As the tide of chemicals born of the Industrial Age has arisen to engulf our environment, a drastic change has come about in the nature of the most serious public health problems.
Rachel Carson, Silent Spring, 1962
Worldwide rates of diabetes and other metabolic diseases have exploded over the last several decades. Globally, more than 170 million individuals currently suffer from diabetes, and this number is projected to reach a staggering 366 million by 2030 (1). This scourge results in significant individual morbidity and mortality while contributing to the economic fragility of healthcare systems across the globe. In the U.S. alone, annual costs associated with diabetes are estimated to be $174 billion (2). As such, every effort must be made to understand the factors underlying this emerging metabolic disaster in order to mitigate its deleterious impact on the individual and society. Recently, an expanding body of scientific evidence has begun to link exposure to synthetic chemicals with a wide variety of diseases, including reproductive tract disorders and neurobehavioral diseases. The present work discusses epidemiological links between chemical exposure and disorders of glucose homeostasis, experimental data demonstrating chemical-induced changes in insulin action, and challenges facing the field of metabolic disruption as well as approaches for addressing those challenges.
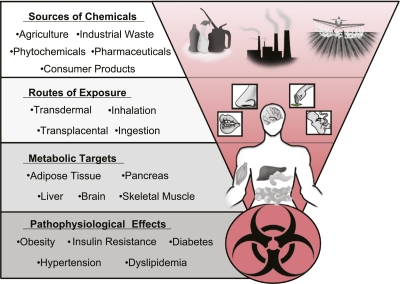
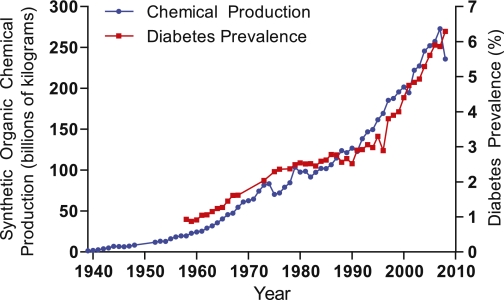
Originally articulated in the early 1990s, the environmental endocrine disruptor theory proposes that some exogenous chemicals interfere with endogenous hormonal axes (3). The recognition of this potential mechanism of action was a paradigm shift in toxicology that had previously focused on a chemical’s capacity to induce acute toxicity or to cause cancer via mutagenesis. The Environmental Protection Agency (EPA) defines an endocrine disrupting chemical (EDC) as “an exogenous agent that interferes with the production, release, transport, metabolism, binding, action, or elimination of natural hormones in the body responsible for the maintenance of homeostasis, reproduction, development, and/or behavior” (4). Putative EDCs include structurally diverse chemicals including organic pollutants, heavy metals, pharmaceuticals, and phytochemicals, with humans exposed through agricultural goods and consumer products, as well as water and air contaminated with industrial waste (Fig. 1). Early studies of EDCs focused on identifying chemicals with the capacity to modulate sex steroid and thyroid hormone signaling; however, recent work suggests that some chemicals may disturb signaling pathways critical for energy homeostasis (5). Despite the potential importance of EDCs in the pathogenesis of metabolic diseases, the contribution of synthetic chemical exposure to the diabetes epidemic remains largely unrecognized and underappreciated even though U.S. diabetes rates have increased in concordance with the national production of synthetic organic chemicals (Fig. 2). While such correlations are crude, emerging data supports a biologically plausible causative link between diabetes and chemical exposure. Here, we present data suggesting a role for some synthetic chemicals in the pathogenesis of diabetes that merits comprehensive efforts to address the contribution of environmental pollutants to this burgeoning metabolic catastrophe.
FIG. 1.
Sources and targets of metabolic disruptors.
FIG. 2.
U.S. synthetic chemical production and diabetes prevalence. Synthetic chemical production in the U.S. from 1939 to 1994 was obtained from the U.S. Tariff Commission reports (72). Production from 1995 to 2008 was extrapolated using the annual index of chemical production published by Chemical & Engineering News from 1989 to 2008 (73,74), with kilograms calculated from linear regression analysis of overlapping data from 1989 to 1994 (r2 = 0.948). Diabetes prevalence was obtained from the Centers for Disease Control and Prevention (75).
Environmental obesogen hypothesis.
An unequivocal contributor to the diabetes epidemic is the metabolic stress induced by rising rates of obesity. Increased adiposity is closely linked to the development of insulin resistance, an important predisposing factor in the development of type 2 diabetes. Over the last several decades, obesity rates have exploded, with more than a third of the adult U.S. population now obese (6). The society-wide accumulation of body fat is undoubtedly a consequence of a widening gap between caloric intake and caloric expenditure resulting from myriad social forces; however, the magnitude and rapidity with which obesity rates have increased raise concerns about other pathogenic factors. In 2002, Baillie-Hamilton (7) proposed a link between the post–World War II increase in synthetic chemical production and the obesity epidemic. This correlation, coupled with experimental evidence demonstrating that certain environmental pollutants induce adipogenesis and weight gain in experimental models, led to the environmental obesogen hypothesis that posits a causative role for synthetic chemicals in the pathogenesis of obesity (rev. in 8).
While environmental obesogens have rightfully received much discussion, it is important to recognize that obesity per se may not lead to abnormalities in glucose homeostasis. An important distinction in obesity research is the differentiation between metabolically deleterious obesity and the “fit fat” (9). Thus, while increased fat mass may contribute to the development of diabetes, obesity is not a necessary or sufficient condition. Insulin resistance can arise independent of obesity, and the onset of frank diabetes necessitates a deficit in β-cell insulin production, as either the primary defect or the failure to compensate for diminished insulin sensitivity. Therefore, the search for pollution-induced diabetes should include a specific focus on compounds with the capacity to induce insulin resistance and/or impair β-cell function.
Epidemiological evidence of diabetogenic pollutants.
