasma lipoprotein clearance in several animal models of diabesity (e.g., Zucker, cp/cp, db/db, ob/ob) (17–22). The insulin-mimetic activity of MEDICA analogs and of ketogenic diets in the diabesity context prompted our interest in FoxO1 as putative target of LCFA/MEDICA.
RESEARCH DESIGN AND METHODS
Animals.
Male guinea pigs (GP) (HsdPoc:DH) weighing 500 g were individually housed with free access to water and standard GP diet (Teklad 2040S). GP were cannulated through the carotid artery and jugular vein under ketamine (75 mg/kg body wt [BW]), xylazine (6 mg/kg BW), and rimadyl (1.5 mg/kg BW) anesthesia. After catheter placement animals were allowed to recover for 7–14 days until exceeding their initial body weight by 80 g. Cannule patency was maintained every 10–14 days by washing with heparinized saline (25 units/mL), followed by clamping the cannules with heparinized glycerol (75% glycerol, 20% saline, and 5% heparin; 5,000 units/mL). Mαα was dosed daily by gavage by stepwise addition of the drug during a period of 2 weeks, reaching a final daily dose of 10 mg/kg BW in 1% carboxymethylcellulose (CMC). The final dose was maintained for a period of 4–8 weeks. Age-matched nontreated animals were dosed by 1% CMC.
Human C-reactive protein (CRP) transgenic mice (hCRPtg) (24) aged 10–12 weeks were fed standard rodent diet (Teklad 2018) with/without 0.06% (w/w) Mαα mixed in the diet or were dosed daily by gavage with 60 mg/kg BW Mαα in 1% CMC or with vehicle. Age-matched SD rats weighing 170–250 g were dosed daily by gavage with 80 mg/kg BW of Mαα in 1% CMC or with vehicle. Upon death, livers were frozen immediately in liquid nitrogen and kept at −70°C.
Animal care and experimental procedures were in accordance with the accredited animal ethics committee of the Hebrew University.
Cultured cells and transfection assays.
HepG2, Hep3B, COS7 cells, and mouse embryo fibroblasts (MEF) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS, 2 mM glutamine, 100 units/mL penicillin, and 0.1 mg/mL streptomycin. H4IIE cells were grown in 1:1 mixture of DMEM and F-12 medium, supplemented with 10% FCS.
COS7 cells cultured in a 24-well plate were transfected with reporter plasmids for FoxO1 [FRE3-TK-LUC], consisting of three copies of the FoxO1 response element (FRE) of the IGFBP1 promoter (0.3 μg) (25), STAT3 [M67-TATA-TK-LUC] (0.3 μg) (26), or GAL4 [GAL4(UAS)5 -LUC] (0.6 μg) as indicated, together with expression plasmids for constitutive STAT3 [FLAG-STAT3-C] (0.05 μg) (26), wild-type GFP-FoxO1(T24, S256, S319) [FoxO1(TSS)] (0.05 μg) (27), GFP-FoxO1(T24A, S256A, S319A) [FoxO1(AAA)] (0.05 μg) (27), GAL4-FoxO1 (Δaa211–655, S256, S319) [GAL4-FoxO1(SS)] (0.05 μg), or GAL4-FoxO1 (Δaa211–655, S256A, S319A) [GAL4-FoxO1(AA)] (0.05 μg) as indicated, using Mirus reagent (Mirus Corporation). The total amount of DNA was kept constant by supplementing with empty plasmid. Six h after transfection, culture medium was replaced with serum-free DMEM containing 0.2% fatty acid-free albumin, supplemented with vehicle, Mαα, or metformin as indicated, and further incubated for 16 h. For insulin treatment, transfected cells were cultured for 24 h in serum-free DMEM with insulin as indicated, being replenished every 12 h. Luciferase values were normalized to β-galactosidase activity.
CHOP+/+ and CHOP−/− MEF (28) cultured in a 24-well plate were transfected with FoxO1 reporter plasmid [FRE3-TK-LUC] (1.65 μg) and FLAG-FoxO1 (AAA) (0.05 μg) expression plasmid using jetPEI (Polyplus Transfection). Six h after transfection the culture medium was supplemented with vehicle or Mαα as indicated, and the cells were further incubated for 40 h before analysis.
Cell lysis, SDS-PAGE, and Western blotting.
Liver and cell lysates were prepared and analyzed by SDS-PAGE/Western blotting (22,29). Presented lanes of the same blot were rearranged in some figures to condense the presentation.
Nuclear extracts and immunoprecipitation.
Sixteen h after transfection with the indicated reporter/expression plasmids cells were washed twice with cold PBS and lysed in lysis buffer A (Hepes [10 mM, pH 8], KCl [10 mM], dithiothreitol [1 mM], EDTA [0.1 mM], EGTA [0.1 mM], NaF [50 mM], NaPPi [5 mM], sodium vanadate [1 mM], bpVphen [40 nM], phenylmethylsulfonylfluoride [1 mM], and protease inhibitor mix [Sigma]), followed by incubation for 15 min on ice. After the addition of 1% NP40, the cells were vortexed for 10 s and centrifuged for 3 min at 3,000g, 4°C. The pellet was resuspended in lysis buffer B (Hepes [20 mM, pH 8], NaCl [400 mM], dithiothreitol [1 mM], EDTA [1 mM], EGTA [1 mM], NaF [50 mM], NaPPi [5 mM], sodium vanadate [1 mM], bpVphen [40 nM], phenylmethylsulfonylfluoride [1 mM], and protease inhibitor mix) and kept on ice for 15 min with continuous shaking. Insoluble material was removed by centrifugation at 13,400g for 5 min. Respective transcription factors were immunoprecipitated by incubating 200 μg nuclear extract with Sepharose A/G beads (Pierce Biotechnology) for 30 min at 4°C (preclearing), followed by incubating the extract overnight at 4°C with Sepharose A/G preloaded with respective antibody. The beads were then washed three times with lysis buffer B and two times with TBS (25 mM Tris, pH 7.2; 150 mM NaCl), and were boiled in SDS sample buffer for 5 min. Immunoprecipitated proteins were analyzed by SDS-PAGE/Western blotting.
Plasmids.
rAMPKα1(D157A) mutant [DN-AMPK] was prepared by mutating rAMPKα1 using the QuikChange Site-Directed Mutagenesis kit (Stratagene, CA) (30). STAT3 [M67-TATA-TK-LUC] reporter plasmid, consisting of four copies of the sequence (GGTTCCCGTAAATGCATCA) and a minimal thymidine kinase promoter (26), and FLAG-STAT3-C expression plasmid were from J.E. Darnell Jr. (Rockefeller University, NY). CHOP expression plasmids (31) were from A. Aronheim (Technion, Israel). pSCT-LAP and pSCT-LIP expression plasmids (32) were from J. Orly (Hebrew University, Israel). GAL4[(UAS)5LUC] construct consisted of five Gal4 Upstream Activator Sequences (UAS) upstream of Luciferase reporter.
