Abstract
We have previously shown that the μ-opioid receptor (MOR) is capable of mediating cross-desensitization of several chemokine receptors including CCR5, but the biochemical mechanism of this process has not been fully elucidated. We have carried out a series of functional and biochemical studies and found that the mechanism of MOR-induced cross-desensitization of CCR5 involves the activation of PKCζ. Inhibition of PKCζ by its pseudosubstrate inhibitor, or its siRNA, or dominant negative mutants suppresses the cross-desensitization of CCR5. Our results further indicate that the activation of PKCζ is mediated through a pathway involving phosphoinositol-dependent kinase-1 (PDK1). In addition, activation of MOR elevates the phosphorylation level and kinase activity of PKCζ. The phosphorylation of PKCζ can be suppressed by a dominant negative mutant of PDK1. We observed that following MOR activation, the interaction between PKCζ and PDK1 is immediately increased based on the analysis of fluorescent resonance energy transfer in cells with the expression of PKCζ-YFP and PDK1-CFP. In addition, cells expressing PKCζ kinase motif mutants (Lys-281, Thr-410, Thr-560) fail to exhibit full MOR-induced desensitization of CCR5 activity. Taken together, we propose that upon DAMGO treatment, MOR activates PKCζ through a PDK1-dependent signaling pathway to induce CCR5 phosphorylation and desensitization. Because CCR5 is a highly proinflammatory receptor, and a critical coreceptor for HIV-1, these results may provide a novel approach for the development of specific therapeutic agents to treat patients with certain inflammatory diseases or AIDS.
Keywords: Chemokines, G protein-coupled receptors (GPCR), Inflammation, Leukocyte, Opiate Opioid, Desensitization
Introduction
There are three structurally related opioid receptors, designated μ, κ, and δ, and it is well established that activation of one or more of these receptors modulates the function of immune cells (1–5). For example, morphine suppresses chemotactic responses and chemokine expression in human and murine cells (6–9). Similarly, Tyr-d-Ala-Gly-N-Me-Phe-Gly-ol (DAMGO),3 a highly selective ligand for the μ-opioid receptor (MOR), suppresses chemokine receptor function in vitro (7, 10–12). Recent work from our laboratories, and others, suggest that heterologous desensitization is the primary mechanism for the inhibitory activity of opioids for chemokine receptor function. Studies reported by Grimm et al. (13, 14) show that activation of μ and δ opioid receptors (MOR and DOR) induced cross-desensitization of the chemokine receptors CCR1, CCR2, CXCR1, and CXCR2, but not FPR (the high affinity receptor for fMLF), resulting in a failure of monocytes and neutrophils to manifest chemotactic responses to chemokines such as CCL2, CCL3, and CCL5 in a dose-dependent manner. Further studies have shown that activation of MOR in HEK293 cells stably expressing both MOR and CCR1, failed to migrate in response to CCL3, a CCR1 ligand (15). More recently, using both primary cells as well as stably transfected cell lines, we have shown that MOR activation induces heterologous desensitization of CCR5 but not CXCR4 (16), a finding which is consistent with the notion that cross-talk among G protein-coupled receptors (GPCRs) is selective (17).
The mechanisms mediating heterologous desensitization between opioid and chemokine receptors are not clearly understood. Our laboratories have previously shown that the MOR-induced cross-desensitization of CCR5 does not result in target receptor endocytosis (10, 13, 14, 16). Evidence from studies of cross-talk between a number of chemokine, opioid, and formyl peptide receptors suggest that multiple processes may contribute to heterologous desensitization. However, the biochemical mechanism of cross-desensitization in most cases involves target receptor phosphorylation (10, 13, 17–19). In addition, studies from several laboratories have shown that second messenger-dependent kinases are required for heterologous desensitization of a number of G protein-coupled receptors (reviewed in Ref. 19), and in particular, the function of protein kinase C (PKC) is typically required for the cross-talk between Gi-coupled receptors.
We are particularly interested in the cross-talk consequences following the activation of MOR, since this receptor is the major receptor, which is activated following heroin or morphine abuse. Moreover, we have given our primary attention to the capacity of this opioid receptor to cross-desensitize CCR5, because this receptor is a critical major HIV co-receptor, and a major participant in inflammatory responses. The biochemical basis for the cross-talk between MOR and CCR5 has not previously been examined, and in the present study, we report that PKCζ, an atypical PKC, plays an important role in the MOR-induced cross-desensitization of CCR5. We employed a specific PKCζ pseudosubstrate inhibitor, a PKCζ siRNA, or PKCζ dominant negative mutants to show that the inhibition of PKCζ reduces the DAMGO-induced desensitization of CCR5. We also demonstrate that the activation of MOR promotes the association between CCR5 and PKCζ, and induces both the kinase activity of PKCζ, as well as the phosphorylation of CCR5 in both transfected cell lines and primary macrophages. Finally, we find that the MOR-induced activation of PKCζ is dependent on the activation of PDK1. Moreover, the activation of PKCζ is associated with the formation of a complex between PKCζ and PDK1.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture
The 300.19 murine pre-B cell line stably expressing FLAG-tagged MOR and CCR5 (designated M13 cells), was cultured in 300.19 medium (RPMI 1640 medium containing 10% fetal bovine serum, 0.1 mm non-essential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, 0.5 mm β-mercaptoethanol) supplemented with 1.5 μg/ml puromycin and 400 μg/ml zeocin. Chinese hamster ovary cells (CHO) stably expressing MOR and FLAG-tagged CCR5 (designated CMC cells), was a gift from Dr. Liu-Chen (Temple University, Philadelphia, PA) (10). The cells were cultured in D10 medium (DMEM medium containing 10% fetal bovine serum, 25 mm HEPES, 2 mm l-glutamine, 200 μg/ml hygromycin, and 200 μg/ml neomycin).
Isolation of Monocytes and Generation of Monocyte-derived Macrophages
Human peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and isolated from buffy coats by density gradient centrifugation using Ficoll-Paque plus® (Amersham Biosciences). Monocytes were isolated from PBMCs using the Midi-MACS® magnetic separation system and monocyte isolation kit II (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. Briefly, cells were incubated with 30 μl of MACS buffer (PBS containing 2 mm EDTA and 0.5% BSA) and 10 μl of FcR blocking reagents per 107 cells. After incubation at 4 °C for 10 min, 30 μl of buffer and 20 μl of anti-biotin microbeads per 107 cells were added. After an additional incubation at 4 °C for 15 min, the cells were washed in 2 ml of buffer, then loaded onto a magnetic separation column. The unlabeled, negatively selected, monocytes were eluted off the column and collected. Monocytes were seeded in 6-well plates and macrophages were generated by incubating these monocytes for 7 days in R10 medium (RPMI 1640 containing 10% fetal bovine serum, 25 mm HEPES, 2 mm l-glutamine, and 0.1% gentamycin) supplemented with 100 ng/ml recombinant human macrophage colony-stimulating factor (M-CSF; PeproTech, Rocky Hill, NJ).
M13 Transfection
Cell transfection was performed using a Nucleofector following the manufacturer's instructions (Amaxa Inc., Gaithersburg, MD). In brief, cells were passaged for 2 days in 300.19 medium without antibiotics. Cells were then resuspended at 1 × 106 cells/100 μl in nucleofector solution V and mixed with 2.5 μg construct DNA, or siRNA (Santa Cruz Biotechnology Inc. Santa Cruz, CA), followed by electroporation using the Amaxa Nucleofector with the U-015 program.
