Abstract
Meningococci are facultative-pathogenic bacteria endowed with a set of adhesins allowing colonization of the human upper respiratory tract, leading to fulminant meningitis and septicemia. The Neisseria adhesin NadA was identified in about 50% of N. meningitidis isolates and is closely related to the Yersinia adhesin YadA, the prototype of the oligomeric coiled-coil adhesin (Oca) family. NadA is known to be involved in cell adhesion, invasion, and induction of proinflammatory cytokines. Because of the enormous diversity of neisserial cell adhesins the analysis of the specific contribution of NadA in meningococcal host interactions is limited. Therefore, we used a non-invasive Y. enterocolitica mutant as carrier to study the role of NadA in host cell interaction. NadA was shown to be efficiently produced and localized in its oligomeric form on the bacterial surface of Y. enterocolitica. Additionally, NadA mediated a β1 integrin-dependent adherence with subsequent internalization of yersiniae by a β1 integrin-positive cell line. Using recombinant NadA24–210 protein and human and murine β1 integrin-expressing cell lines we could demonstrate the role of the β1 integrin subunit as putative receptor for NadA. Subsequent inhibition assays revealed specific interaction of NadA24–210 with the human β1 integrin subunit. Cumulatively, these results indicate that Y. enterocolitica is a suitable toolbox system for analysis of the adhesive properties of NadA, revealing strong evidence that β1 integrins are important receptors for NadA. Thus, this study demonstrated for the first time a direct interaction between the Oca-family member NadA and human β1 integrins.
Keywords: Adhesion, Bacteria, Cell Adhesion, Integrin, Protein-protein Interactions, Yersinia, Trimeric Autotransporter
Introduction
Neisseria meningitidis is a well-known Gram-negative diplococcus, which is able to colonize the nasopharynx of humans with relatively high frequency. Under certain conditions this pathogen translocates across the mucosal layer of the respiratory tract and causes invasive meningococcal disease (IMD)2 comprising septicemic and/or fulminant meningitis. N. meningitidis is endowed with a broad repertoire of adhesions, which are believed to support colonization and eventually invasion of mucosal epithelial cells. The most extensively investigated adhesins are the type IV pili (Tfp) and the non-pilus adhesins: (i) opacity proteins Opa and Opc and (ii) the autotransporter proteins App (adhesion penetration protein), NhhA (Neisseria hia homolog) and NadA (Neisseria adhesin A) (1). The two last-mentioned adhesins are typical members of the oligomeric coiled-coil adhesin (Oca) family, also known as trimeric autotransporters or as type Vc secretion system, of which the prototype is the trimeric coiled-coil adhesin YadA of enteropathogenic yersiniae (2–5). NadA is produced only by 50% of meningococcal isolates, in particular the nadA gene is obviously present in about 84% of isolates in hypervirulent lineages such as electrophoretic types ET-5, ET-15, and ET-37 (6, 7). Interestingly, the nadA gene of ET-15 meningococci is frequently (68%) disrupted by an IS1301 insertion (8). The C-terminal NadA amino acid sequence is closely related to that of the Yersinia adhesin YadA, which has been shown to present a tripartite structured organization: the N-terminal globular head domain, the intermediate α-helical region capable of forming a homotrimeric coiled-coil stalk also called passenger domain and a highly conserved C-terminal anchor domain (four β-strands inserted into the outer membrane), which is responsible for translocation of the head/stalk region and trimerization of the adhesin (4).
Whereas YadA of enteropathogenic Yersinia species mediates binding to diverse ECM proteins (9–12), epithelial cells, and neutrophils, NadA of N. meningitidis does not bind to ECM proteins but binds to a restricted number of cell types such as Chang cells, HEp-2 or human monocytes/macrophages but fails to bind to HUVEC endothelial cells or human endometrium cell line Hec-1B (13–15).
The large diversity of cell adhesins and the capability of the polysaccharide capsule of N. meningitidis to mask the function of non-pilus adhesins hampers the analysis of the role of individual adhesins for host cell interaction including identification of receptors and prevention of complement lysis. Unraveling the host cell receptor for NadA would be pivotal for a better understanding of the role of NadA in meningococcal pathogenesis, particularly also with respect to the lack of a conventional mouse infection model. This prompted us to develop a novel approach for studying the interaction of neisserial adhesins with host cells and their role in colonization and/or pathogenesis in a mouse infection model by using Yersinia enterocolitica serotype O:8 as heterologous gene carrier strain for meningococcal putative virulence genes. Y. enterocolitica O:8 (strain WA-314 or 8081) might be particularly suitable for this approach because these strains are pathogenic for mice, and their pathogenicity factors are well-known: (i) the virulence plasmid pYV encodes YadA, the type 3 secretion system Ysc-T3SS and a set of Yersinia outer proteins (Yops) with anti-host effector functions, and (ii) the chromosomally encoded invasin (Inv) which is recognized by α5β1 integrins, and (iii) the yersiniabactin system for ferric iron uptake, which is encoded by the High Pathogenicity Island (HPI) and is required for mouse virulence (16, 17). By specific deletion of known virulence determinants Y. enterocolitica can be used as toolbox for studying pathogenicity factors in human serum, cell culture models, and experimentally infected mice. In this study we introduced the nadA gene into Y. enterocolitica to dissect putative virulence functions of NadA in regard of NadA host cell receptor interaction. For the first time we provide strong evidence that the Oca member NadA is directly recognized by β1 integrins and triggers an internalization signal.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
The bacterial strains used in this study are listed in Table 1. Y. enterocolitica strains were grown in Luria-Bertani (LB) or brain heart infusion (BHI) medium at 27 °C. For induction of yadA expression, overnight cultures grown at 27 °C were diluted 1:40 in RPMI 1640 cell culture medium (Invitrogen, Karlsruhe, Germany) and grown at 37 °C for 5 h (4). Escherichia coli strains were cultivated at 37 °C in LB medium. Neisseria species were plated on GC-agar supplemented with Vitox (Oxoid, Hampshire, UK) and grown at 37 °C in 5% CO2.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Source or Ref. |
|---|---|---|
| Y. enterocolitica | ||
| WA-314 | clinical isolate of serotype O:8, carrying virulence plasmid pYVO8 | (42) |
| WA-314 ΔyadA | WA-314 harboring pYVO8-A-0 with yadA replaced by a kanamycin (kmR) resistance cassette | (23) |
| WA-314 ΔyadA:SS | WA-314 harboring pYVO8-SS | This study |
| WA-314 ΔyadA:yadA | WA-314 harboring pYVO8-A-1 with integrated pGPS-A-1, complemented by wild-type yadA | (23) |
| WA-314 ΔyadA:nadA | WA-314 harboring pYVO8-nadA | This study |
| WA-c | plasmidless derivate of WA-314 | (42) |
| WA-c Δinv | inv-negative mutant of WA-c | (43) |
| WA-c Δinv(p) | WA-c Δinv harboring plasmid p (pACYC184 with SalI/SphI fragment of virF) | This study |
| WA-c Δinv(pyadA) | WA-c Δinv harboring plasmid pyadA (plasmid p with XbaI/BamHI fragment of yadA) | This study |
| WA-c Δinv(pnadA) | WA-c Δinv harboring plasmid pnadA (plasmid p with XbaI/BamHI fragment of nadA) | This study |
| WA-c Δinv(pYVO8-SS) | WA-c Δinv harboring pYVO8-SS | This study |
| WA-c Δinv(pYVO8-nadA) | WA-c Δinv harboring pYVO8-nadA | This study |
| N. meningitides | ||
| MC58 | serogroup B, serotype 74, clinical isolate of the ST-32 complex | kindly provided by M. Frosch |
| MC58 ΔsiaD | MC58 with deletion of siaD | (44) |
| MC58 ΔsiaD ΔnadA | MC58 with deletion of siaD and nadA | This study |
| N. gonorrhoeae | ||