Real-time PCR.
Total RNA from liver and cultured cells was prepared and analyzed by RT-PCR as previously described (22). The following primers were used: hCRP sense 5′-TCATGCTTTTGGCCAGACAG-3′ and antisense 5′-GGTCGAGGACAGTTCCGTGT-3′; β-actin sense 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and antisense 5′-CACCTTCTACAATGAGCTGCGTGTG-3′; mouse β-actin sense 5′-GGCTGTATTCCCCTCCATCG-3′ and antisense 5′-CCAGTTGGTAACAATGCCATGT-3′; mouse serum amyloid P (SAP) sense 5′-AGACAGACCTCAAGAGGAAAGT-3′ and antisense 5′-AGGTTCGGAAACACAGTGTAAAA-3′; mouse serum amyloid A (SAA)2 sense 5′-TGGCTGGAAAGATGGAGACAA-3′ and antisense 5′-AAAGCTCTCTCTTGCATCACTG-3′; mouse fibrinogen sense 5′-AGTGTTGTGTCCTACGGGATG-3′ and antisense 5′-CTGAGGAGGTATCGGAAACAGA-3′.
Materials.
Anti- STAT3, FoxO1, P-FoxO1(T24)/P-FoxO3a(T32), rabbit C/EBPβ/LAP, and CHOP antibodies were from Cell Signaling Technology. Anti-tubulin antibody was from Sigma. Anti-mouse C/EBPβ antibody was from Santa Cruz. β-Galactosidase determination kit was from Bio-Rad. Luciferase determination kit was from Biological Industries. hCRP(hs) ELISA kit was from DRG Instruments. Insulin RIA kit was from MD-Biomedicals.
Data analysis.
Significance was analyzed by paired t test or by Mann-Whitney U test.
RESULTS
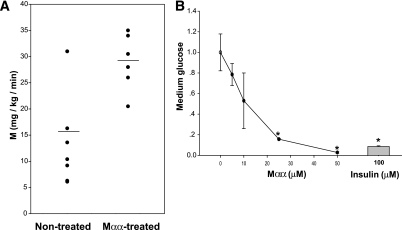
MEDICA analogs have previously been reported to increase total body glucose uptake in several animal models of leptin or leptin receptor defects (17–22). However, because leptin or leptin receptor defects do not account for human diabesity, we were looking for an alternative animal model for studying sensitization to insulin by MEDICA analogs. GP provide a rodent model that is relevant to humans in light of their human-like profile of plasma lipoproteins and lack of liver peroxisomal proliferation in response to peroxisome proliferator--activating receptor-α ligands (33,34). Carbohydrate-restricted high-fat diet also has recently been reported to increase the Quantitative Insulin Sensitivity Check Index (Quicki) (35) of normoglycemic GP (36). Indeed, treatment of GP with the MEDICA analog Mαα [α,α′-tetramethyl hexadecanedioic acid, HOOC-C(CH3)2-(CH2)12-C(CH3)2-COOH] resulted in twofold increase in the rate of infused glucose (M [in mg/min/kg BW]) required to maintain euglycemia under conditions of clamped hyperinsulinemia (Fig. 1A). Moreover, average plasma insulin under hyperinsulinemic clamp conditions (I [ng/mL]) were 14 ± 3 and 20 ± 3 for Mαα-treated and nontreated GP, respectively, implying M-to-I ratio of 2.8 ± 0.7 and 0.8 ± 0.1, respectively (P = 0.011). Furthermore, the insulin-sensitizing efficacy of Mαα actually exceeds the 3.5-fold increase in M/I, since M values for nontreated animals were derived under hyperinsulinemic-euglycemic clamp conditions (plasma glucose 90–106 mg/dL), whereas M values for Mαα-treated animals were derived while maintaining plasma glucose at 67–87 mg/dL, as a result of robust increase in glucose uptake that could not be overcome by maximal rates of glucose infusion. Sensitization to insulin was further demonstrated by dose-dependent insulin-like suppression of dexamethasone/cAMP-induced glucose production in H4IIE hepatocytes by Mαα (Fig. 1B), in line with suppression of glucose-6-phosphatase expression by Mαα (22). These results complement our previous findings indicating sensitization to insulin by Mββ [β,β′-tetramethyl hexadecanedioic acid] in animal models of diabesity (19,20).
FIG. 1.
Insulin-sensitizing activity of Mαα. A: GP were treated with Mαα as described in research design and methods. Fasting plasma glucose amounted to 112 ± 7 mg% and 108 ± 8 mg% in nontreated and Mαα-treated GP, respectively. Fasted GP were primed through the jugular vein cannula with Humulin R insulin in saline/0.2% BSA (fatty acid free), followed by constant infusion of 10–80 mU/kg BW/min for 180 min. Plasma glucose was monitored by Elite glucometer every 10 min and maintained by infusing a solution of 50% glucose in saline at a variable rate, up to 50 μL/min. M, the rate of infused glucose (mg/kg BW/min) required to maintain blood glucose under conditions of clamped hyperinsulinemia. M values for all nontreated animals (n = 7) were derived under hyperinsulinemic-euglycemic clamp conditions (plasma glucose 90–106 mg/dL, plasma insulin 20 ± 3 ng/mL). M values for Mαα-treated animals (n = 6) were derived under hyperinsulinemic-hypoglycemic clamp conditions (plasma glucose 67–87 mg/dL, plasma insulin 14 ± 3 ng/mL), as a result of robust increase in glucose uptake that could not be satisfied by maximal rates of glucose infusion (50 μL/min). Respective means are denoted by line (P < 0.05). B: Lactate/pyruvate-dependent glucose production (arbitrary units) induced by dexamethasone/8-(4-chlorophenylthio)-cAMP was determined in H4IIE cells as previously described (22), in the presence and absence of Mαα and insulin as indicated. Representative experiment in triplicates. Mean ± SE. *Significant as compared with nontreated cells (P < 0.05).
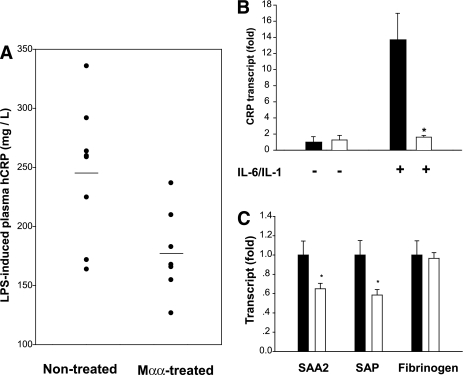
Mαα suppression of the acute phase response induced by LPS/NF-κB/IL-6/STAT3 transduction pathway (reviewed in [37]) has been verified here in vivo in hCRP transgenic mice, which carry a 31-kb ClaI fragment of human genomic DNA consisting of the CRP gene, 17 kb of 5′-flanking sequence, and 11.3 kb of 3′-flanking sequence (24). LPS-induced plasma hCRP was significantly suppressed by in vivo treatment with Mαα (Fig. 2A). Similarly, IL-6/IL-1-induced expression of hCRP (Fig. 2B) or SOCS3 (not shown) was robustly suppressed by Mαα in Hep3B cells, where in contrast with HepG2, hCRP is increased in response to IL-6. Furthermore, IL-6–induced expression of the acute-phase reactants mSAA2 and mSAP was significantly suppressed by in vivo treatment of hCRPtg with Mαα (Fig. 2C), implying Mαα suppression of the acute phase response induced by STAT3.
