Abstract
Aggregation of Tau into amyloid-like fibrils is a key process in neurodegenerative diseases such as Alzheimer. To understand how natively disordered Tau stabilizes conformations that favor pathological aggregation, we applied single-molecule force spectroscopy. Intramolecular interactions that fold polypeptide stretches of ∼19 and ∼42 amino acids in the functionally important repeat domain of full-length human Tau (hTau40) support aggregation. In contrast, the unstructured N terminus randomly folds long polypeptide stretches >100 amino acids that prevent aggregation. The pro-aggregant mutant hTau40ΔK280 observed in frontotemporal dementia favored the folding of short polypeptide stretches and suppressed the folding of long ones. This trend was reversed in the anti-aggregant mutant hTau40ΔK280/PP. The aggregation inducer heparin introduced strong interactions in hTau40 and hTau40ΔK280 that stabilized aggregation-prone conformations. We show that the conformation and aggregation of Tau are regulated through a complex balance of different intra- and intermolecular interactions.
Keywords: Neurodegeneration, Protein Folding, Protein Stability, Single Molecule Biophysics, Tau
Introduction
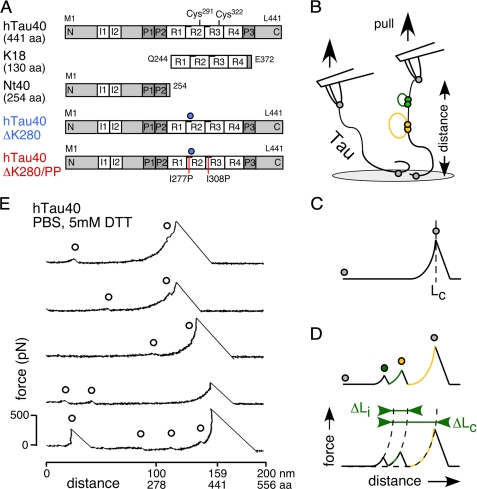
Amyloid forming proteins such as α-synuclein, the prion protein (1), and Tau (2) contain unstructured domains or belong to the family of natively unfolded or intrinsically disordered proteins (IDPs)3 (3). The aggregation of Tau into amyloid-like fibers, known as paired helical filaments (4, 5), is a key process in human protein aggregation diseases that are summarized as tauopathies. In vivo, Tau binds and stabilizes microtubules (MTs) to regulate the cellular MT network. The dissociation of Tau from MTs is controlled by the phosphorylation of Tau at multiple sites (6, 7). The longest human Tau isoform, hTau40 (441 amino acids (aa)), contains a ∼250-aa long N terminus of unknown function, whereas the C terminus comprises the Tau repeat domain, which encompasses four ∼31-aa long semi-conserved repeats (R1 to R4) flanked by proline-rich stretches (Fig. 1A). Both, binding to MTs and fibril assembly are mediated through the Tau repeat domain (8, 9).
FIGURE 1.
Tau isoforms and SMFS setup. A, five investigated isoforms and constructs of the Tau protein. hTau40 (441 aa), the longest human isoform in the central nervous system containing four repeats (4R) and two N-terminal inserts (I1 and I2). K18 (130 aa), consisting of the hTau40 4R domain. Nt40, the 254-aa long N-terminal fragment of hTau40. hTau40ΔK280, a pro-aggregant mutant with a Lys280 deletion (blue circle) in the second repeat (R2) of hTau40. hTau40ΔK280/PP, an anti-aggregant mutant of hTau40ΔK280, in which Ile277 and Ile308 where exchanged against two prolines (red lines). B, for SMFS, Tau proteins adsorbed to amino-functionalized glass supports were picked up by the AFM tip and stretched by the AFM cantilever (probe) until their connection to the tip or the glass (gray closed circles in B-D) ruptured. Tau consists of unstructured protein regions (black lines) with intramolecular interactions (green and yellow filled circles) that fold peptide stretches of different lengths (yellow and green lines). Recording deflection and distance of the cantilever during consecutive approach-retract cycles provides force-distance (F-D) curves of single Tau molecules. C, stretching of a molecule having no intramolecular interactions results in a F-D curve that shows one major detachment peak at the contour length, LC, of the fully extended molecule. D, intramolecular interactions (green and yellow filled circles) can establish force barriers that are detected as additional force peaks in the F-D curve. For every additional force peak, the contour length relative to the detachment peak, ΔLC, and the rupture force was derived. The distance to the next rupture peak, ΔLi, in an F-D curve gives the length of the polypeptide stretch that unravels upon breaking an interaction. E, F-D curves recorded upon stretching single hTau40 molecules in PBS containing 5 mm DTT. The curves show a major detachment peak at the contour length of the Tau molecule (1 aa ≈ 0.36 nm) plus smaller force peaks at shorter contour lengths (open black circles) originating from intramolecular interactions.
As most IDPs, Tau shows a high content of charged aa residues and a low hydrophobicity, which result in an extended solution conformation with a large radius of gyration (10). In solution, Tau has no stable secondary and tertiary structure, as judged by CD and Fourier transform infrared spectroscopy (10). The Stokes radius of Tau increases upon chemical denaturation with urea or guanidine hydrochloride (11, 12) indicating some limited folding. NMR experiments revealed transient secondary structures in hTau40 that partially interact with other polypeptide regions (13). Two hexapeptide motifs, PHF6* in R2 and PHF6 in R3, can adopt β-strand conformation and are predominantly responsible for Tau aggregation into fibrils (9, 14). Using Förster resonance energy transfer (11), the transient “paper clip”-like folding of the C and N termini onto the repeat domain was detected in hTau40. After removing the N- and C-terminal domains, the Tau repeat domain exhibits faster aggregation than full-length Tau (15). This suggests an inhibitory effect of the Tau termini on aggregation. It remains to be determined, which intra- and intermolecular interactions maintain the soluble state of Tau or promote the aggregation of Tau into fibers.
Tau aggregation can be triggered in different ways. In Alzheimer disease, hyperphosphorylated wild-type Tau accumulates in neurofibrillar tangles (16). In frontotemporal dementia, point mutations in the Tau gene lead to malfunction and high aggregation propensity of Tau (17, 18). For example, the “pro-aggregant” deletion mutation ΔK280 triggers pre-tangle aggregation of Tau in mice (19) and leads to spontaneous aggregation of purified Tau (20). In vitro, aggregation of soluble Tau can be triggered by polyanions like heparin, arachidonic acid micelles, acidic peptides, and RNA (21–23). It is thought that these polyanions compensate positive charges of Tau that normally prevent aggregation. Furthermore, Tau fibril assembly is attenuated by a disulfide bridge between Cys291 and Cys322 in the repeat domain (24) and at high ionic strengths (25, 26). This suggests that a complex interplay of interactions guides both fibril formation and fibril growth. Despite the overall hydrophilic nature of Tau, hydrophobic interactions are essential for the integrity of the amyloid-like fibril core (25, 27) that consists of stacked β-strands in the Tau repeat domain. Regardless of the origin of aggregation, Tau fibrils show similar morphologies in electron microscopy (EM) (4, 28) and atomic force microscopy (AFM) (29, 30).
We applied AFM-based single-molecule force spectroscopy (SMFS) to quantify the intramolecular interactions and the unfolding energy landscape of non-aggregated human Tau. Interactions folding polypeptide stretches of ∼19 and ∼42 aa were detected frequently in the repeat domain of hTau40. Diverse interactions randomly folding long polypeptide stretches >100 aa indicated irregular conformations of the terminal ends. The pro-aggregant mutant hTau40ΔK280 stabilized the folding of short polypeptide stretches, whereas the anti-aggregant mutant hTau40ΔK280/PP increased the folding of longer polypeptide stretches. Similarly, buffer conditions attenuating Tau aggregation increased interactions that folded polypeptide stretches >100 aa. Thus, Tau aggregation seems to be supported by specific interactions in the repeat domain and inhibited by irregular interactions of the protein termini. In both hTau40 and hTau40ΔK280, heparin strongly increased the number and strength of interactions. These results support a model in which heparin-induced electrostatic interactions stabilize conformations of Tau that are prone for aggregation. Our results provide insights into competing interactions that prevent or promote Tau folding and aggregation.
EXPERIMENTAL PROCEDURES
Chemicals and Proteins
Heparin (average molecular mass of 9 kDa), GdnHCl, aminopropyl-triethoxy-silane, and DTT were purchased from Sigma. Full-length human Tau isoforms hTau40, hTauΔK280, and hTauΔK280/PP and constructs K18 and Nt40 (Fig. 1D) were expressed in Escherichia coli and purified by heat treatment and ion exchange chromatography (Mono S, GE Healthcare) (31). Protein purities were analyzed by SDS-PAGE, protein concentrations by absorbance at 214 nm.
