Abstract
OBJECTIVE
Adipocyte infiltration of the musculoskeletal system is well recognized as a hallmark of aging, obesity, and type 2 diabetes. Intermuscular adipocytes might serve as a benign storage site for surplus lipid or play a role in disrupting energy homeostasis as a result of dysregulated lipolysis or secretion of proinflammatory cytokines. This investigation sought to understand the net impact of local adipocytes on skeletal myocyte metabolism.
RESEARCH DESIGN AND METHODS
Interactions between these two tissues were modeled using a coculture system composed of primary human adipocytes and human skeletal myotubes derived from lean or obese donors. Metabolic analysis of myocytes was performed after coculture with lipolytically silent or activated adipocytes and included transcript and metabolite profiling along with assessment of substrate selection and insulin action.
RESULTS
Cocultured adipocytes increased myotube mRNA expression of genes involved in oxidative metabolism, regardless of the donor and degree of lipolytic activity. Adipocytes in the basal state sequestered free fatty acids, thereby forcing neighboring myotubes to rely more heavily on glucose fuel. Under this condition, insulin action was enhanced in myotubes from lean but not obese donors. In contrast, when exposed to lipolytically active adipocytes, cocultured myotubes shifted substrate use in favor of fatty acids, which was accompanied by intracellular accumulation of triacylglycerol and even-chain acylcarnitines, decreased glucose oxidation, and modest attenuation of insulin signaling.
CONCLUSIONS
The effects of cocultured adipocytes on myocyte substrate selection and insulin action depended on the metabolic state of the system. These findings are relevant to understanding the metabolic consequences of intermuscular adipogenesis.
Excess body weight promotes insulin resistance, systemic dyslipidemia, and elevated circulating levels of proinflammatory cytokines, all hallmarks of the metabolic syndrome (1,2). Skeletal muscle is a major tissue responsible for insulin-stimulated glucose disposal and a principal target of the foregoing disorders (3–6). These findings have fueled intense interest in the metabolic interplay between adipocytes and skeletal myocytes. On the one hand, adipose tissue protects other cell types from “lipotoxicity” by providing a safe haven for surplus energy. On the other hand, obesity-induced dysregulation of adipocyte lipolysis promotes lipid oversupply to nonadipocytes (7). Moreover, adipose tissue is now well recognized as an endocrine organ that informs the brain and peripheral tissues of changes in whole-body energy status through a network of circulating “adipokines.” These include peptide hormones, such as leptin, adiponectin and resistin, as well as cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (8). Obesity lowers circulating levels of insulin-sensitizing adipokines such as adiponectin while increasing proinflammatory molecules, such as IL-6 and TNF-α (9). In addition to modulating insulin action, these and other adipokines have been shown to directly regulate lipid metabolism in tissues such as skeletal muscle, heart, liver, and pancreas (10–12).
Current understanding of adipocyte–myocyte cross-talk has been shaped in large part by studies examining the metabolic effects of individual adipokine factors on cultured myocytes or isolated muscle strips. By contrast, the goal of this work was to model the complex set of adipocyte-derived signals that regulate skeletal muscle metabolism without confounding effects of other organ systems. To this end, we used an in vitro coculture system wherein myocytes were exposed to a physiologic mixture of free fatty acids and adipokines released by neighboring adipocytes. We examined the net impact of adipocytes on transcriptional programming, fuel selection, and insulin action in cocultured myotubes derived from lean compared with obese donors. Because distinct adipokine factors can either enhance or oppose muscle insulin action, we hypothesized that the interactions between cell types might depend on the metabolic state of the system. In general, our results supported this hypothesis because we found that lipolytically active adipocytes antagonized myocyte glucose utilization and insulin signaling, whereas adipocytes in the basal state had the opposite effect. These findings highlight the potential utility of this model for investigating mechanisms of metabolic dysregulation or identifying suitable strategies for intervention.
RESEARCH DESIGN AND METHODS
Materials.
Sodium oleate, palmitic acid, 3-isobutyl-1-methylxanthine (IBMX), cytochalasin B, and l-carnitine were from Sigma-Aldrich (St. Louis, MO). BSA (fraction V 7.5% cell culture grade) was from Invitrogen (Carlsbad, CA). Nonesterified fatty acid and glycerol were measured using kits from Wako (Richmond, VA) and Sigma-Aldrich, respectively. Adipokines were measured using ELISA kits from Meso Scale Discovery (Gaithersburg, MD). d-[U-14C]Glucose was from Amersham Biosciences (Piscataway, NJ), and [1-14C]oleic acid and [3H]2-d-deoxyglucose were from PerkinElmer Life and Analytical Sciences (Boston, MA).
Cell culture.
Cryopreserved primary human subcutaneous preadipocytes obtained from Zen-Bio (Research Triangle Park, NC) were maintained and differentiated according to the supplier’s specifications. Cells were derived from pooled lots of six female nondiabetic donors 43.3 ± 9.9 years with an average BMI of 27.6 ± 1.1. These cells are functionally similar to noncommercial primary adipocytes (13). Human skeletal myoblasts were isolated from lean or severely obese Caucasian women as described previously (14,15). Myoblasts from four to five subjects with similar demographics were pooled to establish a lean and an obese lot that were used for all experiments. Lean subjects were aged 20.5 ± 1.3 years with a BMI of 22.5 ± 1.9 kg/m2, whereas the obese subjects were aged 29.8 ± 8.1 years with a BMI of 44.5 ± 1.9 kg/m2. Fasting insulin (133.3 ± 18.8 vs. 47.2 ± 4.0 pmol/L) and glucose (5.5 ± 0.4 vs. 4.8 ± 0.1 mmol/L) were higher (P < 0.001) in severely obese subjects compared with lean subjects. Exclusion criteria included hypertension, vascular disease, type 2 diabetes, and medications that could interfere with lipid metabolism. The study was approved by the institutional review board at East Carolina University, and informed and written consent was obtained from all subjects before participation.