| N. gonorrhoeae Ngo OpaCEA | OpaCEA-expressing (Opa52), non-piliated N. gonorrhoeae MS11-B2.1, strain N309 | (45) |
| N. gonorrhoeae Ngo Opa- | Non-opaque, non-piliated N. gonorrhoeae MS11-B2.1, strain N302 | (45) |
| E. coli | ||
| DH5α | endA1 supE44 hsdR17 (rk− mk+) thi-1 recA1gyrA96 relA1 Δ(lacZYA-argF) U169 (ϕ80lacZΔM15) | (46) |
| SM10λpir | thi-1 thr leu tonA lacy supE recA:: RP4–2-TC:: Mu-Kan (λ pir), Kmr | (47) |
| BL21 (DE3) | protein expression strain; F–dcm ompT hsdS (rB– mB–) gal λ(DE3) | (48) |
Strain Construction
To express full-length nadA in Y. enterocolitica strain WA-314 ΔyadA, the nadA gene (allele 1) encoding the mature NadA protein (lacking the leader peptide sequence amino acids 1–23) was amplified by polymerase chain reaction (PCR) from N. meningitidis strain MC58 (gene ID: 904134) using the oligonucleotide primers MC58–1f (TAC TAG AGC TCG CCA CAA GCG ACG ACG ATG, SacI site) and N-1089r (TAC TAG AGC TCT TAC CAC TCG TAA TTG ACG C, SacI site) (bp position 1 refers to bp position 7 = second ATG in the nadA gene ID: 904134) (Table 2). The resulting DNA fragment was digested with SacI and cloned into pGPS-SS, resulting in pGPS-nadA. This plasmid was transformed into E. coli SM10 and subsequently transferred by conjugation into WA-314 ΔyadA. The transconjugants were selected for integration of pGPS-nadA into pYV-A-0 resulting in WA-314 ΔyadA:nadA construct. The pYVO8-nadA plasmid from WA-314 ΔyadA:nadA and the pYVO8-SS plasmid from WA-314 ΔyadA:SS were additionally transformed into WA-c Δinv resulting in WA-c Δinv(pYVO8-nadA) and WA-c Δinv(pYVO8-SS). For generation of nadA- or yadA-expressing Y. enterocolitica strains lacking the pYV plasmid and chromosomally encoded invasin, the pYV plasmid-cured and invasin-negative WA-c Δinv strain was used. To clone nadA and yadA genes carrying the yadA promoter and terminator region and the sequence encoding the YadA LP, nadA, and yadA were amplified by PCR from WA-314 ΔyadA:nadA or WA-314 ΔyadA:yadA, respectively, using the oligonucleotide primers A-144f (TTA ATC TAG ATA GTG CTG TTT TTT GCA TG, XbaI) and A-119r (AAT TGG ATC CAA CTG AAA CCA TGA TAA AAA GC, BamHI). After digestion DNA fragments were cloned into the plasmid pACYC184:virF (p) and transferred into WA-c Δinv resulting in WA-c Δinv(pnadA) and WA-c Δinv(pyadA). pACYC184:virF was generated by amplifying the Yersinia transcriptional activator gene virF from strain WA-314 with the oligonucleotide primers virF-151f (AAT AGC ATG CTT GCC AGT CAC CTA ATAC C, SphI) and virF-86r (AAT AGT CGA CTT GCT CAT CCC ATT GAA TC, SalI) digested with SphI and SalI and cloned into pACYC184 plasmid.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or Ref. |
|---|---|---|
| pGP704 | Mobilizable suicide vector, R6K2 replicon, requires π proteins in trans from λpir positive strain | (47) |
| pUC-A-1 | pUC13 with 5 kb EcoRI/HindIII fragment of pYVO8 from WA-314, carrying yadA gene | (23) |
| pUC-SS | pUC-A-1 derivative, carrying the yadA signal sequence (bp 1–87) | This study |
| pGP-SS | pGP704 with EcoRI/SphI fragment of pUC-SS | This study |
| pGPS-SS | 1,8 kb spectinomycin (spcR) resistance cassette in EcoRI site of pGP-SS | This study |
| pGPS-nadA | pGPS-SS with nadA MC58 bp 75–1095 | This study |
| pYVO8-A-0 | pYVO8, yadA negative, kmR resistance cassette inserted in PstI site of yadA by allelic exchange | (23) |
| pYVO8-SS | pYVO8-A-0 with integrated pGPS-SS | This study |
| pYVO8-A-1 | pYVO8-A-0 with integrated pGPS-A-1, wild type yadA | (23) |
| pYVO8-nadA | pYVO8-A-0 with integrated pGPS-nadA | This study |
| pACYC184 | cloning vector pACYC184 | (49) |
| p | pACYC184 with virF (plus 151 bp upstream and 86 bp downstream of virF) | This study |
| pnadA | p with nadA MC58 (bp 75–1095), yadA promoter (144 bp upstream of yadA), yadA encoding LP (bp 1–90) and yadA terminator (119 bp downstream of yadA) | This study |
| pyadA | p with yadA (bp 1–1266), yadA promoter (144 bp upstream of yadA) and yadA terminator (119 bp downstream of yadA) | This study |
| pET21b+ | Expression with C-terminal His-tag and T7-promotor | Novagen (Darmstadt, Germany) |
| pET21b:nadA | pET21b+ with nadA bp 75–636 | This study |
Generation of Recombinant NadA24–210 Protein for Production of Rabbit Anti-NadA Serum
For production of recombinant NadA protein, the nadA gene (bp 69–630) from N. meningitidis serogroup B strain MC58 was amplified by PCR using the oligonucleotide primers MC58–69f (TAA TTA TCA TAT GGC CAC AAG CGA CGA CGA TG, NdeI) and MC58–630r (ATT ATC TCG AGG GCC GTC TGT TTG GCT TC, XhoI). The DNA fragment was cloned into pET21 vector b+ (Merck, Darmstadt, Germany). After transferring the plasmid into E. coli BL21 (DE3), protein expression was induced at 37 °C by addition of 1 mm IPTG at A600 ∼0.6 and subsequent incubation for additional 4–5 h. The recombinant NadA24–210 protein was purified by affinity chromatography on Ni2+-conjugated chelating fast flow Sepharose 4B resin (GE Healthcare, Munich, Germany). 200–500 μg of purified recombinant NadA24–210 protein were additionally used to immunize rabbits for 91 days according to standard immunization protocol (Pineda Antikörper-Service, Berlin, Germany).
Fluorescent Labeling of Recombinant Proteins
Recombinant NadA24–210 protein and human α5β1 integrin (Millipore, Schwalbach, Germany) were labeled for flow cytometric analysis using Alexa Fluor® Succinimidyl Esters (Invitrogen). 50 μg of NadA24–210 protein or α5β1 integrin were incubated with 0.125 mg Alexa Fluor® Succinimidyl Esters in DMSO and 0.1 m bicarbonate in PBS (NadA24–210)or PBS-0.2% Triton X-100 (α5β1 integrin) for 1.5 h at 20 °C. The proteins were subsequently dialyzed with D-TubeTM Dialyzer Midi (Merck, Darmstadt, Germany) in 4 liters of PBS or PBS-0.2% Triton X-100 overnight, followed by additional dialysis for 3 h. After exchange of dialysis buffer, protein concentration was determined using the Bio-Rad Protein assay (Bio-Rad).
SDS-PAGE and Western Blot Analysis
Outer membrane preparations of Y. enterocolitica were performed as described elsewhere (18) and resuspended in SDS-loading buffer (10% 1 m MgCl2, 4% SDS, 10% glycerin, 5% β-mercaptoethanol, 13% 750 mm Tris pH 6.8, 22% H2O, bromphenol blue). 10 μg of Yersinia outer membrane proteins (OMPs) were boiled at 100 °C for 10 min prior to separation by SDS-PAGE (11% polyacrylamide) and stained with Coomassie solution for visualization of proteins. For immunoblotting separated proteins were transferred onto PVDF-Star Transfer membrane (AppliChem, Darmstadt, Germany). After blocking with 5% milk powder in PBS-T (phosphate-buffered saline, 0.05% Tween) detection of NadA was performed with rabbit anti-NadA serum (1:500) and goat anti-rabbit IgG peroxidase conjugate (1:2000) (Sigma) followed by development with ECL Western Blotting Analysis System (GE Healthcare). Far Western blotting to study the interaction of human integrin with NadA24–210 or Inv397 (O:8) (19) was performed as described by Wu et al. (20). 1 μg of human α5β1 integrin (Millipore, Schwalbach, Germany) was resuspended in SDS-loading buffer without β-mercaptoethanol was separated by SDS-PAGE (11% polyacrylamide) and transferred to PVDF membranes. Immobilized integrins were subsequently renatured by varying concentrations of guanidine-HCl buffer according to the standard protocol (20). The PVDF membrane was blocked with 5% milk powder in PBS-T and incubated with 5 μg of NadA24–210 or Inv397 (O:8) protein overnight. Bound NadA24–210 was detected with anti-NadA serum (1:500) and secondary goat anti-rabbit IgG peroxidase conjugate (1:2000) (Sigma), whereas bound Inv397 (O:8) was detected with anti-Invasin serum (1:5000) (Ingo Autenrieth, Institute of Medical Microbiology and Hygiene, Eberhard Karls University Tübingen, Germany) and secondary goat anti-rabbit IgG peroxidase conjugate (1:2000) (Sigma). Immobilized β1 integrin was detected with anti-human β1 integrin specific antibody MAB1981 (LM534) (Millipore) and secondary anti-rabbit mouse IgG peroxidase conjugate (1:2000) (Sigma).