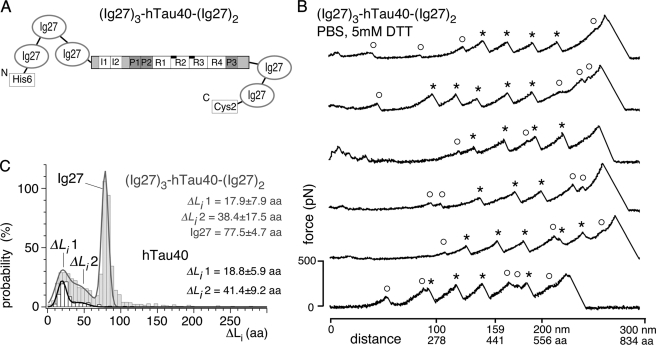
(Ig27)3-hTau40-(Ig27)2 (Fig. 3, supplemental Fig. S2) was generated by cloning hTau40 into a pRSET vector encoding a fusion protein of five identical titin immunoglobulin (Ig27) domains (modified according to Ref. 32), expressed in E. coli BL21(DE3) pLysS, and purified using immobilized metal ion affinity chromatography (HisTrap column, Amersham Biosciences). Purity and yield of (Ig27)3-hTau40-(Ig27)2 were checked by SDS-PAGE and Western blotting using anti-His antibody (Sigma). Ig27 domains were internal references in force-distance (F-D) curves of (Ig27)3-hTau40-(Ig27)2. All Tau samples were stored at −80 °C in PBS containing 1 mm DTT and thawed just before usage to prevent aggregation prior to the experiments.
FIGURE 3.
SMFS of the (Ig27)3-hTau40-(Ig27)2 construct. A, fusion protein (Ig27)3-hTau40-(Ig27)2 in which hTau40 is flanked by three N-terminal and two C-terminal Ig27 domains. The full extension of hTau40 was guaranteed when detecting four or more Ig27 unfolding events in the F-D curve. B, F-D curves attained from stretching single (Ig27)3-hTau40-(Ig27)2 molecules in PBS containing 5 mm DTT show a mixture of Ig27 (*) and hTau40 (○) unfolding events. C, length distribution of unraveled polypeptide stretches, ΔLi, detected in F-D curves of (Ig27)3-hTau40-(Ig27)2 (gray) resemble that detected of isolated hTau40 (black). Triple Gaussian fit to the ΔLi distribution of (Ig27)3-hTau40-(Ig27)2 (gray line; n = 69) reveals interaction contour lengths of ΔLi1 ∼18 aa and ΔLi2 ∼38 aa of the sandwiched hTau40, plus the characteristic contour length of the Ig27 domains of ∼78 aa. A double Gaussian fit to the ΔLi distribution determined for hTau40 only (black line; n = 223) reveals most probable ΔLi of ΔLi1 ∼19 aa and ΔLi2 ∼41 aa. n, gives the number of analyzed F-D curves.
AFM Sample Immobilization for SMFS Experiments
Glass coverslips (Menzel Glaeser, Germany) were piranha etched, amino functionalized by silanization in an aminopropyl-triethoxy-silane gas phase and baked at 80 °C for 30 min. Tau samples were diluted in PBS to a final concentration of 1 μm. For adsorption, ∼20 μl of the Tau solution was placed onto aminopropyl-triethoxy-silane glass for ∼60 min. Excess protein was removed by rinsing the sample with PBS.
SMFS
Tau was stretched using an AFM (Nanoscope IV, Veeco Metrology, Santa Barbara, CA) equipped with a PicoForce module and silicone nitride (Si3Ni4) cantilevers (BL-RC150 VB, Olympus Ltd., Japan; nominal spring constants ∼30 pN/nm; resonance frequencies in buffer ∼8 kHz). Real cantilever spring constants were determined in solution before each experiment using the equipartition theorem (33). Unless stated otherwise, stretching of Tau was performed in freshly prepared buffer (PBS, pH 7.4, 5 mm DTT). The buffer was exchanged every 2 h to account for evaporation and DTT degradation. Buffer with ∼500 mm monovalent ions contained PBS buffer (∼150 mm), 5 mm DTT, and 350 mm NaCl. Buffer of ∼50 mm ion strength consisted of 10 mm Tris, pH 7.4, 5 mm DTT, and 50 mm NaCl. SMFS on hTau40 without DTT was performed in pure PBS solution. For experiments in the presence of heparin, Tau adsorbed to amino-functionalized glass was incubated for 20 min in heparin buffer (PBS, 5 mm DTT, 0.33 mm heparin) and then stretched. For SMFS, the cantilever deflection (force) during approaching and retracting the cantilever stylus was recorded in a F-D curve. F-D curves of Tau were recorded at randomly chosen positions on the surface. The cantilever was pushed onto the glass surface with a force of ∼1 nN for 0–0.5 s to allow unspecific binding of Tau to the cantilever stylus. To initiate the stretching of the attached molecule, the stylus was retracted 200–800 nm from the surface with a constant velocity. Such approach-retract cycles were repeated until a statistically significant amount of F-D curves was obtained. Dynamic SMFS (DFS) experiments were performed in buffer (PBS, 5 mm DTT) at 5 different pulling velocities (104, 249, 497, 1249, and 2490 nm/s for hTau40 and hTau40ΔK280; 104, 256, 497, 1090, and 2490 nm/s for hTau40ΔK280/PP). For each experiment and pulling velocity, at least three different cantilevers were used.
Selection and Analysis of SMFS and DFS Data
The worm-like chain model of elastic polymers (34) describes the force needed to elongate a polymer chain with the contour length, LC. Applying this model to proteins, the polymer persistence length, lP ≈ 0.4 nm, describes the peptide bond length in the polypeptide backbone (monomer length per aa = 0.36 nm) and fits the stretching of most polypeptides by SMFS (36, 37). Using the worm-like chain model, a F-D curve (Fig. 1E) can be transferred into a F-LC curve (supplemental Fig. S1B) by calculating LC for each data point (36). This approach proved useful for handling the large number of F-D curves recorded for proper statistics. 1% of F-D curves could not be described by a worm-like chain using lP of 0.4 nm and were excluded from analysis. F-D curves obtained from hTau40 adsorbed to amino-functionalized glass, plain glass, gold, and mica showed no difference excluding artifacts induced by nonspecific protein-support interactions. Amino-functionalized glass provided the highest yield of F-D curves from full-length Tau and was chosen as supporting surface for the experiments.
Stretching of a non-self-interacting polymer chain results in a F-D curve, in which the force rises non-linearly with the chain extension until the “detachment peak” describes the detachment of the molecule from the support or the cantilever stylus. Sufficiently strong intramolecular interactions established in the Tau polypeptide were detected as additional force peaks in the F-D curve (or spikes in the F-LC curve (36)). A force peak quantifies the force required to rupture the interaction. The distance between two successive force peaks, ΔLi, gives the length of the polypeptide segment that becomes unfolded upon breaking the interaction (Fig. 1D). The relative contour length, ΔLC, denotes the distance of a force peak to the detachment peak. This distance was used to classify the interactions detected in Tau.
Single Tau molecules were picked up by the cantilever stylus at random positions along their polypeptide chain. To analyze the stretching of the entire molecule, we analyzed only F-LC curves having contour lengths of 420 ± 40 aa for hTau40 (441 aa), hTau40ΔK280 (440 aa), and hTau40ΔK280/PP (440 aa), contour lengths of 800 ± 70 aa for the Tau fusion protein (Ig27)3-hTau40-(Ig27)2 (849 aa), contour lengths of 130 ± 20 aa for K18 (130 aa), and contour lengths of 250 ± 30 aa for Nt40 (254 aa). To detect force peaks up to 250 pN (typical forces at which proteins unfold (37, 75)), F-LC curves had to show detachment peaks >250 pN. To calculate density maps (supplemental Figs. S1C, S2, B and C, S3, C and F, and S5, C and F), F-LC curves matching both length and force criteria were manually aligned on their detachment peaks. The position of force peaks in F-LC curves was determined using a custom made procedure (IgorPro, Wavemetrics). Probabilities and most probable positions of rupture peaks were fitted by triple- (hTau40, hTau40ΔK280) and multi-Gaussian functions (hTau40ΔK280/PP) to the ΔLC distributions. Peak combinations (supplemental Fig. S6 and “Materials”) in hTau40, hTau40ΔK280, and hTau40ΔK280/PP were analyzed by counting rupture peaks at ΔLC of 19 ± 6, 42 ± 6, and 73 ± 9 aa in single F-LC curves. Combinations involving other peak distances (ΔLC) were neglected for low statistical relevance (<2% probability). The unfolding energy barriers of interactions at ΔLC values of 19, 42, and 73 aa were determined as described (38) using the DFS data (see supplemental “Materials”).