FIG. 2.
Inhibition of the acute phase response by Mαα. A: Male hCRP transgenic mice were fed for 3 weeks with Mαα (n = 7) mixed in the diet or left untreated (n = 7) as described in research design and methods. LPS (25 μg) was injected intraperitoneally as indicated and the mice were killed 18 h later. LPS-induced plasma hCRP was determined as described in research design and methods. Respective means are denoted by lines (P < 0.05). B: Hep3B cells were used for evaluating Maa effects in suppressing IL6-induced CRP since HepG2 cells fail to respond to IL6. Hep3B cells were incubated for 24 h in the absence (black bars) and presence (white bars) of 200 μmol/L Mαα, 10 ng/mL IL-6, and 1 ng/mL IL-1 as indicated. mRNA normalized to β-actin was quantified by real time PCR as described in research design and methods. mRNA content of nontreated cells is defined as 1.0. Representative experiment in triplicates. Mean ± SE. *Significant as compared with nontreated cells (P < 0.05). C: Male hCRP transgenic mice were dosed daily by gavage for 2 weeks with vehicle (black bars) or 60 mg/kg BW of Mαα (empty bars) as described in research design and methods. IL-6 (500 ng) was injected intraperitoneally, and the mice were killed 18 h later. Mouse hepatic SAA2, SAP, and fibrinogen transcripts normalized to β-actin were determined by real time PCR as described in research design and methods. mRNA content of nontreated animals is defined as 1.0. Mean ± SE (n = 8). *Significant as compared with vehicle-treated mice (P < 0.05).
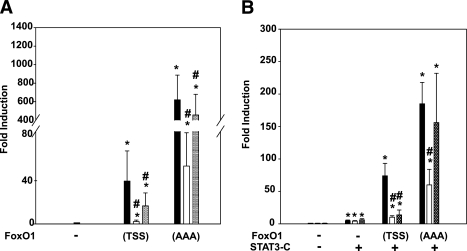
To test whether suppression of FoxO1 function may mediate effects of MEDICA compounds, we used reporter plasmids driven by the FRE of the IGFBP1 gene promoter (25) to probe transcriptional transactivation by FoxO1 or the STAT3-responsive M67 sequence (26) to probe coactivation of STAT3 (Fig. 3). Because FoxO1 transcriptional activity may be affected by its nuclear exclusion as well as by its nuclear transcriptional activity, COS7 cells that lack endogenous FoxO1 were transfected with either an expression plasmid for wild-type foxO1(T24, S256, S319) [FoxO1(TSS)] or with FoxO1(T24, S256A, S319A) mutant [FoxO1(AAA)] that preferentially translocates to the nucleus as a result of loss of the three Akt phosphorylaton sites (T24, S256, S319) (1). Because Mαα transduces sensitization to insulin by activating AMPK, its effect was compared with that of metformin (22). As shown in Fig. 3A, while being similarly overexpressed (not shown), transcriptional transactivation of the FRE reporter increased 600-fold by FoxO1(AAA) as compared with 40-fold increase by wild-type FoxO1(TSS), indicating that transactivation by FoxO1 is indeed strongly affected by its phosphorylation at Akt sites. Transactivation of the FRE reporter by wild-type FoxO1(TSS) was 50% inhibited by metformin, whereas transactivation by the FoxO1(AAA) mutant was only slightly inhibited, albeit significantly (Fig. 3A), implying that suppression of FoxO1 transcriptional activity by metformin may essentially be ascribed to its nuclear exclusion. In contrast with metformin, expression of the FRE reporter, driven by either the wild-type FoxO1(TSS) or the FoxO1(AAA) mutant, was 90% inhibited by Mαα (Fig. 3A), implying inhibition of nuclear FoxO1 transcriptional activity independently of its nuclear export. The robust efficacy of Mαα in suppressing FoxO1 transcriptional activity is further underscored by realizing its high binding affinity to medium albumin (estimated to be >99%, independently of Mαα concentrations in the range of 0 to 0.9 mmol/L [J.B.-T., unpublished results]), resulting in nanomolar concentrations of free Mαα acid in the culture medium.
FIG. 3.
Suppression of FoxO1 transcriptional trans- and coactivation by Mαα and metformin. A: Cos7 cells were transfected with FoxO1 reporter plasmid (FRE3-TK-LUC) and cotransfected with empty (–), FoxO1(TSS), or FoxO1(AAA) expression plasmids as indicated, in the presence of vehicle (black bars), 150 μmol/L Mαα (empty bars), or 2.0 mmol/L metformin (cross-hatched bars) as described in research design and methods. Luciferase activity of cells transfected with an empty plasmid normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with empty-transfected cells (P < 0.05); #significant as compared with respective vehicle-treated cells (P < 0.05). B: Cos7 cells were transfected with STAT3 reporter plasmid (M67-TATA-TK-LUC) and cotransfected with empty (–), STAT3-C, FoxO1(TSS), or FoxO1(AAA) expression plasmids as indicated, in the presence of vehicle (black bars), 150 μmol/L Mαα (empty bars), or 2.0 mmol/L metformin (dotted bars) as described in research design and methods. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with empty-transfected cells (P < 0.05); #significant as compared with respective vehicle-treated cells (P < 0.05).
Suppression of FoxO1 coactivation of STAT3 by Mαα or metformin was verified in COS7 cells transfected with expression plasmids for wild-type FoxO1(TSS) or FoxO1(AAA), together with a STAT3 reporter plasmid and an expression plasmid for constitutively active STAT3 [STAT3-C], which translocates to the nucleus independently of its phosphorylation (26). In line with a previous report (9), transactivation of the STAT3 reporter by STAT3-C was robustly activated by added FoxO1(TSS), and even more so by FoxO1(AAA) (Fig. 3B). Coactivation of STAT3-C by wild-type FoxO1(TSS) was inhibited by Mαα or metformin, whereas coactivation of STAT3-C by FoxO1(AAA) was suppressed by Mαα, but not by metformin, conforming to the profile of the two effectors in the context of the FRE reporter plasmid (Fig. 3A). Hence, although Mαα and metformin may share a similar mode of action in exporting nuclear FoxO1, the transcriptional activity of nuclear FoxO1 is specifically inhibited by Mαα, but not by metformin.
