Abstract
OBJECTIVE
Loss of thrombospondin (TSP)-1 in pancreatic islets has been shown to cause islet hyperplasia. This study tested the hypothesis that endothelial-derived TSP-1 is important for β-cell function.
RESEARCH DESIGN AND METHODS
Islet function was evaluated both in vivo and in vitro. Messenger RNA and protein expression were measured by real-time PCR and Western blot, respectively. The role of endothelial-derived TSP-1 for β-cell function was determined using a transplantation design in which recipient blood vessels either were allowed to grow or not into the transplanted islets.
RESULTS
TSP-1–deficient mice were glucose intolerant, despite having an increased β-cell mass. Moreover, their islets had decreased glucose-stimulated insulin release, (pro)insulin biosynthesis, and glucose oxidation rate, as well as increased expression of uncoupling protein-2 and lactate dehydrogenase-A when compared with control islets. Almost all TSP-1 in normal islets were found to be derived from the endothelium. Transplantation of free and encapsulated neonatal wild-type and TSP-1–deficient islets was performed in order to selectively reconstitute with TSP-1–positive or –negative blood vessels in the islets and supported that the β-cell defects occurring in TSP-1–deficient islets reflected postnatal loss of the glycoprotein in the islet endothelial cells. Treatment of neonatal TSP-1–deficient mice with the transforming growth factor (TGF)β-1–activating sequence of TSP-1 showed that reconstitution of TGFβ-1 activation prevented the development of decreased glucose tolerance in these mice. Thus, endothelial-derived TSP-1 activates islet TGFβ-1 of importance for β-cells.
CONCLUSIONS
Our study indicates a novel role for endothelial cells as functional paracrine support for pancreatic β-cells.
The vasculature traditionally has been regarded mainly as a transport system that mediates metabolic exchange between tissues and blood. However, aside from its transport functions, blood-vessel cells have, in recent years, been recognized to be able to interact with and differentiate adjacent parenchymal cells through paracrine signals during development (1–3). Moreover, they seem to be able to provide mitotic signals during adulthood (4).
To date, there have been few studies (5–7) on whether endothelial cells may directly affect parenchymal function. Islets of Langerhans in the adult have a uniquely dense network of capillaries maintained by constant exposure to vascular endothelial growth factor (VEGF)-A secreted from adjacent β-cells (8,9). The high number of islet capillaries results in each β-cell being located directly adjacent to at least one endothelial cell (10), thereby enabling a direct interaction with endothelium-derived factors. The importance of islet endothelial factors in the control of β-cell proliferation recently has been studied, and both endothelium-derived hepatocyte growth factor (11) and the vascular membrane component laminin (7) seem to be important in this context.
In the current study, we investigated products of purified and isolated islet endothelial cells and thereby found the glycoprotein thrombospondin (TSP)-1 to be highly expressed in the endothelium of islets. TSP-1 is mainly known for its antiangiogenic properties (12) but also may alter the morphology of pancreatic islets and functions as a major activator of transforming growth factor (TGF)β-1 (13). Because TGFβ-1 is known to modulate β-cell function (14,15), we tested the hypothesis that endothelial cell–derived TSP-1 is important to maintain β-cell function postnatally.
RESEARCH DESIGN AND METHODS
TSP-1–deficient (−/−) mice were generated by homologous recombination in 129/Sv-derived embryonic stem cells implanted in C57BL/6 blastocysts. Chimeras were bred to C57BL/6 mice, and heterozygotes were backcrossed (N9) to a C57BL/6 genetic background (99.6%) (16). Wild-type TSP-1 (+/+) or TSP-1–deficient mice aged 0, 10–12, or 52 weeks were allocated to the different studies. All experiments were approved by the animal ethics committee for Uppsala University.
Chemicals.
All chemicals were purchased from Sigma-Aldrich (Irvine, U.K.), unless otherwise mentioned.
Islet morphology.
Pancreata from 10- to 12-week-old wild-type and TSP-1–deficient mice were retrieved, weighed, fixed in 10% (vol/vol) formaldehyde and embedded in paraffin. Sections (5 μm thick) of the pancreata were stained with a guinea pig antibody for insulin (ICN Biomedicals, Aurora, OH) (17) and counterstained with hematoxylin. For each animal, ≥10 tissue sections from all parts of the pancreas were randomly chosen and evaluated. The fraction of the pancreas composed of endocrine tissue was measured by a direct point-counting method (18) and used for calculation of the endocrine mass compensating for differences in pancreatic weight between animals. The β-cell fraction of the islets in wild-type and TSP-1–deficient mice was estimated with a similar point-counting technique. The area of the investigated islets was determined using a computerized system for morphometry (Scion Image, Scion, MD). A total of at least 1,089 intersections were counted in each pancreas.
Oxygen tension measurements.
Oxygen tension in native pancreatic islets of 10- to 12-week-old wild-type and TSP-1–deficient mice were recorded in vivo, as previously described (19).
Twenty-four–hour blood glucose measurements.
Blood glucose measurements were performed at 2-h intervals for 24 h on blood obtained from the cut tip of the tail of 10- to 12-week-old wild-type and TSP-1–deficient mice using test reagent strips (Freestyle; Baxter Travenol, Deerfield, IL). The animals were fed ad libitum during the experiments.
Glucose and insulin tolerance tests.
Glucose or insulin tolerance tests were performed on 10- to 12- or 52-week-old wild-type and TSP-1–deficient mice, as described previously (20).
Islet isolation and culture.
Pancreatic islets from neonatal or 10- to 12-week-old wild-type and TSP-1–deficient C57BL/6 mice, as well as adult WF rats, were prepared by collagenase digestion (21) and maintained free floating in groups of 150 islets at 37°C (air/CO2, 95:5) for 1–3 days in 5 mL culture medium composed of RPMI-1640 medium supplemented with 2 mmol/L l-glutamine, 11 mmol/L glucose, and 10% (vol/vol) FCS.