Coculture.
Human skeletal myoblasts were plated on 12-well tissue culture plates coated with collagen I and maintained in growth media until reaching 80% confluence. Differentiation was induced by changing to a low-serum differentiation media (DFM). Coculture experiments were initiated on day 6 of differentiation. Primary human subcutaneous preadipocytes were seeded and differentiated on inserts from BD Biosciences (San Jose, CA) with a polyethylene terephthalate growth surface containing pores of 3 μmol/L diameter, allowing passage of glucose, free fatty acids, albumin, and a number of cytokines (data not shown). Inserts bearing mature adipocytes were washed and transferred to 12-well plates containing human myotubes and DFM, without affecting adipocyte viability (data not shown). Cocultures were maintained for 24 or 96 h in DFM supplemented with 0.5% BSA and 2 mmol/L l-carnitine ± 100 μmol/L IBMX to stimulate lipolysis. Control myotubes were maintained under identical conditions but without adipocytes. Thus, IBMX exposure was matched in the no-adipocyte control compared with the cocultured myotubes.
Glucose and fatty acid metabolism.
Glucose oxidation and glycogen synthesis rates were determined as described previously (16). Insulin stimulation of glycogen synthesis was assessed after 2 h of serum starvation followed by incubation with α-minimal essential medium (α-MEM) containing d-[UL-14C]glucose ± 100 nmol/L insulin for an additional 2 h. [3H]2-d-Deoxyglucose uptake was measured after 3 h of serum starvation ± 200 nmol/L insulin. Fatty acid oxidation assays were performed with 0.10 mmol/L [1-14C]oleate as described previously (17).
Acylcarnitine profiling and lipid analyses.
Human skeletal myotubes were washed with PBS, placed in liquid nitrogen to flash-freeze the cells, and then sonicated at 2.5 W for 5 s in 250 μL deionized water (18). Acylcarnitines were measured by direct-injection electrospray tandem mass spectrometry, using a Micromass Quattro Micro system equipped with a model 2777 autosampler, a model 1525 high-pressure liquid chromatography solvent delivery system, and a data system running 4.0 MassLynx software (Waters Corporation, Milford, MA). Triacylglycerol and diacylglycerol were extracted in methanol–chloroform and separated by thin-layer chromatography; analysis of esterified fatty acids was performed by gas chromatography methodologies as described previously (19). Ceramides were extracted as described previously (20) and analyzed by flow injection tandem mass spectrometry using a Micromass Quattro spectrometer (Waters Corporation) for precursors of m/z 264.
Real-time PCR.
RNA was extracted from myotubes using RNeasy (Qiagen, Valencia, CA) quantified by RiboGreen (Molecular Probes, Eugene, OR), and cDNA was synthesized from 1 μg RNA using the IScriptcDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Gene expression was determined by real-time quantitative PCR using a Prism 7000 with TaqMan chemistry (Applied Biosystems, Foster City, CA).
Insulin signaling.
After removing adipocytes, myotubes were washed, serum-starved for 5 h, and then incubated ±10 nmol/L insulin for 10 min at 37°C in a humidified atmosphere of 5% CO2 before collecting cell lysates. After SDS-PAGE (Bio-Rad Laboratories) and protein transfer to polyvinylidene difluoride membranes, blots were probed overnight with either anti-phospho-Akt Ser473 or anti-phospho–glycogen synthase kinase-3β (GSK-3β) Ser9 antibody from Cell Signaling Technology (Danvers, MA) followed by incubation with horseradish peroxidase–conjugated Ig-G antibodies and ECL Plus chemiluminescence detection kit. Visualized bands were quantified using ImageQuant software (Amersham Biosciences) and normalized to total protein determined by MemCode Reversible Protein Stain (Thermo Fisher Scientific, Rockford, IL), total Akt, tubulin, and GSK-3β, which were probed after stripping.
RESULTS
In vitro modeling of the adipogenic phenotype of insulin-resistant skeletal muscle.
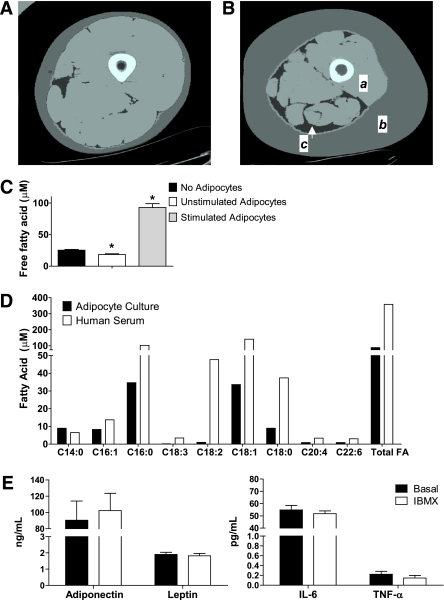
Computed tomography was used to illustrate (Fig. 1A and B) the effect of obesity on the physical relationship between adipose tissue and skeletal muscle of the lower limb (21). Obesity-related expansion of adipose tissue mass was evident in the subcutaneous and intermuscular compartments. This pronounced adipocyte infiltration within and surrounding the thigh muscle underscores the importance of understanding the interplay between adipocyte proliferation and skeletal muscle function. In an attempt to model this pathophysiologic phenomenon in vitro, we developed a coculture system using primary human myotubes and primary human adipocytes. Under basal conditions, the adipocytes decreased free fatty acid levels in the culture medium from a baseline of 25 μmol/L in the myocyte-only control to 18 μmol/L after 24 h of coculture (P < 0.05) (Fig. 1C), presumably reflecting uptake and sequestration of fat into the adipocyte triacylglycerol (TAG) pool. To drive release of free fatty acids from the adipocytes into the coculture medium, we added IBMX as a lipolytic stimulus (Fig. 1C). Under stimulated conditions, the fatty acid concentration of the coculture medium increased approximately fourfold to 93 μmol/L (P < 0.05). Determination of individual free fatty acid species by gas chromatography–mass spectrometry (Fig. 1D) showed that palmitate (c16:0) and oleate (c18:1) were the predominant long-chain fatty acids released by the adipocytes, whereas myristate (c14:0), palmitoleate (c16:1), and stearate (c18:0) were present at lower levels. This fatty acid profile resembled that found in human sera, with the exception of the essential fatty acids, linoleic acid (18:2), and α-linolenic acid (18:3). The human adipocytes also released measurable quantities of prominent adipokines, such as leptin, adiponectin, IL-6, and TNF-α (Fig. 1E), although the levels detected in the culture system were lower than those measured previously in human sera (22). Adipocyte secretion of these adipokines was not altered by IBMX.