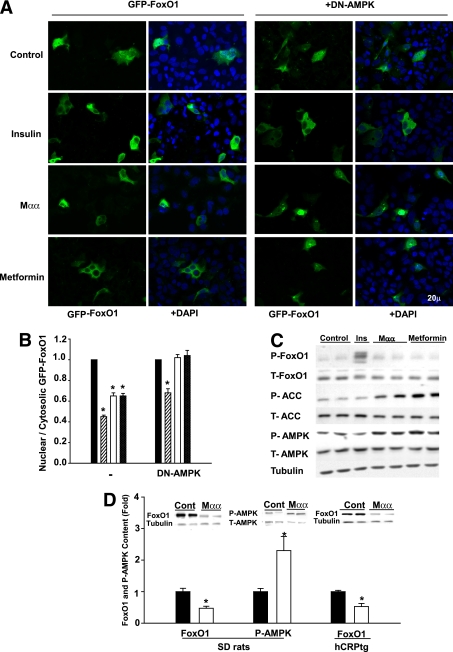
Export of nuclear FoxO1 by Mαα or metformin, as compared with insulin (1), was further pursued in GFP-FoxO1-transfected HepG2 cells by evaluating the nuclear-to-cytosolic ratio of GFP-FoxO1, using fluorescence microscopy. The 1:1 nuclear-to-cytosolic ratio of FoxO1 in HepG2 cells allowed for evaluating translocation in both directions. All three effectors, namely, Mαα, metformin, and insulin, suppressed the nuclear/cytosolic ratio of FoxO1 (Fig. 4A and B), implying its nuclear exclusion. In contrast with insulin, where export of nuclear FoxO1 remained unaffected by coexpression of dominant-negative AMPK (DN-AMPK), that of Mαα or metformin was abrogated by DN-AMPK (Fig. 4A and B), indicating that nuclear exclusion of FoxO1 was driven by Mαα- or metformin-activated AMPK (22). The mode of action of Mαα- or metformin-activated AMPK in exporting nuclear FoxO1 has been probed by the phosphorylation of the T24 consensus Akt site of endogenous FoxO1 in HepG2 cells. Phosphorylation of FoxO1(T24) is required for recruitment of 14–3-3 proteins, which mask nuclear localization signals and promote cytoplasmic localization of FoxO1 (38). In contrast with FoxO1(T24) phosphorylation by insulin, FoxO1(T24) remained unphosphorylated by Mαα or metformin under conditions of activating AMPK (Fig. 4C), implying that in contrast with insulin, nuclear FoxO1 export by AMPK was not transduced by Akt activation. In line with the low stability of FoxO1 as a result of insulin-, shear stress- or AICAR-activated AMPK (39,40), nuclear exclusion of FoxO1 by Mαα-activated AMPK was accompanied by lower FoxO1 content in human umbilical vein epithelial cells (HUVEC) (not shown), as well as in livers of Mαα-treated SD rats and hCRP transgenic mice (Fig. 4D). FoxO1 expression remained unaffected by Mαα (not shown). Hence, nuclear exclusion of wild-type FoxO1(TSS) by Mαα- or metformin-activated AMPK may partly account for their suppression of FoxO1 transcriptional activity.
FIG. 4.
Nuclear exclusion and decrease in cellular FoxO1 induced by Mαα. A and B: HepG2 cells grown on cover slips were transfected with expression plasmids for GFP-FoxO1(TSS) in the absence or presence of DN-AMPK as indicated. After transfection (24 h), the culture medium was changed to serum-free DMEM supplemented with vehicle (black bars), 0.1 μmol/L insulin (hatched bars), 200 μmol/L Mαα (empty bars), or 2.0 mmol/L metformin (cross-hatched bars) as indicated, and further incubated for 24 h before fixation and DAPI staining. A: Representative immunofluorescence slides of GFP-FoxO1 (green) and DAPI-stained nuclei (blue; bar 20 μm). B: Nuclear-to-cytosolic ratio analyzed using fluorescence microscopy (Axioskop). Mean ± SE of three independent experiments. *Significant as compared with vehicle-treated cells (P < 0.05). C: HepG2 cells were incubated for 3 h in serum-free DMEM supplemented with 0.1 μmol/L insulin, 200 μmol/L Mαα, or 2.0 mmol/L metformin as indicated. Cell extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods. Blots were probed with anti- P-FoxO1(T24), FoxO1, P-AMPK(T172), AMPK, P-ACC(S79), ACC, or tubulin antibodies. Representative blots of three independent experiments. D: SD rats were dosed daily by gavage for 2 weeks with vehicle (black bars) or with 80 mg Mαα/kg BW in 1% CMC (empty bars). hCRP transgenic mice were dosed with 0.06% (W/W) Mαα mixed in the diet for a period of 3 weeks (empty bars) or kept untreated (black bars). Liver extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods. Blots were probed with anti-FoxO1 antibodies normalized to tubulin or P-AMPK(T172) antibodies normalized to AMPK. Hepatic FoxO1 content normalized to tubulin and P-AMPK(T172)-to-AMPK ratio of nontreated animals is defined as 1.0. Mean ± SE (n = 4 to 5). *Significant as compared with nontreated animals (P < 0.05). Inset: Representative blots. (A high-quality digital representation of this figure is available in the online issue.)
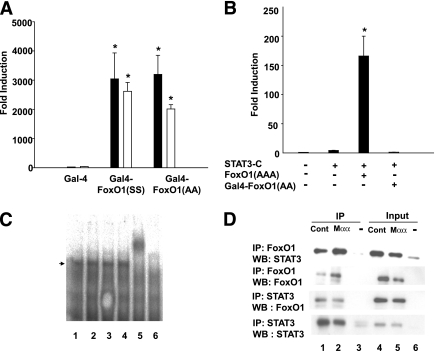
Suppression of the transcriptional activity of nuclear FoxO1 by Mαα was further pursued by defining the FoxO1 domain(s) required for suppressing nuclear FoxO1 activity. Mαα failed to suppress transactivation of a GAL4 reporter plasmid by GAL4-FoxO1(SS) (Δaa211–655, S256, S319) or GAL4-FoxO1(AA) (Δaa211–655, S256A, S319A) expression plasmid chimera, consisting of the GAL4 DNA binding domain (DBD) and nuclear localization signal in-frame with the COOH-terminal transactivation domains of FoxO1 (Fig. 5A). This result indicates that Mαα does not disrupt the function of COOH-terminal transactivation domains in FoxO1, and that NH2-terminal domains of FoxO1 are required for Mαα inhibitory activity in the context of FRE promoters. Similarly, in contrast with coactivation of STAT3 by FoxO1(AAA), the GAL4-FoxO1 (Δaa211–655, S256A, S319A) expression plasmid chimera failed to coactivate STAT3 (Fig. 5B). This result indicates that, in addition to COOH-terminal domains of FoxO1 previously reported to be required for STAT3 coactivation by FoxO1 (9), NH2-terminal elements of FoxO1 are also required for STAT3 coactivation by FoxO1, and hence for STAT3 inhibition by Mαα.