Preparation of Lysates, Immunoprecipitation, and Western Blot Analyses
Cells were serum starved in starvation medium (300.19 medium containing 1% bovine serum albumin without serum). Cells were then treated with DAMGO for the designated times and concentrations for 5 min, and were then lysed in Lysis Buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 2 mm EDTA, complete mini protease inhibitors, phosphatase inhibitor mixture II (Calbiochem, La Jolla, CA) and 2% Triton X-100). After centrifugation at 13,000 rpm for 15 min, the protein concentration of the supernatants was determined using the BCA protein assay reagent (Thermo Fisher Scientific, Pierce). Supernatants of the cell lysates were pre-cleared with protein G-Sepharose beads by incubation at 4 °C for 2 h, followed by centrifugation at 13,000 rpm for 1 min. The supernatants were then incubated with 2 μg of the indicated antibodies and 30 μl of protein G-Sepharose beads overnight at 4 °C. The immunoprecipitated protein complexes were resolved by SDS-PAGE. Proteins were detected using Western blot with the indicated primary antibodies and horseradish peroxidase-conjugated secondary antibodies and SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific, Pierce).
Isolation of Membrane Fractions
Cells treated with 1 μm DAMGO, were suspended in homogenization buffer (10 mm HEPES, 50 mm mannitol, 1 mm EDTA, and protease inhibitors) at 4 °C for 10 min. Cells were mechanically sheared with a 25 gauge needle. Nuclei and cell debris were removed by centrifugation at 3,000 rpm for 5 min. Membranes were collected by ultra-centrifugation at 100,000 × g for 1 h at 4 °C, and dissolved in Lysis Buffer for immunoprecipitation and Western blot analyses.
Flow Cytometry
M13 cells were treated with goat serum at 4 °C for 30 min, washed in FACS buffer (1% BSA in PBS), resuspended in either FACS buffer only or FACS buffer containing biotinylated anti-FLAG (Sigma-Aldrich Corp.) and anti-CCR5-APC (BD Biosciences Pharmingen, Palo Alto, CA) antibodies, and incubated at 4 °C for 30 min. For cells labeled with biotinylated anti-FLAG antibody, a second labeling step was carried out using streptavidin-PE (BD Biosciences Pharmingen) for 30 min at 4 °C. Cells were then washed and resuspended in 400 μl of FACS buffer for flow cytometric analysis using a FACS Calibur flow cytometer (BD Biosciences).
Calcium Flux
M13 cells were incubated in serum-free 300.19 Medium with 5 μm Fura-2 AM (Invitrogen, Molecular Probes, Eugene, OR) for 30 min at room temperature. Calcium flux was measured with a Fluomax-3 fluorescent spectrophotometer (Horiba Jobin Yvon, Inc.) (20). The ratio of fluorescence at 340 and 380 nm was calculated using the DataMax 2.20 software (Horiba Jobin Yvon, Inc.). For heterologous desensitization experiments, the cells were first incubated with 1 μm DAMGO for 5 min before addition of CCL4. For experiments with CTAP (d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2, Tocris Bioscience, Ellisville, MO) and specific inhibitors, cells were pretreated for 5 min before addition of agonists.
Chemotaxis Assay
Chemotaxis was performed using a 48-well chemotaxis chamber (Neuro Probe Inc., Gaithersburg, MD) as previously described (16, 17). In brief, M13 cells were resuspended in 300.19 Binding Medium (RPMI 1640 with 1% bovine serum albumin, 0.1 mm non-essential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, 0.5 mm β-mercaptoethanol, and 20 mm HEPES). Cells were pretreated with DAMGO and/or kinase inhibitors for 30 min at 37 °C and loaded into the upper chemotaxis chambers with 5 μm polycarbonate filter coated with fibronectin (Sigma-Aldrich). CCL4, at the designated concentrations, was loaded into the lower chambers. The chambers were incubated at 37 °C for 5 h. The filters were then washed, fixed, and stained. Cells that had migrated through the filter were counted under 100× magnification in 4 different fields, and results are expressed as the number of cells migrated in response to the chemokine per high power field.
Receptor Phosphorylation
Cells were incubated with phosphate-free DMEM medium at 37 °C for 2 h followed by the treatment with 250 μCi/ml [32P]orthophosphoric acid (H3PO4, PerkinElmer Life Sciences, Boston, MA) for an additional 2 h. CCL5 or DAMGO were added to cells to a final concentration of 10 nm or 1 μm, respectively, and incubated at 37 °C for 25 min. In designated experiments, cells were treated with specific inhibitors for 30 min prior to addition of DAMGO. Cells were then treated with Lysis Buffer at 4 °C for 15 min, and the supernatants were treated with 5 μl of anti-CCR5 antibodies and 25 μl of protein-G-Sepharose beads (GE Healthcare Life Science) for phospho-CCR5 immunoprecipitation. After 24 h, the affinity gel was spun down, washed twice with Lysis Buffer, and subjected to SDS-PAGE. The phosphorylated CCR5 was assessed by autoradiography.
[γ-32P]ATP-PKCζ Kinase Assay
Cells treated with DAMGO were lyzed in Lysis Buffer. CCR5 was immunoprecipitated from 350 μg of the protein extract with anti-CCR5 antibodies or anti-FLAG affinity gel. 100 μm of ATP was then added to the immunoprecipitated protein complexes and incubated for 1 h at 4 °C. The immune complexes were washed twice with Lysis Buffer and subjected to a kinase reaction analysis using 5 μl 10× H1 Kinase Buffer (0.5 m Tris-HCl pH 7.5, 0.1 m MgCl2, and 10 mm DTT), 10 μg histone I protein (Sigma) and 5 μCi of [γ-32P]ATP (Amersham Biosciences/GE Healthcare, Piscataway, NJ). The reaction mixtures were incubated at 30 °C for 45 min, SDS-loading buffer was added and heated at 95 °C for 5 min. The proteins were resolved using SDS-PAGE. The levels of phosphorylated histone H1 protein were evaluated using radioautography or a CycloneTM phosphorimager (Perkin Elmer Life Sciences, Boston, MA).
Colorimetric PKCζ Kinase Assay
PKCζ kinase activity was assessed using the HTscan PKCζ kinase assay kit (Cell Signaling Technology Inc., Danvers, MA) with modification. In brief, cells were treated with the Kinase Lysis Buffer (20 mm Tris-HCl pH 8.0, 15% glycerol, 1% Triton X-100, 5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mm sodium orthovanadate, and 1 mm EGTA). The protein complexes immunoprecipitated as described above were used as the source of enzymes for the analysis of PKCζ kinase activity. Two reactions were performed for each sample in parallel: reaction A, the sample mixed with 40 μm PKCζ pseudosubstrate inhibitor (PSI), and reaction B, without PSI. Samples were incubated at 30 °C for 30 min in Reaction Buffer (25 mmol/liter Tris-HCl pH 7.5, 10 mmol/liter MgCl2, 5 mm β-glycerophosphate, 0.1 mm Na3VO4, 2 mm DTT, 200 μm ATP, 1.5 μm biotinylated kinase peptide substrate). Reactions were stopped with 50 mm EDTA, and reaction mixtures were then transferred to streptavidin-coated strips and incubated at room temperature for 1 h. The phosphorylated peptides were detected by incubation with rabbit anti-phosphorylated peptide antibodies, followed by incubation with HRP-labeled anti-rabbit antibodies, and developed with TMB substrate (R&D Systems, Minneapolis, MN). The TMB reaction was terminated by adding 3 n HCl, and the absorbance was measured using a microplate reader (Thermo Fisher) at 450 nm. The kinase activity of PKCζ is reported as the difference between absorbance of reaction B and A. The data presented are the average of triplicate determinations with standard deviation.