FIG. 1.
Adipocyte-myocyte coculture model. Computed tomography images of the thigh comparing (A) a young healthy female subject with (B) an older, obese female subject. a: Thigh muscle; b: subcutaneous adipose tissue; c: intermuscular adipose tissue. C: Free fatty acid concentration of the culture medium after 24-h exposure to basal (unstimulated) or stimulated (+100 μM IBMX) human adipocytes compared with no adipocytes. D: Individual free fatty acids in culture medium exposed to stimulated adipocytes for 24 h compared with no adipocytes. E: Adipokine concentrations in the culture medium after 24-h exposure to basal (unstimulated) or stimulated (+100 μM IBMX) human adipocytes. Results are expressed as mean ± SEM and representative of at least two experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test. FA, fatty acid. (A high-quality color representation of this figure is available in the online issue.)
Effect of cocultured adipocytes on myocyte glucose disposal.
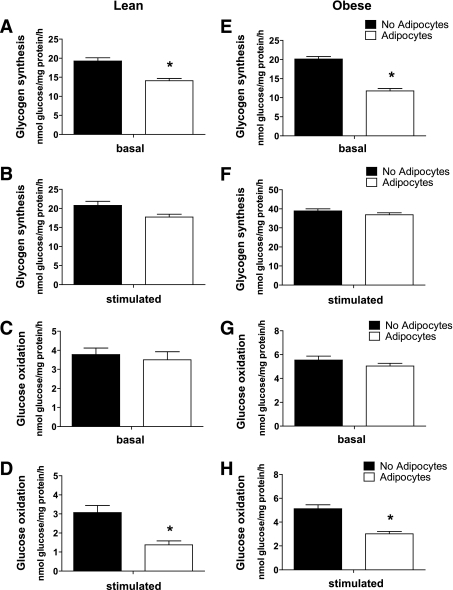
Fully differentiated myotubes were cocultured with differentiated adipocytes for 24 h in the absence or presence of IMBX. To examine myotube adaptation to coculture, the system was then disassembled and metabolic assays were performed in the absence of adipocytes, using fresh culture medium. In the basal (nonstimulated) state, the presence of adipocytes lowered rates of glycogen synthesis (26 and 40%) in myotubes from lean and obese donors, respectively (Fig. 2A and E), but did not affect glucose oxidation (Fig. 2C and G). By contrast, exposure to IBMX-stimulated (lipolytically active) adipocytes decreased glucose oxidation to 53 and 41% in myotubes from lean and obese donors, respectively (Fig. 2D and H); however, glycogen synthesis was maintained at rates similar to those of the control group (Fig. 2B and F).
FIG. 2.
Adipocyte exposure alters glucose metabolism in primary human myotubes. Primary human skeletal myotubes from lean (A–D) and severely obese (E–H) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed, and myotubes were washed and incubated 2 h in α-MEM containing 2 μCi/mL d-[U-14C]glucose. Rates of glycogen synthesis and glucose oxidation were determined by measuring label incorporation into glycogen (A, B, E, and F) and CO2 (C, D, G, and H). Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
Effect of cocultured adipocytes on myocyte fat oxidation.
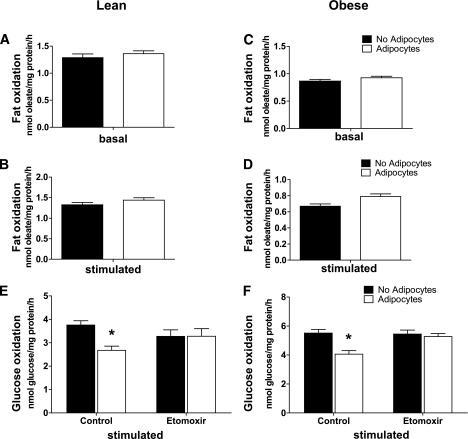
We questioned whether the adipocyte-mediated decline in glucose utilization might be secondary to a reciprocal increase in fat oxidation. As in our previous reports (15,17), rates of fatty acid oxidation were lower in myotubes derived from obese compared with lean donors (Fig. 3A and C); however, 24-h exposure to adipocytes did not affect oxidation rates of exogenously supplied [14C]oleate, regardless of the lipolytic state of the coculture system (Fig. 3A–D). Because glucose metabolism (Fig. 2) was assessed in the absence of exogenously supplied fatty acids, we next sought to determine whether intracellular lipids might have contributed to a shift in substrate selection. To test this possibility, we examined myotube glucose metabolism in the presence of etomoxir, a potent pharmacologic inhibitor of β-oxidation. Indeed, addition of etomoxir during a 2-h incubation with [14C]glucose increased production of radiolabeled CO2 by myocytes that had undergone coculture to rates observed in the control cells (Fig. 3E and F). These results strongly imply that prior exposure of myotubes to lipolytic adipocytes increased accumulation, turnover, and catabolism of intracellular lipids, thereby reducing glucose oxidation.
FIG. 3.