FIG. 5.
FoxO1 domains involved in suppression of FoxO1 by Mαα. A: Cos7 cells were transfected as described in research design and methods with (UAS)5-GAL4 reporter plasmid and with GAL4, GAL4-FoxO1(SS) (Δaa211–655, S256, S319), or GAL4-FoxO1(AA) (Δaa211–655, S256A, S319A) expression plasmids as indicated, in the presence of vehicle (black bars) or 120 μmol/L Mαα (empty bars). Luciferase activity of GAL4-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with GAL4-transfected cells (P < 0.05). Nonsignificant as compared with respective vehicle-treated cells. B: Cos7 cells were transfected with STAT3(M67-TATA-TK-Luciferase) reporter plasmid and with empty (–), STAT3-C, FoxO1(AAA), or GAL4-FoxO1(AA) (Δaa211–655, S256A, S319A) as indicated. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE of three independent experiments. *Significant as compared with STAT3-C–transfected cells (P < 0.05). C: Cos7 cells lacking (lane 6) or overexpressing FoxO1(AAA) (lanes 1–5) were cultured in the presence of vehicle (lanes 1–2) or 150 μM Mαα (lanes 3–5) and in the presence of added anti-FoxO1 antibody (lane 5) as indicated. Labeled double-stranded oligonucleotide containing the FoxO1 binding site of the IGFBP-1 promoter (sense: 5′- cactaGCAAAACAAACTTATTTTGAACAC-3′, antisense: 5′- GTGTGCAAAATAAGTTTGTTTTGctagtg-3′) was prepared by the Klenow fragment of DNA polymerase I and [32P]dCTP. Nuclear extracts prepared from COS7 cells overexpressing FoxO1(AAA) were incubated with the labeled oligonucleotide and subjected to 5% PAGE (29). FoxO1-DNA complex is indicated by an arrow. D: Cos7 cells transfected with empty (–) (lanes 3, 6) or with FoxO1(AAA), and STAT3-C expression plasmids (lanes 1, 4) were cultured in the presence of added 150 μmol/L Mαα (lanes 2, 5) as indicated. Nuclear extracts were immunoprecipitated as described in research design and methods with anti-STAT3 or -FoxO1 antibodies as indicated. Immunoprecipitates (lanes 1–3) and nuclear lysates (input) (lanes 4–6) were subjected to SDS-PAGE followed by Western blotting with anti-STAT3 or -FoxO1 antibodies as indicated. Representative blots of three independent experiments.
Because amino acids 1–211 of FoxO1 consist of part of FoxO1 DBD (aa156–265), including helix3 that interacts directly with FRE, as well as of domains required for intra- and intermolecular protein-protein interactions (41), we also examined whether Mαα might suppress nuclear FoxO1 activity by interfering with its binding to FRE sites and/or its interaction with STAT3. FoxO1 binding to FRE/IGFBP-1 remained unaffected by Mαα as verified by gel-shift analysis using nuclear extracts of Mαα-treated cells (Fig. 5C). Similarly, FoxO1 association with STAT3 analyzed by coimmunoprecipitation remained unaffected by Mαα treatment (Fig. 5D), indicating that Mαα suppression of nuclear FoxO1 activity was not accounted for by abrogating FoxO1 binding to its FRE or to STAT3. In view of these findings, the mode of suppression of nuclear FoxO1 activity by Mαα was further pursued by searching for a third partner that could be recruited by Mαα to FoxO1 or to the FoxO1/STAT3 heterodimer, thereby suppressing trans- and coactivation by nuclear FoxO1, respectively.
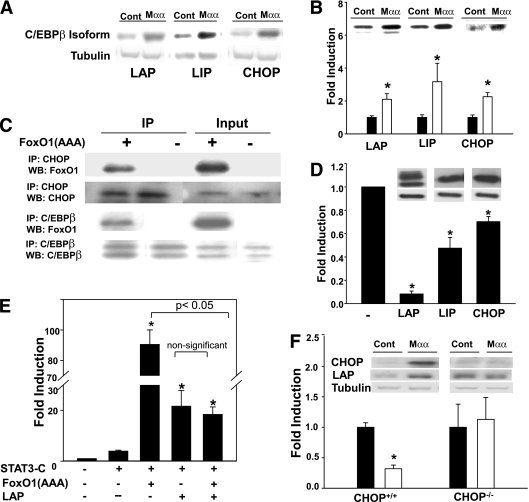
CAAT enhancer-binding protein β (C/EBPβ) (reviewed in [42]) has been previously reported to coactivate STAT3 (43,44) as well as to interact with FoxO1 (45), implying that C/EBPβ family members (e.g., liver activating protein [LAP], liver inhibiting protein [LIP], C/EBP homologous protein [CHOP]) could mediate Mαα suppression of FRE transactivation or STAT3 coactivation by FoxO1. Indeed, LAP and CHOP expression were induced by Mαα (Fig. 6A and B), and both coimmunoprecipitated with nuclear FoxO1 (Fig. 6C). Moreover, FoxO1-induced expression of the FRE reporter plasmid was robustly inhibited by overexpressing LAP, LIP, or CHOP in decreasing order (Fig. 6D), indicating that Mαα-induced C/EBPβ isoforms could suppress transactivation by nuclear FoxO1. Similarly, coactivation of STAT3-C by FoxO1 was completely abrogated by overexpressing LAP (Fig. 6E), indicating that Mαα-induced C/EBPβ isoforms may suppress coactivation of STAT3 by nuclear FoxO1. It is noteworthy that suppression of FoxO1 coactivation of STAT3 by LAP dominates over LAP coactivation of STAT3 (43,44), resulting in overall suppression of STAT3 activity by LAP under conditions of nuclear FoxO1 activity (Fig. 6E).
FIG. 6.