DNA Constructs
The PKCζ and PDK1 coding sequences were amplified by polymerase chain reaction using either PKCζ-GFP plasmid (Clontech, Mountain View, CA) or pCMV-SPORT6-PDK1 (Invitrogen, Full-Length Mammalian Gene Collection Gene ID 4778360), employing flanking primers (PKCζ: forward primer: 5′-CGGAATTCAGATCTCGAGATGG-3′ and reverse 5′-GCTCTAGAACCGACTC CTC-3′; PDK1 forward: 5′-GGAATTCCATATGGCCAGGACCACC-3′ and reverse 5′-GCTCTAGATGCACAGCGGCGTC-3′, respectively, the underlined sequences indicate EcoRI and XbaI restriction sites). These genes were both cloned into the pDNR-Dual donor vector using the Creator DNA cloning kit (Clontech). The PKCζ and PDK1 constructs were transferred from the donor vector to the acceptor vectors pLPS-ECFP or pLPS-EYFP using the Cre recombination reaction. These acceptor vectors were generated from pLPS-3′-EGFP (Clontech) with the substitution of EGFP with an ECFP or EYFP DNA fragment excised from pECFP-N1 or pEYFP-N1 plasmids (Invitrogen) using AgeI and NotI. The PKCζ-EYFP and PDK1-ECFP constructs were transfected into cells using the Nucleofector kit as described above. The CFP and YFP double-positive stable cell lines were obtained following flow cytometry cell sorting. The molecular weight of PKCζ and PDK1 fusion proteins were verified using Western blot with anti-PKCζ (Santa Cruz Biotechnologies) or PDK1 (Cell Signaling Technologies) antibodies.
Fluorescence Microscopy
For M13 cells, images were captured at room temperature using Olympus inverted fluorescent microscope 1X51 at 40× using a SPOT Insight QE CCD microscope digital camera (Diagnostic Instruments, Sterling Heights, MI). Detection of CFP and YFP was performed using CFP (ET 436/20, Dichroic T455LP, Emitter ET 480/40, Chroma Technology, Rockingham VT) and FITC filters. For CMC cells, images were captured using Leica TCS SP5 confocal microscopy in a time lapse manner using 63× oil objective lens with an Argon laser excitation for CFP and YFP at 458 nm and 514 nm, emission at 465–505 nm and 525–600 nm, respectively. The fluorescence intensity in CMC cells was measured using Image J 1.43u. The ratio of fluorescence intensity between cell membrane, and cytosol was calculated and plotted.
Measurement of the Interaction between PKCζ and PDK1 using FRET Analysis
Cells were washed twice with Hank's Buffered Salt Solution (HBSS) containing calcium and magnesium, resuspended in HBSS, and transferred to 4.5 cm disposable polymethyl-methacrylate cuvettes (VWR). The YFP emission was measured at 525 nm by the CFP excitation at 435 nm using a Fluoromax-3 fluorescence spectrophotometer with a 450 watt xenon lamp.
Statistical Analysis
Experimental procedures were performed at least three times, and results were analyzed for statistical significance using the unpaired Student's t test.
RESULTS
A PKCζ Inhibitor Blocks the DAMGO-induced Desensitization of CCR5 in Mediating Calcium Flux and Chemotaxis Responses in M13 Cells
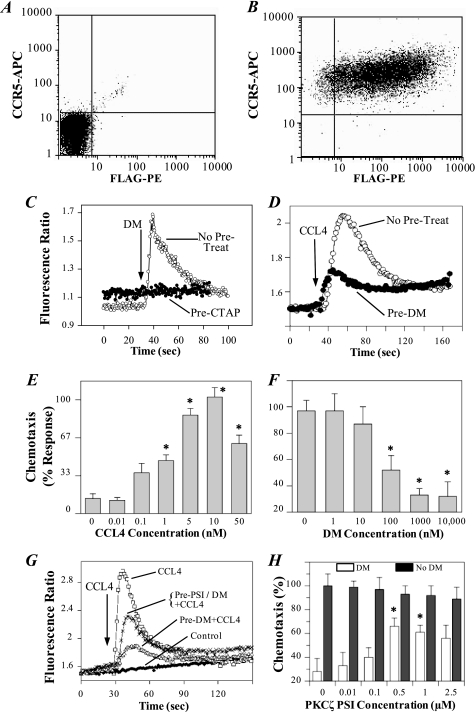
We previously showed that DAMGO can induce CCR5 cross-desensitization in monocytes and CMC cells. CMC cells are derived from the Chinese hamster ovary (CHO) cell line expressing fully functional MOR and Flag-tagged CCR5 receptors, based on radiolabeled binding analysis and receptor-mediated activation of G protein activity (10). However, the molecular mechanism of MOR-induced desensitization of CCR5 is still unclear. To better understand this mechanism, we established the M13 cell line, a mouse pre-B 300.19 cells transfected to stably express both the human MOR and human CCR5 receptors. The reasons for using M13 cells for the mechanistic study in addition to CMC cells include: 1) the mechanism revealed in M13 cells could be more similar to immune cells than CMC cells; 2) M13 cells can be efficiently transfected and highly express the transfected MOR and other genes; 3) the availability of reagents such as RNAi and antibodies that are commercially available for murine cells; 4) M13 cells exhibit strong calcium flux and chemotaxis responses to cytokine stimuli. We performed flow cytometric analysis to examine receptor expression in M13 cells, and the results showed that virtually all (>95%) of these cells co-expressed both MOR and CCR5 (Fig. 1, A and B). The analysis also showed that only a small (<5%) portion of these cells expressed CCR5 in the absence of MOR. These receptors appeared to be fully functional based on the ability of MOR and CCR5 to induce a calcium flux response (Fig. 1, C and D) and chemotaxis (Fig. 1E). Pretreatment with H-d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), a MOR-selective antagonist, blocked the calcium flux induced by DAMGO, confirming that the calcium response to DAMGO was mediated by activation of MOR (Fig. 1C). To determine whether activation of MOR led to a deficit in CCR5 function, cells were treated with DAMGO for 2 min prior to the treatment with CCL4. The results show that pretreatment with 1 μm DAMGO led to a reduction in the calcium flux response to CCL4 (Fig. 1D). The CCL4 induced chemotaxis of M13 was also suppressed by DAMGO (Fig. 1F). DAMGO pretreatment reduced the chemotactic response to CCL4 by 45% with 100 nm DAMGO, and by more than 60% with 1 μm DAMGO. These results showed that activation of MOR induced heterologous desensitization of CCR5.
FIGURE 1.