RESULTS
Human Tau Displays Variable Intramolecular Interactions
Tau molecules fold kinetically unstable β-stranded and α-helical structures in short structural regions (∼20% of the polypeptide) (12). Transient interactions of these short regions may induce the folding of both C and N termini onto the repeat domain (11). To quantify the probability and strength of the interactions stabilizing the folding of Tau, we mechanically stretched single hTau40 molecules by SMFS (Fig. 1B). PBS buffer containing 5 mm DTT provided physiological electrolyte, pH and reducing conditions. 32% of all force-distance (F-D) curves (n = 312) described the stretching of an unstructured polymer followed by its detachment from the AFM stylus or support (Fig. 1C). The remaining 68% F-D curves detected additional force peaks at various stretching distances (Fig. 1E). Each force peak indicated the rupture of an intramolecular interaction that stabilized the folding of a polypeptide segment of hTau40 (Fig. 1B). Upon rupturing the interaction, this polypeptide segment was released (Fig. 1D). Despite whether such an interaction resulted in a secondary structure or not, we used the term “fold” to describe the polypeptide segment stabilized by a certain interaction.
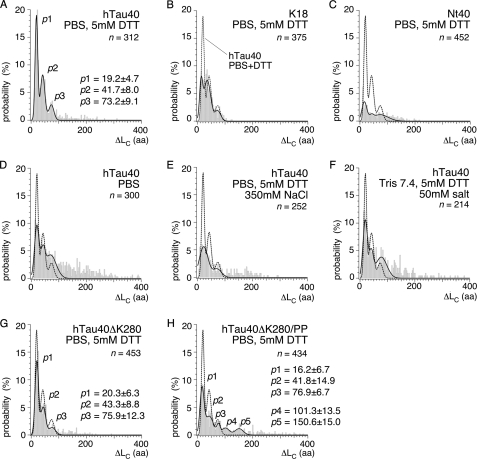
We characterized each rupture peak in the F-D curves by its distance to the detachment force peak, ΔLC (Fig. 2A, supplemental Table S1). In ∼15% of all hTau40 molecules, rupture peaks occurred at various ΔLC positions >100 aa (n = 312). The rupture force of these interactions was 107.6 ± 47.4 pN (most probable ± S.D.; n = 45; pulling velocity 875 nm/s; supplemental Table S2). At shorter ΔLC distances, three prominent force peaks p1, p2, and p3 were reproducibly detected at ΔLC of 19.2 ± 4.7 (most probable ± S.D.; 52%), 41.7 ± 8.0 (34%), and 73.2 ± 9.1 aa (14%), respectively. These interactions ruptured at forces of 90.8 ± 39.8 pN (p1), 77.1 ± 39.2 pN (p2), and 129.8 ± 51.7 pN (p3). The length of the polypeptide segments, ΔLi, that unfolded upon breaking interactions p1 and p2 were determined as 18.5 ± 3.6 aa (n = 269), and the of interaction of p3 was 25.1 ± 1.8 aa (n = 44; supplemental Fig. S1, D and E).
FIGURE 2.
ΔLC distribution of rupture peaks in hTau40, repeat domain construct K18, N-terminal fragment Nt40, and mutant proteins hTau40ΔK280 and hTau40ΔK280/PP. Contour lengths relative to the detachment peak, ΔLC, at which interactions were detected upon mechanically stretching hTau40 (A), K18 (B), and Nt40 (C) molecules in PBS containing 5 mm DTT. The most probable positions of rupture peaks were determined for each condition and construct from triple-Gaussian fits (solid lines in A–H) to the ΔLC distributions. For hTau40 in PBS/DTT (A), the most probable rupture peak positions were determined at p1 ∼19 aa, p2 ∼42 aa, and p3 ∼73 aa. D, contour lengths detected in hTau40 upon stretching in the absence of DTT (pure PBS); E, in buffer of 500 mm ionic strength (PBS/DTT + 350 mm NaCl); and F, in buffer of ∼50 mm ionic strength (Tris + 50 mm NaCl). G, contour lengths in the pro-aggregant mutant hTau40ΔK280; and H, the anti-aggregant mutant hTau40ΔK280/PP upon stretching in PBS + 5 mm DTT. The most probable positions of rupture peaks were determined from Gaussian fits (black lines) to the ΔLC distributions as p1 ∼20 aa, p2 ∼43 aa, and p3 ∼76 aa for hTau40ΔK280 (G), and as p1 ∼16 aa, p2 ∼42 aa, p3 ∼77 aa, p4 ∼101 aa, and p5 ∼151 aa for hTau40ΔK280/PP (H) (supplemental Table S1). n, gives the number of analyzed F-D curves.
To prove that these interactions did not originate from protein-surface adhesion but resembled intramolecular interactions, we stretched hTau40 attached to different supports (mica, gold, and highly ordered pyrolytic graphite; data not shown). These control experiments revealed similar force peak patterns and probabilities as observed for hTau40 attached to amino-functionalized glass (Fig. 2A).
To further investigate the specificity of the detected interactions, we engineered a fusion protein of hTau40 flanked by five identical immunoglobulin 27 (Ig27) molecules, each having 89 residues, termed (Ig27)3-hTau40-(Ig27)2 (Fig. 3A). In F-D curves of this construct, both Ig27 fingerprint and hTau40 force peaks co-occurred (Fig. 3B). The mechanical unfolding of each Ig27 resulted in a characteristic force peak of 226.5 ± 17.8 pN (n = 69, pulling velocity 1000 nm/s; supplemental Fig. S2D) that unfolded a polypeptide stretch of ΔLi = 77.5 ± 4.7 aa (∼28 nm) (Fig. 3C). This is typical for the unfolding of Ig27 (39–42). Observing the unfolding of at least four Ig27 domains (marked asterisk in Fig. 3B) in a F-D curve proved the stretching of the “sandwiched” hTau40. The interactions recorded upon stretching the sandwiched hTau40 (marked ○ in Fig. 3B) ruptured mostly before the first Ig27 at various positions and forces. However, because interactions in hTau40 ruptured at forces (∼50 to 300 pN) similar to Ig27 (∼150 to 250 pN), some hTau40 interactions unfolded between and after the unfolding force peaks of Ig27. The most probable lengths of unfolded polypeptide stretches, ΔLi, were determined as ΔLi1 = 17.9 ± 7.9 aa and ΔLi2 = 38.4 ± 17.5 (n = 69) aa for sandwiched hTau40, and ΔLi1 = 18.8 ± 5.9 aa and ΔLi2 = 41.1 ± 9.2 aa (n = 227) for isolated hTau40 (Fig. 3C). This similarity of hTau40 interactions detected in (Ig27)3-hTau40-(Ig27)2 and isolated hTau40 indicated their specificity for the stretching of individual hTau40 molecules.
Whereas interactions stabilizing the folding of >100-aa long polypeptide stretches revealed diverse force patterns, the three reproducible rupture peaks p1, p2, and p3 at ∼19, ∼42, and ∼73 aa stabilized three folds, fold1, fold2, and fold3, of ∼19-, ∼19-, and ∼25-aa long polypeptide segments, respectively (supplemental Fig. S1F). In the next step we aimed to localize these interactions.
Intramolecular Interactions Predominantly Fold the Repeat Domain
To structurally dissect the interactions of hTau40, we separately stretched the repeat domain construct K18 (130 aa) and the N-terminal part Nt40 (254 aa) of hTau40 (Fig. 1A). About 80% of the K18 molecules (n = 375; supplemental Fig. S3, D–F) showed similar force peak patterns as hTau40 (Fig. 2B). However, the probability to detect these interactions decreased from 52% in hTau40 to 21% in K18 for p1, from 34 to 29% for p2, and from 14 to 10% for p3 (supplemental Table S1). In K18, these interactions ruptured at forces of 142.1 ± 87.6 pN (p1), 112.4 ± 91.5 pN (p2), and 46.5 ± 34.2 pN (p3) (supplemental Table S2). Rupture peaks of K18 detected at ΔLC >120 aa had to be excluded from analysis because they overlapped with nonspecific stylus-support interactions (43) that occur within the first 15 aa (∼5 nm) of F-D curves. In contrast to K18, ∼60% of Nt40 molecules (n = 452) showed no force peaks (Fig. 2C, supplemental Fig. S3, A–C). The remaining 40% F-D curves showed rupture peaks distributed over the entire contour length of Nt40 (∼250 aa). In Nt40, force peaks p1, p2, and p3 were detected with much reduced probability (∼8%, supplemental Table S1) and strength (80.0 ± 20.3 pN (p1), 53.8 ± 66.6 pN (p2), 47.0 ± 32.9 pN (p3), supplemental Table S2). Interactions at ΔLC >100 aa reached forces of 33.8 ± 9.9 pN and were detected with similar probability (0.13/molecule) as for hTau40 (0.15/molecule).
The N-terminal fragment, Nt40, established random interactions of low probability suggesting that it was largely unstructured. The pronounced force peaks p1, p2, and p3 in hTau40 and K18 suggested that fold1, fold2, and fold3 were mainly established in the repeat domain. However, when unfolding the repeat domain construct, K18, the probability to detect fold1, fold2, and fold3 decreased compared with full-length hTau40. This showed that the frequency of interactions in the repeat domain increased in the presence of the termini. To further elucidate these interactions, we next stretched hTau40 in the presence of the disulfide bridge between R2 and R3 (24).