Suppression of FoxO1 by Mαα-induced C/EBPβ isoforms. A: Cos7 cells were cultured for 16 h in the absence or presence of 150 μmol/L Mαα as described in research design and methods. Cellular extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods, using anti-LAP, -LIP, and -CHOP antibodies as indicated. Representative blots for LAP (35/32 kDa), LIP (20 kDa), and CHOP (27 kDa). B: Cos7 cells were cultured in the absence (black bars) or presence (empty bars) of 150 μmol/L Mαα as described in research design and methods. Nuclear extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods, using anti-LAP, -LIP, and -CHOP antibodies as indicated. Loading was controlled by protein/lane. Nuclear LAP, LIP, and CHOP of nontreated cells were defined as 1.0. Mean ± SE for three to four independent experiments for each of the C/EBP isoforms. *Significant as compared with nontreated cells (P < 0.05). Inset: Representative blots. C: Cos7 cells were transfected with empty (–) or FoxO1(AAA) expression plasmids as indicated. Nuclear extracts were immunoprecipitated as described in research design and methods with anti-LAP or -CHOP antibodies as indicated. Immunoprecipitates and cellular lysates (input) were subjected to SDS-PAGE followed by Western blotting with anti-FoxO1, -LAP, or -CHOP antibodies as indicated. Representative blots are shown. D: Cos7 cells were transfected with FoxO1 reporter plasmid (FRE3-TK-Luciferase) and with expression plasmid for FoxO1(AAA) and were cotransfected with empty (–), LAP, LIP, or CHOP expression plasmids as indicated. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments for each C/EBP isoform. *Significant as compared with empty-transfected cells (P < 0.05). Inset: Representative blots of respective C/EBPβ isoforms (upper lane) and tubulin (lower lane), indicating that C/EBPβ isoforms were overexpressed to a similar extent. E: Cos7 cells were transfected with STAT3 reporter plasmid (M67-TATA-TK-Luciferase) and cotransfected with empty (–), STAT3-C, FoxO1(AAA), or LAP expression plasmids as indicated. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with STAT3-C–transfected cells (P < 0.05). F: CHOP+/+ and CHOP−/− MEF were transfected with FoxO1 reporter plasmid (FRE3-TK-Luciferase) and cotransfected with FoxO1(AAA) expression plasmid as described in research design and methods, in the absence (black bars) or presence (empty bars) of 200 μmol/L Mαα. Luciferase activity of vehicle-treated CHOP−/− MEF was 3.2-fold higher as compared with CHOP+/+ cells. Luciferase activity of respective vehicle-treated cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with vehicle-treated cells (P < 0.05). Inset: Mαα-induced expression of LAP and CHOP in CHOP+/+ or CHOP−/− MEF. Representative blots are shown.
The causal role played by C/EBPβ family members in transducing suppression of nuclear FoxO1 by Mαα was verified in CHOP−/− MEF (28), where Mαα failed to induce the expression of LAP (Fig. 6F). In contrast with CHOP+/+ MEF, Mαα failed to suppress FoxO1 in CHOP−/− MEF (Fig. 6F), indicating that inhibition of nuclear FoxO1 activity by Mαα is transduced by Mαα-induced C/EBPβ isoforms.
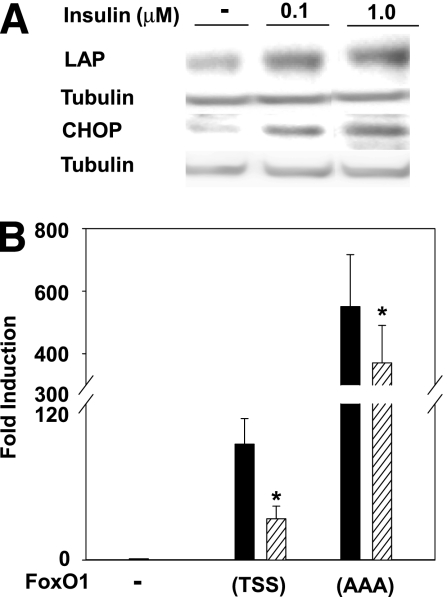
Previous reports concerned with the mode of suppression of FoxO1 by insulin have focused on insulin activity in exporting nuclear FoxO1 by its phosphorylation by activated Akt (1). Suppression of nuclear FoxO1 activity by Mαα-induced C/EBPβ isoforms has prompted us to further pursue similar suppression of nuclear FoxO1 by insulin. Indeed, insulin treatment increased protein levels of LAP and CHOP (Fig. 7A), in line with suppressed transactivation of the FRE/IGFBP-1 reporter plasmid by nuclear FoxO1(AAA) (Fig. 7B) (46). These results indicate that suppression of wild-type FoxO1(TSS) by insulin may be ascribed to both its nuclear export as a result of phosphorylation of its Akt consensus sites as well as inhibition of its nuclear transcriptional activity by insulin-induced C/EBPβ isoforms.
FIG. 7.
Suppression of FoxO1 by insulin-induced C/EBPβ isoforms. A: Cos7 cells were cultured for 24 h in serum-free DMEM in the absence or presence of insulin as indicated. Cellular extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods using anti-LAP, -CHOP, and -tubulin antibodies as indicated. Representative blots are shown. B: Cos7 cells were transfected as described in research design and methods with FoxO1 reporter plasmid (FRE3-TK-Luciferase) and cotransfected with empty (–), FoxO1(TSS), or FoxO1(AAA) expression plasmids in the presence of vehicle (black bars) or insulin (hatched bars) as indicated. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for five independent experiments. *Significant as compared with respective vehicle-treated cells (P < 0.05).
DISCUSSION
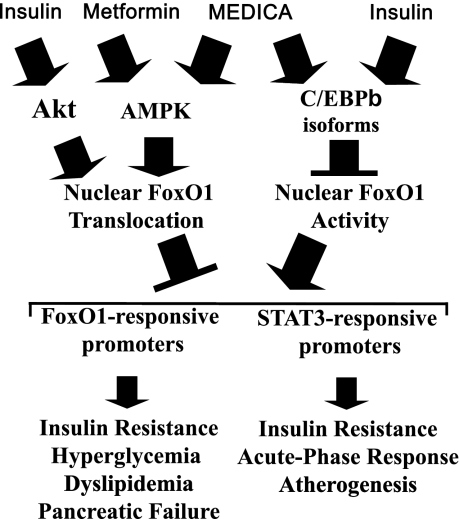
The findings reported here may indicate that suppression of FoxO1 transcriptional activity by Mαα may be ascribed to its nuclear exclusion by Mαα-activated AMPK, complemented by suppression of its nuclear transcriptional activity by Mαα-induced C/EBPβ isoforms (Fig. 8). Suppression of FoxO1 activity by Mαα may indicate that the insulin-sensitizing anti-inflammatory efficacy of MEDICA analogs previously reported in animal models of diabesity (17–22), and further verified here in GP and in hCRP transgenic mice, may partly be accounted for by suppression of FRE-responsive promoters (e.g., G6Pase, PEPCK, MTP, apolipoprotein CIII, CD36, PDK4) by Mαα as well as by suppression of FoxO1 coactivation of STAT3-responsive acute-phase genes (e.g., CRP, SAA, SAP). Suppression of FoxO1 activity by Mαα appears to mimic that of insulin. Indeed, both induce the nuclear exclusion of FoxO1, and further suppress nuclear FoxO1 activity by the induction of C/EBPβ isoforms (Fig. 8). Suppression of nuclear FoxO1 activity by insulin, independently of its nuclear exclusion extends the scope of insulin action beyond its previously reported activity in exporting nuclear FoxO1 (1). In contrast with insulin and Mαα, suppression of FoxO1 activity by metformin appears to be essentially accounted for by promoting nuclear exclusion of FoxO1 by metformin-activated AMPK (Fig. 8). It is worth noting that metformin may also regulate gluconeogenesis by FoxO1-independent mechanisms, including the suppression of CREB/CBP/TORC2 activity (47), or by an AMPK/LKB1-independent decrease in intracellular ATP (48), implying that FoxO1, CREB/CBP/TORC2, and ATP levels may complement each other in controlling hepatic glucose production by metformin.