Islet functional tests.
The capacity of isolated islets from 10- to 12-week-old wild-type or TSP-1–deficient mice for glucose-stimulated or potassium chloride (30 mmol/L)-stimulated insulin release, glucose-stimulated proinsulin biosynthesis, and glucose oxidation was determined in batch-type experiments (for protocol see [6]).
Electron microscopy.
Islets isolated from 10- to 12-week-old wild-type or TSP-1–deficient mice were prepared for electron microscopy (22). Ultrastructural studies of mitochondria and endoplasmic reticulum were performed using an H-7100 transmission electron microscope (Hitachi, Tokyo, Japan).
Determination of reactive oxygen species.
The production of reactive oxygen species (ROS) in groups of 50 wild-type or TSP-1–deficient islets in response to 11.1 or 28 mmol/L glucose with or without the presence of H2O2 was estimated by flow cytometry, as previously described (23).
Gene expression.
Whole islets from 10- to 12-week-old wild-type and TSP-1–deficient mice were used to investigate changes in mRNA expression induced by TSP-1 deficiency. RNA was isolated using Ultraspec (Biotecx Laboratories, Houston, TX), and cDNA synthesis was performed with random nonamers (Sigma-Aldrich) and reverse transcriptase Moloney murine leukemia virus H– (Finnzymes, Espoo, Finland). Amplification was obtained with a Lightcycler system (Roche-Diagnostic, Lewes, U.K.) using a DyNAmo Capillary SYBR Green qPCR kit (Finnzymes). For the primer sequences used, see Table 1. All values were normalized against β-actin.
TABLE 1.
Primer sequences and antibodies
| Gene/antibody | Sense primer (5′–3′) | Antisense primer (5′–3′) | Genebank accession no. |
|---|---|---|---|
| β-Actin | GCTCTGGCTCCTAGCACC | CCACCGATCCACACAGAGTACTTG | NM_007393 |
| Glucokinase | AGGCACGAAGACATAGACAAG | ACCACATCCATCTCAAAGTCC | NM_010292 |
| Insulin | CCATCAGCAAGCAGGTTAT | GGGTGTGTAGAAGAAGCCA | NM_008386 |
| LDH-A | GGTTGCAATCTGGATTCAGCG | TCAGTGCCCAGTTCTGGGTTA | NM_010699 |
| Mitochondrial glycerol phosphate dehydrogenase-2 | GTTGAAGTGAGAAGAGGGGATG | GACAAGCCTGATGTAGAGTGTG | NM_010274 |
| Pyruvate carboxylase | CTACACCAACTACCCTGACAAC | GCTAAGCCCATGTAGTACTCC | NM_008797 |
| PDX-1 | GGTGCCAGAGTTCAGCGCTA | TTGTTTTCCTCGGGTTCCGC | NM_008814 |
| TBP | ACCCTTCACCAATGACTCCTATG | ATGATGACTGCAGCAAATCGC | NM_013684 |
| TSP-1 (mouse) | GGAACGGAAAGACAACACTG | AGTTGAGCCCGGTCCTCTTG | NM_01158 |
| TSP-1 (rat) | GCCGATTCCAGATGATTCC | ACCCGAAAACAAAGCCAG | NM_001013062 |
| UCP-2 | AGCCTACAAGACCATTGCACG | CAGAAGTGAAGTGGCAAGGGA | NM_011671 |
| ERK antibody | |||
| LDH-A antibody | |||
| PDX-1 antibody | |||
| UCP-2 antibody |
All primers were ordered from MWG Biotech (Ebersberg, Germany). Antibodies directed toward LDH-A and PDX-1 were purchased from Cell Signaling (Beverly, MA), whereas ERK antibodies were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Three different UCP-2 antibodies obtained from Santa Cruz Biotechnologies, Millipore (Billerica, MA), and Fitzgerald Industries International (North Acton, MA), respectively, were used.
Western blot and lactate analysis.
Islets from 10- to 12-week-old wild-type and TSP-1–deficient mice were prepared for Western blot, and the protein expression analyzed, as previously described (24). For antibodies used, see Table 1. All values were normalized against total extracellular signal–regulated kinase (ERK). Lactate production from incubated wild-type and TSP-1–deficient islets were determined, as described previously (25).
Isolation and culture of islet endothelial cells.
Outgrowth of islet mesenchymal cells was stimulated using our previously described protocol (11,26). In brief, 20 hand-picked, apparently clean islets isolated from wild-type C57BL/6 mice 10–12 weeks old, or from WF rats of corresponding age, were transferred onto a collagen matrix (1.8 mg collagen type 1 per mL; Collagen, Nutacon, Leimuden, the Netherlands) in a 24-well culture dish. The islets were cultured at 37°C (air/CO2, 95:5) in 1 mL culture medium composed of RPMI-1640 medium supplemented with 11 mmol/L glucose, 20% (vol/vol) FCS, 100 μg endothelial cell growth supplement, and 2 mmol/L l-glutamine. Vascular sprouts grew out from the islets, and the cells were detached with 0.25% (wt/vol) trypsin (Sigma-Aldrich) for <5 min at 37°C. The suspension was washed twice in culture medium. The endothelial cells were extracted from the cell suspension by a dynabead method (27). By the use of Bandeiraea (Griffonia) simplicifolia (BS-1)-coated dynabeads, endothelial cells were separated from contaminating cells, achieving a purity of >90% (11,26). The detailed characterization of the endothelial cells has been described previously (6,26).
Isolation of β-cells.
Islets isolated from wild-type C57BL/6 mice were dissociated with trypsin (Sigma-Aldrich), followed by cell sorting using a fluorescence-activated cell sorter (FACS Calibur; Becton Dickenson, San Jose, CA). The cells were sorted according to autofluorescence at 530 nm, which generates a purity of ~95% β-cells (28).