Data linking diabetes to environmental pollutants have come from a number of epidemiological studies performed in a variety of experimental contexts (Table 1). Environmental disasters such as the chemical plant explosion in Seveso, Italy, have suggested a link between dioxin exposure and diabetes (10), while rice oil contamination in Yucheng, China, has implicated polychlorinated biphenyl ethers (PCBs) and furans (11). Exposure of military personnel to dioxins during the Vietnam War has been associated with a higher prevalence of diabetes and a reduced latency to disease development (12). Several studies of occupational exposure have suggested links between diabetes and organochlorine pesticides (13) or dioxins (14). Recreational contact via consumption of sport fish from the Great Lakes in the U.S. tied diabetes incidence with p,p’-diphenyldichloroethene (DDE), the principle metabolite of the insecticide p,p’-dichlorodiphenyltrichloroethane (DDT) (15). A variety of international studies demonstrated diabetogenic links to organochlorine pollutants (16) and heavy metals (17), with some studies suggesting a specific defect in insulin secretion but not in overall glucose tolerance (18). In addition to diabetes, epidemiological studies have associated various pollutants with other measures of disturbed glucose homeostasis, including prediabetes (impaired fasting glucose and/or impaired glucose tolerance) (16), the metabolic syndrome (19), and insulin resistance (20).
TABLE 1.
Epidemiological data linking EDC exposure to diabetes
| Reference | EDC | Population | Association with diabetes | Notes |
|---|---|---|---|---|
| Morgan et al., Arch Environ Contam Toxicol 1980;9:349–382 | Pesticides | 2,620 pesticide exposed workers from 1971–1977 | Cause-of-death questionnaires addressed to survivors indicated possible association between DDT exposure and diabetes | |
| Lai et al., Am J Epidemiol 1994;139:484–492 | Arsenic | 891 Taiwanese residents exposed to arsenic in 1988 | Abnormal OGTT, medical histories of diagnosed diabetes, and use of diabetes treatments significantly associated with arsenic exposure | Dose-response relationship between arsenic exposure and diabetes prevalence |
| Henriksen et al., Epidemiology 1997;8:252–258 | TCDD | 989 Air Force veterans of Operation Ranch Hand exposed to TCDD | Glucose abnormalities, diabetes diagnosis, and use of diabetic medications associated with TCDD exposure | Significant hyperinsulinemia in exposed nondiabetic subjects |
| Pesatori et al., Occup Environ Med 1998;55:126–131 | TCDD | Large Italian cohort (>230,000) localized in the exposure zones of the 1976 Seveso accident | Mortality study using Poisson regression to assess relative risk determined substantial TCDD exposure correlated to increased diabetes mortality in women | |
| Vena et al., Environ Health Perspect 1998;106:645–653 | TCDD, HCD | International study of 36 cohorts from 12 countries (1939–1992) (>25,000) | Job record data and company questionnaires with biological and environmental measurements suggested possible correlation of TCDD exposure with diabetes | Strongest association found when first exposure was 10–19 years previous to assessment and with duration of exposure of 10–19 years |
| Calvert et al., Occup Environ Med 1999;56:270–276 | TCDD | 281 former workers at two U.S. chemical plants | Cross-sectional study significantly associated individuals with the highest serum lipid–adjusted TCDD concentrations with higher serum glucose levels | |
| Cranmer et al., Toxicol Sci 2000;56:431–436 | TCDD | 69 individuals in Jacksonville, AR, living within 25 miles of the Vertac waste site | Higher fasting plasma insulin levels associated with individuals in the top 10% of TCDD concentrations (>15 ppt) | No associations with TCDD and glucose levels, obesity, or total lipids |
| Bertazzi et al., Am J Epidemiol 2001;153:1031–1044 | TCDD | 15-year follow-up to the 1976 Seveso accident | Mortality study associated an increase in reported diabetes with TCDD exposure in women | |
| Beard et al., Environ Health Perspect 2001;111:724–730 | Pesticides | 1999 Australian pesticide sprayers employed from 1935–1996 | Mortality study and surviving morbidity questionnaire determined increased mortality due to diabetes associated with pesticide exposure | Diabetes more commonly self-reported with occupational herbicide use |
| Fierens et al., Biomarkers 2003;8:529–534 | 17 PCDD/Fs, dioxins, 4 PCBs, 12 PCB markers | 257 environmentally exposed Belgians | Quantification of serum fat from a population-based study determined significantly increased levels of dioxins, PCBs, and PCB markers in diabetic patients | Diabetes risk significantly increased for individuals in the top decile of dioxin concentrations |
| Glynn et al., Environ Health Perspect 2003;111:349–355 | 7 PCBs, 5 OC pesticides | 205 Swedish women | Association study of lifestyle/medical factors and serum PCB levels indicated increased prevalence of diabetes with higher serum PCB concentrations | Serum PCB concentrations also associated with age, body, BMI, diet, and location of residence |
| Rylander et al., Environ Health 2005;4:28 | PCB-153, DDE | 380 male and female Swedish fishers with a Baltic Sea marine diet | Cross-sectional study significantly associated serum PCB-153 and DDE levels with an increased prevalence of diabetes | Association stronger with PCB-153 for men and with DDE for women |
| Lee et al., Diabetes Care 2006;29:1638–1644 | 6 POPs detected in >80% of population | 2,016 adults from the 1999–2002 NHANES | Prevalence of diabetes associated with increased lipid-adjusted serum concentrations of dioxins, PCBs, and organochlorines | Stronger correlations with younger age, obesity, or Mexican American heritage |
| Vasiliu et al., Epidemiology 2006;17:352–359 | PCBs, PBBs | 1,384 individuals from the Michigan PBB cohort | Enrollment questionnaires and serum samples associated serum PCB levels with an increased prevalence of diabetes in women | Exposed overweight and obese men and women had an increased prevalence of diabetes |
| Codru et al., Environ Health Perspect 2007;115:1442–1447 | 101 PCBs, DDE, HCB | 352 adult Native Americans (Mohawk) | Standardized questionnaire and fasting serum samples positively associated the highest tertile of serum HCB levels with diabetes | Nonsignificant associations with PCBs and DDE with diabetes; mirex levels inversely associated with diabetes |
| Cox et al., Environ Health Perspect 2007;115:1747–1752 | OC pesticides | 1,303 adult Mexican Americans from the 1982–1984 HHANES | Self-reported diabetes significantly associated with lipid-adjusted serum DDT levels and serum glucose levels were elevated in individuals exposed to trans-nonachlor and HCH | |
| Everett et al., Environ Res 2007;103:413–418 | HxCDD, PCB, DDT | 1,830 adults from the 1999–2002 NHANES | Diabetes significantly associated with serum PCB 126, DDT, and HxCDD levels. PCB 126 and DDT levels significantly associated with undiagnosed diabetes (HbA1c >6.1%) | |