FIG. 8.
Suppression of FoxO1 by insulin and insulin-mimetics. Suppression of FoxO1 by insulin or Mαα-activated AMPK may be ascribed to its nuclear exclusion and degradation, complemented by suppression of its nuclear transcriptional activity by insulin- or Mαα-induced C/EBPβ isoforms. In contrast with insulin and Mαα, suppression of FoxO1 by metformin is solely accounted for by nuclear exclusion of FoxO1 by metformin-activated AMPK. Suppression of transactivation in the context of FRE-responsive promoters may account for the insulin-sensitizing activity and hypoglycemic hypolipidemic efficacy, whereas suppression of FoxO1 coactivation of STAT3-responsive promoters may account for the insulin-sensitizing activity, anti-inflammatory, and antiatherogenic efficacy of insulin and insulin sensitizers.
The proposed mode of action of MEDICA in suppressing FoxO1 activity is in line with the following findings reported herewith. First, treatment with Mαα resulted in decrease in the nuclear/cytosolic ratio of FoxO1, being abrogated by DN-AMPK. Decreased levels of FoxO1 induced by Mαα-activated AMPK conform to previously reported degradation of FoxO1 by AICAR-activated AMPK in liver cells (40) or by shear stress-activated AMPK in HUVEC (39). The mode of action of AMPK in exporting nuclear FoxO1 by Mαα or metformin is independent of Akt and remains to be investigated. AMPK suppression of FoxO1 activity, independently of Akt, has previously been reported in shear-stressed HUVEC (39). Second, treatment with Mαα, but not with metformin, resulted in suppression of FoxO1(AAA) transcriptional activity, implying suppression of nuclear FoxO1 activity. Finally, transactivation by FoxO1 and coactivation of STAT3 by FoxO1 were inhibited by Mαα-induced C/EBPβ isoforms, whereas failure to induce C/EBPβ isoforms in CHOP−/− cells abrogated the suppression of FoxO1 by Mαα. The mode of induction of C/EBPβ isoforms by Mαα still remains to be determined in terms of expression and degradation of C/EBPβ family members.
It is important to note that the effects of MEDICA compounds and AMPK on the function of FoxO proteins may be isoform specific. Whereas FoxO1 is highly expressed in insulin target tissues involved in regulating the diabetic phenotype, FoxO3a is widely expressed and contributes to the regulation of basic cellular functions, including resistance to oxidative stress (e.g., SOD), cell cycle (e.g., p21, p27), and apoptosis (e.g., BH3-only proteins, TNF-related apoptosis-inducing ligand) (49). Moreover, whereas AMPK suppresses FoxO1 activity and the diabetic phenotype (39,40), AMPK directly phosphorylates COOH-terminal amino acids of FoxO3a, resulting in activating the expression of FoxO3a-dependent genes coding for tumor suppressors, cell cycle arrest, and survival (49). In contrast with AMPK-induced export of nuclear FoxO1, cellular distribution of FoxO3a also was reported to remain unaffected by AMPK (49). These differences between the two FoxO factors in their response to AMPK may reflect their distinct roles in yielding an overall positive response to AMPK and AMPK activators in the FoxO1/metabolic versus FoxO3a/survival contexts.
The insulin-mimetic effects of MEDICA analogs in suppressing FoxO1 activity, combined with the previously reported efficacy of MEDICA analogs in treating insulin resistance, hyperglycemia, diabetic dyslipidemia, and the macrovascular disease of diabesity animal models (17–22), suggest the intriguing possibility that endogenous free LCFA might simulate the antidiabetic insulin-like effects of MEDICA analogs, if allowed to reach high enough intracellular concentrations while avoiding their esterification into downstream lipotoxic products by abrogating the induction of glycerol-3-phosphate acyltransferases (GPAT) by insulin (reviewed in [50]) and by limiting the availability of glycerol-3-phpsphate. Hence, FoxO1 suppression by MEDICA analogs may offer a molecular rationale for the surprising efficacy of carbohydrate-restricted diets in alleviating diabetes (13–15) and may point to the prospects of synthetic substituted LCFA in treating diabetes.
ACKNOWLEDGMENTS
This work was supported in part by the Binational USA Israeli Science Foundation (BSF) (to J.B.-T. and T.G.U.).
J.B.-T. is director in SyndromeX, a company that develops prospective drugs for the metabolic syndrome. No other potential conflicts of interest relevant to this article were reported.
G.Z. researched data and reviewed the manuscript. R.H. researched data and contributed to the design of studies, interpretation of results, and reviewing and editing of the manuscript. M.S., N.M., E.M., E.G., and A.C. researched data and reviewed the manuscript. H.D.D. reviewed the manuscript. T.G.U. contributed to the design of studies, interpretation of results, and reviewing and editing of the manuscript. J.B.-T. contributed to the design of studies and interpretation of results and wrote the manuscript.
The authors thank A. Aronheim (Technion Medical School, Israel) for CHOP MEFs.