Islet cellular localization of TSP-1.
Islet endothelial cells, β-cells or intact islets from wild-type C57BL/6 mice, islet endothelial cells or intact islets from WF rats, and cells from the mouse and rat insulinoma cell lines βTC-6 and INS-1 were washed with PBS and analyzed for TSP-1 mRNA expression (compared with above). In these experiments, β-actin was used as a reference gene; however, normalization against TATA-box–binding protein (TBP) gave similar results. For primer sequences used, see Table 1.
Importance of endothelial-derived TSP-1.
To separately evaluate the contribution of islet endothelial-derived TSP-1 for β-cell function, we performed transplantation experiments of neonatal pancreatic islets that, after isolation from wild-type and TSP-1–deficient mice, had been cultured for 1 day. Wild-type islets were transplanted into either 10- to 12-week-old wild-type or TSP-1–deficient mice. Likewise, islets derived from TSP-1–deficient mice were implanted into either wild-type or TSP-1–deficient mice. For transplantation, groups of 150 neonatal wild-type or TSP-1–deficient islets were syngeneically implanted beneath the capsule of the left kidney in wild-type or TSP-1–deficient C57BL/6 mice. One month posttransplantation, glucose-stimulated insulin secretion of the islet grafts was evaluated by vascular perfusion ex vivo (29,30).
Although precultured and nonencapsulated islets mainly obtain their new vascular system from the recipient after transplantation (19,31), islets encapsulated prior to transplantation lack such blood vessels. A rationale for transplanting encapsulated islets was to provide a control for the possible influence of nonvascular systemic influences on β-cell function in TSP-1–deficient mice. Microencapsulated islets were implanted intraperitoneally, instead of beneath the renal capsule, because the capsules are more easily retrieved for in vitro experiments from the peritoneal cavity. Microcapsules composed of alginate (guluronic acid 73%/mannuronic acid 27%; Pronova Biopolymer, Drammen, Norway), in which divalent cations such as Ca2+ crosslink guluronic acid blocks and thus form a gel, were produced as follows (32,33). The microencapsulated islets were immediately transplanted intraperitoneally into 10- to 12-week-old wild-type or TSP-1–deficient mice. The recipient mice were anesthetized by inhalation of isoflurane (32). A small incision was made in the skin, and the peritoneal membrane and the capsules in ~0.4 mL of saline were then injected into the peritoneal cavity. One to two sutures were required to close the peritoneal membrane, and two to three sutures closed the skin. One month posttransplantation, animals were killed by cervical dislocation; the capsules were retrieved by filling the abdominal cavity with ~5–6 mL cold saline supplemented with 2 mmol/L Ca2+ (balanced salt solution; SBL Vaccindistribution, Stockholm, Sweden) to wash out the capsules. Retrieved capsules were used for measurements of glucose-stimulated insulin release (compared with Supplementary Data 2), with each observation representing one animal.
Treatments with TGFβ-1–activating recombinant proteins.
Two-day-old neonatal wild-type and TSP-1–deficient mice were injected with either the TGFβ-1–activating recombinant protein that is the second type 1 repeat of TSP-1 (TSR2+RFK) or the control protein (TSR2+QFK) (34). The proteins were administered intraperitoneally every morning at a dose of 1 mg/kg. Vehicle (10 mmol/L Tris-HCl, pH 7.6, in 500 mmol/L NaCl)-injected wild-type and TSP-1–deficient animals served as additional controls. Animals were treated for 3 weeks before glucose tolerance tests. Glucose tolerance tests were performed as described in Supplementary Data 1, but glucose was administered intraperitoneally instead of intravenously because of the small size of the animals.
Statistical analysis.
Values are expressed as the means ± SE. When only two groups were compared, an unpaired or paired Student t test was used. Multiple comparisons between parametric data were performed using ANOVA and a Bonferronni post hoc test. Gene expression data were compared using the Mann-Whitney rank-sum test. For all comparisons, P values < 0.05 were considered statistically significant.
RESULTS
Morphology of islets in TSP-1–deficient mice.
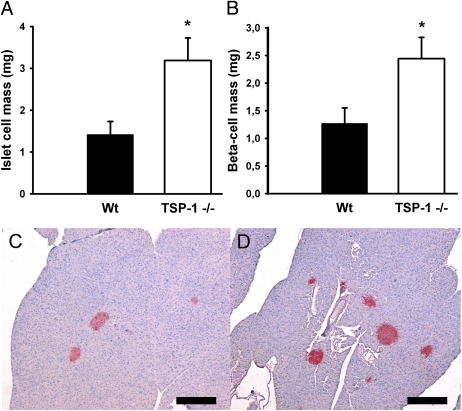
Body weights and pancreas weights were similar in 10- to 12-week-old wild-type and TSP-1–deficient mice. Islet and β-cell masses were higher in TSP-1–deficient mice when compared with controls (Fig. 1A–D).
FIG. 1.
Endocrine (A) and β-cell (B) mass of wild-type (Wt; ■) and TSP-1 (−/−) (□) mice. C and D: Pancreata obtained from a wild-type (C) or TSP-1 (−/−) (D) mouse, respectively, and stained for insulin. Scale bar 300 μm. Values in A and B are means ± SE for five to six experiments in each group. *P < 0.05 when compared with wild-type mice. (A high-quality digital representation of this figure is available in the online issue.)
Oxygen tension in islets in TSP-1–deficient mice.
Oxygen tension levels, as measured in vivo, were similar in pancreatic islets of 10- to 12-week-old wild-type and TSP-1–deficient animals (38.0 ± 1.6 vs. 40.4 ± 1.3 mmHg, respectively, n = 7 animals in each group).