Cell Cultures
Chang cells (Wong-Kilbourne derivate, clone 1–5c-4, human conjunctiva) were maintained in Medium 199 (Invitrogen) supplemented with 10% heat-inactivated FCS. Mouse embryonic cell lines GE-11-β1 (human β1 integrin-positive, epithelial-like), GE-11 (β1 integrin-knock-out, epithelial-like) (21), 2-4-8 (murine β1 integrin-positive, fibroblast-like), 2-4 (β1 integrin-knock-out, fibroblast-like) generously provided by R. Fässler (MPI, Martinsried, Germany), were cultivated in DMEM supplemented with 10% heat-inactivated FCS and 15 mm l-glutamine. β1 integrin-positive GE-11-β1 cells were cultivated under selection pressure of 220 μg/ml zeocin (Invitrogen).
Immunofluorescence Assays
For detection of NadA on the surface of Y. enterocolitica strain WA-314 Δyad:nadA, WA-314 ΔyadA, WA-c Δinv(pnadA), and WA-c Δinv(p) were grown for 6 h at 37 °C in RPMI 1640 medium, harvested by centrifugation, washed with PBS, and diluted to A600 ∼0.1. Unfixed bacteria were coated onto glass slides and incubated with primary polyclonal anti-NadA serum (1:50) and secondary goat anti-rabbit IgG FITC conjugate (Sigma) (1:128) prior to fluorescence microscopic analysis. Epifluorescence microscopy was performed using a Leica Leitz DMRD (Leica, Wetzlar, Germany).
Flow Cytometric Analysis
Surface exposition of β1 (CD29) integrins or α4, α5, α6, and αv integrins on GE-11 and GE-11-β1 cells was demonstrated by FACS analysis. Briefly, 3 × 105 cells were incubated with FITC hamster anti-rat CD29 (BD Pharmingen, Heidelberg, Germany) (1:200) for 1 h at 4 °C and washed twice with PBS. For detection of α4, α5, and αv integrins cells were incubated with 1 μg of mouse anti-human integrin α4 antibody (MAB16983Z), 1 μg of mouse anti-human integrin α5 antibody (MAB1956Z), or mouse anti-human integrin αv antibody (MAB1953Z) (Millipore) for 1 h at 4 °C washed twice with PBS prior to incubation with NL637-conjugated donkey anti-mouse IgG antibody (1:100) (R&D Systems, Wiesbaden-Nordenstadt, Germany) for 1 h at 4 °C. Detection of surface exposed α6 integrins was performed by using 1 μg of rabbit anti-human integrin α6 antibody (MAB1378) and goat anti-rat PE conjugate (1:200) (Sigma-Aldrich).
For binding studies with recombinant Alexa488-labeled NadA24–210 protein, GE-11-β1, GE-11, 2-4-8, or 2-4 cells (3 × 105 cells) were incubated with 1 μg/3 × 105 cells (5 μg/ml) Alexa488-labeled NadA24–210 protein for 1 h at 4 °C, followed by three washing steps with PBS and analysis by flow cytometry. Blocking experiments of GE-11-β1 and GE-11 cells were performed using the blocking rat anti-human β1 integrin antibody AIIB2 (1:40) and mouse anti-human integrin β1 monoclonal antibody LM534 (1:1000) (Millipore). Blocking of α integrin subunits was performed by incubation of cells with 1 μg of mouse anti-human integrin α4 antibody (MAB16983Z), 1 μg of mouse anti-human integrin α5 antibody (MAB1956Z), mouse anti-human integrin αv antibody (MAB1953Z), and 1 μg of rabbit anti-human integrin α6 antibody (MAB1378). Cells were preincubated with indicated antibodies for 1 h at 4 °C, washed twice with PBS and incubated with 1 μg/3 × 105 cells (5 μg/ml) Alexa488-labeled NadA24–210 protein for 1 h at 4 °C. Competition assays were performed by incubation of GE-11-β1 and GE-11 cells with 5 μg/3 × 105 cells (20 μg/ml) of non-labeled NadA24–210 protein for 1 h at 4 °C and subsequent incubation with 5 μg/ml Alexa488-labeled NadA24–210 protein for 1 h at 4 °C and analyzed by flow cytometry. To analyze binding of Alexa488-labeled human α5β1 integrin to nadA-expressing yersiniae, 5 × 105 bacteria grown in RPMI at 37 °C for 4 h were incubated with 1 μg (5 μg/ml) of Alexa488-labeled α5β1 integrin in PBS-0.2% Triton X-100 and 2 mm MnCl2 for 1 h at 4 °C, followed by three washing steps with PBS and flow cytometric analysis. Statistical significance of at least three independent experiments was determined by Student's t test. Flow cytometric analysis was performed using a BD FACS Canto II flow cytometer (BD Pharmingen).
Infection of Cell Monolayers with Y. enterocolitica
For adhesion and invasion assays, 1 × 105 Chang cells per well were seeded in 24-well tissue culture plates overnight and subsequently infected with WA-c Δinv(pnadA), WA-c Δinv(pyadA), or WA-c Δinv(p) with a multiplicity of infection (moi) of 100 in DMEM, and incubated for 3 h at 37 °C in 5% CO2. Non-adherent bacteria were removed by washing cells three times with PBS, and cells were lysed with 1% Triton X-100 in PBS. Serial dilutions of lysed cell supernatants were plated onto LB agar containing chloramphenicol (20 μg/ml) for selection of yersiniae. Quantification of intracellular bacteria was performed by the gentamicin protection assay. For this Chang cells were infected as described above, incubated for 3 h at 37 °C, non-adherent bacteria were removed, and cells were additionally incubated for 90 min in presence of 50 μg/ml gentamicin at 37 °C in 5% CO2. After washing the cell monolayer, intracellular bacteria were released by cell lysis with 1% Triton X-100 in PBS, and the lysates were plated on agar plates. Adherence assays with GE-11-β1 and GE-11 cells were also performed as described for Chang cells, with the exception that cells were infected with a moi of 50 for 1 h at 37 °C in 5% CO2. Statistical significance of at least three independent experiments performed in triplicates was determined by Student's t test.
Infection of Cell Monolayers with N. meningitidis
1 × 105 GE-11-β1 and GE-11 cells per well were seeded in 24-well tissue culture plates and grown to confluency overnight. N. meningitidis strains were grown on GC plates overnight at 37 °C and 5% CO2. Neisseriae were scraped from plates, washed twice with PBS and resuspended in DMEM supplemented with 1% FCS. Afterward, a moi of 100 was adjusted in DMEM supplemented with 1% FCS and adherence was performed for 3 h at 37 °C in 5% CO2. The number of cell-associated bacteria was determined after washing the cell monolayer three times followed by cell lysis of cells with 1% saponin. Serial dilutions of supernatants were plated on GC agar. The number of intracellular bacteria was quantified by gentamicin protection assay (100 μg/ml gentamicin for 1 h at 37 °C in 5% CO2). Statistical significance of at least three independent experiments performed in triplicate was determined by Student's t test.