| Stahlhut et al., Environ Health Perspect 2007;115:876–882 | Phthalates | U.S. men from the 1999–2002 NHANES | Insulin resistance measured by HOMA-IR was associated with three phthalates (MBP, MBzP, MEP) | Four phthalates (MBzP, MEHHP, MEOHP, MEP) associated with increased waist circumference |
| Lee et al., Diabetologia 2007;50:1841–1851 | OC pesticides, PCBs | 721 nondiabetic participants from the 1999–2002 NHANES | Fasting glucose levels and metabolic syndrome significantly associated with increased levels of OC pesticides | PCBs were significantly associated with waist circumference. OC pesticides significantly associated with elevated triacylglycerides |
| Lee et al., Diabetes Care 2007;30:1596–1598 | PCDD/Fs, PCBs, OC pesticides | 1,721 individuals from the 1999–2002 NHANES | Prevalence of diabetes strongly associated with serum concentrations of PCBs and OC pesticides | PCDDs and PCDFs weakly associated with diabetes |
| Lang et al., JAMA 2008;300:1303–1310 | BPA | 1,455 U.S. adults from the 2003–2004 NHANES | Urinary BPA concentrations associated with diabetes prevalence in a dose-dependent manner | |
| Lim et al., Diabetes Care 2008;31:1802–1807 | 5 PDBEs, PBB | 637 adults from the 2003–2004 NHANES | Serum concentrations of various brominated flame retardants correlated with increased prevalence of diabetes with varying dose dependency | PBDE-153 showed an inverted U-shaped association with metabolic syndrome |
| Jørgensen et al., Diabetologia 2008;51:1416–1422 | General POPs | 692 Greenland Inuits sampled from 1999–2002 living on a marine diet | Significant inverse association between POPs and stimulated insulin concentrations and HOMA-B | No association between POP concentration and glucose intolerance or insulin resistance |
| Wang et al., Diabetes Care 2008;31:1574–1579 | PCBs, PCDFs | 1,054 Taiwanese poisoned with PCB-laced rice-bran oil during late 1970s | Blind morbidity follow-up interviews and chloracne diagnoses significantly associated PCB exposure with an increased prevalence of diabetes in women | |
| Turyk et al., Environ Health Perspect 2009;117:1076–1082 | PCBs, DDE | Population of sport fish consumers in the Great Lakes region from 1990s-2005 | Serum concentrations of DDE positively associated with increased diabetes prevalence | No association with total PCB levels |
| Park et al., J Prev Med Public Health 2010;43:1–8 | OC pesticides | 50 South Korean nondiabetic subjects with metabolic syndrome | Community-based health surveys and HOMA-IR measurements associated OC pesticide exposure with metabolic syndrome | Strong dose dependence between heptachlor epoxide and HOMA-IR |
| Ukropec et al., Diabetologia 2010;53:899–906 | PCBs, HCB, DDE, DDT, HCH | 1,220 PCBRISK survey participants from Eastern Slovakia | Abnormal OGTTs and fasting glucose levels associated with serum levels of POPs suggesting dose-dependent increased risk of diabetes and prediabetes | No association between HCB and HCH levels and diabetes |
HCB, hexachlorobenzene; HCD, higher chlorinated dioxins; HCH, hexachlorocyclohexane; HHANES, Hispanic Health and Nutrition Examination Survey; HOMA-B, homeostasis model assessment of β-cell function; HxCDD, hexachlorodibenzo-p-dioxin; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate, NHANES, National Health and Nutrition Examination Survey; OC, organochlorine; OGTT, oral glucose tolerance test; PBB, polybrominated biphenyls; PCDDs, polychlorinated dibenzodioxins; PCDFs, polychlorinated dibenzofurans; PDBE, polybrominated diphenyl ethers.
Many of the above studies focused on specific populations (i.e., those exposed occupationally, recreationally, or through specific industrial accidents) that may not reflect the risk posed to the general population; however, a series of recent investigations have examined the connection between various pollutants and measures of glucose homeostasis using data from the National Health and Nutrition Examination Survey (NHANES), which provides a representative sampling of the U.S. population (21). NHANES-based studies have shown associations between phthalates and various persistent organic pollutants (POPs) with insulin resistance, the metabolic syndrome, and diabetes (20,22). In addition, urinary levels of the ubiquitous plasticizer, bisphenol-A (BPA), have been detected in >95% of the NHANES cohort (23) and positively correlate with diabetes prevalence and other metabolic derangements (24). Thus, there is intriguing evidence suggesting possible connections between pollutants and the development of diabetes.
There are, however, caveats that must be considered in interpreting these studies. One significant challenge is the common use of cross-sectional design to correlate disease prevalence with current EDC levels. Such analyses are particularly problematic for chemicals that metabolize more rapidly and exhibit fewer propensities to bioaccumulate (e.g., BPA and phthalates) because their current levels may differ from concentrations during disease development. Additionally, issues related to coexposures to confounding compounds, selection of control populations, and variability in statistical analyses complicate data interpretation and extrapolation to the general population. Furthermore, there is heterogeneity in the definition of diabetes and insulin resistance used in these studies. Collectively, these challenges underscore the need for expanded longitudinal studies that can follow chemical exposures throughout disease development in order to better relate specific chemicals to the pathogenesis of diabetes.
Evidence of environmental diabetogenic pollutants in animal models.
The shortcomings of epidemiological investigations can be overcome by studying suspected diabetogenic chemicals using animal models. A number of chemicals have been shown to elicit biological effects that alter glucose homeostasis (Table 2). For instance, acute exposure of male mice to BPA was found to reduce the rise in plasma glucose during an intraperitoneal glucose tolerance test; however, sustained exposure (more similar to human exposure) resulted in hyperinsulinemia, a worsening of glucose tolerance, and a concomitant reduction in insulin sensitivity (25). Interestingly, the impairment in insulin action occurred despite a demonstrated increase in β-cell insulin content after both in vivo and in vitro BPA exposure (26). One explanation for these findings is that BPA operates through multiple mechanisms that independently increase insulin synthesis/secretion while simultaneously inducing peripheral insulin resistance. Alternatively, higher insulin levels induced by BPA may result in a compensatory insulin resistance to limit hypoglycemia. Regardless of the process, the overall effects of chronic BPA exposure on glucose homeostasis suggest that it may be a diabetogenic factor (27).
TABLE 2.