The DAMGO-induced cross-desensitization of the calcium flux and chemotaxis response to CCL4 is inhibited by a PKCζ inhibitor. A and B, flow cytometry analysis of the expression of CCR5 (APC) and MOR (FLAG-PE) receptors, using parental 300.19 (A) and the M13 (B) cell lines. C, inhibition of the calcium response of M13 cells to DAMGO (100 nm) by CTAP (10 μm). CTAP was administered for 5 min before stimulation with 100 nm DAMGO. D, DAMGO-induced desensitization of the calcium response of M13 cells induced by CCL4. CCL4 (2 nm) stimulation was carried out 2 min after DAMGO (1 μm) pretreatment. E, chemotaxis analysis of M13 cells was performed with the designated doses of CCL4. *, p < 0.05 versus 0 nm DM control. F, desensitization effect of DAMGO on CCL4-induced chemotaxis. Cells were pretreated for 30 min with the designated doses of DAMGO, followed by chemotaxis against 10 nm CCL4. *, p < 0.05 versus 0 nm DM control. G, PKCζ PSI inhibits the DAMGO-induced CCR5 desensitization, as measured by CCL4-induced calcium mobilization. CCL4 (2 nm) stimulation was carried out 5 min after DAMGO (1 μm) treatment. Alternatively, designated cells were pre-treated with 0.5 μm PKCζ PSI for 5 min, followed by treatment with DAMGO (1 μm) for 5 min prior to stimulation with CCL4 (2 nm). Data are representative of six experiments. H, effect of the PKCζ PSI on the DAMGO-induced suppression of the CCL4 chemotactic response. M13 cells were pretreated with the designated doses of PSI for 30 min, followed by the pretreatment with 1 μm DAMGO. Cells were then subjected to chemotaxis analysis against 10 nm CCL4. Migrated M13 cells were counted in randomly selected high power fields (HPF). Data represent the mean cell number in four HPF as a percentage of the control. Data represent an average of triplicate determinations and are representative of three experiments. *, p < 0.05 versus control. DM, DAMGO.
To explore the role of PKCζ in mediating MOR-induced desensitization of CCR5, we used a selective PKCζ inhibitor to assess whether PKCζ was required for the DAMGO-induced desensitization of CCR5 in mediating a calcium flux induced by CCL4. Cells were pretreated with a myristoylated PKCζ PSI (PKCζ positions 113–129 [SIYRRGARRWRKLYRAN]) (21, 22) for 5 min prior to pretreatment with DAMGO, followed by analysis of the the calcium response to CCL4. The results show that the PKCζ PSI significantly reduced the DAMGO-induced inhibition of the calcium response to CCL4 (Fig. 1G; peak relative fluorescence ratio for controls 2.82 ± 0.1, compared with 1.76 ± 0.07 for DAMGO-treated versus 2.31 ± 0.12 for DAMGO+PSI. This represents a calcium response relative to baseline of 0.12 ± 0.06 versus 0.67 ± 0.05 for DAMGO-treated versus DAMGO+PSI (p < 0.01 for DAMGO versus DAMGO+PSI). The PSI did not affect the CCR5 mediated calcium flux in M13 cells at the 0.5 μm dose (supplemental Fig. S1). We also found the PSI reversed the ability of DAMGO pretreatment to inhibit the chemotaxis response to CCL4. The results show that at an optimal PSI concentration, 0.5 μm, induced a 2-fold increase in the chemotactic response of DAMGO pretreated M13 cells to CCL4 (Fig. 1H). These results suggest that PKCζ plays an important role in mediating the heterologous desensitization between MOR and CCR5.
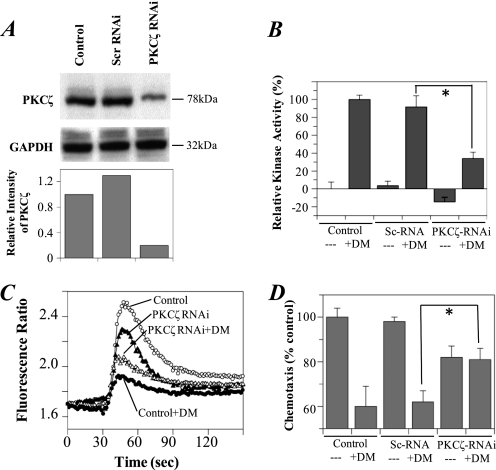
An RNAi for PKCζ Blocks DAMGO-induced Cross-desensitization of CCR5
To further examine the role of PKCζ in the heterologous desensitization pathway between MOR and CCR5, we transfected M13 cells with PKCζ RNAi to “knock-down” endogenous PKCζ protein expression. After treatment with the PKCζ RNAi, the PKCζ protein level was significantly decreased in comparison to the housekeeping protein GAPDH (Fig. 2A). The PKCζ kinase activity was also reduced in these cells (Fig. 2B). The cells with reduced PKCζ protein expression demonstrated a greater calcium flux (Fig. 2C) and chemotaxis (Fig. 2D) response to CCL4 after pretreatment with DAMGO in comparison to cells transfected with the scrambled RNAi or control cells without transfection. These results indicate that PKCζ is required for the biochemical process leading to heterologous desensitization between MOR and CCR5. We also noted a modest reduction in the activity of CCR5 in the presence of the PKCζ-RNAi (Fig. 2D), which may indicate a possible role for PKCζ in CCR5 function.
FIGURE 2.
The suppression of PKCζ activity with RNAi blocks the DAMGO-induced desensitization of calcium responses to CCL4 in M13 cells. Cells were transfected with PKCζ RNAi or a scrambled RNAi. The expression of PKCζ was detected by Western blot with PKCζ antibody, and the relative band intensity (compared with the control) is shown (A). The kinase activity of PKCζ in cells was measured by colorimetric analysis (B) and compared with the cells without transfection as the control. The calcium flux response (C) and chemotaxis (D) induced by 2 nm or 5 nm CCL4 after pretreatment with 1 μm DAMGO (DM) for 5 min. Analysis of data from all experiments shows an average peak calcium response (relative to baseline) of 0.12 ± 0.07 versus 0.49 ± 0.01 (DAMGO versus RNAi+DAMGO; p < 0.01), and 0.80 ± 0.06 versus 0.67 ± 0.35 (Control versus RNAi; p, NS). Data are representative of three experiments. *, p < 0.05 versus control.
The Phosphorylation of CCR5 Induced by DAMGO Treatment Can Be Suppressed by a PKCζ Inhibitor
We have previously shown that MOR-induced desensitization of CCR5 is associated with phosphorylation of CCR5 (16). To examine whether DAMGO induced cross-phosphorylation of CCR5 is mediated by PKCζ, we first examined the serine phosphorylation state of CCR5 in DAMGO-pretreated M13 cells using a Western blotting method (23). M13 cells were treated with CCL4, CCL5, or DAMGO in the presence or absence of PSI, then immunoprecipitated with anti-CCR5 antibodies and blotted with anti-phosphoserine antibodies. We found that CCL4 or CCL5 treatment induced CCR5 serine phosphorylation, which was not substantially changed by PSI (Fig. 3A, upper panel). In contrast, DAMGO treatment induced CCR5 serine phosphorylation, which was suppressed by PSI. Densitometric analysis of the effects of PSI on CCR5 levels (Fig. 3A, upper panel) showed lanes 2 versus 3, 1.0 ± 0.1 versus 0.78 ± 0.19 (NS); lane 4 versus lane 5, 1.47 ± 0.25 versus 1.28 ± 0.35 (NS); and lane 6 versus 7, 0.86 ± 0.07 versus 0.17 ± 0.16 (p < 0.002). The CCR5 and GAPDH in the cell lysates were probed as loading controls, and the results showed that they were expressed at similar levels in all treatment groups (Fig. 3A, lower panel). We also confirmed this finding using a [32P]orthophosphate labeling method (Fig. 3B). Similarly, we found that DAMGO-induced phosphorylation of PKCζ in CMC cells (supplemental Fig. S2). These results suggest that cross-talk between MOR and CCR5 results in PKCζ-dependent phosphorylation of CCR5.
FIGURE 3.
