Abstract
Collagens V and XI comprise a single regulatory type of fibril-forming collagen with multiple isoforms. Both co-assemble with collagen I or II to form heterotypic fibrils and have been implicated in regulation of fibril assembly. The objective of this study was to determine the roles of collagens V and XI in the regulation of tendon fibrillogenesis. Flexor digitorum longus tendons from a haplo-insufficient collagen V mouse model of classic Ehlers Danlos syndrome (EDS) had decreased biomechanical stiffness compared with controls consistent with joint laxity in EDS patients. However, fibril structure was relatively normal, an unexpected finding given the altered fibrils observed in dermis and cornea from this model. This suggested roles for other related molecules, i.e. collagen XI, and compound Col5a1+/−,Col11a1+/− tendons had altered fibril structures, supporting a role for collagen XI. To further evaluate this, transcript expression was analyzed in wild type tendons. During development (E18-P10) both collagen V and XI were comparably expressed; however, collagen V predominated in mature (P30) tendons. The collagens had a similar expression pattern. Tendons with altered collagen V and/or XI expression (Col5a1+/−; Col11a1+/−; Col5a1+/−,Col11a1+/−; Col11a1−/−; Col5a1+/−,Col11a1−/−) were analyzed at E18. All genotypes demonstrated a reduced fibril number and altered structure. This phenotype was more severe with a reduction in collagen XI. However, the absence of collagen XI with a reduction in collagen V was associated with the most severe fibril phenotype. The data demonstrate coordinate roles for collagens V and XI in the regulation of fibril nucleation and assembly during tendon development.
Keywords: Collagen, Connective Tissue, Development, Extracellular Matrix, Extracellular Matrix Proteins, Biomechanics, Collagens V and XI, Ehlers Danlos Syndrome, Tendon
Introduction
Abnormal collagen fibril formation is characteristic of the classic form of Ehlers-Danlos syndrome (EDS).2 Patients with classic EDS (types I and II) have a broad spectrum of generalized connective tissue defects including hyper-extensible skin, fragile skin with wide, depressed, callused scarring, inguinal hernias, and rectal prolapse as well as aortic root dilation and valve prolapse (1, 2). In addition, laxity in the joints, leading to instability and easy dislocation as well as joint hyper-extensibility, is a characteristic feature resulting from dysfunctional tendons and ligaments. More than half of all instances of classic EDS have been linked to heterozygous mutations in the genes for collagen V (3–13). The most common mutation type in classic EDS is one that results in a functional loss of one Col5a1 allele (14, 15).
Collagen V is a fibril-forming collagen. The fibril-forming collagen subfamily includes collagens I, II, III, V, XI, XXIV, and XXVII, and the genes cluster into three distinct clades (16). Collagens I, II, and III are the major components of all collagen fibrils. Collagens V and XI are quantitatively minor collagens found as heterotypic fibrils with collagens I, II, and III and have a regulatory function in fibrillogenesis (17). Collagens XXIV and XXVII have structural differences relative to collagens I, II III, V, and XI, and their specific roles remain to be elucidated.
Collagens V and XI have multiple isoforms: [α1(V)]2α2(V); [α1(V)]3; α1(V)α2(V)α3(V); α1(XI)α2(XI)α3(XI) (18). In addition, there are collagen V and XI hybrids such as [α1(XI)]2α2(V) (19–21). Despite being originally described as separate collagen types, collagens V and XI are now considered a single collagen type with multiple tissue-specific isoforms (17, 18). The major isoforms of collagens V and XI, [α1(V)]2α2(V) and α1(XI)α2(XI)α3(XI), form heterotypic fibrils with collagens I and II in a regulated, tissue-specific manner. Interactions among fibrillar collagens regulate collagen organization in the fibril, resulting in tissue-specific fibril differences.
Collagens V and XI have been shown to regulate fibrillogenesis by nucleating fibril formation. Fibril assembly assays manipulating these collagens in cell culture and in mouse models have demonstrated that reducing the ratio of collagens V and XI to collagens I and II results in fibrils with larger diameters. Reducing collagens V and XI also results in decreased numbers of fibrils assembled (22, 23). A targeted deletion of Col5a1 is embryonic lethal in a mouse model due to cardiovascular failure and a virtual lack of fibril formation in the embryonic mesenchyme (24). This occurs despite the presence of normal collagen I secretion and demonstrates that collagen V is essential for the assembly of collagen I-containing fibrils in this low collagen concentration environment. The heterozygous (Col5a1+/−) mice were haplo-insufficient for collagen V and recapitulated many of the characteristics of classic EDS (25).
Two mouse models in which the production of collagen XI is compromised also demonstrate altered fibrillogenesis. In a mouse model (cho/cho) with naturally ablated Col11a1 alleles, collagen XI is absent. Homozygous animals develop a chondrodysplasia (cho) with cartilage essentially devoid of fibrils, although collagen II is produced normally (26, 27). In Col2a1-null cartilage, collagen II deficiency leads to an up-regulation of collagen I. Collagen I is not produced in normal hyaline cartilage but should form fibrils. However, as in cho/cho mice, the mutant cartilage lacks fibrils. The collagen XI molecule contains an α3(XI) chain that is derived from the Col2a1 gene; therefore, Col2a1-null mice lack this, and fibrils are not assembled (28, 29). These studies corroborate the concept that collagens V and XI have similar regulatory roles in collagen fibrillogenesis.
The purpose of this study is to elucidate specific regulatory roles of collagens V and XI in tendon fibrillogenesis. Specifically, it will be determined whether regulation of tendon fibrillogenesis involves unique, shared, and/or synergistic roles for collagens V and XI in development of the structural and functional properties of tendon.
EXPERIMENTAL PROCEDURES
Animals
Col5a1+/− mice were created by targeted deletion and have been previously described in detail (24). The cho/+ mice also have been characterized (26, 27). The mutation in Col11a1 results in a collagen XI-null homozygous mouse (27). Col5a1+/−, cho/+, and cho/cho mice were obtained from heterozygous matings. The compound mutant mice were obtained by cross-breeding. The genotype of the fetuses was determined as described previously (27, 30). All animal studies were performed in compliance with Institutional Animal Care and Use Committee-approved animal protocols.
Biomechanics
Analyses of P60 flexor digitorum longus (FDL) were done as previously described (31) using six wild type and seven Col5a1+/− littermates. Cross-sectional area of the P60 tendon was calculated from measurements of the width and thickness (32, 33). P60 tendons were glued (using cyanoacrylate) to sandpaper 5 mm apart, and stain lines were placed 2.5 mm apart within the mid-substance to track strain optically (34). Samples were clamped in custom test fixtures, and standard mechanical testing protocols were used including preconditioning, stress relaxation, and ramp to failure as described (35). For all tests, samples were immersed in a 37 °C PBS bath and tested with an Instron 5543 (Instron Corp., Canton, MA). Maximum stress and modulus were calculated as previously described. One way analysis of variance was performed on cross-sectional area, maximum load, maximum stress, stiffness, and elastic modulus comparing across genotype (significance at p ≤ 0.05).
Analyses of mRNA Expression
Total RNA was isolated from pooled FDL tendons from wild type mice. At postnatal day 4 (P4) and P10, 40 FDLs from 20 mice were used for each age. At P30 and P90, 20 FDLs from 10 mice were used at each age. Two independent cDNA preparations at each age were obtained by reverse transcription of total RNA (5 μg) with random primers (High Capacity cDNA Archive kit, Applied Biosystems, Foster City, CA). Semiquantitative RT-PCR was done as described (36, 37).