Cross-linking Repeats R2 and R3 Increases Intramolecular Interactions
The in vitro aggregation rate of Tau decreases in the absence of DTT (24). It is assumed that the Cys291–Cys322 disulfide bridge between R2 and R3 (Fig. 1A) established in oxidizing conditions (no DTT) enables alternate conformations of the repeat domain that disfavor aggregation. The mechanical rupturing of a disulfide bond requires forces of 1–2 nN (44) and could be discernable from the much lower forces stabilizing the folds of Tau (Fig. 1E, supplemental Table S2). In the following we probed the interactions of hTau40 in the absence of DTT.
The average contour length of mechanically stretched hTau40 decreased by ∼34 aa in the absence of DTT (supplemental Fig. S4E). This suggested that the Cys291–Cys322 bond has been formed (24). The frequency of interactions substantially increased from 1.7/molecule in PBS + DTT to 2.5/molecule in the absence of DTT (Fig. 2D). Force peaks with contour lengths of ΔLC >100 aa increased ∼6-fold from 0.15/molecule to 0.96/molecule in the absence of DTT. The wide distribution of these force peaks suggests that reducing the Cys291–Cys322 bond increased the frequency of interactions established between random polypeptide regions. The probability to detect rupture peak p1 (ΔLC ∼19 aa) decreased from 52% in presence of DTT to 29% in the absence of DTT, that for rupture peak p2 (∼42 aa) decreased from 34 to 32%, and that for p3 (∼73 aa) increased from 14 to 22% (supplemental Table S1). Thus, the structural fold1 (∼19 aa) of the Tau repeat region was established less frequently in the presence of the disulfide bond bridging R2 and R3, whereas structural fold2 remained mainly unaffected, and fold3 occurred at slightly increased probability. Next, we characterized electrostatic interactions contributing to the folding of the Tau repeat domain.
Intramolecular Interactions Are Partly Electrostatic
At elevated electrolyte concentrations, counterions start compensating electrostatic interactions of the polypeptide (45). When increasing the monovalent electrolyte concentration from ∼150 mm (PBS, 5 mm DTT) to ∼500 mm (PBS, 5 mm DTT, 350 mm NaCl), the frequency of force peaks in hTau40 decreased from 1.7/molecule to 1.2/molecule (Fig. 2E). Similarly, the probability to detect force peaks p1 and p2 decreased from 52 to 17% and from 34 to 16%, respectively (supplemental Table S1). Decreasing the monovalent electrolyte concentration to ∼50 mm (10 mm Tris-HCl, pH 7.4, 5 mm DTT, 50 mm NaCl; Fig. 2F) increased the overall frequency of force peaks from 1.7/molecule (PBS, 5 mm DTT) to 2.1/molecule, and decreased that of p1 from 52 to 31% and p2 from 34 to 26% (Fig. 2D), respectively. The average forces of p1 and p2 increased for both high (PBS, 5 mm DTT, 350 mm NaCl) and low (10 mm Tris-HCl, 5 mm DTT, 50 mm NaCl) electrolyte concentrations (supplemental Table S2 and Fig. S4, B and C). These results indicate that the stability of the folds detected by force peaks p1 (∼19 aa) and p2 (∼42 aa) partly depend on electrostatic interactions. However, the majority of interactions stabilizing these folds was not affected by the electrolyte concentration and may thus not be of electrostatic nature.
The strength of interactions at p3 and ΔLC >100 aa decreased at ∼500 mm electrolyte concentrations from 129.5 ± 51.7 and 107.6 ± 47.4 pN (PBS, 5 mm DTT) to 55.7 ± 36.4 and 51.7 ± 29.5 pN, respectively (supplemental Table S2 and Fig. S4B). In ∼50 mm NaCl, the probability to detect force peak p3 increased from 14 to 21% and force peaks >100 aa from 15 to 57% (supplemental Table S1). This suggests that interactions forming fold3 and interactions of the termini >100 aa are largely of electrostatic nature. This finding is in agreement with NMR experiments indicating that electrostatic interactions between the N terminus and the hTau40 repeat domain disappear at 600 mm NaCl (12). To specify which interactions of the repeat domain play a role during Tau oligomerization and fibrillization, we investigated mutants that favor or disfavor Tau aggregation.
Mutations Promote Different Interactions and Folds
Deleting Lys280 of repeat R2 leads to spontaneous aggregation of hTau40ΔK280 (20). One hydrophobic and one charged surface of the β-strand formed by the hexapeptide motif PHF6* in R2 favors fibril formation of hTau40ΔK280 (46). To avoid formation of β-strands, the anti-aggregant isoform hTau40ΔK280/PP (19, 20) was generated by exchanging Ile277 of PHF6* and Ile308 of PHF6 in R3 against prolines. In the following we quantified intramolecular interactions of hTau40ΔK280 and hTau40ΔK280/PP.
The distribution of force peaks in F-D curves of hTau40ΔK280 (Fig. 2G and supplemental Fig. S5, A–C) was remarkably similar to that of hTau40 (Fig. 2A). Peaks p1, p2, and p3 occurred at positions and probabilities similar to hTau40 (supplemental Table S1). However, compared with hTau40, the rupture force of p1 increased from 90.8 ± 39.8 to 129.3 ± 60.2 pN and that of p2 from 77.1 ± 39.2 to 102.2 ± 48.1 pN, respectively (supplemental Table S2). In contrast, the rupture force of p3 decreased from 129.8 ± 51.7 to 70.3 ± 17.7 pN, and that of force peaks at ΔLC >100 aa decreased from 107.6 ± 47.4 to 74.6 ± 21.2 pN.
In case of hTau40ΔK280/PP, peaks p1, p2, and p3 occurred at reduced probabilities compared with hTau40 and hTau40ΔK280 (Fig. 2H, supplemental Table S1, and supplemental Fig. S5, D–F). The frequency of interactions folding long polypeptide stretches >100 aa increased from 0.15/molecule to 0.38/molecule (supplemental Fig. S5, A–C). Two new force peaks became prominent at ΔLC of 101.3 ± 13.5 and 150.6 ± 15.0 aa (Fig. 2H).
Mutants hTau40ΔK280 and hTau40ΔK280/PP showed the three force peaks, p1, p2, and p3, at similar contour lengths as hTau40. In the case of hTau40ΔK280, the interaction strength stabilizing fold1 and fold2 increased, whereas that stabilizing fold3 and longer peptide stretches decreased. hTau40ΔK280/PP exhibited a reduced probability of fold1, fold2, and fold3 but favored the folding of long polypeptide stretches. Two prominent folds of hTau40ΔK280/PP involved ∼101- and ∼151-long polypeptide stretches.
Heparin Strengthens Intramolecular Interactions
In vitro aggregation of hTau40 can be induced in the presence of sulfated glycosaminoglycans, such as heparin (47) or other polyanions (22). Sulfated glycosaminoglycans were shown to enhance the phosphorylation of Tau by different kinases (48, 49), to inhibit Tau binding to MTs, and to co-localize with hyperphosphorylated Tau in AD brain tissue (21). To elucidate to which extent heparin-binding changes the interactions in Tau, we characterized hTau40, hTau40ΔK280, and hTau40ΔK280/PP in the presence of 0.33 mm heparin (PBS + DTT + heparin).
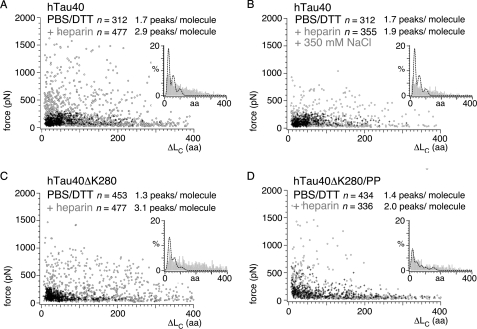
Stretching hTau40 and hTau40ΔK280 in the presence of heparin revealed a significant increase of force peaks from 1.7/molecule (PBS + DTT) to 2.9/molecule for hTau40 (PBS + DTT + heparin; Fig. 4A, supplemental Table S1), and from 1.3/molecule to 3.1/molecule for hTau40ΔK280 (Fig. 4C). In the presence of heparin, the force peaks were distributed over the entire contour length of hTau40 and reached rupture forces up to 1500 pN. The probability to detect force peak p1 (∼19 aa) decreased in hTau40 from 52 to 22%, and in hTau40ΔK280 from 48 to 21% (supplemental Table S1). In contrast, the probability to detect p3 (∼73 aa) increased from 14 to 24% and from 10 to 27%, respectively. Heparin strongly increased the number of interactions detected at ΔLC >100 aa from 0.15/molecule to 1.3/molecule in hTau40 and from 0.14/molecule to 1.87/molecule in hTau40ΔK280. To prove the electrostatic nature of the heparin-induced interactions, we stretched hTau40 in the presence of heparin and ∼500 mm monovalent electrolyte (PBS + DTT + heparin + 350 mm NaCl; Fig. 3B). 90% F-D curves of hTau40 (n = 355) resembled those recorded in ∼500 mm monovalent electrolyte without heparin (PBS + DTT + 350 mm NaCl; supplemental Fig. S4B). Most force peaks with rupture forces >300 pN disappeared and the overall number of force peaks dropped from 2.9/molecule (PBS + DTT + heparin) to 1.9/molecule (PBS + DTT + heparin + 350 mm NaCl).