Effects of adipocyte exposure on fatty acid metabolism in primary human myotubes. Primary human skeletal myotubes from lean (A, B, and E) and severely obese (C, D, and F) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed, and myotubes were washed and incubated 2 h with α-MEM containing 100 μmol/L [14C]oleate (A–D) or 5 mmol/L [14C]glucose (E and F). Label incorporation into CO2 was measured to assess oxidation rates of fatty acid and glucose, respectively. The addition of 100 μmol/L etomoxir during the glucose oxidation assay (E and F) was used to block oxidation of intracellular lipids. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
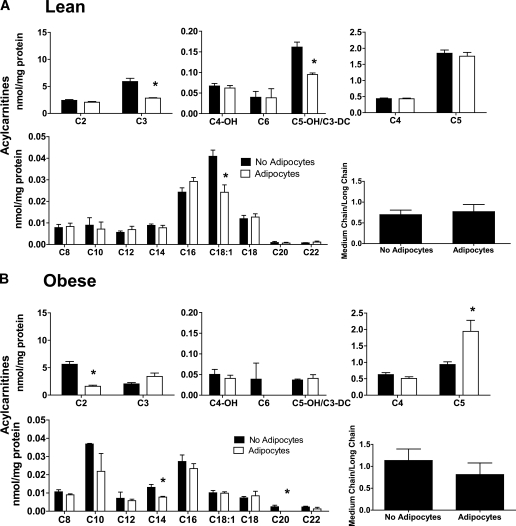
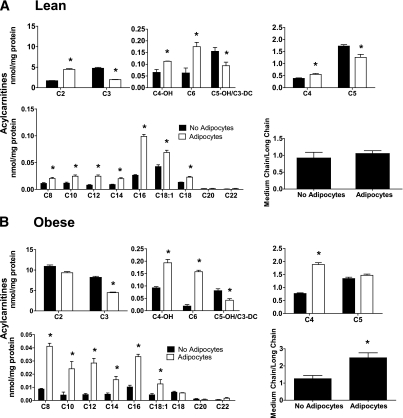
A more comprehensive assessment of mitochondrial metabolism was performed using mass spectrometry–based acylcarnitine profiling. Information gained from this assay can be used to infer changes in metabolic flux and substrate selection without bias as to exogenous versus intracellular sources. Exposure to basal-state adipocytes for 24 h decreased a small subset of acylcarnitine species (Fig. 4A and B), including oleyl carnitine (C18:1) and propionyl-carnitine (C3) in the lean group and acetylcarnitine (C2) in the obese group, fitting with the fatty acid–lowering effect of the adipocytes (Fig. 1C). In the obese group, adipocytes increased myotube levels of isovaleryl-carnitine (C5), a metabolite that derives from leucine catabolism. By contrast, exposure of myotubes to stimulated adipocytes resulted in cellular accumulation of most even-chain acylcarnitines, whereas several odd-chain (amino acid-derived) species declined (Fig. 5A and B). This pattern is consistent with a substrate shift toward increased β-oxidation and reduced catabolism of both glucose (Fig. 2) and amino acids. The acylcarnitine profiles were similar between myotubes from lean and obese donors (Fig. 5A and B); however, the latter cohort displayed marked accumulation of acetylcarnitine (C2), regardless of the treatment. In addition, myotubes from obese donors had a more pronounced increase in medium-chain acylcarnitine levels in response to adipocyte exposure, such that the ratio of total medium- to long-chain acylcarnitines increased from 1.2 in the no adipocyte control group to 2.4 after coculture (Fig. 5B). This profile is consistent with a potential flux limitation at medium-chain acyl-CoA dehydrogenase (23).
FIG. 4.
Adipocyte exposure alters acylcarnitine accumulation in primary human myotubes. Primary human skeletal myotubes from lean (A) and severely obese (B) women were maintained 24 h in the absence or presence of human adipocytes under basal conditions. After 24 h of coculture, adipocytes were removed, myotubes were washed, and cell lysates were prepared for acylcarnitine profiling by mass spectrometry. C3DC and C5OH are isobaric species. The ratio of total medium-chain relative to total long-chain acylcarnitines was calculated to assess potential flux limitations at the medium-chain acyl-CoA dehydrogenase step of β-oxidation. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. Abbreviations reflect carbon chain length (e.g., C2, acetylcarnitine). DC, dicarboxylic acid; OH, hydroxylated species. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
FIG. 5.
Effect of stimulated adipocyte exposure on acylcarnitine accumulation in primary human myotubes. Primary human skeletal myotubes from lean (A) and severely obese (B) women were maintained 24 h in the absence or presence of human adipocytes under stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed, myotubes were washed, and cell lysates were prepared for acylcarnitine profiling by mass spectrometry. C3DC and C5OH are isobaric species. The ratio of total medium-chain relative to total long-chain acylcarnitines was calculated to assess potential flux limitations at the medium-chain acyl-CoA dehydrogenase step of β-oxidation. Results are expressed as mean ± SEM and are representative of at least three experiments performed in triplicate. Abbreviations reflect carbon chain length (e.g., C2, acetylcarnitine). DC, dicarboxylic acid; OH, hydroxylated species. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
Effect of cocultured adipocytes on myocyte gene expression.