Real-time RT-PCR was performed using the LightCycler System (Invitrogen) with the SYBR Green PCR Master Mix (Applied Biosystems) (31) according to the manufacturers' directions. Classic II 18 S internal standard (Ambion) was used as an endogenous control to standardize the amount of sample RNA. A series of 10× dilutions of cDNA mixtures from each developmental time point was used to generate relative standard curves for 18 S for comparison with Col5 or Col11 samples. PCR amplification was done with cDNA derived from 25 ng of RNA input for each sample used as template with primer concentrations of 0.3 μm for collagens V and XI and 0.1 μm for 18 S; the optimal 18 S primer pair/competimer ratio was 1:4. The PCR cycle parameters were 95 °C for 2 min for 1 cycle and 95 °C for 5 s, 58 °C for 15 s, and 72 °C for 20 s for 40 cycles. The optimal MgCl2 concentration used for the PCR was 2 mm.
Antibodies
Antibodies against mouse type V and XI collagen were produced as described previously (24, 38). Anti-type I collagen (Chemicon, AB765p) and anti-actin primary antibodies (Chemicon) were used at 1:10,000.
Immunolocalization Analyses
FDL tendons were dissected from wild type mice at P4, and immunolocalization was done as previously described (39). Tissues were embedded in OCT medium, quick-frozen in an ethanol/dry ice bath, and stored at −80 °C. Frozen sections (6 μm) were cut using an HM 505E cryostat. Indirect immunofluorescence staining was performed as previously described (39). Rabbit anti-mouse collagen V and rabbit anti-mouse collagen XI antibodies were used, each at 1:100. The secondary antibody (Molecular Probes, Eugene, OR) was an Alexa Fluor 568-conjugated goat anti- rabbit IgG used at 1:200. Vectashield mounting solution with DAPI (Vector Laboratories, Inc., Burlingame, CA) was used as a nuclear marker. Negative control samples were incubated identically, except the primary antibody was excluded. Images were captured using a DM5500 Upright microscope system (Leica). Identical conditions and set integration times were used to facilitate comparisons between samples.
Immunoblot Analyses
FDL tendons were collected from wild type mice at P4 and P30. The tissues (20 mg wet weight) were rinsed in PBS and homogenized in a 15-fold excess of extraction buffer (50 mm Tris, pH 8.0, 1 m NaCl, 10 mm EDTA, 10 mm N-ethylmaleimide, and proteinase inhibitor mixture, Roche Applied Science). Collagens were extracted at 4 °C overnight with stirring followed by centrifugation at 14,000 rpm using an Eppendorf centrifuge 5415C (Eppendorf) for 10 min at 4 °C. Total protein content in the sample was determined using a BCA Protein Assay kit (Pierce). Constant total protein for each sample was resolved by SDS-PAGE and transferred to nitrocellulose membrane (GE Healthcare). Immunoblotting was done with anti-collagen I, collagen V, and collagen XI antibodies used at 10 ng/ml for collagen I and 1 μg/ml for collagens V and XI. Actin in each sample was probed with anti-actin antibody (10 ng/ml; Chemicon) as a loading control. Goat anti-rabbit IgG-peroxidase (GE Healthcare) was used as the secondary antibody at 1:3000 with ECL as the detection system (Pierce). For each independent sample, immunoblotting was done at least in duplicate.
Transmission Electron Microscopy
Tendons between embryonic day (E) 18 and post-natal day (P) 30 were analyzed by transmission electron microscopy. Briefly, FDL tendons were dissected and fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 m sodium cacodylate, pH 7.4, with 8.0 mm CaCl2, post-fixed with 1% osmium tetroxide, and en bloc-stained with uranyl acetate, 50% ethanol (3). After dehydration in an ethanol series followed by propylene oxide, the tissue samples were infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA). Thin sections (90 nm) were cut using a Leica UCT ultramicrotome and post-stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. Cross-sections from the mid-plantar regions of FDL tendons were examined at 80 kV using a Tecnai 12 transmission electron microscope equipped with a Gatan Ultrascan US1000 2K digital camera. For each genotype, 2–7 tendons from 2–6 different animals at E18 and 4 specimens at P30 were analyzed.
Fibril Diameter Distribution
Fibril diameters were measured using a RM Biometrics-Bioquant Image Analysis System (Nashville, TN). Digital images were randomly chosen, masked, and analyzed at a final magnification of 60,000× for E18 and 15,000× for P30. For measurements at E18, 2 fields per image each with a constant area of 0.18 μm2 were utilized. Each field of view contained fibrils in cross-section and was free of cell processes. The count per field was 54–94 fibrils measured, with 34 images measured for wild type (total fibril count of 2643), 39 images for the Col5a1+/− genotype (total fibril count of 2124), 41 images for Col11a1+/− (total fibril count of 1974), 58 images for Col5a1+/−,Col11a1+/− (total fibril count of 1252), 43 images for Col11a1−/− (total fibril count of 2050), and 57 images for Col5a1+/−, Col11a1−/− (total fibril count of 1094). For measurements at P30, 1 field per image with an area of 2.75 μm2 and containing cross-section fibrils free of cell processes was utilized. The count per field was 74–177 fibrils measured, with 22 fields measured for wild type (total fibril count of 2499), 32 for the Col5a1+/− genotype (total fibril count of 3409), and 62 for Col5a1+/−,Col11a1+/− (total fibril count of 5582).
RESULTS
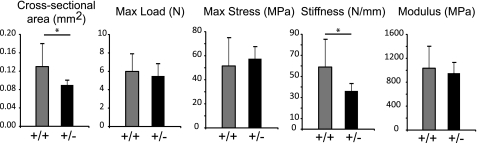
Tendon function in a Col5a1+/− mouse model of classic Ehlers Danlos syndrome was analyzed. The biomechanical properties of mature (P60) Col5a1+/- and wild type control FDL tendons were analyzed (Fig. 1). The cross-sectional areas of Col5a1+/− tendons were significantly smaller than the wild type tendons. The maximum load, maximum stress, and the modulus were not significantly different. However, a significant decrease in stiffness between Col5a1+/− and wild type tendons was observed. This decrease in stiffness is consistent with the hyper-extensible joint phenotype observed in patients with classic EDS.
FIGURE 1.
Altered biomechanical properties in Col5a1+/− mouse tendons. Cross-sectional area, maximum load, maximum stress, stiffness, and modulus were measured in P60 FDL tendons from Col5a1+/+ and Col5a1+/− mice. Cross-sectional areas were significantly decreased in Col5a1+/− tendons compared with wild type tendons. There was a significant reduction in stiffness in Col5a1+/− tendons compared with control tendons, consistent with increased elasticity. The maximum load, stress, and modulus were comparable in both genotypes. Asterisk, p < 0.05.