FIGURE 4.
Heparin-induced interactions in hTau40, hTau40ΔK280, and hTau40ΔK280/PP. Scatter plots of rupture forces detected upon stretching hTau40 (A), hTau40ΔK280 (C), and hTau40ΔK280/PP (D) in PBS containing 5 mm DTT in the absence (black crosses in A and B, n = 312; C, n = 453; and D, n = 434; pulling velocity 875 nm/s) and presence (open gray circles in A, n = 477; C, n = 244; and D, n = 336; pulling velocity 1000 nm/s) of 0.33 mm heparin. Insets show the distributions of ΔLC in the presence (gray bars) of heparin. For comparison, the fits to the ΔLC distributions in the absence of heparin (black dashed lines) are shown. B, scatter plot of force peaks detected when stretching hTau40 in the presence of 0.33 mm heparin and ∼500 mm electrolyte concentrations (PBS + 5 mm DTT + 350 mm NaCl + 0.33 mm heparin; open gray circles (B, n = 355). Heparin binding induced a large number of high force interactions (300–1500 pN) over the full range of contour lengths in hTau40 (A) and hTau40ΔK280 (C). The ensemble of interactions in hTau40ΔK280/PP (D) showed only minor changes. Addition of heparin at ∼500 mm electrolyte reversed most of the heparin-induced interactions in B. n, gives the number of analyzed F-D curves.
When stretching hTau40 and hTau40ΔK280 in the presence of heparin, the rupture force distributions tailed toward high forces of ∼1500 pN (Fig. 4, A and C, insets). In contrast, the force peak pattern and the rupture forces of hTau40ΔK280/PP showed only minor deviations upon addition of heparin (Fig. 4D).
Exposure of hTau40 and hTau40ΔK280 to heparin increased the frequency of strong electrostatic interactions established along the entire polypeptide chain. The heparin-induced decrease in force peaks that unravel short peptide stretches was similarly observed at enhanced electrolyte concentrations (PBS, 5 mm DTT, 350 mm NaCl; Fig. 2E) and may, thus, be attributed to the ionic character of heparin.
Energy Landscape of Folded Conformations
The force required to unfold a polypeptide depends on the applied force-loading rate (50, 51). Quantifying the most probable unfolding forces over a range of force-loading rates enables estimation of the width and height of the free unfolding energy barrier stabilizing a folded structure. From this barrier, the kinetic and mechanical properties of the folded structure can be derived (38, 52) (supplemental Fig. S7A). In the following, we determined the energy barriers of the three folds, fold1, fold2, and fold3, detected by force peaks p1, p2, and p3. Therefore, we unfolded hTau40, hTau40ΔK280, and hTau40ΔK280/PP at five different pulling velocities. Fitting of the DFS spectra (supplemental Fig. S7, B–L) approximates the parameters characterizing the unfolding energy barriers (see “Experimental Procedures” and supplemental “Materials”).
In hTau40, the equilibrium unfolding rates, k0, of structure fold1, fold2, and fold3 ranges were determined as 0.12 s−1 (fold1), 0.17 s−1 (fold2), and 0.13 s−1 (fold3). These rates slightly decreased in hTau40ΔK280 ranging from 0.05 s−1 (fold1) to 0.06 s−1 (fold2 and fold3). Accordingly, the transition barrier heights, ΔG‡, varied in hTau40 between 20.2 kBT (fold2) and 20.5 kBT (fold1 and fold3), and ranged from 21.2 kBT (fold2 and fold3) to 21.5 kBT (fold1) in hTau40ΔK280 (supplemental Table S3). In hTau40ΔK280/PP, the unfolding rates and barrier heights of fold1, fold2, and fold3 were similar to hTau40 and hTau40ΔK280 (supplemental Table S3, supplemental Fig. S7, K–M).
The distance, xu, between folded and transition state approximates the width of the unfolding energy barrier (supplemental Fig. S7A). Together, ΔG‡ and xu were used to estimate the spring constants, k, and mechanical properties of the folded structures. Fold1 had a xu of ∼0.2 nm in hTau40, and a xu of ∼0.1 nm in hTau40ΔK280 and hTau40ΔK280/PP. Thus, k of fold1 increased ∼3-fold from 4.2 N/m in hTau40 to 14.5 N/m in hTau40ΔK280 and 13.9 N/m in hTau40ΔK280/PP. In contrast, k of fold2 and fold3 decreased from 13.7 and 13.9 N/m in hTau40 to 5.4 and 4.8 N/m in hTau40ΔK280, and to 5.4 and 1.5 N/m in hTau40ΔK280/PP (supplemental Table S3), respectively.
Compared with hTau40, the three folds of the repeat domain showed a slightly increased lifetime (∼1/k0) in hTau40ΔK280. In hTau40ΔK280 and hTau40ΔK280/PP fold1 exhibited a higher structural rigidity, whereas fold2 and fold3 showed lower rigidity than in hTau40. Both results suggest that the deletion of Lys280 stabilized fold1 and softened fold2 in the repeat domain of hTau40. However, the proline insertions that prevent hTau40ΔK280/PP from aggregation did not affect fold1 and fold2. In contrast, fold3 of the hTau40ΔK280/PP mutant showed a ∼3-fold increased lifetime and a ∼9-fold reduced rigidity (∼κ), indicating a softening of fold3.
DISCUSSION
Different Interaction Sites Contribute to Order and Disorder
Intrinsic disorder plays a pivotal role for the aggregation of proteins into amyloid-like structures (53). Only ∼70% of hTau40 molecules established intramolecular interactions strong enough (>10 pN) to be detected by SMFS. The force peak pattern of Tau appeared highly variable. Reproducibly occurring force peaks reflected reproducibly folded structures that correlated with the hTau40 repeat domain. Randomly distributed force peaks reflected interactions dispersed along the polypeptide. These insights confirm the IDP character of Tau, which is largely characterized by random coil-like conformations with a propensity to adopt certain residual conformations (54).
Hierarchy of Folding
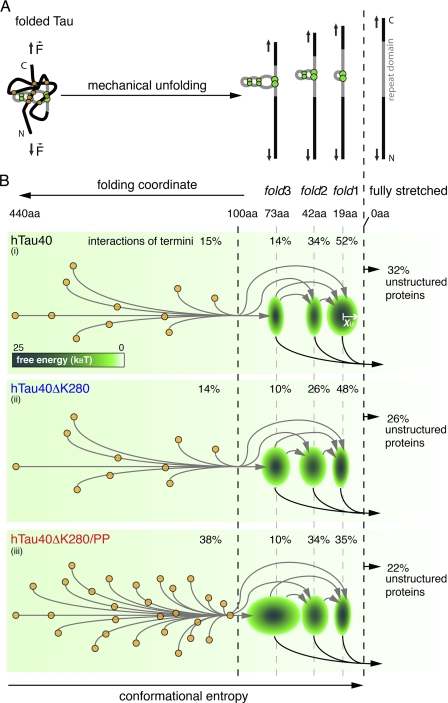
The sequence in which serial interactions of a polypeptide rupture during mechanical unfolding relies on their interaction strengths (51). Generally, weak interactions rupture before stronger ones. However, if a structure stabilized by a weak interaction is embedded in a more stable structure, the stronger interaction ruptures before the weaker one. In hTau40, interactions folding the shorter polypeptide stretches fold1 (p1, ∼19 aa, ∼90 pN) and fold2 (p2, ∼42 aa, ∼80 pN) required lower unfolding forces than unfolding the longer polypeptide stretches of fold3 (p3, ∼73 aa, ∼130 pN) and >100 aa (∼100 pN) (supplemental Table S2). Thus, fold1 and fold2 were embedded into fold3, which was stabilized by stronger interactions (supplemental Fig. S1F). Force peaks detected at contour lengths >100 aa reflect interactions of the Tau termini. Our findings likely combine two current Tau folding models. One model proposes various interactions of transient small structures (6 β-strands, 2 α-helices, 3 polyproline II helices (PPII)) formed in the Tau repeats and the flanking regions, which are spaced by <50 aa (12). The other paper clip model (11) describes the transient interaction of the Tau termini with the repeat domain and with each other. This paper clip folding of Tau involves longer polypeptide stretches of ≥100 aa.
Specific and Unspecific Interactions of the Repeat Domain
Approximately 70% of full-length Tau molecules established interactions that fold 19- (fold1) and 42-aa (fold2) long polypeptide stretches of the repeat domain. At high (500 mm) and low (50 mm) monovalent electrolyte concentrations, the frequency of these two characteristic folds decreased (Fig. 1, E and F, supplemental Table S1). The frequency of fold3 (∼73 aa) decreased slightly with increasing electrolyte and increased with decreasing electrolyte concentration. This indicates that fold1, fold2, and fold3 are stabilized by electrostatic and by other interactions, e.g. hydrophobic or polar ones. Electrostatic interactions folding the repeat domain may involve negatively charged residues in R4 (Glu338, Glu342, Asp345, Asp348) and positive charges such as Lys267/His268, Lys298/His299, His329/His330/Lys331, and His362 (9).