DAMGO induces phosphorylation of CCR5 in M13 cells. M13 cells were serum starved and then pretreated with PKCζ PSI (25 μm) for 30 min prior to stimulation with DAMGO (1 μm). CCR5 was immunoprecipitated with anti-CCR5 antibodies, then detected by Western blot analysis with anti-phosphoserine antibodies (A, upper panel). The total CCR5 in cell lysates was determined by Western blot analysis with anti-CCR5 antibody (A, middle panel). GAPDH in total cell lysates (TCL) was detected with anti-GAPDH antibodies as a loading control (A, lower panel). Data are representative of three experiments. B, M13 cells were radio-labeled with [γ-32P]orthophosphoric acid, and then treated with or without DAMGO (DM; 1 μm) for 25 min in the presence or absence of PSI. CCR5 was immunoprecipitated with anti-CCR5 antibody from total cell lysates. The immunoprecipitated complexes were subjected to SDS-PAGE and the phosphoryated CCR5 was detected using audioradiography. Data are representative of five experiments.
Phosphorylated PKCζ Associates with CCR5
We found that the level of phosphorylated PKCζ increased in M13 cells after DAMGO pretreatment (Fig. 4A, upper panel). To examine whether the phosphorylated PKCζ forms a complex with CCR5, we immunoprecipitated CCR5 complexes from the M13 cell lysates, then probed with monoclonal antibodies, which recognize PKCζ at the phosphorylated Thr-410 site. We found that the association between this phosphorylated form of PKCζ and CCR5 increased after DAMGO treatment (Fig. 4A, middle panel). In addition, the phosphorylated PKCζ is increased in the membrane fraction, and associated with CCR5 in both M13 cells (Fig. 4B) and CMC cells (supplemental Fig. S3) after 5 min of treatment with DAMGO. These results indicated an increase in the association of CCR5 with phospho-PKCζ after MOR activation at the cell membrane.
FIGURE 4.
Phosphorylated PKCζ associates with CCR5 upon treatment with DAMGO. A, M13 cells were serum starved and then treated with 1 μm DAMGO for the indicated time periods. Cells were then lysed and immunoprecipitated (IP) with anti-PKCζ-T410 or anti-CCR5. Phosphorylated PKCζ (PKCζ-T410) and total CCR5 in the immunoprecipitated complex were detected by immunoblotting (IB) with the respective antibodies. A control blot from an immunoprecipitation with an irrelevant antibody (anti-GFP) is also presented. B, M13 cells were treated with 1 μm DAMGO for 0 or 5 min, and then membrane fractions (MF) were isolated, and CCR5 was immunoprecipitated with anti-CCR5 antibody from total cell lysates (TCL) or the purified membrane fraction. Western blotting analysis was performed to detect the levels of phosphorylated PKCζ (Thr-410). Data are representative of five experiments.
DAMGO Stimulates PKCζ Kinase Activity
The association between PKCζ and CCR5 suggests that PKCζ is in close proximity to CCR5 and enables PKCζ to phosphorylate CCR5. Because the PKCζ phosphorylation, particularly at the Thr-410 residue site, is required for its activation (24), and we found that Thr-410 is phosphorylated in cells after DAMGO treatment, to confirm that the activity of PKCζ is indeed increased in cells, we further analyzed the kinase activity of PKCζ using a colorimetric PKCζ kinase assay. This assay specifically measures the kinase activity of PKCζ, and the absorbance correlates with PKCζ protein concentration, and shows a linear dose curve to recombinant PKCζ protein (supplemental Fig. S4). By analyzing the kinase activities of immunoprecipitated PKCζ, we found the kinase activity of PKCζ was elevated within 30 s following treatment with DAMGO in M13 cells, and gradually increased further during the 5 min analysis (Fig. 5A). Moreover, our analysis showed that the DAMGO-induced activation of PKCζ was dose dependent (Fig. 5B).
FIGURE 5.
Activation of PKCζ in M13 cells induced by DAMGO. A, M13 cells were treated with 1 μm DAMGO for the designated time periods. B, M13 cells were treated with the designated concentrations of DAMGO for 5 min. Kinase activity of PKCζ was measured by colorimetric kinase analysis. Each data point represents an average of triplicate assays. Data are representative of three experiments. *, p < 0.01. Elevated PKCζ kinase activity is detected in immunoprecipitated CCR5 complexes in DAMGO-treated M13 cells. M13 (C) or CMC (D) or monocyte-derived macrophage (MDM) cells (E) were treated with or without 1 μm DAMGO for 5 min. PKCζ kinase activity of protein complexes immunoprecipitated with anti-CCR5 or anti-PKCζ antibodies was measured using colorimetric kinase analysis. Anti-GFP antibody was used as a negative control (irrelevant antibody) for the immunoprecipation. Each data point represents an average of triplicate assays. F, M13 cells were treated with or without 1 μm DAMGO for 5 min, and the kinase activity of PKCζ in CCR5 protein complexes which were immunoprecipitated with anti-CCR5 antibody was determined in the presence or absence of 2.5 μm PSI using a [γ-32P]ATP kinase analysis. Data are representative of five experiments. *, p < 0.01 versus beads only.
To test whether the PKCζ associated with CCR5 is also in an activated state, we analyzed the PKCζ kinase activity in immunoprecipitated complexes with CCR5. After M13 cells were treated with 100 nm DAMGO for 5 min, CCR5 protein complexes were immunoprecipitated with anti-CCR5 antibody, and the PKCζ kinase activity was determined (Fig. 5C). We found that the kinase activity of PKCζ associated with CCR5 was increased, based on comparison with the anti-GFP antibody immunoprecipitation control, indicating that the CCR5 associated-PKCζ is activated in M13 cells after DAMGO treatment.
We further performed experiments using a [γ-32P]ATPase kinase assay. M13 cells were treated with 1 μm DAMGO for 2 min and the kinase activity of PKCζ in the immunoprecipitated CCR5 complexes was analyzed using histone-I as a substrate and detected by autoradiography. Here again, we found that PKCζ kinase activity significantly increased with DAMGO treatment for 2 min (Fig. 5F). The kinase activity was substantially suppressed by the treatment with the PKCζ PSI, consistent with the specificity of PKCζ activity. Thus, these experiments suggest that treatment with DAMGO activates PKCζ.
To confirm the phosphorylation findings just described describe a general regulatory mechanism of cross-talk between MOR and CCR5, we analyzed the kinase activities in CCR5 immunoprecipitated complexes from both CMC cells and primary macrophages. These primary cells express both endogenous MOR and CCR5. Here again, we found that the kinase activities of CCR5 are elevated in both CMC and primary macrophages using the colorimetric assay (Fig. 5, D and E). Collectively, the results suggest a common mechanism in which MOR activation induces PKCζ activation, and activated PKCζ is associated with CCR5.
The PDK1 Pathway Is Involved in the MOR-induced Activation of PKCζ
To determine the pathway involved in PKCζ activation, we examined whether DAMGO-induced PKCζ activation is dependent on PDK1, a kinase with the reported capacity to activate PKCζ (25). We examined the phosphorylation status of PDK1 in DAMGO-treated cells, and we found that the level of PDK1 phosphorylation was increased within 1 min following DAMGO administration (Fig. 6A). To examine the involvement of PDK1 in PKCζ activation, we established a PDK1-deficient M13 cell line by transfecting M13 cells with a molecular construct that yields expression of kinase-dead (KD-PDK1 which bears a mutatation in the PIF pocket (L155E). Western blot analysis (Fig. 6B) shows that KD-PDK1 is highly expressed. Moreover, the calcium flux and chemotaxis results (Fig. 6, C and D) show that the cells expressing KD-PDK1 failed to exhibit the typical DAMGO-induced cross-desensitization observed in the parental M13 cells, or M13 cells expressing empty vector.