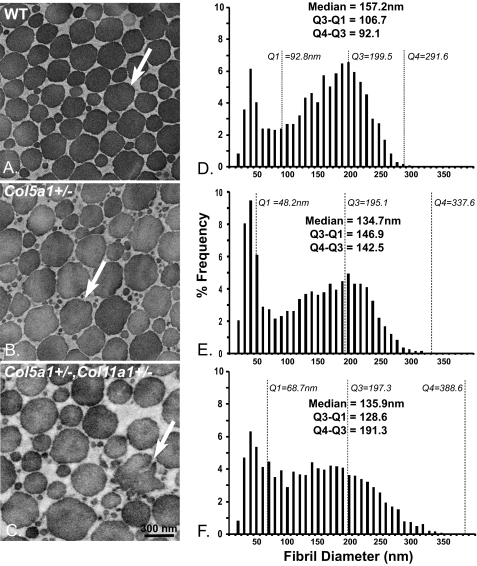
To address structure/function relationships, collagen fibril structure was analyzed in the tendons from Col5a1+/− and wild type mice at P30 (Fig. 2, A and B). The haplo-insufficient Col5a1+/− tendons had fibril structures comparable with wild type controls, with both genotypes showing a heterogeneous population of fibrils. The wild type tendons had near circular fibril cross-sections with the Col5a1+/− tendons composed of fibrils with less regular fibril cross-section profiles. In addition, there were larger numbers of small diameter fibrils in the Col5a1+/− tendons. This result was unexpected based on the fibril phenotype observed in the dermis of the Col5a1+/− animals (24, 25) and from patients with classic Ehlers Danlos syndrome (40). To examine potential regulatory interactions involving collagen V and XI isoforms, an analysis of fibril structure was done on tendons from mice haplo-insufficient in both Col5a1 and Col11a1 (Col5a1+/−,Col11a1+/−) (Fig. 2C). The compound heterozygous mice had larger diameter fibrils compared with the wild type controls. In addition, there was a subpopulation of large, structurally aberrant fibrils in the compound heterozygous mice. This phenotype is comparable with the dermal phenotype observed in patients with classic EDS and in the Col5a1+/− mouse model. Comparable results were obtained from P90 tendons (data not shown). The tendons from Col11a1+/− mice also were analyzed, and the fibril diameter distribution and fibril morphology were comparable with that of Col5a1+/− mice. However, the diameter distribution in Col11a1+/− tendons has a larger percentage of small diameter fibrils with first quartile (Q1) = 37.7 versus 48.2 nm, and the resultant shift in the median 103.2 versus 134.7 nm. The remaining parameters were comparable (supplemental Fig. S1).
FIGURE 2.
Mature Col5a1+/− tendons demonstrate a fibril structure comparable with wild type controls, whereas Col5a1+/−,Col11a1+/− tendons have an EDS-like fibril phenotype. A and B, collagen fibril structure was analyzed in the tendons from Col5a1+/− and wild type mice at P30 using transmission electron microscopy. Fibril structures in Col5a1+/− tendons and wild type controls were comparable, with each showing a heterogeneous population of fibrils with normal, near circular fibril cross-sections (arrow). However, there was an increased number of small and of large diameter fibrils in the mutant mice. In addition, the fibril cross-sectional profiles were less regular in the Col5a1+/− tendons (arrow). C, fibril structure in the compound heterozygotes appears comparable with the Col5a+/− tendon, but fibril cross-sectional profiles are more irregular. In addition, there is a subpopulation of structurally aberrant fibrils (arrow). D–F, fibril diameter distributions of tendons at P30 had a broad, heterogeneous population of fibrils with a bimodal distribution in wild type, Col5a1+/−, and Col5a1+/−,Col11a1+/− mice. Both sets of mutant tendons demonstrate an increased number of small diameter fibrils relative to the wild type controls. In addition, both mutant genotypes developed a shoulder composed of larger fibrils with a heterogeneous distribution of diameters. This shoulder was substantially better developed in the compound heterozygous versus Col5a1+/− tendon.
The fibril diameter distributions were analyzed at P30 for wild type, Col5a1+/−, and compound Col5a1+/−,Col11a1+/− tendons (Fig. 2, D–F). All three genotypes contained two distinct fibril subpopulations; a narrow distribution of small diameter fibrils and a heterogeneous distribution of larger diameter fibrils. Both mutant genotypes demonstrated an increased number of small diameter fibrils relative to the wild type control tendons. This is illustrated by the decrease in the first quartile (Q1) values: 92.8 nm in the wild type tendon and 48.2 and 69.7 nm in the Col5a1+/−, and Col5a1+/−,Col11a1+/− tendons, respectively. In addition, both mutant genotypes demonstrated an increase in the range, with fourth quartile (Q4) values increasing from 292 nm in the wild type to 338 and 389 nm in the Col5a1+/− and Col5a1+/−,Col11a1+/− tendons, respectively. This was the result of the development of a right-hand shoulder composed of large diameter fibrils in the mutant tendons. The Q3 value and increased interquartile range (Q4-Q3) define these differences; Q3 remains relatively constant in tendons from all 3 genotypes at 195–200 nm. Q4-Q3 is 92 nm in wild type tendons compared with 142 and 191 nm in Col5a1+/− and Col5a1+/−,Col11a1+/− tendons, respectively. This is consistent with a subpopulation of larger, heterogeneous fibrils in the mutant tendons.
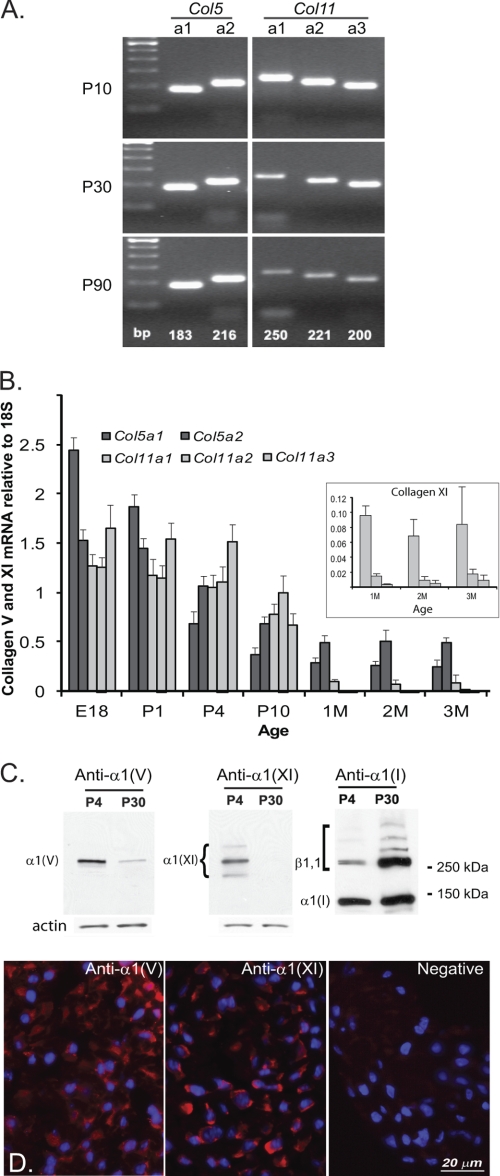
The relatively normal fibril phenotype in the Col5a1+/− haplo-insufficient tendon and the defects in the Col5a1+/−,Col11a1+/− tendons suggested a cooperative regulatory interaction in tendon fibrillogenesis that is absent in the dermis of Col5a1+/− mice. To address this, collagen V and XI α-chain expression was analyzed during mouse tendon development, focusing on the ubiquitous α1(V)2α2(V) and α1(XI)α2(XI)α3(XI) isoforms. All 5 α-chains were demonstrated during tendon development and maturation (P10 to P90) using a qualitative PCR analysis (Fig. 3A). The expression was further examined from E18 to P90 using quantitative real time PCR (Fig. 3B). These transcript analyses indicated comparable expression of the collagen V and XI genes required for the two different isoforms during early tendon development (E18 to P10). However, at P30-P90, collagen XI α-chains had low expression relative to collagen V α-chains. These analyses indicate that a number of different isoforms is possible during tendon development. To determine whether the collagen proteins were present, α1(V) and α1(XI) chain expression during tendon development was analyzed using immunoblots (Fig. 3C). Both α-chains were present during early tendon development (P4); however, at P30 only the α1(V) chain was present. Immunofluorescence localization analysis at P4 demonstrated reactivity for α1(V)- and α1(XI)-associated with tendon fibroblasts (Fig. 3D).