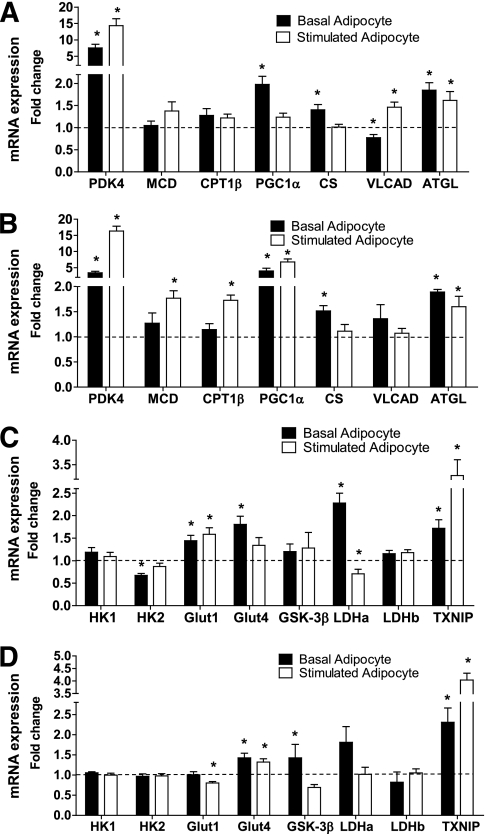
In previous studies, we found that exposure of cultured myotubes to high concentrations of fatty acid increases expression of genes involved in β-oxidation (24). In the current study, we found a similar effect of adipocyte exposure. Thus, the coculture condition induced myotube mRNA expression of several genes that promote fatty acid catabolism (Fig. 6A and B), including pyruvate dehydrogenase kinase 4 (PDK4), peroxisome proliferator–activated receptor γ coactivator 1-α (PGC1-α), very long-chain acyl-CoA dehydrogenase, and adipocyte triglyceride lipase (ATGL). This effect was most pronounced when lipolysis was stimulated. However, it is noteworthy that several genes, including PDK4, PGC1-α, and ATGL, were upregulated even in the basal condition, suggesting that adipocyte-derived factors in addition to free fatty acids contribute to transcriptional remodeling of the myotubes. Genes involved in glucose metabolism were also affected by adipocyte coculture (Fig. 6C and D). The coculture condition increased myotube mRNA expression of the glucose transporters 1 and 4, as well as lactate dehydrogenase α, but lowered mRNA expression of the glycolytic enzyme hexokinase 2. These changes were most pronounced when myotubes from lean subjects were exposed to basal adipocytes. It is also notable that lipolytically stimulated adipocytes caused a three- to fourfold increase in myotube expression of thioredoxin interacting protein, a redox-regulatory protein that has been implicated as a negative regulator of glucose homeostasis (25). In general, the transcriptional responses were similar between the lean and obese groups, with a few subtle distinctions. For example, adipocyte-mediated upregulation of malonyl-CoA decarboxylase, carnitine palmitoyltransferase 1β (CPT1β), PGC1-α, and thioredoxin-interacting protein was more robust in the obese cohort, whereas the effects on the glucose transporters were more pronounced in the lean group.
FIG. 6.
Adipocyte exposure alters myotube mRNA expression. Primary human skeletal myotubes from lean (A and C) and severely obese (B and D) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed and myotubes were washed and harvested for RNA extraction. Myotube expression of candidate mRNAs involved in fatty acid oxidation (A and B) or glucose metabolism (C and D) was measured by real-time quantitative PCR and normalized to mRNA levels of 18S. Data are expressed as mean ± SEM of the fold change in mRNA abundance relative to the control cells that were not exposed to adipocytes (dashed line). Results are representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test. CS, citrate synthase; Glut1, glucose transporter 1; Glut4, glucose transporter 4; HK1, hexokinase 1; HK2, hexokinase 2; LDHa, lactate dehydrogenase α; LDHb, lactate dehydrogenase β; MCD, malonyl-CoA decarboxylase; VLAD, very long-chain acyl-CoA dehydrogenase; TXNIP, thioredoxin-interacting protein.
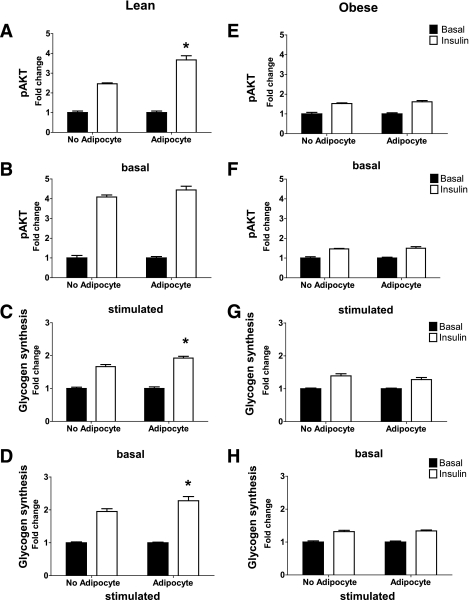
Effect of cocultured adipocytes on myocyte insulin action.
In myotubes from lean donors, 24-h exposure to basal adipocytes enhanced insulin-stimulated phosphorylation of Akt at Ser473 and rates of glycogen synthesis by 1.5- and 1.2-fold, respectively (P < 0.05) (Fig. 7A and C). In the no-adipocyte control group, pretreatment with IBMX amplified insulin-mediated activation of Akt by 66% compared with the no IBMX condition. Likewise, the addition of IBMX resulted in a 17% enhancement of insulin-stimulated glycogen synthesis. In the stimulated condition, the presence of adipocytes did not affect pAkt but did result in a modest 17% enhancement of insulin-stimulated glycogen synthesis (Fig. 7B and D), possibly because of glucose repartitioning from oxidation to storage (Fig. 2). A similar but nonsignificant trend was observed with insulin-stimulated phosphorylation of GSK-3β (Supplementary Fig. 1A and B). Exposure of myotubes to cocultured adipocytes did not affect insulin-stimulated glucose uptake (Supplementary Fig. 2A). Compared with the lean group, insulin responsiveness in myotubes from obese donors was markedly impaired (Fig. 7E–H, Supplementary Fig. 1C and D). It is noteworthy that neither IBMX stimulation nor the presence of adipocytes was sufficient to enhance insulin action in the obese cohort.
FIG. 7.