RESULTS
Expression of nadA in Y. enterocolitica
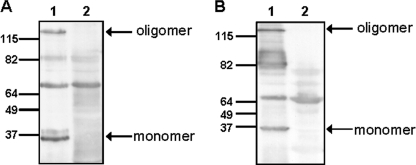
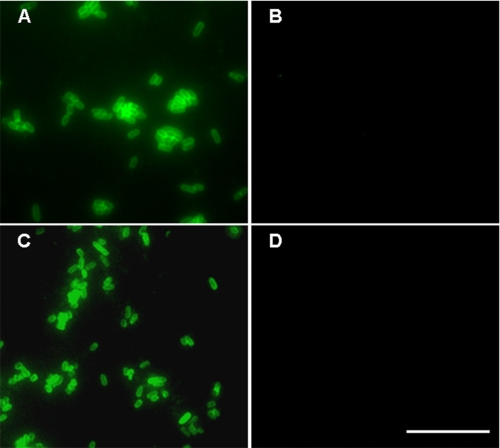
To study the functional role of NadA in Y. enterocolitica under YadA promoter conditions we fused the upstream and proximal portion of yadA comprising its promoter region and leader peptide (LP) encoding region with the nadA gene sequence encoding the mature NadA protein (Fig. 1). The nadA gene was cloned into the suicide plasmid pGPS-SS and integrated into the pYV-A-0 plasmid of Y. enterocolitica strain WA-314 ΔyadA, via homologous recombination. Additionally, full-length nadA encoding the processed NadA together with the yadA promoter, LP and terminator regions was ligated into the plasmid pACY184:virF (p). The resulting plasmid pnadA was transferred into the pYV-cured invasin-negative Y. enterocolitica strain WA-c Δinv resulting in WA-c Δinv(pnadA). Expression of nadA in both strains was confirmed by Western blot analysis. Outer membrane fractions of WA-c Δinv(pnadA) incubated at 100 °C showed a high molecular mass protein at ∼120 kDa corresponding to the oligomeric form of NadA (Fig. 2A, lane 1) and a low molecular mass band at ∼35 kDa corresponding to the monomeric form of NadA (Fig. 2A, lane 1), which were absent in WA-c Δinv(p) (Fig. 2A, lane 2). Localization of NadA in the outer membrane could also be demonstrated with strain WA-314 ΔyadA:nadA (Fig. 2B, lane 1), lacking in the WA-314 ΔyadA control strain (Fig. 2B, lane 2). Oligomeric NadA produced by Y. enterocolitica had the same electrophoretic mobility in SDS-PAGE as NadA produced by unencapsulated N. meningitidis MC58 ΔsiaD strain (data not shown), indicating that NadA is likely not further post-translationally modified by N. meningitidis and Y. enterocolitica. Localization of NadA in the outer membrane could additionally be confirmed by immunofluorescence microscopy of unfixed yersiniae revealing NadA exposition on the surface of strain WA-314 ΔyadA:nadA and WA-c Δinv(pnadA) (Fig. 3). These results demonstrate that full-length NadA produced by Y. enterocolitica forms heat-stable oligomers and is efficiently transported across the outer membrane and exposed probably in its trimeric form on the surface of Y. enterocolitica similar to YadA.
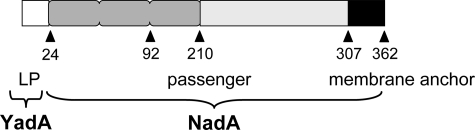
FIGURE 1.
Schematic representation of full-length NadA protein (allele 1) produced by Y. enterocolitica. The YadA leader peptide (LP), the predicted NadA passenger domain and the membrane anchor domain are shown (alignment according to (4)). Numbers refer to amino acid residues of the protein.
FIGURE 2.
Expression of nadA in Y. enterocolitica. Western blot analysis of outer membrane fractions (10 μg) incubated at 100 °C. A, WA-c Δinv(pnadA) (lane 1); WA-c Δinv(p) (lane 2). B, WA-314 Δyad:nadA (lane 1); WA-314 ΔyadA (lane 2). The assays were performed using rabbit anti-NadA serum and secondary peroxidase-conjugated antibody. The arrows indicate the oligomeric and monomeric form of NadA.
FIGURE 3.
Surface localization of NadA in Y. enterocolitica. Immunofluorescence microscopy showing localization of NadA on the surface of Y. enterocolitica. A, WA-314 ΔyadA:nadA; B, WA-314 ΔyadA (negative control); C, WA-c Δinv(pnadA); D, WA-c Δinv(p) (negative control). Unfixed bacteria were incubated with rabbit anti-NadA serum and secondary anti-rabbit FITC antibody. Scale bar, 10 μm.
nadA-expressing Yersiniae Do Not Interact with Extracellular Matrix (ECM) Proteins
Previously it has been demonstrated that NadA produced by N. meningitidis or E. coli does not bind to ECM proteins (13). Therefore we investigated the ability of full-length NadA produced on the surface of Y. enterocolitica to mediate interaction with immobilized collagen type I, fibronectin, and matrigel, respectively, using an ELISA technique (22). As expected NadA-positive yersiniae failed to bind any of the tested ECM proteins in contrast to YadA-positive yersiniae which are known to bind to different ECM proteins (11, 23) (supplemental Fig. S1).
nadA-expressing Yersiniae Mediate Adhesion to and Invasion into Chang Cells
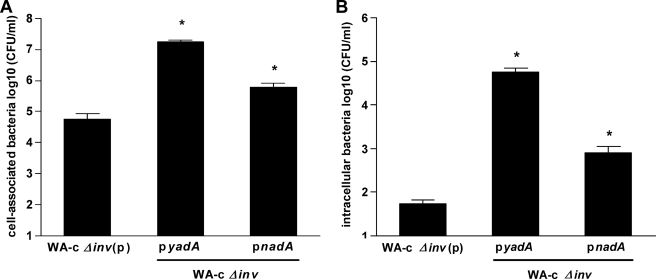
Cell-association and internalization was analyzed for nadA-expressing inv-negative yersiniae and human Chang cells. Thus, Chang cell monolayers were infected with different derivatives of WA-c Δinv yersiniae to investigate the role of NadA in Y. enterocolitica cell monolayer interaction. Quantification of cell-associated bacteria revealed a ∼300-fold higher adhesion capacity for YadA-positive yersiniae as well as an ∼11-fold higher adhesion capacity for NadA-positive yersiniae to Chang cells, compared with the control strain WA-c Δinv(p) (yersiniae background control) (Fig. 4A). Using the gentamicin protection assay we also determined the number of internalized yersiniae. As shown in Fig. 4B YadA-positive (∼1000-fold) and NadA-positive yersiniae (∼15-fold) showed also significantly increased uptake into Chang cells compared with WA-c Δinv(p). These results demonstrate that both YadA and NadA mediate adherence and induce internalization of yersiniae into Chang cells with NadA being probably a weaker adhesin than YadA.
FIGURE 4.
Role of nadA-expressing yersiniae in adhesion and invasion into Chang cells. Chang cell monolayers were infected with WA-c Δinv(p) (negative control), WA-c Δinv(pyadA), and WA-c Δinv(pnadA) for 3 h (moi 100). Shown are (A) total cell-associated bacteria (including both intra- and extracellular bacteria), and (B) intracellular bacteria determined using gentamicin protection assays. The number of cell-associated and intracellular bacteria is expressed as log10 colony forming units per ml (CFU/ml). Data are expressed as the means ± S.E. of the mean of at least three independent experiments. *, p < 0.0383 versus WA-c Δinv(p).
nadA-expressing Yersiniae Do Not Bind Soluble CEACAM-GFP Constructs
N. meningitidis and N. gonorrhoeae express members of the Opa protein family which facilitate interaction with several host cell types (24, 25). OpaHS proteins mediate attachment and invasion into several epithelial cell lines via heparin sulfate proteoglycans, whereas OpaCEA proteins interact with host cell receptors of the CEACAM family (26). Therefore, we tested the ability of nadA-expressing yersiniae to bind to CEACAM 1, 3, 5, 6, and 8. Hence, yersiniae strains WA-c Δinv(pnadA), WA-c Δinv(pyadA), WA-c Δinv(p) (negative control), a non-opaque N. gonorrhoeae strain (Ngo Opa-) and an isogenic, OpaCEA-expressing N. gonorrhoeae strain (Ngo OpaCEA; CEACAM-binding positive control) were incubated with recombinant GFP-tagged CEACAM1, CEACAM3, CEA, CEACAM6, or CEACAM8 extracellular, N-terminal domains. Binding of the fluorescent receptor domains to the microorganisms was analyzed by flow cytometry according to the protocol of Kuespert et al. (27). We detected no interaction of recombinant CEACAMs with nadA-or yadA-expressing yersiniae, nor with the non-opaque N. gonorrhoeae strain. In contrast, the OpaCEA-expressing N. gonorrhoeae strain showed marked interaction with CEACAM1, CEACAM3, CEA, and CEACAM6 (supplemental Fig. S2).