FIGURE 3.
Differential expression of collagens V and XI during tendon development. A, qualitative transcript analysis of collagen V and XI α-chain gene expression from P10 to P90 indicates that a number of different isoforms are possible during tendon development. B, quantitative transcript analysis of collagen V and XI α-chain genes from E18 to P90 quantitatively depicts the different isoforms possible throughout development, showing a shift from early expression of both collagen V and XI α-chains to preferential expression of collagen V beginning at 1 month. All five α-chain genes decrease with development. The inset shows only the expression of the collagen XI chains with an expanded scale at 1–3 months. C, immunoblot analyses of α1(V) and α1(XI) chain expression during tendon development detected the α1 chains of collagens I, V, and XI. The position of cross-linked collagen I α-chains including β1,1 is indicated by the square bracket. Molecular weight markers are presented on the right. D, immunolocalization of collagens V and XI in early development shows reactivity for α1(V) and α1(XI) throughout the P4 tendon.
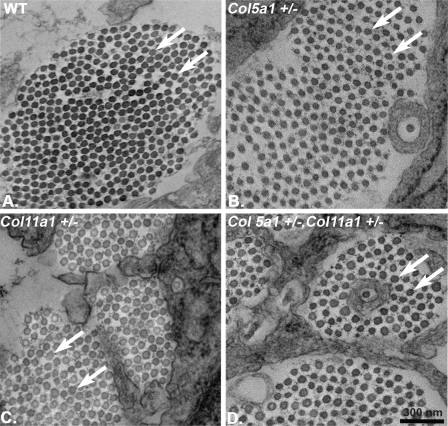
The regulatory roles of collagens V and XI in nucleation of protofibril assembly were analyzed during early tendon development (E18), where protofibril assembly is the dominant stage in fibrillogenesis. An analysis comparable with that described for the P30 tendons was done using tendons from wild type, both Col5a1 and Col11a1 heterozygous, and compound heterozygous mice. The results were analogous to those described for the P30 tendons. Both Col5a1+/− and Col11a1+/− tendons had fibrils with structures comparable with the wild type control mice (Fig. 4). However, the compound heterozygote (Col5a1+/−,Col11a1+/−) mice had more heterogeneous fibril diameters and larger fibrils, demonstrating a phenotype comparable with that observed in the mature compound heterozygous mutant mice.
FIGURE 4.
Compound heterozygous (Col5a1+/−,Col11a1+/−) mice demonstrate a fibril phenotype in early tendon development. Col5a1+/− and Col11a+/− tendons show normal fibril morphology and a small increase in fibril diameter relative to wild type tendons (arrows in A–C). In contrast, compound heterozygous mice have larger diameter fibrils that are beginning to display irregular fibril cross-sections (arrows in D) compared with both the wild type and haplo-insufficient mice. Transmission electron micrographs are of cross-sections from E18 mouse FDL tendons.
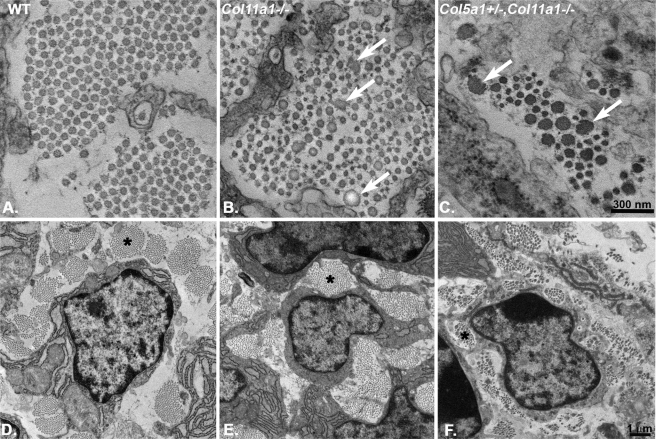
Fibril structure was analyzed in collagen XI-null (Col11a1−/−) or collagen XI-null and collagen V haplo-insufficient (Col11a1−/−,Col5a1+/−) tendons (Fig. 5). Both genotypes resulted in a severe fibril phenotype, with the compound mutant being significantly more severe. The Col11a1−/− tendons were composed of fibrils with a heterogeneous distribution of fibril diameters. In addition, there was a subpopulation of large diameter fibrils with aberrant fibril structures, i.e. the fibril cross-sections were irregular. When the effect of reducing collagen V in the absence of collagen XI was analyzed in the compound Col11a1−/−,Col5a1+/− tendons, the same basic fibril phenotype was observed but was considerably more severe than in the collagen XI-null tendon, the diameters were more heterogeneous, and the subpopulation of structurally aberrant fibrils accounted for a higher percentage of the total fibrils. Overall, the basic tendon architecture, i.e. organization of fibrils into fibers associated with tendon fibroblasts, was disrupted in both genotypes (Fig. 5, E and F). Tendon architecture is recognizable but disorganized in Col11a1−/−,Col5a1+/− tendons (Fig. 5F) with an obvious decrease in the number of fibrils compared with the wild type controls.
FIGURE 5.
A severe collagen XI-null fibril phenotype is enhanced by reduction in collagen V, indicating synergistic regulatory roles. The loss of collagen XI in Col11a1−/− tendons (B and C) is associated with abnormally large fibrils with aberrant structures (arrows) compared with wild type controls (A). A deficiency in collagen XI coupled with a reduction in collagen V results in an increased severity of the fibril phenotype, including a substantial decrease in the number of fibrils (C). The disruption in fibrillogenesis results in disrupted fiber assembly (D–F, asterisks) and overall tendon structure. Again, this is more severe in the compound mutant tendons. Transmission electron micrographs of E18 mouse tendons in cross-section are shown. D–F are low magnification micrographs of regions comparable with those in A–C, respectively.
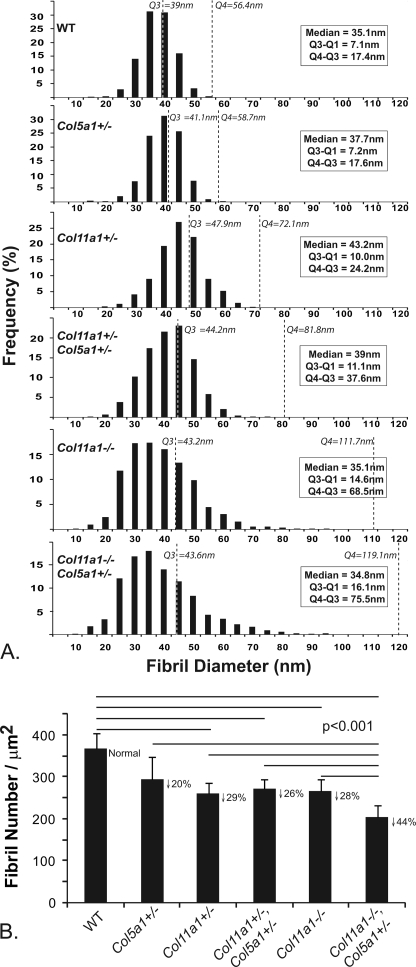
The fibril diameter distributions were analyzed in tendons with altered collagen V and XI expression at E18 (Fig. 6A). The results of the single heterozygous and compound heterozygous mice were analogous to those observed in P30 tendons. All three genotypes demonstrated a shift to larger diameter fibrils seen as an increase in the median from 35 nm in the wild type control to up to 43 nm in the mutant tendons. In addition, a broadening of the diameter distribution was observed as an increase in the interquartile range (Q3-Q1) from 7 to 11 nm. The Col11a1−/− and Col11a1−/−,Col5a1+/− tendons demonstrated an even broader distribution with Q3-Q1 increasing to 15 and 16 nm, respectively. All of the tendons with reduced collagen XI developed a right-hand shoulder on the distribution consistent with the structurally abnormal fibril population. The Q3 values shifted from 39 nm in wild type tendons to 43–48 nm in tendons with reduced collagen XI. The heterogeneity and size of fibrils in this subpopulation, defined by Q4-Q3, increased from 24 nm in Col11a1+/− and 38 nm in Col11a1+/−, Col5a1+/− to 69 nm in Col11a1−/− and 76 nm in Col11a1−/−,Col5a1+/− tendons.