The stability of the most frequent folds, fold1 and fold2, increased in hTau40ΔK280 and hTau40ΔK280/PP (supplemental Table S2). Thus, the ΔK280 mutation strengthened fold1 and fold2, presumably through the enhanced β-strand character of PHF6* in R2. The two proline mutations of hTau40ΔK280/PP, located near the N-terminal ends of PHF6* in R2 and PHF6 in R3 (Fig. 1A), did not affect the stability of fold1 and fold2 (supplemental Table S2). However, the frequency of fold1 and fold2 decreased in hTau40ΔK280/PP, whereas that of fold3 and longer polypeptide stretches (>100 aa) increased (supplemental Table S1). It may be concluded that interactions introduced by the proline substitutions stabilize protein conformations that prevent Tau aggregation, far beyond the local disruption of the β-structure in R2.
Our results indicate that interactions stabilizing fold1 and fold2 involve the PHF6* β-strand, in which the ΔK280 mutation locates and elevates the propensity to form β-stranded structures. A plausible explanation for our findings is provided by an intramolecular stacking of β-strands in repeats R2, R3, and R4 (9). Assuming an anti-parallel stack of β-strands (Ser285–Gly304 for R2 stacked with R3, Ser316–Gly335 for R3 stacked with R4; Fig. 1A), the mechanical unfolding of the repeat domain would unravel polypeptide segments having the length (ΔLi ∼20 aa) of fold1 and fold2 (supplemental Fig. S1D). Interestingly, the rupture forces of fold1 (∼90 pN) and fold2 (∼70 pN) in hTau40 resemble those observed for the mechanical unzipping of anti-parallel β-strands (55). However, the precise structural localization of fold1 and fold2 remains unclear. It may be possible to locate these folds by applying SMFS to Tau mutants, in which amino acid residues of the repeat domain are systematically manipulated.
N-terminal Interactions Prevent Tau Aggregation
All full-length Tau isoforms exhibited irregularly distributed interactions that folded polypeptide stretches >100 aa (Fig. 2, A, G, and H, and supplemental Table S1). Randomly distributed interactions observed for the N-terminal fragment of hTau40 are consistent with the unstructured projection of the N-terminal half of Tau when bound to MTs (56, 57) and its brush-like protrusion from Tau fibers (58, 59). Such random-coil behavior may originate from the high charge density and low hydrophobicity of the N terminus (60). About 50% of “randomly established” interactions depended on electrolytes indicating that they were partly electrostatic. Similarly, electrostatic interactions established between the positively charged repeat domain and proline-rich regions, and negatively charged regions in the N- and C termini (Fig. 1A) are thought to fold hTau40 into a paper clip conformation (11). Whereas interactions of the N and C termini with the repeat domain inhibit Tau aggregation (61, 62), the C-terminal truncation of hTau40, facilitated in vivo by caspase (63), accelerates Tau aggregation (15).
The number of dispersed interactions stabilizing polypeptide stretches >100 aa increased ∼6-fold when forming the disulfide bond between repeats R2 and R3 (Fig. 2D, supplemental Table S1, supplemental Fig. S4D). The most probable rupture force of these interactions decreased from ∼110 to ∼70 pN in the absence of DTT (supplemental Table S2). Thus, the intact disulfide bond favors Tau conformations that enhance weak unspecific interactions of the termini. Opening of the disulfide bond by addition of DTT favors intermolecular interactions that accelerate Tau aggregation in vitro (24, 64). Accordingly, unspecific interactions of the C and N termini, which prevent hTau40 from aggregation, increased in the absence of DTT. Similarly, the formation of such unspecific interactions was supported at elevated electrolyte concentrations of 500 mm (Fig. 2E) that hinder Tau aggregation (25). Low electrolyte concentrations (50 mm), which prevent Tau molecules from binding to each other in the absence of heparin (26), increased the interactions at p3 (∼73 aa) and ΔLC of ∼130 aa (Fig. 2F). These two interactions may, thus, favor conformations of soluble Tau that prevent aggregation. Similarly, the frequency of unspecific interactions stabilizing polypeptide stretches >100 aa was ∼2-fold increased in the anti-aggregant mutant hTau40ΔK280/PP compared with wild-type and pro-aggregant mutant Tau.
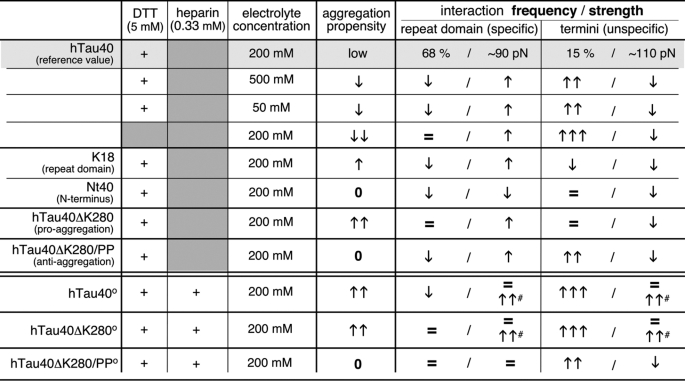
Our data support a mechanistic model in which unspecific weak electrostatic interactions stabilize a variety of protein conformations that prevent Tau from aggregating (Table 1, Fig. 5A). In conditions that disfavor aggregation, Tau establishes specific short (≤40 aa) and unspecific long (>100 aa) polypeptide folds. The balance of these interactions stabilizing short and long polypeptide stretches changes in conditions that favor Tau aggregation. At aggregation conditions, interactions stabilizing long polypeptide stretches are reduced and interactions stabilizing the repeat domain dominate (Table 1). This mechanism shows the complexity of interactions guiding the (mal)function of Tau. It remains to be shown whether such balance of intramolecular interactions folding different polypeptide stretches resembles a general mechanism for regulating the misfolding and aggregation of amyloidogenic IDPs (65–67).
TABLE 1.
Interrelation of aggregation propensities and interactions for hTau40 in different buffer conditions, hTau40 constructs, and pro- and anti-aggregant mutants of hTau40
Interaction frequencies determined by SMFS for hTau40 in PBS/DTT were taken as reference values to indicate the increase (arrows pointing upwards) and decrease (arrows pointing downwards) of interaction frequencies. The number of arrows depicts the amount of increase and decrease (0 indicates no aggregation, = 0–20% indicates in-/decrease, one arrow indicates 21–100% in-/decrease, two arrows indicate 101–500% in-/decrease, three arrows indicate >500% in-/decrease). Changes of interactions in the presence of 0.33 mm heparin (°) are given in respect to the Tau isoform in absence of heparin (high-force interactions in presence of heparin are marked #).
FIGURE 5.
Model of the unfolding energy landscape of full-length human Tau. A, scheme showing the main unfolding intermediates when stretching full-length Tau protein. Tau conformations (left) stabilized by interactions of the termini (orange circles) and the repeat domain (green circles) are forced into fully stretched conformations (right). Thereby, Tau unfolds stepwise taking the main unfolding intermediates stabilized by fold3, fold2, and fold1. B, schematic energy landscapes for the stretching of hTau40 (i), hTau40ΔK280 (ii), and hTau40ΔK280/PP (iii). The three main intramolecular interactions p1, p2, and p3 (green ellipses) establish energy wells in the unfolding landscape of Tau. The widths (x axis) of the three ellipses at 19 (p1), 42 (p2), and 73 aa (p3) estimate the widths xu of the energy wells stabilizing fold1, fold2, and fold3. The depth of every energy well, ΔG‡, is indicated by the color-coded scale bar. Different Tau conformations show different combinations of interactions in the termini and the repeat domain and stepwise unfold through various unfolding pathways (arrows in B, i, ii, and iii). Weak interactions of the termini break before the three abundant folds, fold1, fold2, and fold3, in the repeat domain. The larger number of unspecific, long peptide folds and the low stiffness of the fold3 interaction in hTau40ΔK280/PP (iii) may protect this Tau mutant from establishing aggregation conformations. In contrast, the ∼19- (fold1) and ∼42-aa (fold2) interactions are strengthened in hTau40ΔK280 (ii) and may thus be important for the aggregation of Tau.
Aggregation Accelerator Heparin Changes Molecular Conformations of Tau
Heparin and other polyanions accelerate the in vitro aggregation of hTau40 and hTau40ΔK280 (21, 68, 69). Heparin exposes a homogenous high density of negative charges along its sugar backbone and binds via nonspecific electrostatic interactions to proteins of opposite charges (70). The binding sites of heparin are suggested in the Tau repeat domain and the up- and downstream flanking P2 and R′ regions (71, 72), where lysine and arginine residues expose positive charges (Fig. 1A).