NadA24–210 Protein Binds to Human and Murine β1 Integrin-expressing Cell Lines
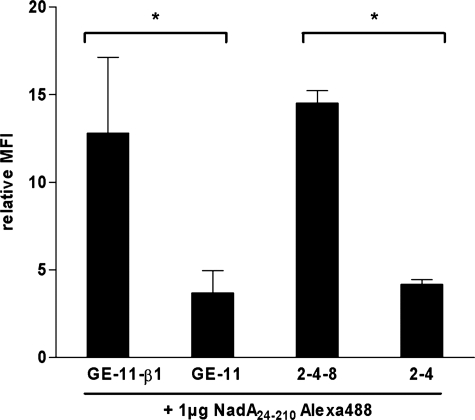
To analyze whether recombinant NadA24–210 protein (representing the supposed NadA binding module) interacts with human β1 integrins we analyzed NadA interaction with murine embryonic epithelial-like GE-11-β1 (human β1 integrin-positive), GE-11 (human β1 integrin-negative), fibroblast-like 2-4-8 (mouse β1 integrin-positive) and 2-4 (mouse β1 integrin-negative) cell lines using Alexa488-labeled NadA24–210 for flow cytometric binding studies. Flow cytometric analysis revealed that NadA24–210 had a ∼3-fold increased binding capacity to human β1 integrin-expressing GE-11-β1 cells, compared with the corresponding β1 integrin-negative GE-11 cells (Fig. 5). In addition, similar results were obtained for interaction of NadA24–210 and murine β1 integrin-expressing 2-4-8 cells showing also higher binding capacity (∼3-fold) than to 2-4 cells (Fig. 5).
FIGURE 5.
Interaction of Alexa488-labeled NadA24–210 protein with epithelial-like and fibroblast-like mouse cells. Epithelial-like GE-11 (human β1 integrin-negative) and GE-11-β1 (human β1 integrin-positive) cells and fibroblast-like 2-4-8 (murine β1 integrin-positive), and 2-4 (murine β1 integrin-negative) cells were incubated with Alexa488-labeled NadA24–210 protein (1 μg/3 × 105 cells) for 1 h at 4 °C and analyzed by flow cytometry. The MFI was related to untreated cells. Data are expressed as the means ± S.E. of at least three independent experiments. *, p < 0.0055.
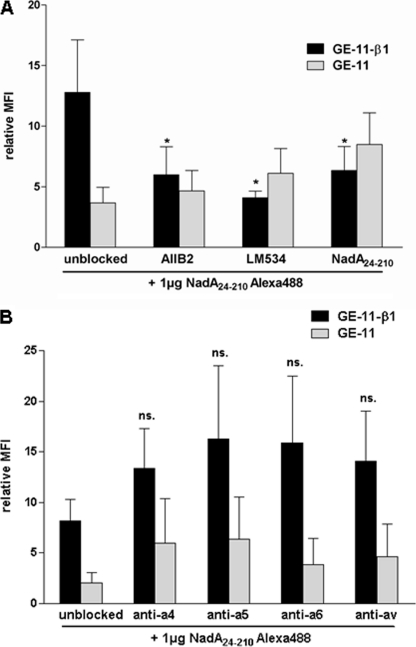
To analyze the specificity of the NadA24–210-GE-11-β1 interaction we applied β1-integrin specific blocking antibodies or unlabeled NadA24–210 protein for competition with Alexa488-labeled NadA24–210 protein. GE-11-β1 and GE-11 cells were preincubated with β1 integrin specific antibodies AIIB2, LM534, or unlabeled NadA24–210 prior to incubation with Alexa-488-labeled NadA24–210 and analyzed by flow cytometry. We could show that incubation of GE-11-β1 cells with the β1-integrin blocking antibodies AIIB2 or LM534 reduced binding of Alexa488-labeled NadA24–210 protein significantly of about 50% (Fig. 6A) To exclude interference of binding of AIIB2 or LM534 antibodies to recombinant Alexa488 NadA24–210 protein, respectively, GE-11 cells were simultaneously preincubated with the AIIB2 and LM534 antibodies showing no effect on NadA background binding (Fig. 6A). Specificity was additionally verified by competition assays with unlabeled NadA24–210, resulting in significantly reduced (∼50%) interaction of Alexa488-labeled NadA24–210 and GE-11-β1 cells as well (Fig. 6A). These data suggest that NadA24–210 binds specifically to the β1 integrin subunit of human β1 integrin-expressing GE-11-β1 cells. As β1 integrins form heterodimers with different α-subunits we also investigated the potential involvement of different α-subunits in interaction with NadA24–210. Therefore GE-11 and GE-11-β1 cells were first screened for α4, α5, α6 and αv subunit presentation. As shown in Table 3, GE-11 and GE-11-β1 showed similar amounts of αv on their cell surface whereas α4, α5, and α6 integrin expression was significantly increased on GE-11-β1 cells compared with GE-11 cells. Furthermore, blocking of α integrin subunits by preincubation of GE-11 and GE-11-β1 with mouse anti-human integrin α4, α5, α6, or αv antibody did not reduce interaction of Alexa-488 labeled NadA24–210 protein significantly compared with unblocked cells (Fig. 6B).
FIGURE 6.
Inhibition of interaction of Alexa488-labeled NadA24–210 protein with GE-11-β1 cells by using anti-β1 integrin antibodies, unlabeled NadA24–210 protein and anti-α integrin antibodies. Epithelial-like GE-11 (human β1 integrin-negative) and GE-11-β1 (human β1 integrin-positive) cells were treated with indicated β1 integrin specific antibodies (AIIB2 1:4; LM534 1:200) or unlabeled NadA24–210 protein (5 μg/3 × 105 cells) (A), or with α4, α5, α6, or αv integrin specific antibodies (1 μg α4: MAB16983Z, α5: MAB1956Z, αv:MAB1953Z, α6: MAB1378) (B) prior to incubation with Alexa488-labeled NadA24–210 protein (1 μg/3 × 105 cells) and analyzed by flow cytometry. The MFI was related to untreated cells. Data are expressed as the means ± S.E. of at least three independent experiments. *, p < 0.0462, versus unblocked control values.
TABLE 3.
Flow cytometric detection of α integrin subunits on the surface of GE-11 and GE-11-β1 cells
| α Integrin antibody | GE-11-β1 |
GE-11 |
|---|---|---|
| Relative FITC-Mean (×-fold) compared to unstained cells | ||
| Anti-α4 | 12.2 ± 3.5a | 4.3 ± 2.2 |
| Anti-α5 | 37.2 ± 12.6a | 12.8 ± 0.6 |
| Anti-α6 | 176.9 ± 54.6a | 37.1 ± 10.6 |
| Anti-αv | 31.5 ± 9.5 | 18.9 ± 7.3 |
a p < 0.0167, versus GE-11 cells.
NadA24–210 Protein Directly Binds to the Human β1 Integrin Subunit
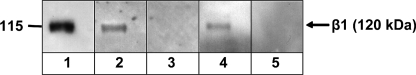
To further substantiate receptor-ligand interactions of NadA24–210 and β1 integrins we performed Far Western blotting. Thus, purified human α5β1 integrin (prey protein) was separated by SDS-PAGE, transferred onto membranes, renatured, and incubated with NadA24–210 (bait protein). Interaction of NadA24–210 with α5 or β1 integrin was detected with rabbit anti-NadA serum and showed one single high molecular mass band at 120 kDa which represents the β1 integrin subunit (Fig. 7, lane 2). As Yersinia Invasin is known to bind directly to α5β1 integrins (28), the Inv397 (O:8) protein was used as positive control and showed the same high molecular mass band at 120 kDa after detection with anti-Invasin serum (Fig. 7, lane 4). Comparison with a Far Western blot performed without NadA or Invasin bait protein, detected with an anti-β1 integrin-specific antibody, revealed also one single band at 120 kDa representing the β1 integrin subunit (Fig. 7, lane 1). We therefore conclude that recombinant NadA24–210 protein directly interacts with the β1 integrin subunit of the α5β1 integrin heterodimer.
FIGURE 7.