FIGURE 6.
Decreases in collagens V and XI lead to dysfunctional regulation of fibrillogenesis and a decreased number of fibrils assembled in early tendon development. A, fibril diameter distributions from E18 tendons with altered collagen V and XI expression are shown. The reduction in collagens V and XI was associated with changes in the diameter distributions relative to wild type controls. All mutant tendons except Col5a1+/− demonstrated the development of a right-hand shoulder with larger and more heterogeneous fibril diameters. This is illustrated by the increase in the range of the last quartile (Q4-Q3) that increases from 17 nm in wild type tendons to 76 nm in Col11a1−/−,Col5a1+/− tendons, with the other genotypes being intermediate. B, shown is analysis of fibril number in tendons with altered collagen V and XI expression. A decrease in fibril density of ∼20% is seen in Col5a1+/− tendons. Col11a1+/−, compound Col11a1+/−,Col5a1+/−, and Col11a1−/− tendons demonstrated a significant decrease in fibril number relative to wild type tendons of ∼26–29%. In contrast, the Col11a1−/−,Col5a1+/− tendons showed an ∼44% decrease. These data are consistent with nucleation and assembly of fewer fibrils. Horizontal lines indicate pairs of data that were significantly different from each other (p < 0.001) in analysis of variance. The down arrow and percentage indicate the % decrease in fibril density for each genotype compared with wild type controls.
In addition, the number of fibrils assembled in tendons from the different genotypes was determined (Fig. 6B). All the tendons with reduced collagen V and XI demonstrated a reduction in the number of fibrils assembled. In the Col5a1+/− tendons there was a 20% reduction in fibril number, but this was not statistically significant. However, the reduction in fibril number was statistically significant (p < 0.001) in all other genotypes compared with the wild type controls. The Col11a1+/−, the Col11a1+/−,Col5a1+/−, and the Col11a1−/− tendons had a 26–29% reduction in fibril number. In contrast, the Col11a1−/−,Col5a1+/− tendons demonstrated a 44% reduction in fibril number. This reduction was significant relative to all other genotypes with reduced collagen V and XI (p < 0.001). These results are consistent with differential/coordinate roles for collagens V and XI in the regulation of fibril nucleation and initial assembly in early tendon development.
DISCUSSION
In the current study coordinate regulatory roles for collagens V and XI in fibril nucleation and initial assembly during tendon development were identified. This is the first demonstration of coordinate regulation involving collagens V and XI. Collagen V has been shown to regulate these early steps in dermis (25) and cornea (23), whereas a role for collagen XI has been demonstrated in cartilage (27). These data suggested that regulation of these key steps involved tissue-specific expression of collagen V or XI isoforms. The data presented herein indicate additional complexity in these regulatory interactions.
Tendon Structure and Function in a Col5a1+/− Mature Mouse Model of Classic EDS
Biomechanical testing of Col5a1+/− tendons suggested increased elasticity as indicated by a decrease in tissue stiffness. This functional phenotype is consistent with the joint laxity and hyper-extensibility seen in patients with the classic form of EDS (1). Our hypothesis was that the structural basis of this functional defect would be related to alterations in fibril structure comparable with those described in the dermis of this mouse model (25) and from patient biopsies (40). In both cases the dermis was composed of fibrils with larger diameters and a subpopulation that was large and structurally aberrant with irregular fibril cross-sectional profiles. In addition, fewer fibrils were assembled in the mouse model. It was expected that these changes, indicative of dysfunctional regulation of nucleation and initial fibril growth, also would be associated with the tendons that are compositionally similar to the dermis. The results in the tendon indicate relatively normal fibril structure in the mature Col5a1+/− mice. However, the biomechanical analysis documented a significant decrease in the cross-sectional area of the tendons from Col5a1+/− compared with wild type mice. The decrease in tendon size can be explained by a decrease in collagen V resulting in the nucleation and assembly of fewer fibrils. This is supported by assembly of fewer fibrils in the Col5a1+/− dermis and cornea with no change in collagen I relative to the control mice (22–24). The decreased stiffness observed in the tissue but not at the material level could be explained by the development of smaller tendons in the mutant mice.
Overall, the structural analysis demonstrating normal fibril structure in the Col5a1+/− tendon provided novel, unexpected results that suggested to us that regulation of tendon fibril nucleation and initial assembly involved other interactions in addition to collagen V. We hypothesized that collagen XI was a good candidate. The rationale supporting this is based on comparable regulatory roles for collagen XI in regulating the nucleation and assembly of collagen II-containing fibrils in cartilage (27, 41). Also in cartilage heterozygous for collagen XI (cho/+) two fibril subpopulations were observed; one comparable with wild type controls and a second larger, heterogeneous subpopulation (42). In addition, collagen XI is expressed during development in a variety of tissues (30, 43, 44). Finally, collagen XI was shown to interact with collagen I to regulate fibril assembly in in vitro assays (45). This work demonstrated that collagens I and XI formed heterotypic fibrils and that in vitro co-assembly of collagens I and XI resulted in the assembly of smaller diameter fibrils. Collagen XI facilitated nucleation with mixtures showing reduced lag times. All of these data suggested the potential for collagen V and XI regulatory roles in tendon development. The analysis of compound Col5a1+/−,Col11a1+/− tendons demonstrated a fibril phenotype comparable with that seen in the Col5a1+/− dermis and EDS patients, which supported this hypothesis.
Collagens V and XI in Tendon Development
The developmental expression of genes for collagen V and XI supported regulatory interactions involving both collagen V and XI forms. The transcripts for all five of the α-chains making up the major collagen V and XI isoforms, [α1(V)]2α2(V) and α1(XI)α2(XI)α3(XI), were comparably expressed during the early stages of tendon development (E18-P10) when the nucleation and subsequent assembly of immature protofibrils is the predominant assembly step (17, 46). However, in the mature tendon, α-chain transcripts for collagen V chains were dominant. This supports a coordinate role for collagens V and XI in early development. The collagen α1(XI) transcript was expressed at reduced levels but above base line in the mature tendon. Hybrid molecules composed of α-chains of collagens V and XI have been identified (19–21, 47). The expression of an α1(XI) transcript leaves open the possibility of a role for a hybrid collagen V and XI molecule, i.e. [α1(XI)]2α2(V) in both mature and developing tendons. The α1(XI) chain was not observed in our immunoblots in mature tendons; however, the sensitivity of the assay does not allow us to rule out a potential role for this form.
