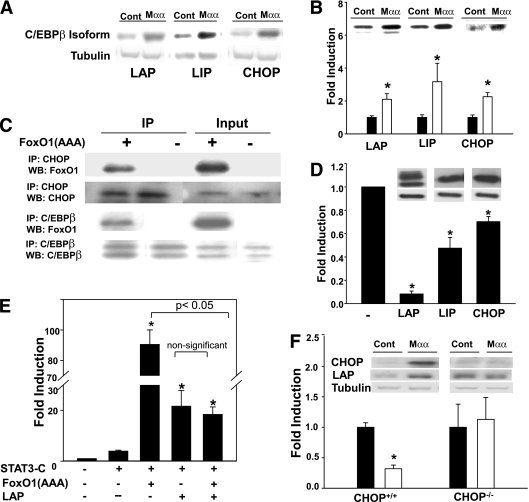
FIG. 6.
Suppression of FoxO1 by Mαα-induced C/EBPβ isoforms. A: Cos7 cells were cultured for 16 h in the absence or presence of 150 μmol/L Mαα as described in research design and methods. Cellular extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods, using anti-LAP, -LIP, and -CHOP antibodies as indicated. Representative blots for LAP (35/32 kDa), LIP (20 kDa), and CHOP (27 kDa). B: Cos7 cells were cultured in the absence (black bars) or presence (empty bars) of 150 μmol/L Mαα as described in research design and methods. Nuclear extracts were subjected to SDS-PAGE followed by Western blotting as described in research design and methods, using anti-LAP, -LIP, and -CHOP antibodies as indicated. Loading was controlled by protein/lane. Nuclear LAP, LIP, and CHOP of nontreated cells were defined as 1.0. Mean ± SE for three to four independent experiments for each of the C/EBP isoforms. *Significant as compared with nontreated cells (P < 0.05). Inset: Representative blots. C: Cos7 cells were transfected with empty (–) or FoxO1(AAA) expression plasmids as indicated. Nuclear extracts were immunoprecipitated as described in research design and methods with anti-LAP or -CHOP antibodies as indicated. Immunoprecipitates and cellular lysates (input) were subjected to SDS-PAGE followed by Western blotting with anti-FoxO1, -LAP, or -CHOP antibodies as indicated. Representative blots are shown. D: Cos7 cells were transfected with FoxO1 reporter plasmid (FRE3-TK-Luciferase) and with expression plasmid for FoxO1(AAA) and were cotransfected with empty (–), LAP, LIP, or CHOP expression plasmids as indicated. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments for each C/EBP isoform. *Significant as compared with empty-transfected cells (P < 0.05). Inset: Representative blots of respective C/EBPβ isoforms (upper lane) and tubulin (lower lane), indicating that C/EBPβ isoforms were overexpressed to a similar extent. E: Cos7 cells were transfected with STAT3 reporter plasmid (M67-TATA-TK-Luciferase) and cotransfected with empty (–), STAT3-C, FoxO1(AAA), or LAP expression plasmids as indicated. Luciferase activity of empty-transfected cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with STAT3-C–transfected cells (P < 0.05). F: CHOP+/+ and CHOP−/− MEF were transfected with FoxO1 reporter plasmid (FRE3-TK-Luciferase) and cotransfected with FoxO1(AAA) expression plasmid as described in research design and methods, in the absence (black bars) or presence (empty bars) of 200 μmol/L Mαα. Luciferase activity of vehicle-treated CHOP−/− MEF was 3.2-fold higher as compared with CHOP+/+ cells. Luciferase activity of respective vehicle-treated cells normalized to β-galactosidase was defined as 1.0. Mean ± SE for three independent experiments. *Significant as compared with vehicle-treated cells (P < 0.05). Inset: Mαα-induced expression of LAP and CHOP in CHOP+/+ or CHOP−/− MEF. Representative blots are shown.