Far Western blotting of human α5β1 integrin and recombinant NadA24–210 protein. 1 μg of human α5β1 integrin was separated by SDS-PAGE and transferred onto nitrocellulose membranes. Integrins were renatured and detected with anti-β1 integrin antibody (lane 1), NadA specific antibody (lane 3), or Invasin specific antibody (lane 5) and peroxidase-conjugated secondary antibody. Additionally, renatured integrins were incubated with 5 μg of NadA24–210 protein and detected with anti-NadA serum and peroxidase-conjugated secondary antibody (lane 2), or 5 μg of Inv397 (O:8) protein and detected with anti-Invasin serum and peroxidase-conjugated secondary antibody (lane 4). The arrow indicates the size of the β1 integrin subunit at 120 kDa.
nadA-expressing Yersiniae Directly Bind to Human α5β1 Integrin
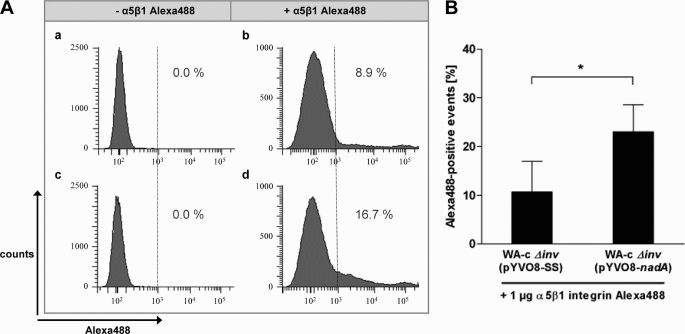
To further analyze whether the observed direct interaction between NadA and human β1 integrins can also be detected with oligomeric NadA expressed on the cell surface of yersiniae, we used Alexa488-labeled recombinant human α5β1 integrin for cytometric binding studies. As shown in Fig. 8, the NadA-positive strain WA-c Δinv(pYV-nadA) demonstrated a significantly higher binding (∼2.15-fold) of Alexa488-labeled human α5β1 integrin compared with the NadA-negative strain WA-c Δinv(pYV-SS), confirming the direct interaction between NadA localized on the bacterial surface of Y. enterocolitica and human α5β1 integrins.
FIGURE 8.
Binding of Alexa488-labeled human α5β1 integrin to nadA-expressing yersiniae. 5 × 105 WA-c Δinv(pYV-nadA) (NadA-positive) or WA-c Δinv(pYV-SS) (NadA-negative, negative control) yersiniae were incubated with or without 1 μg of Alexa488-labeled human α5β1 integrin in PBS-0.2% Triton X-100 and 2 mm MnCl2 (5 μg/ml) for 1 h at 4 °C and analyzed by flow cytometry. A, histograms display the result from one representative experiment. a, WA-c Δinv(pYVO8-SS) without addition of Alexa488-labeled α5β1 integrin. b, WA-c Δinv(pYVO8-SS) with addition of Alexa488-labeled α5β1 integrin. c, WA-c Δinv(pYVO8-nadA) without addition of Alexa488-labeled α5β1 integrin. d, WA-c Δinv(pYVO8-nadA) with addition of Alexa488-labeled α5β1 integrin. Percents of Alexa488-positive events are displayed. B, Alexa488-positive events are expressed as the means ± S.E. of four independent experiments. *, p < 0.0416.
NadA-specific Cell Adhesion and Invasion Is Not Detectable in N. meningitidis
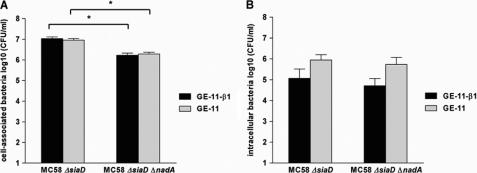
NadA has previously been described as an invasin, mediating invasion of nadA-expressing E. coli and meningococci into human Chang cells (13). To test whether β1 integrins are involved in mediating adhesion and invasion of N. meningitidis we used the unencapsulated N. meningitidis strain MC58 ΔsiaD and the isogenic nadA mutant MC58 ΔsiaD ΔnadA for cellular infection of GE-11-β1 and GE-11 cell monolayers for 3 h (moi 100). As shown in Fig. 9A, we found that the nadA-positive strain MC58 ΔsiaD showed significantly higher numbers of cell-associated bacteria compared with the isogenic nadA mutant MC58 ΔsiaD ΔnadA. Nevertheless, no significant difference for β1 integrin-positive GE-11-β1 and β1 integrin-negative GE-11 cells could be observed for the strain MC58 ΔsiaD and MC58 ΔsiaD ΔnadA, respectively. This demonstrates that nadA-expressing unencapsulated meningococci have higher binding capacity to GE-11 and GE-11-β1 cells, but this effect seems not to be solely dependent on the presence of β1 integrins. We also investigated the role of NadA in N. meningitidis in cell invasion of GE-11-β1 and GE-11 cells by using MC58 ΔsiaD and MC58 ΔsiaD ΔnadA in gentamicin protection assays. Interestingly, both strains showed higher numbers of intracellular bacteria for GE-11 cells, compared with GE-11-β1 cells (Fig. 9B). This indicates that probably not only β1 integrins are exploited by Neisseria but also other receptors, which might be present on GE-11 cells in higher amounts than on GE-11-β1 cells. However, comparing MC58 ΔsiaD and MC58 ΔsiaD ΔnadA, no significant contribution of NadA for cell entry either into GE-11 or into GE-11-β1 cells could be detected.
FIGURE 9.
Role of NadA in adhesion and invasion of N. meningitidis. For determination of cell-associated bacteria GE-11-β1 and GE-11 cell monolayers were infected for 3 h (moi 100) with unencapsulated N. meningitidis strain MC58 ΔsiaD and the isogenic nadA mutant MC58 ΔsiaD ΔnadA. For determination of intracellular bacteria GE-11-β1 and GE-11 cell monolayers were additionally treated with gentamicin. Shown are (A) total cell-associated bacteria (including both intra- and extracellular bacteria), and (B) intracellular bacteria determined using gentamicin protection assays. The number of cell-associated and intracellular bacteria is expressed as log10 colony forming units per ml (CFU/ml). Data are expressed as the means ± S.E. of the mean of at least three independent experiments. *, p < 0.0001.
nadA-expressing Yersiniae Mediate Adhesion and Invasion into Human and Mouse β1 Integrin-expressing Cells
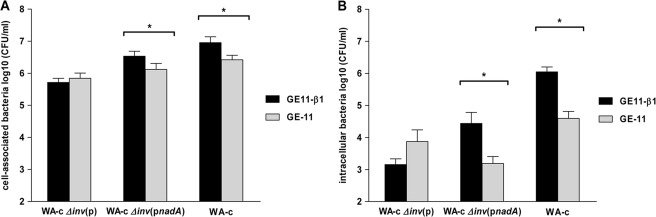
As N. meningitidis is endowed with diverse surface exposed bacterial adhesins probably masking a NadA-β1 integrin-dependent colonization of human cells under applied in vitro conditions, we used the Yersinia model to test whether β1 integrins are involved in mediating adherence and entry of nadA-expressing yersiniae. Therefore, we quantified the number of cell-associated and intracellular bacteria after infection of GE-11-β1 and GE-11 cells. GE-11-β1 and GE-11 cells were incubated with WA-c Δinv(pnadA), WA-c Δinv(p) or the invasin-positive WA-c strain (Inv/β1 integrin- mediated invasion) (29, 30) for 1 h and an moi of 50 and analyzed for cell-association and intracellular bacteria. We found that the nadA-expressing strain WA-c Δinv(pnadA) showed a significantly higher number (∼2.6-fold) of cell-associated bacteria for β1 integrin-positive GE-11-β1 cells than for β1 integrin-negative GE-11 cells. This result is in accordance with the result obtained for the invasin-positive WA-c strain showing also increased cell-association (∼3.5-fold) for GE-11-β1 cells compared with GE-11 cells. The control strain WA-c Δinv(p) showed weak interaction with GE-11-β1 and GE-11 cells compared with WA-c Δinv(pnadA) and WA-c (Fig. 10A). Concerning β1 integrin-mediated internalization of nadA-expressing yersiniae into GE-11-β1 and GE-11 cells, gentamicin protection assays revealed that the number of intracellular WA-c Δinv(pnadA) bacteria was significantly higher (∼18-fold) for β1 integrin-positive cells than for β1-negative cells. This result resembles that of the invasin-positive strain WA-c, showing higher numbers of intracellular bacteria (∼28-fold) in presence of human β1 integrins in comparison to β1 integrin-negative cells (Fig. 10B). These results confirm that β1 integrins appear to function as receptors for NadA, supporting β1 integrin-dependent adhesion and internalization and emphasize the advantage of yersiniae expressing NadA as the only effective cell adhesin to demonstrate function and specificity of a neisserial adhesin.