FIGURE 6.
DAMGO activates PKCζ through a PDK1-dependent pathway. A, M13 cells were treated with 1 μm DAMGO for the indicated time periods, and PDK1 was immunoprecipitated from the cell lysates. The phosphorylated PDK1 was detected using Western blot analysis with anti-phospho-PDK1 (Tyr373/376) antibody. Data are representative of three experiments. B, cells were transfected with KD-PDK1 or empty vector. The expression of KD-PDK1 and PDK1 were detected by Western blot with PDK1 antibody. The calcium flux response (C) or chemotaxis (D) of M13 cells stably expressing KD-PDK1, empty vector, or cells without transfection, were induced with 2 nm or 10 nm CCL4 after pretreatment with 1 μm DAMGO treatment for 5 min. Analysis of data from all experiments shows an average peak calcium response (relative to baseline) of 0.20 ± 0.01 versus 0.57 ± 0.13 (Vector+DAMGO versus KD-PDK1+DAMGO; p < 0.05), and 0.69 ± 0.15 versus 0.92 ± 0.13 (Vector versus KD-PDK1; p, NS). Data represent an average of triplicate determinations and are representative of three experiments. *, p < 0.05 versus control + DAMGO.
Activation of MOR Promotes the Interaction between PKCζ and PDK1
To examine the molecular action of PDK1 in activating PKCζ upon DAMGO treatment, we measured the physical interaction of PDK1 and PKCζ using M13 and CMC cells, which stably express PDK1-CFP and PKCζ-YFP. These fusion proteins permit interaction studies using fluorescence resonance energy transfer (FRET) analysis. Cell imaging showed that PDK1-CFP ubiquitously distributes in cells. PKCζ-YFP predominately distributes in the cytoplasm (Fig. 7, A and D), which is similar to the distribution pattern of endogenous PDK1 and PKCζ (26, 27). The results suggest that following the DAMGO treatment, PKCζ initiates translocation to the cellular membrane (Fig. 7, A and D), and the translocation is apparent as early as 10 min following opioid treatment (Fig. 7, D and E). These distribution patterns were also exhibited in transfected CMC cells (Fig. 7, D and E). Western blot analysis showed that PKCζ-YFP (105 kDa) or PDK1-CFP (92 kDa) were highly expressed in M13 cells with transfection of either PKCζ-YFP or PDK1-CFP but not in the untransfected cells or cells with transfection of CFP, or YFP (Fig. 7B). We measured the interaction between PKCζ-YFP and PDK1-CFP using FRET analysis. The FRET signal was substantially increased within 45 s of DAMGO treatment (Fig. 7C), while the fluorescence intensity in M13 cells with CFP and YFP expression did not show detectable elevation. These results clearly indicate that there was an enhanced interaction between PDK1 with PKCζ following MOR activation.
FIGURE 7.
DAMGO treatment promotes the interaction between PKCζ-YFP and PDK1-CFP in M13 cells. M13 cells were co-transfected with pLPS-EYFP-PKCζ and pLPS-ECFP-PDK1. A, cells were imaged by inverted fluorescence microscopy with either a YFP filter or CFP filter. The distribution of PKCζ-YFP was primarily cytoplasmic while PDK1-CFP was distributed in both the cytoplasm and nucleus. B, expression of PKCζ in M13 cells following transfection with the designated constructs. M13 cells were transfected with pEYFP-N1 or/and pECFP-N1 or pLPS-EYFP-PKCζ or/and pLPS-ECFP-PDK1. PKCζ and PKCζ-YFP were detected by Western blot analysis with anti-PKCζ or anti-PDK1 antibody. GAPDH was employed as a loading control, and was detected with anti-GAPDH antibody. C, M13 cells with either CFP and YFP expression (lower line), or PDK1-CFP and PKCζ-YFP expression (upper line), were analyzed for PDK1 and PKCζ interaction by measuring CFP and YFP FRET using fluorospectrophotometry with excitation at 435 nm and emission at 525 nm. Cells were treated with 1 μm DAMGO at 30 s. Data are presented as a normalized moving averaged trend line. D, CMC cells expressing PKCz-YFP and PDK1-CFP were imaged by confocal microscopy following treatment with 100 nm DAMGO and imaged at the designated times (min). E, fluorescence intensity at the cell membrane, versus the cytoplasm and nucleus, was measured using Image J. The ratio of fluorescence intensity between these two regions at each time point was calculated and plotted. Data are representative of three experiments.
Kinase-deficient PKCζ Mutants Fail to Support Cross-desensitization of CCR5
We examined the ability of three kinase-deficient PKCζ mutants to participate in the heterologous desensitization of CCR5. We generated mutants for three critical residues for these studies including Lys-281, an essential amino acid for kinase activity in the ATP binding region of the kinase domain, Thr-410 in the activation loop and the phosphorylation site for PDK1, and Thr-560 in the turn motif which is the autophosphorylation site and its phosphorylation is also crucial for the activation (reviewed in Ref. 25). We transfected M13 cells to stably express PKCζ mutants K281M, T410A, and T560A. We also included the T410E mutation, since this substitution results in an enzyme which retains most of the activity of the wild-type form. Cells were also transfected with wildtype PKCζ or pcDNA3.0 empty vector as controls. Western blot analysis showed that these proteins were highly expressed (Fig. 8A). The kinase activities (Fig. 8B) of cells bearing PKCζ K281M, T410A, or T560A mutations in M13 cells were lower than those of cells without transfection or transfected with vector control, while wild type and T410E-expressing cells demonstrated kinase activities, which were greater than control. The results (Fig. 8C) showed that after DAMGO treatment, the calcium flux response of M13 cells with K281M, T410A, and T560A mutations were restored to normal (or elevated) levels in response to CCL4, while the T410E mutant and wild type expressing cells exhibited the typical reduced calcium flux responses following DAMGO pre-treatment (Fig. 8C). Consistent with these calcium flux results, DAMGO treatment of cells expressing K281M, T410A, and T560A mutants failed to result in the typical inhibition of the CCR5 chemotactic response, when compared with wild type, T410E, non-transfected, or vector transfected control cells (Fig. 8D). These results show that PKCζ residues Lys-281, Thr-410, and Thr-560, with established roles in the activity of this kinase, are also necessary for PKCζ to mediate DAMGO-induced cross-desensitization of CCR5.
FIGURE 8.
Effect of PKCζ mutants on the MOR activation-induced CCR5 desensitization. A, cells were transfected with indicated PKCζ mutants, wild type (WT), or empty vector (vector) or without transfection (control). The expression of PKCζ variants were detected by Western blot with PKCζ antibody. B, kinase activity of PKCζ was measured by colorimetry and compared with the cells without transfection as the control. The calcium flux response (C) or chemotaxis (D) of these M13 cells was induced by 2 nm or 10 nm CCL4 after pretreatment with 1 μm DAMGO treatment for 5 min. Data are representative of three experiments. Analysis of data from all experiments shows an average peak calcium response (relative to baseline) of 0.23 ± 0.02 versus 0.93 ± 0.04 (WT versus K281M; p < 0.01), 0.23 ± 0.02 versus 1.12 ± 0.08 (WT versus T410A; p < 0.01), 0.23 ± 0.02 versus 1.08 ± 0.03 (WT versus T560A; p < 0.01), 0.23 ± 0.02 versus 0.27 ± 0.07 (WT versus T410E; p = NS). Cells subjected to chemotaxis analysis were counted in randomly selected high-power fields (HPF). Data represent the mean cell number in four HPF as a percentage of the control. Data represent an average of triplicate determinations and are representative of three experiments. *, p < 0.05 versus control + DAMGO.