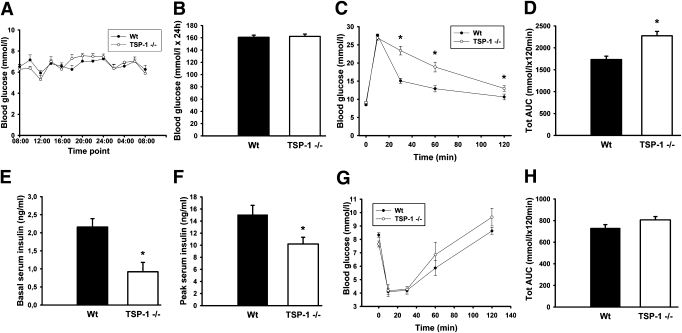
Glucose and insulin tolerance tests.
Ten- to 12-week-old TSP-1–deficient mice had normal blood glucose concentrations throughout the day (Fig. 2A and B) but showed impaired glucose tolerance after intravenous glucose administration (Fig. 2C and D). Serum insulin levels both before and 10 min after glucose administration in the glucose tolerance test were lower in TSP-1–deficient mice when compared with wild-type mice (Fig. 2E and F). There were no differences in glucose tolerance and insulin secretion between males and females or between 10- to 12- and 52-week-old TSP-1–deficient mice (data not shown). Insulin tolerance tests revealed no differences between wild-type and TSP-1–deficient mice (Fig. 2G and H).
FIG. 2.
Functional characteristics in vivo of wild-type (Wt; ● or ■) and TSP-1 (−/−) (○ or □) mice. A: Blood glucose during 24 h. B: Area under the curve (AUC) for blood glucose measurements during 24 h. C: Intravenous glucose tolerance test. D: AUC for blood glucose concentrations during the intravenous glucose tolerance test. E and F: Serum insulin before (E) and 10 min after (F) glucose injection in the intravenous glucose tolerance test. G: Insulin tolerance test. H: Total AUC for blood glucose concentrations during the insulin tolerance test. Values are means ± SE for 5–12 experiments in each group. *P < 0.05 when compared with wild-type mice. Tot, total.
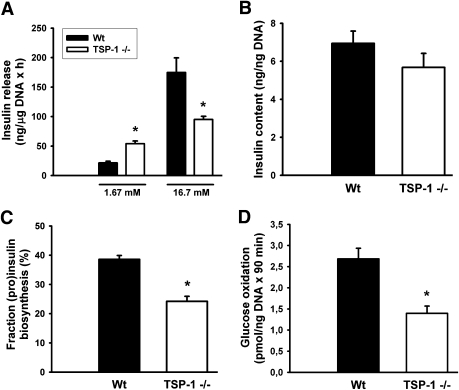
Islet function in vitro.
At low glucose concentrations (1.67 mmol/L), pancreatic islets derived from 10- to 12-week-old TSP-1–deficient mice secreted more insulin than controls (Fig. 3A). However, in response to an increased glucose concentration (16.7 mmol/L), there was an impaired insulin response. Potassium chloride (30 mmol/L) induced similar insulin release from wild-type and TSP-1–deficient islets (ratios 22.5 ± 4.9 [n = 4] vs. 22.4 ± 9.8 [n = 4] for wild-type and TSP-1–deficient islets, respectively, in high and low potassium concentrations). Islet insulin content was similar when comparing TSP-1–deficient islets and wild-type islets, and that held true also when compensating for islet DNA content (Fig. 3B). Glucose-stimulated (pro)insulin biosynthesis was impaired in the TSP-1–deficient islets (Fig. 3C), as was the rate of glucose oxidation at high glucose (Fig. 3D).
FIG. 3.
Functional characteristics in vitro of wild-type (Wt; ■) and TSP-1 (−/−) (□) islets. A: Glucose-stimulated insulin release. B: Islet insulin content. C: (Pro)insulin biosynthesis as percentage of total protein biosynthesis. D: Glucose oxidation. Values are means ± SE for six experiments in each group. *P < 0.05 when compared with wild-type islets.
Ultrastructural analysis and ROS generation.
No changes in the number or structure of mitochondria, or of the endoplasmic reticulum, could be observed by electron microscopy in β-cells of TSP-1–deficient islets when compared with wild-type islets (Supplementary Data 1). Likewise, there was no difference in ROS production between wild-type and TSP-1–deficient islets after exposure to 11.1 or 28 mmol/L glucose (Supplementary Data 2).
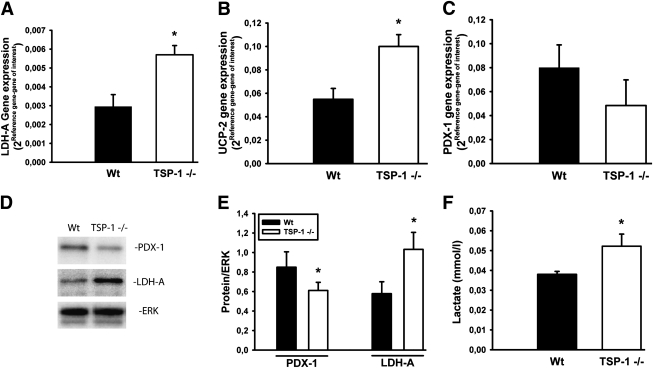
Islet mRNA and protein expression.
Islets derived from 10- to 12-week-old TSP-1–deficient mice had an increased mRNA expression of both lactate dehydrogenase (LDH)-A and uncoupling protein (UCP)-2 when compared with control islets (Fig. 4A and B). Moreover, they showed a tendency for decreased gene expression of pancreatic duodenal homeobox gene (PDX)-1 (P = 0.076) (Fig. 4C). In contrast, there were no differences in the gene expression of pyruvate carboxylase, glucokinase, mitochondrial glycerol-phosphate dehydrogenase-2, and insulin (data not shown). Immunoblotting confirmed increased protein levels of LDH-A and decreased levels of PDX-1, whereas lack of specific antibodies (cross-reactivity) prevented analysis of UCP-2 protein (Fig. 4D and E). Lactate concentrations were higher in the medium of incubated TSP-1–deficient islets than control islets (Fig. 4F).