Animal studies demonstrating EDC-induced changes in glucose homeostasis
| Author | EDC | Model system | Disruption of glucose homeostasis |
|---|---|---|---|
| Weber et al., Toxicology 1991;66:133–144 | TCDD | Wild-type male Sprague Dawley rats | Injection of 25 μg/kg TCDD resulted in decreased activity of PEPCK and G-6-Pase after 2 and 8 days of treatment, respectively. |
| Liu et al., Mol Pharmacol 1995;47:65–73 | TCDD | Wild-type male C57BL/6 and DBA/2J mice | A single dose of 116 μg/kg i.p. TCDD resulted in the significant decrease in glucose transport in adipose tissue and brain after 24 h that was sustained for at least 30 days. The effect was AhR mediated. |
| Gayathri et al., Indian J Med Res 2004;119:139–144 | DEHP | Wild-type female Wistar Kyoto rats | Administration of 75 μg/kg DEHP every other day for 14 days resulted in a decrease in serum insulin and cortisol as well as liver glycogen; blood glucose was increased. The effects were reversible upon stopping treatment. |
| Alonso-Magdalena et al., Environ Health Perspect 2006;114:106–112 | BPA | Wild-type male Swiss albino OF1 mice | Administration of a single 10 μg/kg dose of BPA produced a rapid rise in plasma insulin and a corresponding decrease in plasma glucose; however, 4-day treatment with 100 μg/kg/day of BPA impaired glucose tolerance on an intraperitoneal glucose tolerance test and reduced the hypoglycemic effect of insulin in an insulin tolerance test. |
| Hoppe and Carey, Obesity 2007;15:2942–2950 | Penta-BDE | Wild-type male Sprague Dawley rats | Daily gavage of 14 mg/kg penta-BDE for 4 weeks resulted in a 30% increase in isoproterenol-stimulated lipolysis and a 59% decrease in insulin-stimulated glucose oxidation in adipocytes. |
| Alonso-Magdalena et al., PLoS One 2008;3:e2069 | BPA | Wild-type male Swiss albino OF1 mice and ERα and ERβ KO mice | Administration of 100 μg/kg BPA twice per day for 4 days resulted in a significant increase in β-cell insulin content that was ERα dependent. Isolated islets treated with 1 nmol/L BPA had an increase in insulin content. |
| Sato et al., Toxicol Appl Pharmacol 2008;229:1019 | TCDD | Wild-type male C57BL/6 and AhR KO mouse | Oral administration of 500 ng/kg TCDD once a day for 18 days resulted in significantly increased CYP1A1 expression in the liver and changes in energy metabolism gene expression that was AhR-mediated. |
| Ruzzin et al., Environ Health Perspect 2010;118:465–471 | General POPs | Wild-type male Sprague Dawley rats | Administration of a crude fish oil diet for 28 days resulted in systemic insulin resistance, visceral fat accumulation, and hepatosteatosis. Several genes regulating hepatic lipid metabolism were altered. Isolated POP classes impaired insulin-stimulated glucose uptake in 3T3-L1 adipocytes. |
| Fried et al., Drug Chem Toxicol 2010;33:261–268 | TCDD | Wild-type male Sprague Dawley rats | Diabetic rats (high-fat diet/streptozotocin treatment) dosed with 12.8 μg/kg TCDD had significantly reduced serum glucose levels by day 8 of treatment. |
| Zuo et al., Environ Toxicol 2011;26:79–85 | TBT | Wild-type male KM mice | Oral administration once every 3 days for 45 days of 0.5–50 μg/kg TBT resulted in body weight gain, hepatic steatosis, hyperinsulinemia, hyperleptinemia, and a reduction in hepatic adiponectin levels in a dose-dependent fashion. |
BDE, bromodiphenyl ether; DEHP, di(2-ethylhexyl)-phthalate; G-6-Pase, glucose-6-phosphatase.
Other pollutants also disrupt glucose homeostasis in experimental models. Exposure of rats to the flame retardant polybrominated diphenyl ether significantly increased lipolysis while reducing insulin-stimulated glucose uptake (28). Diethylhexyl phthalate, a common plasticizer, reduced insulin levels and raised serum glucose levels in exposed rats (29), while mice treated with tributyl tin (TBT), a fungicide and antifouling agent, demonstrated hepatic steatosis and hyperinsulinemia (30). Recently, rats fed fish oil naturally contaminated with a variety of POPs demonstrated impaired glucose homeostasis, with several chemicals in the contaminated fish oil found to suppress insulin-stimulated glucose uptake in 3T3-L1 adipocytes (31).
These results are similar to findings that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) treatment of primary murine adipose tissue impaired insulin-stimulated glucose uptake, likely by reducing glucose transporter 4 transcript levels (32). In a separate model, mice exposed to TCDD had reduced glucokinase gene expression (33), predicting a rise in blood glucose levels analogous to that seen in maturity-onset diabetes of the young type 2. Others have suggested that the diabetogenic effects of TCDD are mediated through an antagonism of peroxisome proliferator–activated receptor-γ (PPARγ) action (34) or through upregulation of the inflammatory adipokine tumor necrosis factor-α (TNF-α) in adipocytes (35). While these data are consistent with epidemiological observations linking TCDD exposure to diabetes, other studies have shown that TCDD has hypoglycemic effects. In a rat model of diabetes incorporating high-fat diet coupled with streptozotocin treatment, TCDD treatment reduced plasma glucose levels (36). However, this study may reflect an alternative metabolic disruption of quasi-starvation mediated through TCDD suppression of gluconeogenesis via inhibition of PEPCK (37). Furthermore, the hypoglycemic effects of TCDD occurred at concentrations within an order of magnitude of the known lethal dose for rat. The apparent incongruence between hypoglycemic and hyperglycemic observations likely reflects dose-dependent effects. Such findings underscore the need for mechanistic studies over wide concentration ranges that reflect both variability in human exposure and the potential for different mechanisms to predominate at different concentrations.
Putative diabetogenic mechanisms
Traditional endocrine disruption.
Historically, EDC research has focused on the ability of exogenous chemicals to modulate the activity of classic nuclear hormone receptors, including those for estrogens, androgens, and thyroid hormone. Several of these pathways appear to be critically important for energy regulation in general and glucose homeostasis in particular. For example, knockout models of aromatase and the estrogen receptor-α demonstrate the capacity of estrogens to augment glucose tolerance and insulin sensitivity (38,39). However, the effects of estrogen on insulin action may be context-dependent, as conditions associated with estrogen levels that are both high (e.g., pregnancy) and low (e.g., menopause) correlate with insulin resistance. BPA is known to have estrogenic properties, and as mentioned, prolonged treatment of male mice with this EDC induces changes consistent with a diabetic phenotype (25). Furthermore, the augmentation in β-cell insulin content after BPA exposure appears to be a direct result of its estrogenic properties, as the effect was not observed in estrogen receptor-α–knockout animals (26). Because estrogens can have divergent effects on insulin action, estrogenic EDCs may modulate insulin action differently depending on the background hormonal milieu. Thus, the experimental effects may differ between males and females as well as among females at various stages of their reproductive lives (i.e., prepubertal, postmenopausal, or reproductive age).