DISCUSSION
In the present study, we systematically analyzed the role of PKCζ in the MOR-induced desensitization of CCR5. Our results demonstrate that activated MOR up-regulates the activity of PKCζ through PDK1. PDK1 interacts with and phosphorylates PKCζ, and the activated PKCζ then phosphorylates CCR5 through its interaction with CCR5 at the cell membrane (Fig. 9). In the present study immunoprecipitated CCR5 from lysates, or cell membrane preparations, of cells treated with DAMGO, were analyzed for PKCζ-specific kinase activities in the protein complexes by either a radioisotope method, or by a non-radioactive colorimetric assay. The results show that the activation of MOR induces an elevation in PKCζ kinase activity, which can be inhibited by a PKCζ specific pseudosubstrate inhibitor. Immunoprecipitation analysis indicates that the phosphorylated and activated form of PKCζ directly associates with CCR5. These results suggest that PKCζ participates in the the heterologous desensitization of CCR5 through a phosphorylation mechanism. To our best knowledge, this is the first report showing that PKCζ mediates cross-desensitization between GPCRs by this mechanism.
FIGURE 9.
Proposed model of the pathway for MOR-induced PKCζ-mediated CCR5 desensitization. Activation of MOR with DAMGO administration activates G-protein β, which induces activation of PI3K and promotes the activation and phosphorylation of PDK1. Phosphorylated PDK1 acts to phosphorylate PKCζ to enable PKCζ translocation to the cell membrane, and then the recruited PKCζ phosphorylates CCR5. The recruited PKCζ supplements the complex of phosphorylated PKCζ and CCR5. The phosphorylation of CCR5 is suppressed by the PKCζ pseudosubstrate inhibitor (PSI), its dominant negative mutant K281M, T410A, T560A, or its RNAi, which prevents the phosphorylation and desensitization of CCR5.
In this study, we propose a mechanism of PKCζ-mediated CCR5 desensitization in which MOR is activated by DAMGO, and this is followed by induction of PDK1 activation. We found that overexpression of KD-PDK1, a kinase-dead mutant of PDK1, decreases MOR-induced CCR5 desensitization. Previous studies have demonstrated that PDK1 can directly phosphorylate PKCζ (28). Finally, we also demonstrated a rapid association of PDK1-CFP and PKCζ-YFP following MOR activation, based on FRET analysis. Collectively, these results suggest a signaling pathway in which MOR induces PDK1 activation, leading to association between PDK1 and PKCζ, and this results in PKCζ activation.
As a general mechanism, our evaluation of both M13 and CMC transfected cell lines, as well as primary monocyte-derived macrophages, shows that the association between CCR5 and PKCζ is increased after MOR activation. The activated PKCζ may translocate to the cell membrane following MOR activation. PKCζ has previously been shown to translocate to the cellular membrane upon activation of this kinase with either ceramide in astocytes (27), or by activation of opioid receptors using met-enkephalin (a non-selective opioid agonist), in HEK293 cells (15).
The PKC family includes twelve serine-threonine kinases, which are subdivided into three groups based on structural homology. Classical PKCs (α, β1, β2, and γ) can be regulated by both calcium and diacylglycerol (DAG), while novel (δ, ϵ, θ, and η) and atypical PKCs (ζ and ι/λ) are calcium independent, and only the activity of novel but not atypical PKCs can be activated by DAG.
Structurally, PKCζ consists of a Phox-Bim1 (PB1) domain in the N terminus, a pseudosubstrate (PS) region, a C1 domain, and a Ser/Thr kinase domain in the C terminus. The kinase domain includes an ATP binding region, an activation loop, a turn motif and a hydrophobic motif. In the ATP binding region, Lys-281 is essential for kinase activity. Amino acid substitutions of Lys-281 have been used as kinase-defective dominant-negative forms of PKCζ. Thr-410 in the activation loop is phosphorylated by PDK1, and T410A loses enzymatic activity, while the Glu mutant T410E, which may mimick phsophorlated Thr, retains kinase activity (25, 29). PKCζ Thr-560 in the turn motif is the autophosphorylation site, and its phosphorylation is also crucial for kinase activation (24).
Previous reports have suggested that PKC family members are involved in the cross-desensitization of CCR5 following activation of the high affinity formyl peptide receptor (FPR) in monocyte-derived immature dendritic cells (30). In these studies, W peptide, a potent agonist of FPR, induced phosphorylation of CCR5 in a PKC-dependent (staurosporine-inhibitable) manner (31). Work carried out using various partially selective chemical inhibitors of the PKCs have suggested that either PKCδ or PKCζ appear to be necessary for opioid receptor-induced cross-desensitization of CCR1 (15). In contrast, the CXCR1-induced cross-desensitization of CCR5 appears to be dependent on PKCϵ (31). Moreover, DAMGO treatment stimulates PKCϵ translocation to the cell membrane and induces MOR homologous desensitization in SH-SY5Y neuroblastoma cells (32). The variable use of PKC isoforms may enable the GPCRs to selectively cross-talk with other GPCRs, depending on the precise PKC family member that is induced, and the ability of the potential target receptor to serve as a substrate for that PKC member.
PKCζ can be involved in some of the signaling responses mediated via other GPCRs. For example, PKCζ is involved in the polymorphonuclear leukocyte integrin-dependent adhesion and chemotaxis in responses to formyl peptide and CXCL8 (33). Indeed, it would appear that PKCζ may participate in integrin activation pathways, based on studies showing that integrin-dependent signaling can result in PKCζ activation through the recruitment of small GTPases including Rho and cdc42 and subsequent recruitment and activation of this PKC (34). However, the results in the present report show that PKCζ-PSI fails to inhibit the calcium and chemotactic responses through CCR5. Taken together, these studies suggest that CCR5 may carry out signaling processes, which are less dependent on PKCζ than are the pathways initiated by the receptors for CXCL8 or formyl peptide.
It should be acknowledged that signal transduction pathways other than the PDK1 and PKCζ pathway described here, may also play a role in MOR induced CCR5 desensitization. For example, other PKCs can also activate MAP kinases through phosphorylation of the serine/threonine protein kinase c-Raf (35), and activated MAP kinases can in turn activate G protein-coupled receptor kinases (GRKs) (36). It is conceivable that GRKs may contribute to the desensitization of CCR5 by cross-phosphorylation (37), which would provide a pathway for PKC-mediated indirect phosphorylation of CCR5.
It has been found that adaptors or scaffold proteins are required for PKCζ to achieve functional specificity and plasticity in various cellular processes. For example, PKCζ indirectly phosphorylates the IL-4 receptor (IL-4R) by being recruited to the IL-4R complex through an interaction with the tyrosine kinase Janus kinase-1 (Jak1). Once recruited, Jak1 phosphorylates the IL-4Rα chain, creating a docking site for the SH2 domain of Stat6, which is then recruited and phosphorylated by the receptor-bound Jak1 (38).