FIG. 4.
mRNA and protein expression in wild-type (Wt; ■) and TSP-1 (−/−) (□) islets. mRNA expression of LDH-A (A), UCP-2 (B), and PDX-1 (C). D and E: Protein expression of LDH-A and PDX-1. F: Lactate concentration in incubation medium of 30 islets exposed to 95% air/5% CO2 for 2 h at 37°C. Values are means ± SE for 5–12 experiments in each group. *P < 0.05 when compared with wild-type islets.
Expression of TSP-1 in islets.
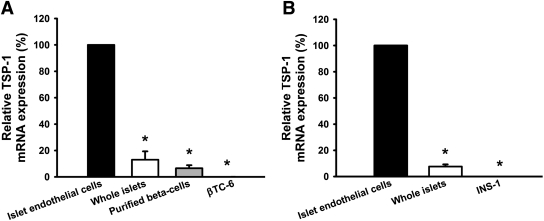
Islet endothelial cells of both 10- to 12-week-old mice (Fig. 5A) and rats (Fig. 5B) had more than 15 times higher expression of mRNA for TSP-1 than whole islets and purified β-cells. In β-cell lines derived from mouse (βTC-6) and rat (INS-1) no TSP-1 mRNA expression at all could be detected.
FIG. 5.
TSP-1 mRNA expression in mouse (A) and rat (B) islet cells or whole islets. Values are means ± SE for four to eight experiments in each group. *P < 0.05 when compared with islet endothelial cells.
Importance of endothelium-derived TSP-1.
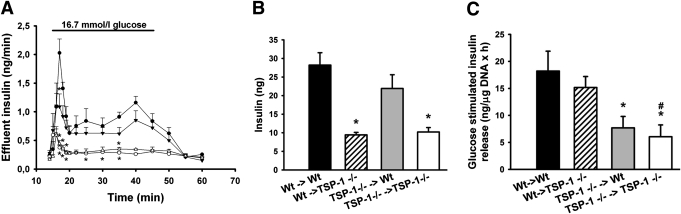
Nonencapsulated neonatal islets were investigated in perfusion experiments 1 month after syngeneic transplantation beneath the renal capsule of adult mice. When challenged with high glucose (16.7 mmol/L), wild-type islets transplanted into TSP-1–deficient mice secreted much less insulin than when implanted to wild-type mice (Fig. 6A and B). Moreover, TSP-1–deficient islets implanted into TSP-1–deficient mice secreted much lower amounts of insulin in response to glucose than if instead implanted to wild-type mice (Fig. 6A and B). In fact, TSP-1–deficient islets implanted to wild-type mice responded to glucose as rigorously as the wild-type islets implanted to the same hosts (Fig. 6A and B).
FIG. 6.
Functional studies 1 month posttransplantation of neonatal islets transplanted nonencapsulated to the renal subcapsular space or intraperitoneally after encapsulation. A: Glucose-stimulated insulin release of islet grafts composed of nonencapsulated islets; islets transplanted from wild-type (Wt) to wild-type animals (●), from wild-type to TSP-1 (−/−) animals (○), from TSP-1 (−/−) to wild-type animals (▼), and from TSP-1 (−/−) to TSP-1 (−/−) animals (△). B: Area under the curve for insulin secretion from islet grafts composed of nonencapsulated islets during high-glucose (16.7 mmol/L) conditions, islets transplanted from wild-type to wild-type animals (■), from wild-type to TSP-1 (−/−) animals (▨), from TSP-1 (−/−) to wild-type animals ( ), and from TSP-1 (−/−) to TSP-1 (−/−) animals (□). C: Glucose-stimulated (16.7 mmol/L) insulin release from intraperitoneally transplanted encapsulated islets. Values are means ± SE for four to seven experiments in each group. *P < 0.05 when compared with wild-type transplanted to wild-type; #P < 0.05 when compared with wild-type transplanted to TSP-1 (−/−).
), and from TSP-1 (−/−) to TSP-1 (−/−) animals (□). C: Glucose-stimulated (16.7 mmol/L) insulin release from intraperitoneally transplanted encapsulated islets. Values are means ± SE for four to seven experiments in each group. *P < 0.05 when compared with wild-type transplanted to wild-type; #P < 0.05 when compared with wild-type transplanted to TSP-1 (−/−).
Encapsulated neonatal wild-type islets were investigated in batch-type experiments after retrieval 1 month after syngeneic intraperitoneal transplantation into adult mice. When challenged with high glucose (16.7 mmol/L), wild-type islets secreted similar amounts of insulin when retrieved from TSP-1–deficient or wild-type hosts (Fig. 6C). In contrast, TSP-1–deficient islets responded poorly to a high glucose concentration irrespective of whether they were retrieved from the wild-type or TSP-1–deficient hosts (Fig. 6C).
Treatment with a TGFβ-1–activating protein.