REFERENCES
- 1.Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal 2011;14:649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel VT, Choi CS, Phillips TG, et al. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 2006;55:2042–2050 [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab 2007;6:208–216 [DOI] [PubMed] [Google Scholar]
- 4.Bastie CC, Nahlé Z, McLoughlin T, et al. FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem 2005;280:14222–14229 [DOI] [PubMed] [Google Scholar]
- 5.Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 2008;118:2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altomonte J, Cong L, Harbaran S, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest 2004;114:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 2002;110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene 2008;27:2289–2299 [DOI] [PubMed] [Google Scholar]
- 9.Kortylewski M, Feld F, Krüger KD, et al. Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem 2003;278:5242–5249 [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Muñoz G, Martin-Ventura JL, Hernandez-Vargas P, et al. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:525–531 [DOI] [PubMed] [Google Scholar]
- 11.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 2000;275:15985–15991 [DOI] [PubMed] [Google Scholar]
- 12.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 2002;277:42394–42398 [DOI] [PubMed] [Google Scholar]
- 13.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–2090 [DOI] [PubMed] [Google Scholar]
- 14.Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc 2008;108:91–100 [DOI] [PubMed] [Google Scholar]
- 15.Westman EC, Yancy WS, Jr, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond) 2008;5:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 2010;1801:209–214 [DOI] [PubMed]
- 17.Bar-Tana J, Ben-Shoshan S, Blum J, et al. Synthesis and hypolipidemic and antidiabetogenic activities of β,β,β’,β’-tetrasubstituted, long-chain dioic acids. J Med Chem 1989;32:2072–2084 [DOI] [PubMed] [Google Scholar]
- 18.Bar-Tana J, Rose-Kahn G, Frenkel B, Shafer Z, Fainaru M. Hypolipidemic effect of β, β’-methyl-substituted hexadecanedioic acid (MEDICA 16) in normal and nephrotic rats. J Lipid Res 1988;29:431–441 [PubMed] [Google Scholar]
- 19.Mayorek N, Kalderon B, Itach E, Bar-Tana J. Sensitization to insulin induced by β,β’-methyl-substituted hexadecanedioic acid (MEDICA 16) in obese Zucker rats in vivo. Diabetes 1997;46:1958–1964 [DOI] [PubMed] [Google Scholar]
- 20.Russell JC, Shillabeer G, Bar-Tana J, et al. Development of insulin resistance in the JCR:LA-cp rat: role of triacylglycerols and effects of MEDICA 16. Diabetes 1998;47:770–778 [DOI] [PubMed] [Google Scholar]
- 21.Russell JC, Amy RM, Graham SE, Dolphin PJ, Wood GO, Bar-Tana J. Inhibition of atherosclerosis and myocardial lesions in the JCR:LA-cp rat by β, β’-tetramethylhexadecanedioic acid (MEDICA 16). Arterioscler Thromb Vasc Biol 1995;15:918–923 [DOI] [PubMed] [Google Scholar]
- 22.Za’tara G, Bar-Tana J, Kalderon B, et al. AMPK activation by long chain fatty acyl analogs. Biochem Pharmacol 2008;76:1263–1275 [DOI] [PubMed] [Google Scholar]
- 23.Kalderon B, Sheena V, Shachrur S, Hertz R, Bar-Tana J. Modulation by nutrients and drugs of liver acyl-CoAs analyzed by mass spectrometry. J Lipid Res 2002;43:1125–1132 [DOI] [PubMed] [Google Scholar]
- 24.Szalai AJ, McCrory MA. Varied biologic functions of C-reactive protein: lessons learned from transgenic mice. Immunol Res 2002;26:279–287 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Gan L, Pan H, et al. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem 2002;277:45276–45284 [DOI] [PubMed] [Google Scholar]
- 26.Besser D, Bromberg JF, Darnell JE, Jr, Hanafusa HA. A single amino acid substitution in the v-Eyk intracellular domain results in activation of Stat3 and enhances cellular transformation. Mol Cell Biol 1999;19:1401–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan L, Zheng W, Chabot JG, Unterman TG, Quirion R. Nuclear/cytoplasmic shuttling of the transcription factor FoxO1 is regulated by neurotrophic factors. J Neurochem 2005;93:1209–1219 [DOI] [PubMed] [Google Scholar]
- 28.Zinszner H, Kuroda M, Wang XZ, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 1998;12:982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheena V, Hertz R, Berman I, Nousbeck J, Bar-Tana J. Transcriptional suppression of human microsomal triglyceride transfer protein by hypolipidemic insulin sensitizers. Biochem Pharmacol 2005;70:1548–1559 [DOI] [PubMed] [Google Scholar]
- 30.Bronner M, Hertz R, Bar-Tana J. Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor alpha by AMP-activated protein kinase. Biochem J 2004;384:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidenfeld-Baranboim K, Bitton-Worms K, Aronheim A. TRE-dependent transcription activation by JDP2-CHOP10 association. Nucleic Acids Res 2008;36:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman E, Yivgi-Ohana N, Sher N, Bell M, Eimerl S, Orly J. Transcriptional activation of the steroidogenic acute regulatory protein (StAR) gene: GATA-4 and CCAAT/enhancer-binding protein β confer synergistic responsiveness in hormone-treated rat granulosa and HEK293 cell models. Mol Cell Endocrinol 2006;252:92–101 [DOI] [PubMed] [Google Scholar]
- 33.Fernandez ML. Guinea pigs as models for cholesterol and lipoprotein metabolism. J Nutr 2001;131:10–20 [DOI] [PubMed] [Google Scholar]
- 34.Roberts RA. Peroxisome proliferators: mechanisms of adverse effects in rodents and molecular basis for species differences. Arch Toxicol 1999;73:413–418 [DOI] [PubMed] [Google Scholar]
- 35.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 36.Leite JO, DeOgburn R, Ratliff JC, et al. Low-carbohydrate diet disrupts the association between insulin resistance and weight gain. Metabolism 2009;58:1116–1122 [DOI] [PubMed] [Google Scholar]
- 37.He G, Karin M. NF-κB and STAT3—key players in liver inflammation and cancer. Cell Res 2011;21:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Gan L, Pan H, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 2004;378:839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixit M, Bess E, Fisslthaler B, et al. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res 2008;77:160–168 [DOI] [PubMed] [Google Scholar]
- 40.Barthel A, Schmoll D, Krüger KD, Roth RA, Joost HG. Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology 2002;143:3183–3186 [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Marshall CB, Yamamoto K, et al. Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J Mol Biol 2008;384:590–603 [DOI] [PubMed] [Google Scholar]
- 42.Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol 2007;17:318–324 [DOI] [PubMed] [Google Scholar]
- 43.Milosavljević T, Lazić T, Uskoković A, Petrović M, Grigorov I. Expression of the rat liver haptoglobin gene is mediated by isoforms of C/EBPalpha, -β and -δ proteins. Gen Physiol Biophys 2003;22:181–190 [PubMed] [Google Scholar]
- 44.Bogojević D, Mihailović M, Petrović M, Dinić S, Ivanović-Matić S, Poznanović G. Acute-phase-dependent binding affinity of C/EBPbeta from the nuclear extract and nuclear matrix towards the hormone response element of the alpha2-macroglobulin gene in rat hepatocytes. Gen Physiol Biophys 2003;22:279–285 [PubMed] [Google Scholar]
- 45.Christian M, Zhang X, Schneider-Merck T, et al. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein beta in differentiating human endometrial stromal cells. J Biol Chem 2002;277:20825–20832 [DOI] [PubMed] [Google Scholar]
- 46.Tsai WC, Bhattacharyya N, Han LY, Hanover JA, Rechler MM. Insulin inhibition of transcription stimulated by the forkhead protein Foxo1 is not solely due to nuclear exclusion. Endocrinology 2003;144:5615–5622 [DOI] [PubMed] [Google Scholar]
- 47.He L, Sabet A, Djedjos S, et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009;137:635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foretz M, Hébrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greer EL, Oskoui PR, Banko MR, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007;282:30107–30119 [DOI] [PubMed] [Google Scholar]
- 50.Gimeno RE, Cao J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res 2008;49:2079–2088 [DOI] [PubMed] [Google Scholar]