Altered Collagen V and XI Expression Alters Fibril Assembly during Early Tendon Development
Comparable with the data from the mature mice, tendons from Col5a1+/− mice at E18 had relatively normal structures, whereas the Col11a1+/− tendons assembled larger and more irregular fibrils. These differences between heterozygous collagen V and XI tendons indicate synergistic rather than redundant roles. The compound heterozygous and single heterozygous tendons all assembled fewer fibrils than in the wild type controls. However, the single and compound mutants demonstrated a comparable reduction, again suggesting that the dysfunctional regulation is not the result of two closely related collagens with redundant regulatory roles.
Collagens V and XI Coordinately Regulate Fibril Nucleation and Assembly during Early Tendon Development
Tendons from Col11a1−/− mice had a fibril phenotype that was more severe than that observed in the compound heterozygous mice, with fewer and larger fibrils. There also was a distinct subpopulation of structurally aberrant fibrils with irregular fibril cross-sections or profiles. The effect of reducing collagen V by half in the absence of collagen XI was extremely severe. There was a significant drop in the number of fibrils assembled compared with all other genotypes. This indicates a synergistic role in control of fibril nucleation. In addition, fibril structure was markedly altered, with a greater number of large diameter fibrils with abnormal structures. This can be interpreted as an unregulated assembly of collagen I when the regulatory interactions involving collagens V and XI have become limiting. A completion of this analysis will require the studies of the collagen V-null tendon. Due to the critical role in nucleation within the embryonic environment, where there is a relatively low collagen concentration, loss of collagen V results in an embryonic lethal phenotype at E10, and these studies are not possible using the current mouse models. Development of conditional models will be required to address this issue.
Tendon Fibrillogenesis
Tendons are established at E14.5 of mouse development when fibrillogenesis begins and E18 is a period when fibrillar extracellular matrix is recognizable (48–50). From E14.5 to ∼P4, the tendon is composed of a homogeneous population of short, small diameter protofibrils. In contrast, the mature tendon contains fibrils with a heterogeneous population of large diameter fibrils (46). The assembly of mature fibrils requires linear and lateral growth of preformed protofibrils (17).
Nucleation initiates protofibril assembly resulting in the assembly of the short, small diameter protofibrils. Protofibrils are immature collagen fibrils with a typical 67-nm periodicity. However, they are short, 3–10 μm in length with tapered ends (51). The protofibrils are deposited and organized into fibers in the developing matrix. Second, the protofibrils assemble into mature, long, larger diameter fibrils via linear and lateral growth from the preformed protofibrils (52–54). Regulation of these steps involves changing expression patterns and interactions with fibril-associated collagens with interrupted triple helices (FACIT collagens), i.e. collagens XII and XIV and small leucine-rich proteoglycans, i.e. decorin, biglycan, fibromodulin, and lumican (31, 36, 46, 55, 56). These growth steps are blocked in the tendon until around P4–10 when significant linear and lateral growth is first observed (46).
Our model of tendon fibrillogenesis involves regulatory interactions at sequential steps (17). In the studies presented, fibril parameters at E18 are determined primarily by mechanisms involved in the regulation of protofibril nucleation and assembly. Although at P30, mature fibril parameters are determined by the structure of the building blocks, i.e. protofibrils as well as from mixing of subpopulations during lateral growth (25).
At E18, protofibril nucleation predominates relative to the other steps in fibril assembly. Genetic reductions in collagen V and/or XI in tendons at E18 resulted in abnormalities in three parameters; 1) the number of protofibrils, 2) changes in the diameter of protofibrils, and 3) alterations resulting in less cylindrical protofibrils, i.e. irregular cross-sections or profiles. These data indicate a mechanism whereby collagens V and XI regulate these parameters. The nucleators, i.e. collagens V and XI are directly responsible for determining the number of protofibrils assembled and the protofibril diameter. Collagens V and/or XI interact with collagen I and nucleate protofibril assembly, subsequently becoming incorporated into the heterotypic fibrils (22, 41, 57) thereby only functioning in one round of assembly. Therefore, reduced fibril number is a direct result of decreased collagen V and XI content. Protofibril diameter also is a regulated by collagens V and XI. It is possible that collagens V and XI have different interactions with collagen I resulting in unique composite structures that would result in different fibril structures (45). A decrease in nucleation events and constant collagen I tissue content results in more collagen I assembled into each protofibril resulting in larger diameters. Data from dermis indicate that there is a limit to protofibril diameter as collagen V is reduced (25). It is unclear whether this is due to intrinsic molecular properties dictated by molecular interactions or to the presence of fibril associated macromolecules such as small leucine-rich proteoglycans or FACIT collagens or some combination. A reduction in collagen V resulted in protofibrils with larger diameters and near circular fibril cross-sections. However, a second large, structurally aberrant subpopulation that was negative for anti-collagen V immunoreactivity was assembled. This suggests that limiting nucleation events leads to unregulated utilization of collagen I (24, 25). The heterozygous tendons all have near circular protofibril cross-sections with larger diameters than controls. This indicates that nucleation has not become limiting, and all collagen I can be assembled into fewer, larger diameter protofibrils. However, in the Col11a1−/− and Col11a1−/−,Col5a1+/− tendons a subpopulation of structurally aberrant protofibrils is assembled. Our interpretation is that this is a result of nucleation becoming limiting resulting in an unregulated utilization or sequestering of collagen I.
Mature fibrils are assembled by growth from protofibrils involving end-to-end and lateral fusion (17, 57, 58). Therefore, the structure of mature fibrils would be influenced by the structure of the protofibrils as well as incorporation of the structurally abnormal fibrils into the growth process. The irregular fibril cross-sections observed in the mature Col5a1+/−,Col11a1+/− could be the result from the incorporation of protofibrils with abnormal structure into the lateral growth step required during assembly of mature fibrils. We have demonstrated that this occurs in the dermis of the Col5a1+/− mouse model of EDS (25). In addition, branching fibrils were demonstrated during mouse tendon development resulting from fusion of portions of adjacent fibrils (59). It is likely that the lateral association and fusion involving regions of adjacent fibrils results from the lateral growth of protofibrils. Clearly, these fusions involving abnormal fibrils would also impact mature fibril structure. Lateral growth is controlled via interactions with leucine-rich proteoglycans (36, 46, 55, 56). These interactions prevent lateral fibril growth in early tendon development so the altered fibrils observed at E18 are likely to result solely from dysfunctional regulation of nucleation, initial fibril assembly, and perhaps collagen processing.