Coculture exposure for 24 h enhances myotube insulin response. Primary human skeletal myotubes from lean (A–D) and severely obese (E–H) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. Adipocytes were removed, and myotubes were washed and incubated with α-MEM ± 100 nmol/L insulin for 10 min. Protein extracts were used for immunoblot analysis of Akt Ser473 phosphorylation, corrected for total protein loading using Memcode staining. Measurement of total Akt protein showed no treatment effects. Rates of glycogen synthesis were measured after a 2-h incubation with α-MEM containing 5 mmol/L [14C]glucose ± 100 nmol/L insulin. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
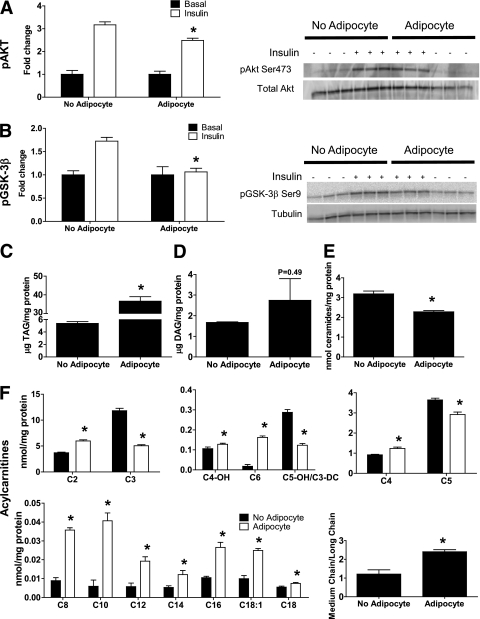
Myocyte responses to prolonged adipocyte exposure.
Because obesity-related metabolic derangements develop secondary to chronic overnutrition, we questioned whether prolonged exposure to lipolytic adipocytes might provoke a more diabetogenic outcome. Therefore, we exposed myotubes from lean subjects to stimulated coculture conditions for 96 h, which caused a modest but significant 21% reduction in insulin-stimulated Akt phosphorylation (Fig. 8A) and a decline in GSK-3β phosphorylation (Fig. 8B), without affecting glucose uptake (Supplementary Fig. 2B). Prolonged exposure to adipocytes caused a marked 6.8-fold increase in myotube TAG content compared with the 35% increase (P < 0.05) that occurred after 24 h of coculture (Fig. 8C, Supplementary Fig. 3). Although diacylglycerol levels trended higher in cocultured myotubes, differences between groups did not reach statistical significance (Fig. 8D, Supplementary Fig. 3), and concentrations of total ceramides (Fig. 8E) and several subspecies (Supplementary Fig. 3) were 29% lower after coculture (P < 0.05). We then examined concurrent changes in the cellular acylcarnitine profile. Compared with the short-term (24-h) exposure (Fig. 5A), 96 h of coculture resulted in a more robust accumulation of medium-chain acylcarnitines (Fig. 8F), resembling the profile of myotubes from obese subjects (Fig. 5B).
FIG. 8.
Chronic coculture exposure dampens myotube insulin response. Primary human skeletal myotubes from lean women were maintained 96 h in the absence or presence of human adipocytes under stimulated (100 μmol/L IBMX) conditions. Adipocytes were removed, and myotubes were washed and incubated with α-MEM ± 100 nmol/L insulin for 10 min. A: Protein extracts were used for immunoblot analysis of Akt Ser473 phosphorylation, corrected for total protein loading using Memcode staining. Measurement of total Akt protein showed no treatment effects. B: GSK-3β Ser9 phosphorylation, corrected for total protein using tubulin staining. C–F: Myotube extracts were used for measurement of total triacylglycerol (C), total diacylglycerol (D), total ceramide (E), and acylcarnitine profiling (F). C3DC and C5OH are isobaric species. The ratio of total medium-chain relative to total long-chain acylcarnitines was calculated to assess potential flux limitations at the medium-chain acyl-CoA dehydrogenase step of β-oxidation. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate, except for the TAG, DAG, and ceramides, which represent two independent experiments performed in quadruplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test. Abbreviations reflect carbon chain length (e.g., C2, acetylcarnitine). DC, dicarboxylic acid; OH, hydroxylated species.
DISCUSSION
This investigation examined metabolic reprogramming of primary human skeletal myotubes from lean and obese donors in response to cocultured adipocytes. The most salient outcome of the study was that the adipocytes encouraged myotubes to use fatty acids rather than glucose as an oxidative fuel. The adipocyte-induced shift in substrate preference was mediated at multiple levels. First, the coculture conditions increased mRNA abundance of several genes that promote fatty acid catabolism, including PDK4, PGC-1α, very long-chain acyl-CoA dehydrogenase, and ATGL. This effect was strongest when myocytes were exposed to lipolytically active adipocytes, consistent with the notion that fatty acids and their metabolites induce muscle expression of oxidative enzymes by acting as ligands for the PPAR family of transcription factors (26). However, it is noteworthy that changes in gene expression occurred even under lipolytically silent conditions, suggesting that adipocyte-derived agents other than fatty acids can affect myocyte remodeling at the genomic level.
Although cocultured myotubes were transcriptionally primed for β-oxidation, a measurable switch in fuel selection occurred only when a surfeit of fatty acids was made available via lipolytic stimulation. This conclusion stems from both the acylcarnitine profiles and the coincident decline in glucose oxidation. Cellular levels of acylcarnitines, which are byproducts of mitochondrial catabolism, fluctuate in response to changes in substrate availability and flux limitations at specific metabolic enzymes (23). Long-chain acylcarnitines are produced by the outer mitochondrial membrane enzyme, CPT1, which catalyzes the first committed step in β-oxidation. The increase in myocyte long-chain acylcarnitines on coculture with stimulated adipocytes could reflect increased fatty acid availability, increased CPT1 activity, or a flux limitation immediately downstream of this enzyme. It is noteworthy that the CPT1 inhibitor (etomoxir) abolished adipocyte-induced suppression of myotube glucose oxidation, even though the assay was performed after the coculture system was disassembled. Taken together, these observations suggest that persistent trafficking of fatty acids from perimuscular adipocytes to intramyocellular storage pools (Supplementary Fig. 3) contribute to reduced glucose utilization in the obese state (27,28).