Androgens also appear to modulate insulin sensitivity. For example, emerging data suggests that low androgen levels in men correlate with insulin resistance. In the TIMES2 trial, testosterone treatment of hypogonadal men with diabetes or the metabolic syndrome improved insulin sensitivity as assessed by homeostasis model assessment of insulin resistance (HOMA-IR) (40). In contrast, exposure to androgens can also adversely affect glucose tolerance. Rhesus monkeys prenatally exposed to androgens show evidence of insulin resistance, with the females having features consistent with the polycystic ovarian syndrome (PCOS) phenotype (41). In humans, insulin resistance is an important clinical feature of PCOS. Interestingly, recent data suggests that women with PCOS have higher levels of BPA than control subjects, and among these PCOS patients, BPA levels correlated with measures of insulin resistance (42). Various synthetic chemicals have the capacity to function as both androgen agonists and antagonists (43), suggesting their capacity to disrupt glucose homeostasis. Importantly, these data also emphasize the potential importance of the timing, context, and relative balance of EDCs on the overall impact of chemical exposure on diabetes risk.
Given the central role of thyroid hormone in energy metabolism, disruption of normal thyroid hormone action may facilitate the development of a diabetic phenotype. Many chemicals can disrupt the thyroid hormone axis (44), and levels of several thyroid disruptors have been correlated with diabetes in epidemiological studies, including PCBs (45). Likewise, glucocorticoids are known modulators of energy metabolism, and recent data suggest that some EDCs may have the capacity to stimulate signaling through the glucocorticoid receptor (46) or by altering glucocorticoid synthesis or activation (47,48). EDCs with glucocorticoid-like activity would be predicted to diminish insulin sensitivity and foster a diabetic phenotype.
Other ligand-activated nuclear hormone receptors are important for energy regulation and have been implicated as EDC targets. Of particular interest are EDCs activating the PPARs. For example, TBT promotes adipogenesis by stimulating PPARγ and its obligate heterodimeric partner retinoid X receptor (RXR) in mouse models (49) and human mesenchymal stem cell cultures (50). Conversely, TCDD inhibits adipogenesis through a suppression of PPARγ (51). The proadipogenic effects of TBT and other EDCs serve as the basis for the environmental obesogen hypothesis. Nevertheless, while PPARγ promotes fat accumulation, its activation also increases insulin sensitivity; this is the rationale for using thiazolidinediones to treat diabetes. Despite this, TBT may impair insulin sensitivity (30); however, this may reflect its promiscuous activation of heterodimeric partners of RXR other than PPARγ. EDC-mediated effects on nuclear hormone signaling are emerging as important mechanisms of metabolic disruption; however, work remains to clarify whether these compounds alter signaling directly at the ligand binding site or whether indirect mechanisms such as coactivator/corepressor recruitment, ligand activation, allosteric effects, targeted receptor degradation, or others are the principle modes of action.
Cross-talk between xenobiotic signaling and metabolism.
In addition to the traditional hormone receptors, the superfamily of ligand-activated nuclear hormone receptors includes several members that function primarily in the sensing and detoxification of foreign compounds, i.e., xenobiotics. These include the aryl hydrocarbon receptor (AhR), the pregnane X receptor, and the constitutive androstane receptor. In addition to their role in the induction of drug metabolizing enzymes, these receptors have modulating effects on lipid and glucose metabolism through their interaction with a wide array of other nuclear receptors (e.g., thyroid hormone receptor, glucocorticoid receptor, PPARα, PPARγ) and transcription factors (e.g., CREB, FOXO1, PGC1α) involved in energy regulation; moreover, the xenobiotic receptors appear to influence inflammatory responses (52). Interestingly, AhR was originally identified as the receptor for dioxin, one of the chemicals most frequently associated with diabetes in epidemiological studies. It is intriguing to speculate that xenobiotic receptors evolved in part to adjust metabolic pathways to environmental stressors, and that the proliferation of anthropogenic chemicals in the environment has overwhelmed these adaptive processes, thereby contributing to the onset of metabolic diseases.
Epigenetic changes.
In line with the “developmental origins of adult disease” hypothesis, a recent emphasis in EDC research has been focused on the effects of in utero and early postnatal chemical exposure on the genesis of adult diseases through modulation of the epigenome. Exposure to a variety of pollutants appears to modify the epigenome (53), and concerning evidence demonstrates that chemical-induced epigenetic changes can be heritable. In a rat model, exposure of pregnant dams to the fungicide vinclozolin led to transgenerational epigenetic modifications into at least the F4 generation (54). Intriguingly, there is now data demonstrating the epigenetic regulation of various genes influencing metabolic diseases, including diabetes (55). While links between EDC exposure and epigenetic alterations of genes controlling energy metabolism have yet to be described, current evidence supports the contention that exposure to EDCs may influence the metabolic state of an individual, with the potential for these effects to be transmitted to subsequent generations.
Alternative mechanisms.
EDC effects on other molecular mechanisms implicated in the development of diabetes, e.g., inflammation and oxidative stress, have only recently been considered. For example, PCB-77 has been shown to promote expression of the proinflammatory adipokines interleukin-6 (IL-6) and TNF-α, leading to impaired insulin signaling in endothelial cells (56). BPA treatment of human adipose tissue explants also augments secretion of IL-6 and TNF-α while simultaneously inhibiting the release of the insulin-sensitizing adipokine adiponectin (57). Interestingly, the detoxification of exogenous chemicals by the cytochrome P450 enzymes generates oxidative stress, which may promote hepatic insulin resistance due to the liver’s dual role in energy and drug metabolism (58). Other diabetogenic mechanisms such as induction of endoplasmic reticulum stress, implicated in arsenic-induced β-cell apoptosis (59), are intriguing but remain poorly studied. With the plethora of structurally diverse compounds present in the environment, these and additional mechanisms may be relevant in the disruption of energy regulation. Characterizing the relevant mechanisms is critical for identifying potential pharmaceutical targets to treat environmentally induced diabetes.