FIGURE 10.
Role of nadA-expressing yersiniae in adhesion and invasion. GE-11-β1 and GE-11 cell monolayers were infected with WA-c Δinv(p) (negative control), WA-c Δinv(pnadA), and WA-c (positive control) for 1 h (moi 50). Shown are (A) total cell-associated bacteria (including both intra- and extracellular bacteria), and (B) intracellular bacteria determined using gentamicin protection assays. The number of cell-associated and intracellular bacteria is expressed as log10 colony forming units per ml (CFU/ml). Data are expressed as the means ± S.E. of the mean of at least three independent experiments. *, p < 0.041.
DISCUSSION
Y. enterocolitica is a suitable bacterial pathogen to investigate fundamental aspects of virulence including bacterial adhesion, invasion, subversion of the innate immune defense, mechanisms of extracellular survival and multiplication in the mouse infection model (31–33). The major pathogenicity determinants (virulence plasmid pYV, invasin gene inv, HPI), their gene products and pathogenicity functions have been well characterized. Therefore, it is conceivable that pathogenicity factors of non-mouse virulent pathogens similar to Yersinia ones, can be studied in Yersinia by genetic replacement. In this study this approach has been applied to the neisserial adhesin NadA by replacement of the yadA gene by nadA. By fusing the coding sequence of mature NadA with the promoter region and the coding region of the N-terminal signal sequence of yadA we could demonstrate NadA production, secretion, insertion into the outer membrane and surface exposition of NadA by Y. enterocolitica. This is remarkable as the genus Neisseria belongs to the β subdivision of Proteobacteria in contrast to Yersinia belonging to the γ subdivision and suggests a certain degree of functional autonomy of Oca family members. Previously it has been demonstrated that NadA forms heat-stable oligomers (6). This characteristic of Oca family members could also be demonstrated with yersiniae expressing nadA. A typical function of Yersinia YadA is binding to extracellular matrix (ECM) proteins. We could demonstrate that NadA produced by yersiniae does not contribute to binding to ECM proteins (matrigel, fibronectin, and collagen type I). However, we could demonstrate that NadA as well as YadA mediate adhesion to Chang cells and triggering of internalization by using a pYV- and inv deleted Y. enterocolitica mutant. These latter results are in agreement with experiments using E. coli expressing nadA, as previously shown (13). Moraxella catarrhalis Oca family member UspA1 and Neisseria Opa proteins are known to be recognized by CEACAMs, which are expressed by diverse host cells (34). By using soluble recombinant GFP-tagged CEACAMs and Y. enterocolitica expressing yadA or nadA and N. gonorrhoeae as controls we could demonstrate by flow cytometry that neither NadA nor YadA are recognized by CEACAM 1, 3, 5, 6, or 8, respectively. Previously it has been demonstrated that NadA induces chemokine IL-8 production of diverse host cell types similar to Yersinia Inv which is recognized by β1 integrins (14, 35–37). This prompted us to check whether NadA could also interact with β1 integrins. Interaction of NadA and β1 integrins could be substantiated by using recombinant NadA protein covering the NadA binding domain (NadA24–210) in binding studies with epithelial-like GE-11 (human β1 integrin-negative and GE-11-β1 (human β1 integrin-positive) or fibroblast-like 2-4 (mouse β1 integrin-negative) and 2-4-8 (mouse β1 integrin-positive) cells. We could clearly demonstrate by flow cytometry that NadA24–210 binds to human and mouse β1 integrins.
The specificity of NadA24–210 binding to human β1 integrins could further be corroborated by blocking experiments with anti-human β1 monoclonal antibodies. Additional blocking experiments with different anti-human α monoclonal antibodies further confirmed interaction of NadA24–210 with the β1 integrin subunit, whereas the α subunit seems not to be involved. Interaction of NadA and β1 integrins was further analyzed by Far Western blotting using recombinant NadA (NadA24–210) as “prey” and β1 integrin as “bait”, revealing direct interaction of NadA with the β1 integrin subunit. Direct interaction of NadA and β1 integrins could additionally be verified for nadA-expressing, invasin-negative yersiniae, and Alexa488-labeled human α5β1 integrin, revealing that native NadA localized on the bacterial surface is involved in binding to human β1 integrins. Additionally, we compared the interaction of pYV-negative Y. enterocolitica expressing nadA or inv in β1 integrin-specific invasion, respectively, with epithelial-like cells derived from β1 integrin-knock-out mouse embryonal (GE-11) cells and GE-11 cells transfected with human β1 integrins (GE-11-β1 cells). Inv and NadA both significantly contributed to β1 integrin-mediated adhesion and internalization. These results were compared with non-encapsulated N. meningitidis MC58 ΔsiaD and a double mutant MC58 ΔsiaD ΔnadA. Surprisingly, when the non-encapsulated isogenic pair MC58 ΔsiaD/MC58 ΔsiaD ΔnadA was compared for cell invasion, we found a higher rate of neisserial invasion for β1 integrin-negative GE-11 cells which was independent of the presence of NadA, whereas for adhesion there was a weak significant effect in favor of NadA-β1 interaction with GE-11-β1 cells. This result shows that probably because of the presence of multiple adhesins of N. meningitidis the identification of neisserial adhesin-specific host receptors is severely restricted unless a heterologous well-defined bacterial host/carrier is used for expression of the respective adhesins. In conclusion, the NadA binding analysis using Y. enterocolitica (Δinv mutant) as heterologous expression system for nadA and the recombinant NadA binding module structure in conjunction with β1 integrin Far Western blotting and defined isogenic pairs of β1 integrin-positive and β1 integrin-negative cell lines revealed for the first time that the NadA head domain interacts specifically with β1 integrins. The β1 integrin subunit might thus function as host cell receptor for N. meningitidis expressing nadA gene. Therefore NadA is the first adhesin of the Oca family which directly interacts with the β1 integrin subunit.
Bacterial adhesin-β1 integrin interactions have been described for several pathogens colonizing and/or invading the mucosal epithelium of the gastrointestinal or the respiratory tract including Yersinia species and E. coli. Surface β1 integrin expressing cells such as M cells of the Peyer's patches (PP) and nasal-associated lymphoid tissue (NALT) are recognized by bacterial adhesins resulting in bacterial translocation across the mucosal layer and triggering the release of chemokines such as IL-8/CXCL8 (38–41). Moreover, antigen-presenting dendritic cells (DCs), macrophages and neutrophils, which have been recruited to bacterial entry sides, also express α4β1 and/or α5β1 integrins. In analogy to invasin-expressing Yersinia it is not unlikely that N. meningitidis interacts through NadA with αβ1 integrins of M-cells of the NALT and with DCs, macrophages and neutrophils of the submucosa (14, 24, 36).
Supplementary Material
Acknowledgments
We thank Christof Hauck and Kathrin Kuespert from the University of Konstanz for kindly providing cell culture supernatants containing GFP-tagged Igv-like domains of different CEACAMs. Reinhard Fässler from the Max-Planck-Institut for Biochemistry in Munich is gratefully acknowledged for his gift of cell lines GE-11, GE-11-β1, 2-4, and 2-4-8. We also thank Matthias Frosch and Ulrich Vogel for providing us with Neisseria strains and Ingo Autenrieth for the anti-Invasin serum. We also want to express our thanks to Gisela Anding for excellent technical assistance.
This work was supported by the Friedrich-Baur-Stiftung, the Munich Centre for Integrated Protein Science (CIPSM) and by the German Research Foundation (DFG) Grant SFB594. This work was also supported in part by the DFG Grant SFB479 (to O. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- IMD
- invasive meningococcal disease
- moi
- multiplicity of infection
- Oca
- oligomeric coiled-coil adhesin
- Tfp
- type IV pili
- LP
- leader peptide
- NadA
- Neisseria adhesin A
- ECM
- extracellular matrix.