Exposure to nonstimulated adipocytes lowered myotube levels of selected acylcarnitine species, possibly reflecting a reduction in metabolic load. Under these conditions, cocultured adipocytes potentiated insulin stimulation of pAkt and glycogen synthesis (Fig. 7). This enhancement might have occurred secondary to changes in fuel metabolism or the actions of insulin-sensitizing adipokines (10). By contrast, prolonged exposure (96 h) to the lipolytic condition resulted in a shift toward an insulin-resistant phenotype, evidenced by a modest but significant attenuation of insulin-stimulated Akt phosphorylation. The severity of adipocyte-induced insulin resistance in the current investigation was modest compared with studies in which cultured myotubes were incubated with high concentrations of palmitate (29,30). This discrepancy could stem from multiple factors, including the nature of the lipid load. We reason that the coculture model bears closer resemblance to in vivo physiology because myotubes are exposed to a steady release of mixed fatty acids, rather than a large bolus of a single fatty acid. Last, we found that adipocyte-induced perturbations in glucose metabolism progressed from substrate switching during short-term coculture to impaired Akt signaling after longer exposure. This scenario is similar to the development of diet-induced insulin resistance in both animals and humans (31,32).
To our knowledge, only one other group has examined insulin signaling in human myocytes after coculture with adipocytes (33,34). Investigators reported that 48 h of coculture caused a mild form of insulin resistance resulting in reduced stimulation of insulin receptor substrate-1 and Akt. A subsequent study found that impaired insulin signaling in human myotubes incubated with adipocyte-conditioned medium was reversed by pharmacologic inhibition of inhibitor κB kinase, thereby implicating inflammatory stress as an underlying mechanism (32). These earlier studies did not evaluate different lipolytic conditions or changes in myocyte energy metabolism. In the current study, adipocyte stimulation proved to be a determining factor that influenced metabolic outcomes. Secretion of several prominent adipokines was unchanged by IBMX (Fig. 1), although the compound could have affected other cytokines and peptide hormones known to act on muscle (35,36). Nonetheless, our data strongly imply that adipocyte lipolysis contributed to changes in myotube energy metabolism. This result is relevant to reports that have identified dysregulated lipolysis as an early feature of the metabolic syndrome (37,38). Our observation that relatively low concentrations of adipocyte-derived fatty acids were sufficient to coerce myotubes into lipid oxidation underscores the potential importance of localized lipolysis in provoking glucose disuse.
The reciprocal relationship between lipid and glucose oxidation was first described by Randle et al. (39,40) as the “glucose-fatty acid cycle,” which holds that β-oxidation suppresses glycolysis and pyruvate oxidation by inhibiting hexokinase, phosphofructokinase, and pyruvate dehydrogenase (40). Another popular theory to explain obesity-induced glucose intolerance proposes that fatty acid oversupply causes cellular accumulation of insulin-desensitizing lipid molecules, such as diacylglycerol and ceramides (28,41–42). However, it is noteworthy that myotube content of these specific lipid species was either unchanged or decreased after prolonged exposure to the coculture conditions, despite marked elevations in triacylglycerol levels. Moreover, myotubes from obese subjects remained profoundly insulin resistant even when grown in the absence of fatty acids or adipocytes, again hinting at alternative mechanisms. Recent animal studies suggested that lipid-induced antagonism of glucose disposal might stem from a mitochondrial-derived signal that closely associates with intramuscular acylcarnitine accumulation (24,43). In light of these earlier reports, it is noteworthy that the current study also revealed an intriguing connection between impaired insulin signaling after 96-h exposure to lipolytic adipocytes and myocyte accumulation of medium-chain acylcarnitines. Likewise, the insulin-resistant phenotype of the obese myotubes was accompanied by a more pronounced accumulation of short- and medium-chain acylcarnitines. Although evidence that acylcarnitines play a direct role in mediating glucose intolerance is lacking, these metabolites might serve as markers of mitochondrial events that are known to influence insulin signaling, such as production of reactive oxygen species or perturbations in redox balance (25,44,45).
Whereas the current investigation was executed using pooled lots of human myocytes, our previous work revealed compelling between-group differences when analyses were performed using cell cultures established from individual donors (15,46). In aggregate, these studies have shown that myotubes from obese compared with lean donors have lower rates of complete fat oxidation, increased acylcarnitine accumulation, elevated rates of TAG synthesis on lipid exposure, and impaired insulin-stimulated phosphorylation of Akt. A novel and potentially important finding from the coculture model is that the insulin-sensitizing effects of basal adipocytes were observed only in myotubes derived from lean donors, possibly reflecting an inherent resistance to antidiabetic adipokines in myotubes isolated from obese donors. This interpretation is supported by previous studies showing that myotubes from obese donors are refractory to the salutary effects of adiponectin (47,48).
In conclusion, we have developed and comprehensively characterized a novel coculture system designed to mimic the physiologic interactions between skeletal myofibers and intermuscular adipocytes. One caveat of the model is that the lipolytic agent (IBMX) used to increase cAMP levels in adipocytes has a similar effect on other cell types and was found in the current study to enhance myocyte insulin responsiveness (Fig. 7). Thus, it is noteworthy that our reference to a stimulated state refers to both compartments of the model. Although IBMX might have modified myocyte responses to the coculture, we reason that the stimulated condition is physiologically relevant given that obesity is often accompanied by a heightened sympathetic tone (49–51). Studies using this model provided new and important insights regarding the net metabolic impact of local adipocytes on neighboring myocytes. This work sets the stage for future investigations seeking to elucidate the molecular connections between increased adiposity and muscle dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health, National Institute on Aging Grant R01-AG-028930 (D.M.M. and W.E.K.); the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants R44-DK-07425302 (B.M.B.), R01-DK-56112 (J.A.H.), and R01-DK-073284 (C.L.K.); and the American Heart Association (J.-P.K.).