Challenges in endocrine/metabolic disruption research.
As alluded to above, there are a number of challenges limiting our understanding of the impact of synthetic chemicals on metabolic diseases that relate to the chemicals themselves, the exposed individuals, and the experimental approach used to study EDC effects on glucose homeostasis (Table 3). The tens of thousands of unique chemicals released into the environment create an enormous analytical challenge in quantifying human exposure while the physical properties of some compounds contribute to their bioaccumulation and persistence in human tissues long after the exposure has terminated. This contributes to the near ubiquity of certain EDCs in the U.S. population (e.g., hexachlorobenzene and DDE) (23) and raises important questions about the threshold of exposure necessary to elicit a disease phenotype. The experimental challenge is further complicated by the lack of clear structure-function relationships that preclude in silico prediction of adverse health effects, thereby necessitating the use of bioassays to characterize the physiological effects of chemical exposure.
TABLE 3.
Challenges in endocrine/metabolic disruption of glucose homeostasis
| Challenges related to the chemicals |
| Number of structurally diverse compounds to which humans are exposed |
| Measurement of chemicals in metabolically-relevant tissues |
| Lack of clear structure-function relationships |
| Multiple mechanisms of action for a single chemical |
| Effects mediated by a chemical’s metabolites |
| Chemical breakdown differing by route of exposure |
| Interactions among chemicals |
| Additive, antagonistic, and synergistic effects |
| Interactions between chemicals and endogenous metabolites |
| Persistence of chemicals |
| Ubiquity of exposure to some chemicals |
| Challenges related to exposed individuals |
| Interindividual genetic susceptibility to EDCs |
| Differences in EDC target genes |
| Differences in genes regulating EDC metabolism |
| Coexisting diabetes risk factors |
| Obesity, high-fat diet, sedentary lifestyle, family history |
| Medical comorbidities |
| Pharmaceutical agents/medications |
| Hormonal status |
| Women versus men |
| Prepubertal versus reproductive age versus postmenopausal |
| Eugonadal versus hypogonadal |
| Challenges related to experimental design and approaches |
| Cross-sectional versus longitudinal epidemiological design |
| Single chemical approaches versus analyses of mixtures |
| Additive, antagonistic, and synergistic effects |
| Nonmonotonic dose-response relationships |
| Failure of cell culture or animal models to recapitulate human physiology |
| Background hormonal milieu of experimental animals |
| Effect of timing of exposure |
| in utero or early postnatal versus adult exposure |
| Transgenerational effects |
| Phytochemical content of animal feed |
Inter-individual variation in gene-environment interactions may also modify the deleterious effects of synthetic chemicals. For example, the adverse consequences of occupational exposure to pesticides among Costa Rican banana farmers was found to be influenced by whether they inherited “favorable” or “unfavorable” metabolizing genes (60). Other predisposing factors, such as obesity or a family history of diabetes, may also accentuate the diabetogenic effects of some chemicals (61), while high-fat diets may augment exposure to lipophilic EDCs. As discussed, EDCs that modulate sex steroid action may have divergent metabolic effects depending on the background hormonal milieu leading to sexually dimorphic effects that are also influenced by changes over the life span. Finally, evidence of in utero programming and transgenerational effects suggest that an individual’s disease-related exposure may have taken place before birth or even in a prior generation.
Lastly, experimental design may influence the observed biological effect or fail to accurately recapitulate real-world scenarios. Particularly vexing are mixtures of compounds that may exert additive, antagonistic, or even synergistic biological effects. Consequently, the ultimate metabolic phenotype may differ considerably from studies of single chemicals in isolation. Furthermore, nonmonotonic dose-response relationships are seen with some chemicals (e.g., BPA [62] and polybrominated diphenyl ethers [19]), thus mandating studies across wide concentration ranges that also account for effects at extremely low doses. Finally, animal models and humans may have divergent responses to EDCs (63), and the phytochemical content of animal feeds modulates EDCs effects (64). Thus, careful selection of experimental models and appropriate controls are critical for understanding the metabolic effects of synthetic chemicals.
Diabetes and environmental injustice.
One of the profound tragedies of the diabetes epidemic is its disproportionate effect on minority groups and the economically disadvantaged. National survey data from 2007–2009 show that 11.8% of Hispanics and 12.6% of non-Hispanic blacks self-identified as having diabetes compared with only 7.1% of non-Hispanic whites (65). Furthermore, rates of diabetes are inversely related to household income and education level (66). This heightened risk may reflect disproportionate exposure to chemical pollutants among these groups (67). Some studies have specifically linked chemical exposure with diabetes among high-risk groups, including Mexican Americans (68) and Native Americans (69), while others have found stronger associations between POPs and diabetes among Hispanics (22). This strengthened association may reflect coordinate exposure to other diabetes-promoting chemicals, increased susceptibility to metabolic disruption (e.g., in 60), or an interaction between diabetogenic chemicals and other predisposing risk factors, e.g., diet or limited access to healthcare. The greater synthetic chemical exposure of poor and minority groups is likely a consequence of many factors; however, one important contributor is the historical construction of chemical production facilities and toxic waste sites in poor communities (70). The causality of these intertwined relationships is difficult to dissect; nevertheless, the synergy of coexisting poverty, poor education, and pollution likely contributes to the pathogenesis of metabolic diseases.
Strategies for change.
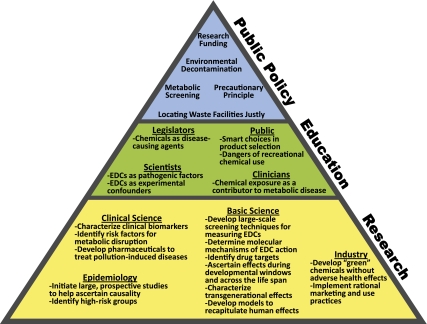
Mitigation of the detrimental effects of pollution on metabolic health will require the development and implementation of comprehensive strategies, including expanded research programs, improved regulation and public policy, and education efforts (Fig. 3). The EPA has recently begun analyzing synthetic chemicals for endocrine-disrupting effects through the Endocrine Disruptor Screening Program. This initiative should be expanded to include analyses of metabolic disruption in order to specifically identify diabetogenic chemicals with significant emphasis placed on characterizing the molecular mechanisms in order to better identify health-threatening chemicals, to anticipate additive/synergistic effects of mixtures, and to determine potential pharmaceutical targets. Susceptibility across the life span should be assessed with special emphasis on critical developmental windows and transgenerational effects.