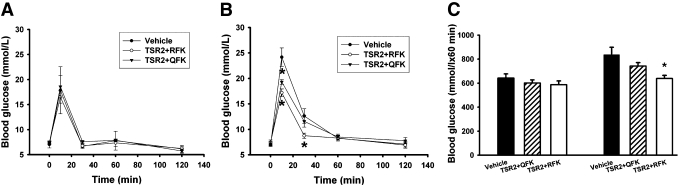
To explore the role of TSP-1 as an activator of TGFβ-1, wild-type and TSP-1–deficient mice were treated with recombinant proteins that contain the second type 1 repeat of TSP-1. The wild-type recombinant protein (TSR2+RFK) includes the sequence RFK, which is essential for activation of TGFβ-1 by TSP-1 (35). The control recombinant protein is identical except that the first arginine in the sequence is mutated to glutamine, rendering the protein incapable of activating TGFβ-1. In wild-type mice, there were no differences in response to a glucose load between animals treated for 3 weeks after birth with TSR2+RFK, TSR2+QFK, or vehicle only (Fig. 7A and C). In contrast, neonatal TSP-1–deficient mice treated for 3 weeks with the TGFβ-1–activating recombinant protein (TSR2+RFK) responded better in the glucose tolerance tests than corresponding mice treated with TSR2+QFK or vehicle only (Fig. 7B and C). Treatment with TSR2+RFK prevented the development of decreased glucose tolerance in TSP-1–deficient mice when compared with wild-type mice (Fig. 7C).
FIG. 7.
Glucose tolerance test of wild-type (A and C) and TSP-1–deficient (B and C) mice treated with vehicle (● or ■), control protein TSR2+QFK (▼ or ▨), or TGFβ-1–activating protein TSR2+RFK (△ or □) daily for 3 weeks. All values are expressed as means ± SE for four to nine experiments in each group. *P < 0.05 when compared with vehicle.
DISCUSSION
In the current study, we sought to examine the cell-specific expression and functional importance of the glycoprotein TSP-1 in pancreatic islets. TSP-1 is an extracellular matrix–bound factor and was the first naturally occurring inhibitor of angiogenesis to be reported (36). It regulates angiogenesis through binding to the CD36 receptor on endothelial cells by inhibition of matrix metalloproteinase-9 and by activation of TGFβ-1 (37). It was later shown to be involved in many other processes in the body, including the regulation of extracellular matrix function, blood clot formation, and immune responses (13,37–39).
When TSP-1–deficient mice were first characterized, they were found to have an almost normal morphological phenotype (13). An increased number of blood vessels were, however, discerned in the pancreatic islets of such mice, whereas blood vessel numbers in their exocrine tissue were unaffected (13). The islet blood vessels also seem to be fully functional, as assessed in the present article by islet oxygen tension measurements. Indeed, an additional increase in oxygen tension when compared with wild-type mice is impossible because of already achieved equilibrium with pO2 in venous blood (compared with [40]). It is interesting to note that the increased islet blood vessel density was associated with islet hyperplasia. The importance of TSP-1 for islet function has, however, not been investigated. In the current study, we observed a decreased glucose tolerance in TSP-1–deficient mice, despite their increased islet and β-cell mass. However, the impaired glucose tolerance did not seem to reflect increased insulin resistance because a decreased insulin response to a glucose load was observed both in vivo and in vitro. There did not seem to be a defect in β-cell ATP-sensitive K+ channel or postchannel activity because the islets responded normally to high potassium levels. However, a more detailed investigation of isolated TSP-1–deficient islets showed that they had an impaired capacity to oxidate glucose, which indicates a perturbed mitochondrial function, although ultrastructural analysis showed normal mitochondria. Other changes in mitochondrial function were indicated by the gene expression studies, in which TSP-1–deficient islets were observed to have an upregulation of UCP-2. UCP-2 may uncouple mitochondrial oxidative respiration by catalyzing a mitochondrial inner-membrane H+ leak that bypasses ATP synthase leading to decreased glucose-induced ATP production and a decreased ability of glucose to inhibit ATP-sensitive K+ channels (41). Because of the cross-reactivity of all tested antibodies, this could, however, not be confirmed at the protein level. Additional functional defects may be caused by the increased LDH-A activity in the TSP-1–deficient islets, which enhances catalyzation of pyruvate into lactate. Increased lactate production in TSP-1–deficient islets also was found. This yields less ATP production and insulin secretion when compared with the aerobic metabolism of pyruvate (42). Moreover, the upregulation of LDH-A in β-cells is likely to stimulate insulin secretion at low glucose concentrations (43), which also was observed in the TSP-1–deficient mice. Functional defects also may be caused by decreased protein levels of the β-cell differentiation marker PDX-1. It is interesting to note that despite normal insulin mRNA levels and insulin content, we found a decreased capacity for (pro)insulin biosynthesis in TSP-1–deficient islets. Several of the functional changes observed in TSP-1–deficient islets are similar to those described in islets of type 2 diabetic animal models (44). Glucose toxicity seems unlikely to explain the alterations because although the animals had a decreased glucose tolerance, their blood glucose levels during normal life for 24 h when fed ad libitum were similar to wild-type animals. Likewise, no increased endoplasmic reticulum stress or ROS activity in the β-cells of TSP-1–deficient mice seemed to occur.