SMFS showed that heparin introduces a large number of strong interactions in hTau40 and hTau40ΔK280 requiring rupture forces up to ∼1500 pN (Fig. 4, A and C). These interactions were randomly distributed along the polypeptide chain of Tau and superimposed on the interactions established in the absence of heparin. High electrolyte concentrations (500 mm monovalent ions) cancelled most heparin-induced “high force” interactions (Fig. 4B), which are thus assumed to be electrostatic in nature. Stabilization of certain molecular conformations upon substrate binding is a common mechanism for IDPs to fulfill variable functions (73). Because heparin catalyzes Tau aggregation into fibrils, we assume that the strong interactions established in the presence of heparin force Tau into conformations that favor the assembly of β-strand motifs in the repeat domain with those of other Tau molecules. This model of heparin-induced Tau aggregation is based on conformational restrictions of monomeric Tau, regardless of the a priori aggregation propensity of the Tau isoform. It also applies to the pro-aggregant mutant hTau40ΔK280 and explains the elevated aggregation speed of hTau40ΔK280 in the presence of heparin.
The interactions of the anti-aggregant mutant hTauΔK280/PP did not change in the presence of heparin. We conclude that the proline mutations prevented heparin to establish strong interactions with hTauΔK280/PP (Fig. 5B). This agrees with the finding that heparin fails to induce aggregation of hTau40ΔK280/PP (19). Apparently, both β-strand breaking proline substitutions (Fig. 1A) efficiently suppress interactions between repeat domains of two adjacent hTau40ΔK280/PP molecules, which are essential for aggregation (20).
The Unfolding Energy Landscape of Tau
DFS experiments can describe the unfolding free energy barriers stabilizing a folded protein. The sequence of all rupture events in a F-D curve describes the unfolding barriers taken by the protein funneling along the unfolding energy landscape (74). We observed that Tau establishes multiple combinations of interactions that stabilize different unfolding intermediates. The three force peaks at p1, p2, and p3, which quantify the interactions stabilizing the three folds fold1, fold2, and fold3, occurred independently of each other (supplemental “Materials” and Fig. S6). Tau can thus unfold via one, two, or all three of these unfolding intermediates (Fig. 5). These prominent unfolding intermediates were superimposed by highly variable interactions of the termini that folded long polypeptide stretches of >100 aa and induced a heterogeneous set of conformations. Such highly dispersed interactions of low probability introduce many energy wells into the unfolding energy landscape. Each of these wells potentially traps a conformational substrate of Tau. At conditions that disfavor aggregation in vitro, such as elevated electrolyte concentrations and absence of DTT, these interactions became more frequent resulting in a rugged unfolding energy landscape of Tau (Table 1).
Similarly, the anti-aggregant mutant hTau40ΔK280/PP strongly increased the frequency of interactions stabilizing longer polypeptide stretches. Thus, this mutant exhibits a rougher energy landscape surface with an increased number of energy wells compared with hTau40. We conclude that interactions folding long polypeptide stretches create energy wells that trap Tau conformations that prevent aggregation. In contrast, the pro-aggregant mutant hTau40ΔK280 strengthened and favored interactions stabilizing the folds of the repeat domain but did not alter interactions folding longer polypeptide stretches. Interactions that fold the repeat domain, thus, appear to stabilize Tau conformations that promote aggregation (Table 1).
Heparin introduced numerous strong electrostatic interactions in both hTau40 and hTau40ΔK280. These interactions occurred in addition to the ones folding the repeat domain and the termini in the absence of heparin. We assume that heparin-induced interactions promote Tau aggregation by stabilizing conformations that favor interactions between the hexapeptide motifs PHF6* and PHF6 in the repeat domains of adjacent Tau molecules. Being kinetically trapped in such aggregation prone conformations would substantially increase the probability for intermolecular interactions of Tau and, thus, initiate aggregation. Furthermore, the conformational restriction of Tau by heparin binding may also provide better access for kinases to the phosphorylation sites in Tau. This would explain the heparin-induced increase in phosphorylation and the co-localization of heparin with hyperphosphorylated Tau in vivo (21, 48, 49).
Supplementary Material
Acknowledgments
We thank S. Brand and I. Lindner for excellent technical assistance, C. Stange for help with protein purification, and J. Biernat, E.-M. Mandelkow, and J. Helenius for expert advice and discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft and ETH Zürich (to D. J. M.), the Max-Planck Society (to D. J. M. and E. M.), and VW Foundation Grant 1/82544 (to E. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3, Figs. S1–S7, and “Materials.”
- IDP
- intrinsically disordered protein
- aa
- amino acid
- AFM
- atomic force microscopy
- DFS
- dynamic force spectroscopy
- hTau40
- longest human Tau isoform (441 aa)
- hTau40ΔK280
- Lys280 deletion mutant of hTau40
- hTau40ΔK280/PP
- hTau40ΔK280 carrying mutations I277P and I308P
- MT
- microtubule
- R1
- repeat 1 of the hTau40 repeat domain
- R2
- repeat 2 of the hTau40 repeat domain
- R3
- repeat 3 of the hTau40 repeat domain
- R4
- repeat 4 of the hTau40 repeat domain
- SMFS
- single-molecule force spectroscopy
- F-D
- force-distance
- N
- newton
- K18
- repeat domain protein construct of hTau40
- Nt40
- N-terminal protein construct of hTau40.
REFERENCES
- 1. Chien P., Weissman J. S., DePace A. H. (2004) Annu. Rev. Biochem. 73, 617–656 [DOI] [PubMed] [Google Scholar]
- 2. Skrabana R., Sevcik J., Novak M. (2006) Cell Mol. Neurobiol. 26, 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uversky V. N. (2002) Protein Sci. 11, 739–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kidd M. (1963) Nature 197, 192–193 [DOI] [PubMed] [Google Scholar]
- 5. Crowther R. A. (1990) Biochim. Biophys. Acta 1096, 1–9 [DOI] [PubMed] [Google Scholar]
- 6. Landrieu I., Lacosse L., Leroy A., Wieruszeski J. M., Trivelli X., Sillen A., Sibille N., Schwalbe H., Saxena K., Langer T., Lippens G. (2006) J. Am. Chem. Soc. 128, 3575–3583 [DOI] [PubMed] [Google Scholar]
- 7. Lippens G., Sillen A., Landrieu I., Amniai L., Sibille N., Barbier P., Leroy A., Hanoulle X., Wieruszeski J. M. (2007) Prion 1, 21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirokawa N., Shiomura Y., Okabe S. (1988) J. Cell Biol. 107, 1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mukrasch M. D., Biernat J., von Bergen M., Griesinger C., Mandelkow E., Zweckstetter M. (2005) J. Biol. Chem. 280, 24978–24986 [DOI] [PubMed] [Google Scholar]
- 10. Schweers O., Schönbrunn-Hanebeck E., Marx A., Mandelkow E. (1994) J. Biol. Chem. 269, 24290–24297 [PubMed] [Google Scholar]
- 11. Jeganathan S., von Bergen M., Brutlach H., Steinhoff H. J., Mandelkow E. (2006) Biochemistry 45, 2283–2293 [DOI] [PubMed] [Google Scholar]
- 12. Mukrasch M. D., Bibow S., Korukottu J., Jeganathan S., Biernat J., Griesinger C., Mandelkow E., Zweckstetter M. (2009) PLoS Biol. 7, e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mukrasch M. D., Markwick P., Biernat J., Bergen M., Bernadó P., Griesinger C., Mandelkow E., Zweckstetter M., Blackledge M. (2007) J. Am. Chem. Soc. 129, 5235–5243 [DOI] [PubMed] [Google Scholar]
- 14. von Bergen M., Friedhoff P., Biernat J., Heberle J., Mandelkow E. M., Mandelkow E. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5129–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin H., Kuret J. (2006) FEBS Lett. 580, 211–215 [DOI] [PubMed] [Google Scholar]
- 16. Goedert M., Spillantini M. G., Hasegawa M., Jakes R., Crowther R. A., Klug A. (1996) Cold Spring Harbor Symp. Quant. Biol. 61, 565–573 [PubMed] [Google Scholar]
- 17. Goedert M., Spillantini M. G. (2000) Biochim. Biophys. Acta 1502, 110–121 [DOI] [PubMed] [Google Scholar]
- 18. Wolfe M. S. (2009) J. Biol. Chem. 284, 6021–6025 [DOI] [PubMed] [Google Scholar]
- 19. Eckermann K., Mocanu M. M., Khlistunova I., Biernat J., Nissen A., Hofmann A., Schönig K., Bujard H., Haemisch A., Mandelkow E., Zhou L., Rune G., Mandelkow E. M. (2007) J. Biol. Chem. 282, 31755–31765 [DOI] [PubMed] [Google Scholar]
- 20. von Bergen M., Barghorn S., Li L., Marx A., Biernat J., Mandelkow E. M., Mandelkow E. (2001) J. Biol. Chem. 276, 48165–48174 [DOI] [PubMed] [Google Scholar]
- 21. Goedert M., Jakes R., Spillantini M. G., Hasegawa M., Smith M. J., Crowther R. A. (1996) Nature 383, 550–553 [DOI] [PubMed] [Google Scholar]
- 22. Kampers T., Friedhoff P., Biernat J., Mandelkow E. M., Mandelkow E. (1996) FEBS Lett. 399, 344–349 [DOI] [PubMed] [Google Scholar]
- 23. Pérez M., Valpuesta J. M., Medina M., Montejo de Garcini E., Avila J. (1996) J. Neurochem. 67, 1183–1190 [DOI] [PubMed] [Google Scholar]
- 24. Schweers O., Mandelkow E. M., Biernat J., Mandelkow E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8463–8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedhoff P., Schneider A., Mandelkow E. M., Mandelkow E. (1998) Biochemistry 37, 10223–10230 [DOI] [PubMed] [Google Scholar]
- 26. Wischik C. M., Edwards P. C., Lai R. Y., Roth M., Harrington C. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 11213–11218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andronesi O. C., von Bergen M., Biernat J., Seidel K., Griesinger C., Mandelkow E., Baldus M. (2008) J. Am. Chem. Soc. 130, 5922–5928 [DOI] [PubMed] [Google Scholar]
- 28. Berriman J., Serpell L. C., Oberg K. A., Fink A. L., Goedert M., Crowther R. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9034–9038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wegmann S., Jung Y. J., Chinnathambi S., Mandelkow E. M., Mandelkow E., Muller D. J. (2010) J. Biol. Chem. 285, 27302–27313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moreno-Herrero F., Pérez M., Baró A. M., Avila J. (2004) Biophys. J. 86, 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barghorn S., Davies P., Mandelkow E. (2004) Biochemistry 43, 1694–1703 [DOI] [PubMed] [Google Scholar]
- 32. Steward A., Toca-Herrera J. L., Clarke J. (2002) Protein Sci. 11, 2179–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butt H. J., Jaschke M. (1995) Nanotechnology 6, 1–7 [Google Scholar]
- 34. Marko J. F., Siggia E. D. (1995) Macromolecules 28, 8759–8770 [Google Scholar]
- 35. Deleted in proof.