FIG. 3.
Strategies for addressing environmental disruption of metabolism.
Risk analysis can be enhanced by identifying groups with exposures to high-risk chemicals and achieving a better understanding of factors predisposing to disruptions in energy homeostasis, including genetic polymorphisms linked to detoxification and metabolic pathways. Exposure analysis must be expanded beyond urine and serum to include lipid-rich organs that bioaccumulate POPs (e.g., brain and adipose) as well as tissues relevant to in utero and early postnatal exposure (e.g., human breast milk, cord blood, and placental tissue). Beyond direct measurement of EDCs, development of clinical biomarkers will facilitate identification of chemical-exposed individuals who can then be monitored prospectively for the development of diabetes, enhancing efforts to establish causality.
The new toxicology paradigm of endocrine and metabolic disruption mandates a transformation in regulatory policy to limit the production and use of chemicals that threaten metabolic health. Specific attention should be paid to banning chemicals with long-lasting efforts due to either their environmental persistence or ability to induce transgenerational effects. When doubts persist about a chemical’s metabolic impact, the “precautionary principle” should be adopted and its use restricted. Whenever possible, decontamination of environments and individuals exposed to metabolic disruptors should be pursued to limit ongoing exposure. For individuals with exposures that cannot be cleared, drugs should be developed to modulate the specific pathways responsible for pollution-induced diabetes. Finally, scientists and clinicians must become advocates in educating lawmakers and the public about the threat of metabolic disruptors as well as the means to limit their impact through sound government policy and smart consumer choices.
Conclusions.
In 1962, Rachel Carson warned of the health threat posed by environmental pollution (71). Nearly 50 years later, evidence suggests that human exposure to synthetic chemicals may be contributing to the burgeoning diabetes epidemic. While the revolution in synthetic chemistry has facilitated vast improvements in our quality of life, these benefits increasingly appear to have come with a hidden cost. This paradox of progress now mandates a reassessment of how our consumption habits negatively impact our metabolic health in order to devise effective strategies to limit the significant individual and societal toll of diabetes.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health/National Institute of Environmental Health Sciences Grant K08ES019176 (to R.M.S.).
No potential conflicts of interest relevant to this article were reported.
B.A.N. and R.M.S. wrote, reviewed, and edited the manuscript.
Due to reference constraints, the authors regret that some literature could not be cited.
The authors thank Rachel P. Delaney of the U.S. Environmental Protection Agency Andrew W. Breidenbach Environmental Research Center Library in Cincinnati, Ohio, for research assistance; Yuxi Lin of the University of Chicago for editorial comments; and Paul A. Volden of the University of Chicago Committee on Molecular Metabolism and Nutrition for assistance with illustrations.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. In 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 3.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 1993;101:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Environmental Protection Agency Special Report on Environmental Endocrine Disruption: An Effects Assessment and Analysis. Washington, D.C., U.S. Environmental Protection Agency, 1997 [Google Scholar]
- 5.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 2011;73:135–162 [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 7.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 2002;8:185–192 [DOI] [PubMed] [Google Scholar]
- 8.Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol 2009;304:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008;168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 10.Bertazzi PA, Consonni D, Bachetti S, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol 2001;153:1031–1044 [DOI] [PubMed] [Google Scholar]
- 11.Wang SL, Tsai PC, Yang CY, Leon Guo Y. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care 2008;31:1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology 1997;8:252–258 [DOI] [PubMed] [Google Scholar]
- 13.Beard J, Sladden T, Morgan G, Berry G, Brooks L, McMichael A. Health impacts of pesticide exposure in a cohort of outdoor workers. Environ Health Perspect 2003;111:724–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvert GM, Sweeney MH, Deddens J, Wall DK. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med 1999;56:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect 2009;117:1076–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ukropec J, Radikova Z, Huckova M, et al. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia 2010;53:899–906 [DOI] [PubMed] [Google Scholar]
- 17.Lai MS, Hsueh YM, Chen CJ, et al. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol 1994;139:484–492 [DOI] [PubMed] [Google Scholar]
- 18.Jørgensen ME, Borch-Johnsen K, Bjerregaard P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia 2008;51:1416–1422 [DOI] [PubMed] [Google Scholar]
- 19.Lim JS, Lee DH, Jacobs DR., Jr Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003-2004. Diabetes Care 2008;31:1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 2007;115:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention National Health and Nutrition Examination Survey. Atlanta, GA, Centers for Disease Control and Prevention, 2010 [Google Scholar]
- 22.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care 2006;29:1638–1644 [DOI] [PubMed] [Google Scholar]
- 23.National Health and Nutrition Examination Survey. NHANES 2003-2004 Laboratory Files. Atlanta, GA, Centers for Disease Control and Prevention, 2010 [Google Scholar]
- 24.Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008;300:1303–1310 [DOI] [PubMed] [Google Scholar]
- 25.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 2006;114:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alonso-Magdalena P, Ropero AB, Carrera MP, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 2008;3:e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso-Magdalena P, Ropero AB, Soriano S, Quesada I, Nadal A. Bisphenol-A: a new diabetogenic factor? Hormones (Athens) 2010;9:118–126 [DOI] [PubMed] [Google Scholar]
- 28.Hoppe AA, Carey GB. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring) 2007;15:2942–2950 [DOI] [PubMed] [Google Scholar]
- 29.Gayathri NS, Dhanya CR, Indu AR, Kurup PA. Changes in some hormones by low doses of di (2-ethyl hexyl) phthalate (DEHP), a commonly used plasticizer in PVC blood storage bags & medical tubing. Indian J Med Res 2004;119:139–144 [PubMed] [Google Scholar]
- 30.Zuo Z, Chen S, Wu T, et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environ Toxicol 2011;26:79–85 [DOI] [PubMed] [Google Scholar]
- 31.Ruzzin J, Petersen R, Meugnier E, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 2010;118:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu PC, Matsumura F. Differential effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the “adipose- type” and “brain-type” glucose transporters in mice. Mol Pharmacol 1995;47:65–73 [PubMed] [Google Scholar]