No potential conflicts of interest relevant to this article were reported.
J.-P.K. designed and performed the experiments and wrote the manuscript; D.S. and R.D.S. performed the experiments; W.E.K. and J.A.H. contributed to the experiments and manuscript preparation; J.B.N., Y.R.L.-C., and B.M.B. provided materials and contributed to the discussion; K.E. and C.L.K. contributed to lipid analysis; and D.M.M. contributed to experimental design and writing and editing the manuscript.
The authors thank the dedicated staff of the Metabolomics and Biomarker Core of the Sarah W. Stedman Nutrition and Metabolism Center and Dr. Timothy Koves (Duke University) for their contributions.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0427/-/DC1.
REFERENCES
- 1.Haslam DW, James WPT. Obesity. Lancet 2005;366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 2.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005;111:1448–1454 [DOI] [PubMed] [Google Scholar]
- 3.Després J.-P. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006;38:52–63 [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981;30:1000–1007 [DOI] [PubMed] [Google Scholar]
- 5.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 [DOI] [PubMed] [Google Scholar]
- 6.Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord 2003;27(Suppl. 3):S6–S11 [DOI] [PubMed] [Google Scholar]
- 7.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–229 [DOI] [PubMed] [Google Scholar]
- 8.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab 2003;14:137–145 [DOI] [PubMed] [Google Scholar]
- 9.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 2005;33:1078–1081 [DOI] [PubMed] [Google Scholar]
- 10.Dyck DJ, Heigenhauser GJF, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186:5–16 [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–946 [DOI] [PubMed] [Google Scholar]
- 12.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab 2000;11:212–217 [DOI] [PubMed] [Google Scholar]
- 13.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 2002;51:2929–2935 [DOI] [PubMed] [Google Scholar]
- 14.Muoio DM, Way JM, Tanner CJ, et al. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 2002;51:901–909 [DOI] [PubMed] [Google Scholar]
- 15.Consitt LA, Bell JA, Koves TR, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 2010;59:1407–1415. Epub 2010 Mar 3 [DOI] [PMC free article] [PubMed]
- 16.Kim J-Y, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 2000;279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- 17.Kim J-Y, Koves TR, Yu G-S, et al. Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab 2002;282:E1014–E1022 [DOI] [PubMed] [Google Scholar]
- 18.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10:268–274 [DOI] [PubMed] [Google Scholar]
- 19.Kien CL, Everingham KI, D Stevens R, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005;36:207–224 [DOI] [PubMed] [Google Scholar]
- 21.Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab 2008;295:E407–E412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS ONE 2010;5:e9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hove JL, Zhang W, Kahler SG, et al. Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: diagnosis by acylcarnitine analysis in blood. Am J Hum Genet 1993;52:958–966 [PMC free article] [PubMed] [Google Scholar]
- 24.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005;280:33588–33598 [DOI] [PubMed] [Google Scholar]
- 25.Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muoio DM, MacLean PS, Lang DB, et al. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR δ. J Biol Chem 2002;277:26089–26097 [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, Mintun MA, Watkins SC, et al. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J Clin Invest 1996;97:2705–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cline GW, Magnusson I, Rothman DL, Petersen KF, Laurent D, Shulman GI. Mechanism of impaired insulin-stimulated muscle glucose metabolism in subjects with insulin-dependent diabetes mellitus. J Clin Invest 1997;99:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 1999;274:24202–24210 [DOI] [PubMed] [Google Scholar]
- 30.Chavez JA, Knotts TA, Wang L-P, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 2003;278:10297–10303 [DOI] [PubMed] [Google Scholar]
- 31.Chokkalingam K, Jewell K, Norton L, et al. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: an important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 2007;92:284–292 [DOI] [PubMed] [Google Scholar]
- 32.Hoy AJ, Brandon AE, Turner N, et al. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am J Physiol Endocrinol Metab 2009;297:E67–E75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietze D, Koenen M, Röhrig K, Horikoshi H, Hauner H, Eckel J. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 2002;51:2369–2376 [DOI] [PubMed] [Google Scholar]
- 34.Dietze D, Ramrath S, Ritzeler O, Tennagels N, Hauner H, Eckel J. Inhibitor κB kinase is involved in the paracrine crosstalk between human fat and muscle cells. Int J Obes Relat Metab Disord 2004;28:985–992 [DOI] [PubMed] [Google Scholar]
- 35.Sell H, Laurencikiene J, Taube A, et al. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009;58:2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Famulla S, Lamers D, Hartwig S, et al. Pigment epithelium-derived factor is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes 2010 Oct 12 [Epub ahead of print] [DOI] [PubMed]
- 37.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 2005;19:471–482 [DOI] [PubMed] [Google Scholar]
- 38.Bays HE, González-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368 [DOI] [PubMed] [Google Scholar]
- 39.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 40.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998;14:263–283 [DOI] [PubMed] [Google Scholar]
- 41.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999;103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 43.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 44.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci U S A 2009;106:17787–17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulver MW, Berggren JR, Carper MJ, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2005;2:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAinch AJ, Steinberg GR, Mollica J, et al. Differential regulation of adiponectin receptor gene expression by adiponectin and leptin in myotubes derived from obese and diabetic individuals. Obesity (Silver Spring) 2006;14:1898–1904 [DOI] [PubMed] [Google Scholar]
- 48.Bruce CR, Mertz VA, Heigenhauser GJF, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes 2005;54:3154–3160 [DOI] [PubMed] [Google Scholar]
- 49.Shigetoh Y, Adachi H, Yamagishi S-i, et al. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am J Hypertens 2009;22:151–155 [DOI] [PubMed] [Google Scholar]
- 50.Huggett RJ, Burns J, Mackintosh AF, Mary DASG. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 2004;44:847–852 [DOI] [PubMed] [Google Scholar]
- 51.Grassi G, Dell’Oro R, Quarti-Trevano F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 2005;48:1359–1365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.