- 36. Puchner E. M., Franzen G., Gautel M., Gaub H. E. (2008) Biophys. J. 95, 426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janshoff A., Neitzert M., Oberdörfer Y., Fuchs H. (2000) Angew. Chem. Int. Ed. Engl. 39, 3212–3237 [DOI] [PubMed] [Google Scholar]
- 38. Bippes C. A., Zeltina A., Casagrande F., Ratera M., Palacin M., Muller D. J., Fotiadis D. (2009) J. Biol. Chem. 284, 18651–18663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kellermayer M. S., Smith S. B., Granzier H. L., Bustamante C. (1997) Science 276, 1112–1116 [DOI] [PubMed] [Google Scholar]
- 40. Rief M., Gautel M., Oesterhelt F., Fernandez J. M., Gaub H. E. (1997) Science 276, 1109–1112 [DOI] [PubMed] [Google Scholar]
- 41. Gräter F., Shen J., Jiang H., Gautel M., Grubmüller H. (2005) Biophys. J. 88, 790–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu H., Isralewitz B., Krammer A., Vogel V., Schulten K. (1998) Biophys. J. 75, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Butt H. J., Jaschke M., Ducker W. (1995) Bioelectrochem. Bioener. 38, 191–201 [Google Scholar]
- 44. Grandbois M., Beyer M., Rief M., Clausen-Schaumann H., Gaub H. E. (1999) Science 283, 1727–1730 [DOI] [PubMed] [Google Scholar]
- 45. Israelachvili J. N. (2011) in Intermolecular and Surface Forces (Israelachvili J. N. ed) 3rd Ed, Academic Press, San Diego, CA [Google Scholar]
- 46. von Bergen M., Barghorn S., Biernat J., Mandelkow E. M., Mandelkow E. (2005) Biochim. Biophys. Acta 1739, 158–166 [DOI] [PubMed] [Google Scholar]
- 47. Hasegawa M., Crowther R. A., Jakes R., Goedert M. (1997) J. Biol. Chem. 272, 33118–33124 [DOI] [PubMed] [Google Scholar]
- 48. Brandt R., Lee G., Teplow D. B., Shalloway D., Abdel-Ghany M. (1994) J. Biol. Chem. 269, 11776–11782 [PubMed] [Google Scholar]
- 49. Mawal-Dewan M., Sen P. C., Abdel-Ghany M., Shalloway D., Racker E. (1992) J. Biol. Chem. 267, 19705–19709 [PubMed] [Google Scholar]
- 50. Bell G. I. (1978) Science 200, 618–627 [DOI] [PubMed] [Google Scholar]
- 51. Evans E. (2001) Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 [DOI] [PubMed] [Google Scholar]
- 52. Sapra K. T., Park P. S., Palczewski K., Muller D. J. (2008) Langmuir 24, 1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uversky V. N. (2010) FEBS J. 277, 2940–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uversky V. N. (2002) Eur. J. Biochem. 269, 2–12 [DOI] [PubMed] [Google Scholar]
- 55. Carrion-Vazquez M., Oberhauser A. F., Fisher T. E., Marszalek P. E., Li H., Fernandez J. M. (2000) Prog. Biophys. Mol. Biol. 74, 63–91 [DOI] [PubMed] [Google Scholar]
- 56. Santarella R. A., Skiniotis G., Goldie K. N., Tittmann P., Gross H., Mandelkow E. M., Mandelkow E., Hoenger A. (2004) J. Mol. Biol. 339, 539–553 [DOI] [PubMed] [Google Scholar]
- 57. Schaap I. A., Hoffmann B., Carrasco C., Merkel R., Schmidt C. F. (2007) J. Struct. Biol. 158, 282–292 [DOI] [PubMed] [Google Scholar]
- 58. Mandelkow E., von Bergen M., Biernat J., Mandelkow E. M. (2007) Brain Pathol. 17, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4506–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosenberg K. J., Ross J. L., Feinstein H. E., Feinstein S. C., Israelachvili J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7445–7450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horowitz P. M., LaPointe N., Guillozet-Bongaarts A. L., Berry R. W., Binder L. I. (2006) Biochemistry 45, 12859–12866 [DOI] [PubMed] [Google Scholar]
- 62. Abraha A., Ghoshal N., Gamblin T. C., Cryns V., Berry R. W., Kuret J., Binder L. I. (2000) J. Cell Sci. 113, 3737–3745 [DOI] [PubMed] [Google Scholar]
- 63. Gamblin T. C., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A. L., Lu M., Fu Y., Garcia-Sierra F., LaPointe N., Miller R., Berry R. W., Binder L. I., Cryns V. L. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10032–10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yao T. M., Tomoo K., Ishida T., Hasegawa H., Sasaki M., Taniguchi T. (2003) J. Biochem. 134, 91–99 [DOI] [PubMed] [Google Scholar]
- 65. Bertoncini C. W., Jung Y. S., Fernandez C. O., Hoyer W., Griesinger C., Jovin T. M., Zweckstetter M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1430–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bertoncini C. W., Fernandez C. O., Griesinger C., Jovin T. M., Zweckstetter M. (2005) J. Biol. Chem. 280, 30649–30652 [DOI] [PubMed] [Google Scholar]
- 67. Klein-Seetharaman J., Oikawa M., Grimshaw S. B., Wirmer J., Duchardt E., Ueda T., Imoto T., Smith L. J., Dobson C. M., Schwalbe H. (2002) Science 295, 1719–1722 [DOI] [PubMed] [Google Scholar]
- 68. Barghorn S., Biernat J., Mandelkow E. (2005) Methods Mol. Biol. 299, 35–51 [DOI] [PubMed] [Google Scholar]
- 69. Maeda S., Sahara N., Saito Y., Murayama M., Yoshiike Y., Kim H., Miyasaka T., Murayama S., Ikai A., Takashima A. (2007) Biochemistry 46, 3856–3861 [DOI] [PubMed] [Google Scholar]
- 70. Capila I., Linhardt R. J. (2002) Angew. Chem. Int. Ed. Engl. 41, 391–412 [DOI] [PubMed] [Google Scholar]
- 71. Mukrasch M. D., von Bergen M., Biernat J., Fischer D., Griesinger C., Mandelkow E., Zweckstetter M. (2007) J. Biol. Chem. 282, 12230–12239 [DOI] [PubMed] [Google Scholar]
- 72. Sibille N., Sillen A., Leroy A., Wieruszeski J. M., Mulloy B., Landrieu I., Lippens G. (2006) Biochemistry 45, 12560–12572 [DOI] [PubMed] [Google Scholar]
- 73. Wright P. E., Dyson H. J. (2009) Curr. Opin. Struct. Biol. 19, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schlierf M., Rief M. (2006) Biophys. J. 90, L33–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oberhauser A. F., Carrion-Vasquez M. (2008) J. Biol. Chem. 283, 6617–6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.