During tendon development, initial collagen assembly occurs in close association with the fibroblast surface (57, 60, 61). We can speculate that during normal development collagens V and/or XI are tethered to the fibroblast surface through direct, e.g. with integrins, or indirect, e.g. with fibronectin interactions. This possibility is supported by a recent analysis indicating that the NH2 non-collagenous domain of collagen V interacts with numerous matrix proteins that could generate such interactions (62). Alterations in collagens V and XI would compromise control of initial protofibril assembly and deposition by freeing the process from its normal regulatory domain. For instance, mutations in tenascin X lead to an EDS phenotype in the presence of normal collagen V (63, 64). However, the phenotype is relatively mild. The mechanism may be related to the uncoupling of fibril assembly from the fibroblast surface resulting from the absence of an indirect link to the fibroblast. The overall effect is dysregulation of protofibril assembly and ultimately defective tendon structure and function. Dissociation from the cell surface could impact regulation in several ways. First, a non-tethered nucleator would be expected to be less efficient in nucleating assembly of collagen I. In addition, having the initial assembly steps in a microdomain at the cell surface provides a mechanism whereby other processes important in fibril assembly such as procollagen processing can be fully integrated under cellular control. The efficiency of procollagen processing with altered collagen V and/or XI expression needs to be determined. It is known that retention of the N-propeptide of collagen I results in irregular fibril cross-sectional profiles in EDS patients with defects in processing of the NH2-propeptide of procollagen I as well as other genetic diseases with this defect (2, 65, 66). In vitro analyses also show defective regulation of cross-sectional profiles with retention of the procollagen I NH2-propeptide and more severe defects when both fully and partially processed collagen were present in the assay (67). This could at least partially explain the altered structure observed at E18 before the initiation of lateral fibril growth, with the reduction of collagens V and/or XI resulting in less efficient procollagen processing when the process is untethered from the fibroblast surface. Collagen V and XI interactions with the tendon fibroblast in this manner also allow the cellular positioning of newly assembled fibrils during development. The severe disruption of tendon architecture observed supports this suggestion.
Overall, our data indicate that coordinate interactions involving collagens V and XI regulate early steps in fibrillogenesis during tendon development. A mechanism involving synergistic interaction of collagens V and XI controlling fibril nucleation is proposed. This would provide regulation of initial protofibril assembly, growth of mature fibrils, and tendon function.
Supplementary Material
Acknowledgments
We gratefully acknowledge Diana Menezes, Biao Zuo, and Sheila Adams for expert technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AR044745 (NIAMS; to D. E. B.) and AR050950 (NIAMS, supporting the Penn Center for Musculoskeletal Disorders (to L. J. S.)). This work was also supported by National Research Service Award Post Doctoral Fellowship AR056937 (to S. M. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- EDS
- Ehlers-Danlos syndrome
- FDL
- flexor digitorum longus.
REFERENCES
- 1. Beighton P. (1992) in McKusick's Heritable Disorders of Connective Tissue (Beighton P. ed) pp. 189–251, Mosby, St. Louis, MO [Google Scholar]
- 2. Steinmann B. (2002) in Connective Tissue and Its Heritable Disorders (Royce P. ed) pp. 431–523, Wiley-Liss, Inc., New York, NY [Google Scholar]
- 3. Sokolov B. P., Prytkov A. N., Tromp G., Knowlton R. G., Prockop D. J. (1991) Hum. Genet. 88, 125–129 [DOI] [PubMed] [Google Scholar]
- 4. Wordsworth B. P., Ogilvie D. J., Sykes B. C. (1991) Br. J. Rheumatol. 30, 173–177 [DOI] [PubMed] [Google Scholar]
- 5. Burrows N. P., Nicholls A. C., Yates J. R., Gatward G., Sarathachandra P., Richards A., Pope F. M. (1996) J. Invest. Dermatol. 106, 1273–1276 [DOI] [PubMed] [Google Scholar]
- 6. Nicholls A. C., Oliver J. E., McCarron S., Harrison J. B., Greenspan D. S., Pope F. M. (1996) J. Med. Genet. 33, 940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toriello H. V., Glover T. W., Takahara K., Byers P. H., Miller D. E., Higgins J. V., Greenspan D. S. (1996) Nat. Genet. 13, 361–365 [DOI] [PubMed] [Google Scholar]
- 8. Wenstrup R. J., Langland G. T., Willing M. C., D'Souza V. N., Cole W. G. (1996) Hum. Mol. Genet. 5, 1733–1736 [DOI] [PubMed] [Google Scholar]
- 9. Burrows N. P., Nicholls A. C., Yates J. R., Richards A. J., Pope F. M. (1997) Clin. Exp. Dermatol. 22, 174–176 [PubMed] [Google Scholar]
- 10. De Paepe A., Nuytinck L., Hausser I., Anton-Lamprecht I., Naeyaert J. M. (1997) Am. J. Hum. Genet. 60, 547–554 [PMC free article] [PubMed] [Google Scholar]
- 11. Michalickova K., Susic M., Willing M. C., Wenstrup R. J., Cole W. G. (1998) Hum. Mol. Genet. 7, 249–255 [DOI] [PubMed] [Google Scholar]
- 12. Richards A. J., Martin S., Nicholls A. C., Harrison J. B., Pope F. M., Burrows N. P. (1998) J. Med. Genet. 35, 846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouma P., Cabral W. A., Cole W. G., Marini J. C. (2001) J. Biol. Chem. 276, 13356–13364 [DOI] [PubMed] [Google Scholar]
- 14. Schwarze U., Atkinson M., Hoffman G. G., Greenspan D. S., Byers P. H. (2000) Am. J. Hum. Genet. 66, 1757–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wenstrup R. J., Florer J. B., Willing M. C., Giunta C., Steinmann B., Young F., Susic M., Cole W. G. (2000) Am. J. Hum. Genet. 66, 1766–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boot-Handford R. P., Tuckwell D. S. (2003) Bioessays 25, 142–151 [DOI] [PubMed] [Google Scholar]
- 17. Birk D. E., Bruckner P. (2011) in The Extracellular Matrix: An Overview (Mecham R. P. ed) pp. 77–155, Springer-Verlag New York Inc., New York [Google Scholar]
- 18. Hoffman G. G., Branam A. M., Huang G., Pelegri F., Cole W. G., Wenstrup R. M., Greenspan D. S. (2010) Matrix Biol. 29, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleman J. P., Hartmann D. J., Ramirez F., van der Rest M. (1992) Eur. J. Biochem. 210, 329–335 [DOI] [PubMed] [Google Scholar]
- 20. Mayne R., Brewton R. G., Mayne P. M., Baker J. R. (1993) J. Biol. Chem. 268, 9381–9386 [PubMed] [Google Scholar]
- 21. Wu J. J., Weis M. A., Kim L. S., Carter B. G., Eyre D. R. (2009) J. Biol. Chem. 284, 5539–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birk D. E. (2001) Micron 32, 223–237 [DOI] [PubMed] [Google Scholar]