- 33.Sato S, Shirakawa H, Tomita S, et al. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol Appl Pharmacol 2008;229:10–19 [DOI] [PubMed] [Google Scholar]
- 34.Remillard RB, Bunce NJ. Linking dioxins to diabetes: epidemiology and biologic plausibility. Environ Health Perspect 2002;110:853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiumi S, Yoshida M, Azuma T, Yoshida K, Ashida H. 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs an insulin signaling pathway through the induction of tumor necrosis factor-alpha in adipocytes. Toxicol Sci 2010;115:482–491 [DOI] [PubMed] [Google Scholar]
- 36.Fried KW, Guo GL, Esterly N, Kong B, Rozman KK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) reverses hyperglycemia in a type II diabetes mellitus rat model by a mechanism unrelated to PPAR gamma. Drug Chem Toxicol 2010;33:261–268 [DOI] [PubMed] [Google Scholar]
- 37.Weber LW, Lebofsky M, Stahl BU, Gorski JR, Muzi G, Rozman K. Reduced activities of key enzymes of gluconeogenesis as possible cause of acute toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in rats. Toxicology 1991;66:133–144 [DOI] [PubMed] [Google Scholar]
- 38.Takeda K, Toda K, Saibara T, et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol 2003;176:237–246 [DOI] [PubMed] [Google Scholar]
- 39.Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 2006;49:588–597 [DOI] [PubMed] [Google Scholar]
- 40.Jones TH, Arver S, Behre HM, et al. TIMES2 Investigators. Testosterone Replacement in Hypogonadal Men With Type 2 Diabetes and/or Metabolic Syndrome (the TIMES2 Study). Diabetes Care 2011;34:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab 2000;85:1206–1210 [DOI] [PubMed] [Google Scholar]
- 42.Kandaraki E, Chatzigeorgiou A, Livadas S, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab 2011;96:E480–E484 [DOI] [PubMed] [Google Scholar]
- 43.Urbatzka R, van Cauwenberge A, Maggioni S, et al. Androgenic and antiandrogenic activities in water and sediment samples from the river Lambro, Italy, detected by yeast androgen screen and chemical analyses. Chemosphere 2007;67:1080–1087 [DOI] [PubMed] [Google Scholar]
- 44.Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and thyroid hormone physiology. Biochem Pharmacol 2010;79:939–947 [DOI] [PubMed] [Google Scholar]
- 45.Everett CJ, Frithsen IL, Diaz VA, Koopman RJ, Simpson WM, Jr, Mainous AG., 3rd Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999-2002 National Health and Nutrition Examination Survey. Environ Res 2007;103:413–418 [DOI] [PubMed] [Google Scholar]
- 46.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010;18:1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atanasov AG, Tam S, Röcken JM, Baker ME, Odermatt A. Inhibition of 11 beta-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochem Biophys Res Commun 2003;308:257–262 [DOI] [PubMed] [Google Scholar]
- 48.Atanasov AG, Nashev LG, Tam S, Baker ME, Odermatt A. Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids. Environ Health Perspect 2005;113:1600–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grün F, Watanabe H, Zamanian Z, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 2006;20:2141–2155 [DOI] [PubMed] [Google Scholar]
- 50.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol 2010;24:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanlon PR, Ganem LG, Cho YC, Yamamoto M, Jefcoate CR. AhR- and ERK-dependent pathways function synergistically to mediate 2,3,7,8-tetrachlorodibenzo-p-dioxin suppression of peroxisome proliferator-activated receptor-gamma1 expression and subsequent adipocyte differentiation. Toxicol Appl Pharmacol 2003;189:11–27 [DOI] [PubMed] [Google Scholar]
- 52.Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm 2008;5:35–41 [DOI] [PubMed] [Google Scholar]
- 53.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005;308:1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology 2006;147:5515–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 2009;58:2718–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Lv X, Du Y. Inflammatory response and insulin signaling alteration induced by PCB77. J Environ Sci (China) 2010;22:1086–1090 [DOI] [PubMed] [Google Scholar]
- 57.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol 2009;304:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011;50:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu TH, Su CC, Chen YW, et al. Arsenic induces pancreatic beta-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett 2011;201:15–26 [DOI] [PubMed] [Google Scholar]
- 60.Au WW, Sierra-Torres CH, Cajas-Salazar N, Shipp BK, Legator MS. Cytogenetic effects from exposure to mixed pesticides and the influence from genetic susceptibility. Environ Health Perspect 1999;107:501–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujiyoshi PT, Michalek JE, Matsumura F. Molecular epidemiologic evidence for diabetogenic effects of dioxin exposure in U.S. Air force veterans of the Vietnam war. Environ Health Perspect 2006;114:1677–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 2009;30:75–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feige JN, Gerber A, Casals-Casas C, et al. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ Health Perspect 2010;118:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heindel JJ, vom Saal FS. Meeting report: batch-to-batch variability in estrogenic activity in commercial animal diets—importance and approaches for laboratory animal research. Environ Health Perspect 2008;116:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention 2011 National Diabetes Fact Sheet. Atlanta, GA, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 66.Espelt A, Borrell C, Roskam AJ, et al. Socioeconomic inequalities in diabetes mellitus across Europe at the beginning of the 21st century. Diabetologia 2008;51:1971–1979 [DOI] [PubMed] [Google Scholar]
- 67.Quintero-Somaini AQM. Hidden Danger: Environmental Health Threats in the Latino Community National Resources Defense Council, Ed. Washington, DC, National Resources Defense Council, 2004 [Google Scholar]
- 68.Cox S, Niskar AS, Narayan KM, Marcus M. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: Hispanic health and nutrition examination survey, 1982-1984. Environ Health Perspect 2007;115:1747–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO; Akwesasne Task Force on Environment Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect 2007;115:1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brulle RJ, Pellow DN. Environmental justice: human health and environmental inequalities. Annu Rev Public Health 2006;27:103–124 [DOI] [PubMed] [Google Scholar]
- 71.Carson R. Silent Spring. New York, Houghton Mifflin, 1962 [Google Scholar]
- 72.U.S. Tariff Commission, Ed. Synthetic Organic Chemicals: United States Production and Sales. Washington, DC, Government Printing Office; 1939–1994 [Google Scholar]
- 73.Gains beat losses. In Chemical & Engineering News. Washington, DC, American Chemical Society, 2000, p. 50–56 [Google Scholar]
- 74.Output Declines in U.S. Europe. In Chemical & Engineering News. Washington, DC, American Chemical Society, 2010, p. 54–62 [Google Scholar]
- 75.Centers for Disease Control and Prevention, Ed. Long-term Trends in Diabetes. Atlanta, GA, 2009 [Google Scholar]