We and others have previously observed the presence of TSP-1 in rodent and human islets at both the mRNA (11,30,45,46) and protein levels (30) and found it to be expressed at least by the endothelial cells (11,46). Immunohistochemical staining for the presence of TSP-1 has indicated a diffuse staining of the islets, likely a result of sequestration of the glycoprotein to the islet matrix (11). To determine the cellular origin of TSP-1 in islets, we analyzed mRNA levels in islet endothelial cells, intact whole islets, purified β-cells, and β-cell–derived cell lines. Of interest, in both rats and mice, TSP-1 mRNA levels were high in the islet endothelial cells, whereas TSP-1 expression in intact islets and β-cells purified to ~95% was much lower. In the mouse and rat β-cell–derived cell lines, βTC-6 and INS-1, the presence of mRNA for TSP-1 was undetectable. Although our gene expression analyses indicated that the majority, if not all, of the TSP-1 in pancreatic islets is of endothelial origin, it cannot be excluded that the TSP-1 that is important for β-cell function is derived from other islet cell types. For this purpose, we designed an experiment in which neonatal pancreatic islets from wild-type and TSP-1–deficient mice were isolated, cultured, and then transplanted into either adult wild-type or TSP-1–deficient mice. Islets cultured prior to transplantation have been previously shown to derive most, if not all, of their new vascular system from the recipient (19,31) (i.e., TSP-1–deficient islets implanted to wild-type mice acquire mainly TSP-1–positive blood vessels and wild-type islets implanted to TSP-1–deficient mice acquire TSP-1–negative blood vessels). Neonatal islets were used for transplantation because morphological changes with increased islet mass and vascular density in TSP-1–deficient mice were previously shown to be prevented by treatment with the TGFβ-1–activating sequence of TSP-1 from birth (13). Of interest, TSP-1–deficient islets implanted to wild-type mice had normal function when evaluated 1 month posttransplantation. In contrast, wild-type islets implanted to TSP-1–deficient mice displayed similar dysfunction as TSP-1–deficient islets implanted to TSP-1–deficient mice. These data suggested that it was indeed TSP-1 in blood vessels that was of importance for β-cell function; however, the results could be a result of a possible negative systemic milieu in TSP-1–deficient mice. We therefore conducted experiments in which the neonatal islets were microencapsulated before transplantation. This procedure prevents the ingrowth of recipient blood vessels, and, instead, donor blood vessels remain (Supplementary Data 3) (47). As a result, encapsulated TSP-1–deficient islets will be deficient of TSP-1 after transplantation into wild-type mice and only contain donor TSP-1–negative endothelial cells. In these experiments, we observed that TSP-1–deficient islets implanted to wild-type mice were dysfunctional 1 month posttransplantation, whereas wild-type islets implanted to TSP-1–deficient mice functioned as well as wild-type islets implanted to wild-type mice. These experiments were therefore consistent with our hypothesis that TSP-1 derived from endothelial cells is important for β-cell function, whereas the systemic milieu of TSP-1–deficient mice did not, per se, seem to be negative for β-cell function.
To investigate whether the islet dysfunction and decreased glucose tolerance in the TSP-1–deficient animals were a result of decreased TGFβ-1 activation or whether they reflected other mechanism(s), we treated neonatal TSP-1–deficient mice with the TGFβ-1–activating sequence of TSP-1 for 3 weeks, followed by glucose tolerance tests. Treatment with the recombinant protein TSR2+RFK normalized the response of TSP-1–deficient mice to glucose, whereas no effects at all of the treatment were observed in wild-type mice. At least part of the effects mediated by endothelial TSP-1 on glucose homeostasis therefore seems to be caused by TGFβ-1 activation.
In pancreatic islets transplanted to the liver or beneath the renal capsule (17,19), there is a markedly decreased vascular density, which opens the possibility that some of the islet functional defects and gene expression changes that occurs after transplantation (48–50) may reflect impaired endothelial cell influence. Indeed, we recently showed better revascularization of pancreatic islets partially or totally deficient of TSP-1 (30). When TSP-1 expression was only transiently decreased by small interfering RNA during the initial 1- to 2-week revascularization phase, vascular engraftment was improved and associated with increased islet graft function when evaluated 1 month posttransplantation. There also seems to be a deterioration of the islet vasculature in long-standing experimental type 2 diabetes (45). Nevertheless, identification of the molecular signals required for adequate β-cell function may facilitate our quest for a better understanding of the pathogenesis of diabetes. The current study identifies a novel role for islet endothelium as a functional support for the surrounding parenchymal cells, the β-cells, by local production of the glycoprotein TSP-1.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Research Council (55X-15043), the Juvenile Diabetes Research Foundation, the European Foundation for the Study of Diabetes/Novo, the Novo Nordisk Foundation, the Wallenberg Foundation, the Swedish Diabetes Association, StemTherapy, AFA, the Swedish Juvenile Diabetes Fund, the Anér Foundation, the Åke Wiberg Foundation, and the Family Ernfors Fund. No other potential conflicts of interest relevant to this article were reported.
J.O. researched data, contributed to discussion, and wrote the manuscript. D.M. researched data. M.J. contributed to discussion. G.C. researched data. J.L. researched data and contributed to discussion. N.W. contributed to discussion. P.-O.C. researched data, contributed to discussion, and wrote the manuscript. All authors reviewed and edited the manuscript.
The skilled technical assistance of Astrid Nordin, Birgitta Bodin, and Eva Törnelius (all from the Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden) is gratefully acknowledged. The authors are also grateful to Dr. Sara Bohman (Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden) for valuable instructions on islet microencapsulation.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0277/-/DC1.