- 23. Segev F., Héon E., Cole W. G., Wenstrup R. J., Young F., Slomovic A. R., Rootman D. S., Whitaker-Menezes D., Chervoneva I., Birk D. E. (2006) Invest. Ophthalmol. Vis. Sci. 47, 565–573 [DOI] [PubMed] [Google Scholar]
- 24. Wenstrup R. J., Florer J. B., Brunskill E. W., Bell S. M., Chervoneva I., Birk D. E. (2004) J. Biol. Chem. 279, 53331–53337 [DOI] [PubMed] [Google Scholar]
- 25. Wenstrup R. J., Florer J. B., Davidson J. M., Phillips C. L., Pfeiffer B. J., Menezes D. W., Chervoneva I., Birk D. E. (2006) J. Biol. Chem. 281, 12888–12895 [DOI] [PubMed] [Google Scholar]
- 26. Seegmiller R., Fraser F. C., Sheldon H. (1971) J. Cell Biol. 48, 580–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y., Lacerda D. A., Warman M. L., Beier D. R., Yoshioka H., Ninomiya Y., Oxford J. T., Morris N. P., Andrikopoulos K., Ramirez F. (1995) Cell 80, 423–430 [DOI] [PubMed] [Google Scholar]
- 28. Li S. W., Prockop D. J., Helminen H., Fässler R., Lapveteläinen T., Kiraly K., Peltarri A., Arokoski J., Lui H., Arita M. (1995) Genes Dev. 9, 2821–2830 [DOI] [PubMed] [Google Scholar]
- 29. Aszódi A., Chan D., Hunziker E., Bateman J. F., Fässler R. (1998) J. Cell Biol. 143, 1399–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lincoln J., Florer J. B., Deutsch G. H., Wenstrup R. J., Yutzey K. E. (2006) Dev. Dyn. 235, 3295–3305 [DOI] [PubMed] [Google Scholar]
- 31. Ansorge H. L., Meng X., Zhang G., Veit G., Sun M., Klement J. F., Beason D. P., Soslowsky L. J., Koch M., Birk D. E. (2009) J. Biol. Chem. 284, 8427–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin T. W., Cardenas L., Soslowsky L. J. (2005) J. Biomech. 38, 99–105 [DOI] [PubMed] [Google Scholar]
- 33. Lin T. W., Cardenas L., Glaser D. L., Soslowsky L. J. (2006) J. Biomech. 39, 61–69 [DOI] [PubMed] [Google Scholar]
- 34. Derwin K. A., Soslowsky L. J., Green W. D., Elder S. H. (1994) J. Biomech. 27, 1277–1285 [DOI] [PubMed] [Google Scholar]
- 35. Christner P. J., Gentiletti J., Peters J., Ball S. T., Yamauchi M., Atsawasuwan P., Beason D. P., Soslowsky L. J., Birk D. E. (2006) J. Invest. Dermatol. 126, 595–602 [DOI] [PubMed] [Google Scholar]
- 36. Ezura Y., Chakravarti S., Oldberg A., Chervoneva I., Birk D. E. (2000) J. Cell Biol. 151, 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young B. B., Gordon M. K., Birk D. E. (2000) Dev. Dyn. 217, 430–439 [DOI] [PubMed] [Google Scholar]
- 38. Oxford J. T., Doege K. J., Horton W. E., Jr., Morris N. P. (1994) Exp. Cell Res. 213, 28–36 [DOI] [PubMed] [Google Scholar]
- 39. Zhang G., Young B. B., Birk D. E. (2003) J. Anat. 202, 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vogel A., Holbrook K. A., Steinmann B., Gitzelmann R., Byers P. H. (1979) Lab. Invest. 40, 201–206 [PubMed] [Google Scholar]
- 41. Blaschke U. K., Eikenberry E. F., Hulmes D. J., Galla H. J., Bruckner P. (2000) J. Biol. Chem. 275, 10370–10378 [DOI] [PubMed] [Google Scholar]
- 42. Xu L., Flahiff C. M., Waldman B. A., Wu D., Olsen B. R., Setton L. A., Li Y. (2003) Arthritis Rheum. 48, 2509–2518 [DOI] [PubMed] [Google Scholar]
- 43. Yoshioka H., Iyama K., Inoguchi K., Khaleduzzaman M., Ninomiya Y., Ramirez F. (1995) Dev. Dyn. 204, 41–47 [DOI] [PubMed] [Google Scholar]
- 44. Smith S. M., Birk D. E. (2010) Exp. Eye Res., doi: 10.1016/j.exer.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hansen U., Bruckner P. (2003) J. Biol. Chem. 278, 37352–37359 [DOI] [PubMed] [Google Scholar]
- 46. Zhang G., Young B. B., Ezura Y., Favata M., Soslowsky L. J., Chakravarti S., Birk D. E. (2005) J. Musculoskelet. Neuronal. Interact. 5, 5–21 [PubMed] [Google Scholar]
- 47. Fernandes R. J., Weis M., Scott M. A., Seegmiller R. E., Eyre D. R. (2007) Matrix Biol. 26, 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J., Schweitzer R. (2007) Development 134, 2697–2708 [DOI] [PubMed] [Google Scholar]
- 49. Watson S. S., Riordan T. J., Pryce B. A., Schweitzer R. (2009) Dev. Dyn. 238, 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schweitzer R., Zelzer E., Volk T. (2010) Development 137, 2807–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Birk D. E., Zycband E. I., Winkelmann D. A., Trelstad R. L. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 4549–4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Birk D. E., Zycband E. (1994) J. Anat. 184, 457–463 [PMC free article] [PubMed] [Google Scholar]
- 53. Birk D. E., Nurminskaya M. V., Zycband E. I. (1995) Dev. Dyn. 202, 229–243 [DOI] [PubMed] [Google Scholar]
- 54. Birk D. E., Zycband E. I., Woodruff S., Winkelmann D. A., Trelstad R. L. (1997) Dev. Dyn. 208, 291–298 [DOI] [PubMed] [Google Scholar]
- 55. Ameye L., Aria D., Jepsen K., Oldberg A., Xu T., Young M. F. (2002) FASEB J. 16, 673–680 [DOI] [PubMed] [Google Scholar]
- 56. Zhang G., Ezura Y., Chervoneva I., Robinson P. S., Beason D. P., Carine E. T., Soslowsky L. J., Iozzo R. V., Birk D. E. (2006) J. Cell. Biochem. 98, 1436–1449 [DOI] [PubMed] [Google Scholar]
- 57. Birk D. E., Linsenmayer T. F. (1994) in Extracellular Matrix Assembly and Structure (Yurchenco P. D., Birk D. E., Mecham R. P. eds) Academic Press, Inc, New York [Google Scholar]
- 58. Graham H. K., Holmes D. F., Watson R. B., Kadler K. E. (2000) J. Mol. Biol. 295, 891–902 [DOI] [PubMed] [Google Scholar]
- 59. Starborg T., Lu Y., Huffman A., Holmes D. F., Kadler K. E. (2009) Scand. J. Med. Sci. Sports 19, 547–552 [DOI] [PubMed] [Google Scholar]
- 60. Birk D. E., Trelstad R. L. (1986) J. Cell Biol. 103, 231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Canty E. G., Lu Y., Meadows R. S., Shaw M. K., Holmes D. F., Kadler K. E. (2004) J. Cell Biol. 165, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Symoens S., Renard M., Bonod-Bidaud C., Syx D., Vaganay E., Malfait F., Ricard-Blum S., Kessler E., Van Laer L., Coucke P., Ruggiero F., De Paepe A. (2010) Biochem. J. 433, 371–381 [DOI] [PubMed] [Google Scholar]
- 63. Mao J. R., Taylor G., Dean W. B., Wagner D. R., Afzal V., Lotz J. C., Rubin E. M., Bristow J. (2002) Nat. Genet. 30, 421–425 [DOI] [PubMed] [Google Scholar]
- 64. Bristow J., Carey W., Egging D., Schalkwijk J. (2005) Am. J. Med. Genet. C Semin. Med. Genet. 139C, 24–30 [DOI] [PubMed] [Google Scholar]
- 65. Colige A., Nuytinck L., Hausser I., van Essen A. J., Thiry M., Herens C., Adès L. C., Malfait F., Paepe A. D., Franck P., Wolff G., Oosterwijk J. C., Smitt J. H., Lapière C. M., Nusgens B. V. (2004) J. Invest. Dermatol. 123, 656–663 [DOI] [PubMed] [Google Scholar]
- 66. Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. (1971) Eur. J. Biochem. 23, 533–543 [DOI] [PubMed] [Google Scholar]
- 67. Hulmes D. J., Kadler K. E., Mould A. P., Hojima Y., Holmes D. F., Cummings C., Chapman J. A., Prockop D. J. (1989) J. Mol. Biol. 210, 337–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.