REFERENCES
- 1. Virji M. (2009) Nat. Rev. Microbiol. 7, 274–286 [DOI] [PubMed] [Google Scholar]
- 2. Cotter S. E., Surana N. K., St. Geme J. W., 3rd (2005) Trends Microbiol. 13, 199–205 [DOI] [PubMed] [Google Scholar]
- 3. Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala'Aldeen D. (2004) Microbiol. Mol. Biol. Rev. 68, 692–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoiczyk E., Roggenkamp A., Reichenbecher M., Lupas A., Heesemann J. (2000) EMBO J. 19, 5989–5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linke D., Riess T., Autenrieth I. B., Lupas A., Kempf V. A. (2006) Trends Microbiol. 14, 264–270 [DOI] [PubMed] [Google Scholar]
- 6. Comanducci M., Bambini S., Brunelli B., Adu-Bobie J., Aricò B., Capecchi B., Giuliani M. M., Masignani V., Santini L., Savino S., Granoff D. M., Caugant D. A., Pizza M., Rappuoli R., Mora M. (2002) J. Exp. Med. 195, 1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Comanducci M., Bambini S., Caugant D. A., Mora M., Brunelli B., Capecchi B., Ciucchi L., Rappuoli R., Pizza M. (2004) Infect. Immun. 72, 4217–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elias J., Vogel U. (2007) J. Clin. Microbiol. 45, 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamm A., Tarkkanen A. M., Korhonen T. K., Kuusela P., Toivanen P., Skurnik M. (1993) Mol. Microbiol. 10, 995–1011 [DOI] [PubMed] [Google Scholar]
- 10. Tahir Y. E., Kuusela P., Skurnik M. (2000) Mol. Microbiol. 37, 192–206 [DOI] [PubMed] [Google Scholar]
- 11. Heise T., Dersch P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roggenkamp A., Ackermann N., Jacobi C. A., Truelzsch K., Hoffmann H., Heesemann J. (2003) J. Bacteriol. 185, 3735–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capecchi B., Adu-Bobie J., Di Marcello F., Ciucchi L., Masignani V., Taddei A., Rappuoli R., Pizza M., Aricò B. (2005) Mol. Microbiol. 55, 687–698 [DOI] [PubMed] [Google Scholar]
- 14. Franzoso S., Mazzon C., Sztukowska M., Cecchini P., Kasic T., Capecchi B., Tavano R., Papini E. (2008) J. Leukoc. Biol. 83, 1100–1110 [DOI] [PubMed] [Google Scholar]
- 15. Mazzon C., Baldani-Guerra B., Cecchini P., Kasic T., Viola A., de Bernard M., Aricò B., Gerosa F., Papini E. (2007) J. Immunol. 179, 3904–3916 [DOI] [PubMed] [Google Scholar]
- 16. Cornelis G. R. (2002) J. Cell Biol. 158, 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schubert S., Rakin A., Heesemann J. (2004) Int. J. Med. Microbiol. 294, 83–94 [DOI] [PubMed] [Google Scholar]
- 18. Mack D., Heesemann J., Laufs R. (1994) Med. Microbiol. Immunol. 183, 217–227 [DOI] [PubMed] [Google Scholar]
- 19. Wiedemann A., Linder S., Grassl G., Albert M., Autenrieth I., Aepfelbacher M. (2001) Cell Microbiol. 3, 693–702 [DOI] [PubMed] [Google Scholar]
- 20. Wu Y., Li Q., Chen X. Z. (2007) Nat. Protoc. 2, 3278–3284 [DOI] [PubMed] [Google Scholar]
- 21. Gimond C., van Der, Flier A., van Delft S., Brakebusch C., Kuikman I., Collard J. G., Fässler R., Sonnenberg A. (1999) J. Cell Biol. 147, 1325–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackermann N., Tiller M., Anding G., Roggenkamp A., Heesemann J. (2008) J. Bacteriol. 190, 5031–5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roggenkamp A., Neuberger H. R., Flügel A., Schmoll T., Heesemann J. (1995) Mol. Microbiol. 16, 1207–1219 [DOI] [PubMed] [Google Scholar]
- 24. McNeil G., Virji M., Moxon E. R. (1994) Microb. Pathog. 16, 153–163 [DOI] [PubMed] [Google Scholar]
- 25. Virji M., Makepeace K., Moxon E. R. (1994) Mol. Microbiol. 14, 173–184 [DOI] [PubMed] [Google Scholar]
- 26. Hauck C. R., Meyer T. F. (2003) Curr. Opin. Microbiol. 6, 43–49 [DOI] [PubMed] [Google Scholar]
- 27. Kuespert K., Weibel S., Hauck C. R. (2007) J. Microbiol. Methods 68, 478–485 [DOI] [PubMed] [Google Scholar]
- 28. Isberg R. R., Leong J. M. (1990) Cell 60, 861–871 [DOI] [PubMed] [Google Scholar]
- 29. Isberg R. R., Hamburger Z., Dersch P. (2000) Microbes Infect. 2, 793–801 [DOI] [PubMed] [Google Scholar]
- 30. Wong K. W., Isberg R. R. (2005) PLoS. Pathog. 1, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong K. W., Isberg R. R. (2005) Curr. Opin. Microbiol. 8, 4–9 [DOI] [PubMed] [Google Scholar]
- 32. Cornelis G. R. (2006) Nat. Rev. Microbiol. 4, 811–825 [DOI] [PubMed] [Google Scholar]
- 33. Heesemann J., Sing A., Trülzsch K. (2006) Curr. Opin. Microbiol. 9, 55–61 [DOI] [PubMed] [Google Scholar]
- 34. Kuespert K., Pils S., Hauck C. R. (2006) Curr. Opin. Cell Biol. 18, 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grassl G. A., Kracht M., Wiedemann A., Hoffmann E., Aepfelbacher M., von Eichel-Streiber C., Bohn E., Autenrieth I. B. (2003) Cell Microbiol. 5, 957–971 [DOI] [PubMed] [Google Scholar]
- 36. Kolb-Mäurer A., Unkmeir A., Kämmerer U., Hübner C., Leimbach T., Stade A., Kämpgen E., Frosch M., Dietrich G. (2001) Infect. Immun. 69, 6912–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulte R., Grassl G. A., Preger S., Fessele S., Jacobi C. A., Schaller M., Nelson P. J., Autenrieth I. B. (2000) FASEB J. 14, 1471–1484 [DOI] [PubMed] [Google Scholar]
- 38. Corr S. C., Gahan C. C., Hill C. (2008) FEMS Immunol. Med. Microbiol. 52, 2–12 [DOI] [PubMed] [Google Scholar]
- 39. Heritage P. L., Underdown B. J., Arsenault A. L., Snider D. P., McDermott M. R. (1997) Am. J. Respir. Crit. Care Med. 156, 1256–1262 [DOI] [PubMed] [Google Scholar]
- 40. Kiyono H., Fukuyama S. (2004) Nat. Rev. Immunol. 4, 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park H. S., Francis K. P., Yu J., Cleary P. P. (2003) J. Immunol. 171, 2532–2537 [DOI] [PubMed] [Google Scholar]
- 42. Heesemann J., Keller C., Morawa R., Schmidt N., Siemens H. J., Laufs R. (1983) J. Infect. Dis. 147, 107–115 [DOI] [PubMed] [Google Scholar]
- 43. Ruckdeschel K., Roggenkamp A., Schubert S., Heesemann J. (1996) Infect. Immun. 64, 724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurzai O., Schmitt C., Claus H., Vogel U., Frosch M., Kolb-Mäurer A. (2005) Cell Microbiol. 7, 1319–1334 [DOI] [PubMed] [Google Scholar]
- 45. Kupsch E. M., Knepper B., Kuroki T., Heuer I., Meyer T. F. (1993) EMBO J. 12, 641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanahan D. (1983) J. Mol. Biol. 166, 557–580 [DOI] [PubMed] [Google Scholar]
- 47. Miller V. L., Mekalanos J. J. (1988) J. Bacteriol. 170, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wood W. B. (1966) J. Mol. Biol. 16, 118–133 [DOI] [PubMed] [Google Scholar]
- 49. Chang A. C., Cohen S. N. (1978) J. Bacteriol. 134, 1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuespert K., Hauck C. R. (2009) Methods Mol. Biol. 470, 57–65 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.