REFERENCES
- 1.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001;294:559–563 [DOI] [PubMed] [Google Scholar]
- 2.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med 2003;9:661–668 [DOI] [PubMed] [Google Scholar]
- 3.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567 [DOI] [PubMed] [Google Scholar]
- 4.LeCouter J, Moritz DR, Li B, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 2003;299:890–893 [DOI] [PubMed] [Google Scholar]
- 5.Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor–A is essential for islet vascularization, revascularization, and function. Diabetes 2006;55:2974–2985 [DOI] [PubMed] [Google Scholar]
- 6.Johansson A, Lau J, Sandberg M, Borg LA, Magnusson PU, Carlsson PO. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia 2009;52:2385–2394 [DOI] [PubMed] [Google Scholar]
- 7.Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and beta cell proliferation. Dev Cell 2006;10:397–405 [DOI] [PubMed] [Google Scholar]
- 8.Lammert E, Gu G, McLaughlin M, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 2003;13:1070–1074 [DOI] [PubMed] [Google Scholar]
- 9.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 2006;290:H560–H576 [DOI] [PubMed] [Google Scholar]
- 10.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 1982;31:883–889 [DOI] [PubMed] [Google Scholar]
- 11.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 2006;147:2315–2324 [DOI] [PubMed] [Google Scholar]
- 12.Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 2000;6:41–48 [DOI] [PubMed] [Google Scholar]
- 13.Crawford SE, Stellmach V, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 1998;93:1159–1170 [DOI] [PubMed] [Google Scholar]
- 14.Sjöholm A, Hellerström C. TGF-beta stimulates insulin secretion and blocks mitogenic response of pancreatic beta-cells to glucose. Am J Physiol 1991;260:C1046–C1051 [DOI] [PubMed] [Google Scholar]
- 15.Smart NG, Apelqvist AA, Gu X, et al. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol 2006;4:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawler J, Sunday M, Thibert V, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 1998;101:982–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 2002;51:1362–1366 [DOI] [PubMed] [Google Scholar]
- 18.Weibel E. Practical methods for biological morphometry. In Stereological Methods. Weibel E, Ed. London, Academic Press, 1979, p. 15 [Google Scholar]
- 19.Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab 2002;87:5418–5423 [DOI] [PubMed] [Google Scholar]
- 20.Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves β-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes 2006;55:367–374 [DOI] [PubMed] [Google Scholar]
- 21.Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 1978;14:397–404 [DOI] [PubMed] [Google Scholar]
- 22.Borg LA, Schnell AH. Lysosomes and pancreatic islet function: intracellular insulin degradation and lysosomal transformations. Diabetes Res 1986;3:277–285 [PubMed] [Google Scholar]
- 23.Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol 2002;190:75–82 [DOI] [PubMed] [Google Scholar]
- 24.Mokhtari D, Myers JW, Welsh N. The MAPK kinase kinase-1 is essential for stress-induced pancreatic islet cell death. Endocrinology 2008;149:3046–3053 [DOI] [PubMed] [Google Scholar]
- 25.Carlsson PO, Nordin A, Palm F. pH is decreased in transplanted rat pancreatic islets. Am J Physiol Endocrinol Metab 2003;284:E499–E504 [DOI] [PubMed] [Google Scholar]
- 26.Mattsson G, Danielsson A, Kriz V, Carlsson PO, Jansson L. Endothelial cells in endogenous and transplanted pancreatic islets: differences in the expression of angiogenic peptides and receptors. Pancreatology 2006;6:86–95 [DOI] [PubMed] [Google Scholar]
- 27.Jackson CJ, Garbett PK, Nissen B, Schrieber L. Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J Cell Sci 1990;96:257–262 [DOI] [PubMed] [Google Scholar]
- 28.Van de Winkle M, Maes E, Pipeleers D. Islet cell analysis and purification by light scatter and autofluorescence. Biochem Biophys Res Commun 1982;107:525–532 [DOI] [PubMed] [Google Scholar]
- 29.Korsgren O, Jansson L, Andersson A. Effects of hyperglycemia on function of isolated mouse pancreatic islets transplanted under kidney capsule. Diabetes 1989;38:510–515 [DOI] [PubMed] [Google Scholar]
- 30.Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes 2008;57:1870–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 2005;54:2287–2293 [DOI] [PubMed] [Google Scholar]
- 32.Bohman S, Andersson A, King A. No differences in efficacy between noncultured and cultured islets in reducing hyperglycemia in a nonvascularized islet graft model. Diabetes Technol Ther 2006;8:536–545 [DOI] [PubMed] [Google Scholar]
- 33.King A, Sandler S, Andersson A, Hellerström C, Kulseng B, Skjåk-Braek G. Glucose metabolism in vitro of cultured and transplanted mouse pancreatic islets microencapsulated by means of a high-voltage electrostatic field. Diabetes Care 1999;22(Suppl. 2):B121–B126 [PubMed] [Google Scholar]
- 34.Miao WM, Seng WL, Duquette M, Lawler P, Laus C, Lawler J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and -independent mechanisms. Cancer Res 2001;61:7830–7839 [PubMed] [Google Scholar]
- 35.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59–69 [DOI] [PubMed] [Google Scholar]
- 36.Good DJ, Polverini PJ, Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 1990;87:6624–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol 2004;36:961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today 1993;14:131–136 [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 1999;274:13586–13593 [DOI] [PubMed] [Google Scholar]
- 40.Carlsson PO, Jansson L, Palm F. Unaltered oxygen tension in rat pancreatic islets despite dissociation of insulin release and islet blood flow. Acta Physiol Scand 2002;176:275–281 [DOI] [PubMed] [Google Scholar]
- 41.Chan CB, De Leo D, Joseph JW, et al. Increased uncoupling protein-2 levels in β-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes 2001;50:1302–1310 [DOI] [PubMed] [Google Scholar]
- 42.Sekine N, Cirulli V, Regazzi R, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem 1994;269:4895–4902 [PubMed] [Google Scholar]
- 43.Ishihara H, Wang H, Drewes LR, Wollheim CB. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J Clin Invest 1999;104:1621–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laybutt DR, Glandt M, Xu G, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time: adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem 2003;278:2997–3005 [DOI] [PubMed] [Google Scholar]
- 45.Li X, Zhang L, Meshinchi S, et al. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes 2006;55:2965–2973 [DOI] [PubMed] [Google Scholar]
- 46.Cantaluppi V, Biancone L, Romanazzi GM, et al. Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant 2006;6:2601–2611 [DOI] [PubMed] [Google Scholar]
- 47.Bohman S. Microencapsulation of pancreatic islets: a non-vascularised transplantation model. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 2008;396:1–39 [Google Scholar]
- 48.Mattsson G, Jansson L, Nordin A, Andersson A, Carlsson PO. Evidence of functional impairment of syngeneically transplanted mouse pancreatic islets retrieved from the liver. Diabetes 2004;53:948–954 [DOI] [PubMed] [Google Scholar]
- 49.Lau J, Mattsson G, Carlsson C, et al. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes 2007;56:1544–1550 [DOI] [PubMed] [Google Scholar]
- 50.Ahn YB, Xu G, Marselli L, et al. Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia 2007;50:334–342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.