In controlling cell polarity, a ternary complex of PAR-2, PAR-6, and PKCζ or PKCλ is formed. Disruption of the protein complexes by overexpression of kinase-negative PKCζ and PAR-6 suppresses cell polarity (reviewed in Ref. 24). In addition, PKCζ associates with the nerve growth factor p75 receptor, and this association appears to require the participation of a scaffold protein, designated sequestosome-1 (p62) (39). The binding of PKCζ to p62 involves the interactions between PB1 domains within both PKCζ and p62. PB1 domains have also been identified in other scaffold proteins such as Partitioning Defective-6 (Par-6) and MAPK/ERK kinase (MEK)-5 (40). In this way, p62 may potentially bind to a number of proteins involved in various biological processes. Recent evidence suggests that p62 plays a role in inflammation, neurogenesis, and T cell differentiation, by virtue of the heterodimerization of p62 with proteins such as PKCζ, PKCλ/τ, receptor-Interacting protein (RIP-1), and TNF receptor-associated factor-6 (TRAF-6) (41). p62 interacts with the atypical PKCs (PKCζ and PKCλ/ι) but not with any of the other closely related PKC family members. However, currently it is not clear whether the process of binding of phosphorylated PKCζ to CCR5 may require p62, or another scaffold protein(s) or kinases to mediate receptor phosphorylation.
The activation of MOR is relevant to the situation that occurs with heroin abuse, and the consequences for CCR5 function in this setting is an important issue for further analysis. It should be pointed out that morphine, a natural heroin metabolite, exhibits agonist activity which is distinct in some respects from some other MOR-selective agonists such as DAMGO. For example, morphine fails to induce a significant degree of MOR internalization, in contrast to methadone, fentanyl, endomorphin 2, or DAMGO (42, 43). On the other hand, morphine is able to activate GIRK channels via MOR at a level, which is not significantly different than methadone or DAMGO (44). These studies suggest that these agonists exhibit differential signaling activities, or biased agonism (45).
We believe our findings have implications for the possible development of PKCζ-based therapeutic strategies for the treatment of HIV-1 infections. We have previously shown that DAMGO-induced desensitization of CCR5 inhibits the capacity of human macrophages to be infected with R5 strains of HIV-1, in the absence of detectable internalization of CCR5 (16). Based on the present results, it appears that the PKCζ-mediated phosphorylated form of CCR5 may be unable to function as a fully active co-receptor for this virus. Additional experiments to address the biochemical basis for the failure of macrophages to be infected with HIV-1 following MOR-induced cross-desensitization of CCR5 are currently underway in our laboratories. Moreover, given the clear role of CCR5 in inflammatory responses, and inflammatory disease conditions, we suggest that the present studies take on additional significance. The down-regulation of the functional activity of CCR5 following MOR activation, and the predominant role of PKCζ in mediating this process, suggest that a PKCζ-related therapeutic may be developed, which could be used to treat inflammatory disease states.
Supplementary Material
Acknowledgments
We thank Drs. Jodene Moore and Arthur G. Balliet for assistance with flow cytometric analyses, as well as Christine Happel, Matthew Finley, and David Kaminsky for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants DA-14230, DA-25532, P01DA-23860, P30DA-13429, T32DA-07237, and DA-06650 from NIDA.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- DAMGO
- Tyr-d-Ala-Gly-N-Me-Phe-Gly-ol
- APC
- allophycocyanin
- GPCR
- G-protein-coupled receptor
- GRK
- G-protein-coupled receptor kinase
- M-CSF
- macrophage colony-stimulating factor
- MOR
- μ-opioid receptor
- PE
- phycoerythrin
- PKCζ
- protein kinase Cζ
- PSI
- pseudosubstrate inhibitor.
REFERENCES
- 1. McCarthy L., Wetzel M., Sliker J. K., Eisenstein T. K., Rogers T. J. (2001) Drug Alcohol Depend. 62, 111–123 [DOI] [PubMed] [Google Scholar]
- 2. Sharp B. M., Roy S., Bidlack J. M. (1998) J. Neuroimmunol. 83, 45–56 [PubMed] [Google Scholar]
- 3. Peterson P. K., Molitor T. W., Chao C. C. (1998) J. Neuroimmunol. 83, 63–69 [DOI] [PubMed] [Google Scholar]
- 4. Madden J. J., Whaley W. L., Ketelsen D. (1998) J. Neuroimmunol. 83, 57–62 [DOI] [PubMed] [Google Scholar]
- 5. Eisenstein T. K., Hilburger M. E. (1998) J. Neuroimmunol. 83, 36–44 [DOI] [PubMed] [Google Scholar]
- 6. Hu S., Chao C. C., Hegg C. C., Thayer S., Peterson P. K. (2000) J. Psychopharmacol. 14, 238–243 [DOI] [PubMed] [Google Scholar]
- 7. Pérez-Castrillón J. L., Pérez-Arellano J. L., García-Palomo J. D., Jiménez-López A., De Castro S. (1992) Immunopharmacology 23, 57–61 [DOI] [PubMed] [Google Scholar]
- 8. Ruff M. R., Wahl S. M., Mergenhagen S., Pert C. B. (1985) Neuropeptides 5, 363–366 [DOI] [PubMed] [Google Scholar]
- 9. Tubaro E., Avico U., Santiangeli C., Zuccaro P., Cavallo G., Pacifici R., Croce C., Borelli G. (1985) Int. J. Immunopharmacol. 7, 865–874 [DOI] [PubMed] [Google Scholar]
- 10. Chen C., Li J., Bot G., Szabo I., Rogers T. J., Liu-Chen L. Y. (2004) Eur. J. Pharmacol. 483, 175–186 [DOI] [PubMed] [Google Scholar]
- 11. Choi Y., Chuang L. F., Lam K. M., Kung H. F., Wang J. M., Osburn B. I., Chuang R. Y. (1999) In Vivo. 13, 389–396 [PubMed] [Google Scholar]
- 12. Szabo I., Rogers T. J. (2001) Adv. Exp. Med. Biol. 493, 75–79 [DOI] [PubMed] [Google Scholar]
- 13. Grimm M. C., Ben-Baruch A., Taub D. D., Howard O. M., Wang J. M., Oppenheim J. J. (1998) Ann. N.Y. Acad. Sci. 840, 9–20 [DOI] [PubMed] [Google Scholar]
- 14. Grimm M. C., Ben-Baruch A., Taub D. D., Howard O. M., Resau J. H., Wang J. M., Ali H., Richardson R., Snyderman R., Oppenheim J. J. (1998) J. Exp. Med. 188, 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang N., Hodge D., Rogers T. J., Oppenheim J. J. (2003) J. Biol. Chem. 278, 12729–12736 [DOI] [PubMed] [Google Scholar]
- 16. Szabo I., Wetzel M. A., Zhang N., Steele A. D., Kaminsky D. E., Chen C., Liu-Chen L. Y., Bednar F., Henderson E. E., Howard O. M., Oppenheim J. J., Rogers T. J. (2003) J. Leukoc. Biol. 74, 1074–1082 [DOI] [PubMed] [Google Scholar]
- 17. Szabo I., Chen X. H., Xin L., Adler M. W., Howard O. M., Oppenheim J. J., Rogers T. J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10276–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ali H., Richardson R. M., Haribabu B., Snyderman R. (1999) J. Biol. Chem. 274, 6027–6030 [DOI] [PubMed] [Google Scholar]
- 19. Steele A. D., Szabo I., Bednar F., Rogers T. J. (2002) Cytokine Growth Factor Rev. 13, 209–222 [DOI] [PubMed] [Google Scholar]
- 20. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 21. Kazanietz M. G., Areces L. B., Bahador A., Mischak H., Goodnight J., Mushinski J. F., Blumberg P. M. (1993) Mol. Pharmacol. 44, 298–307 [PubMed] [Google Scholar]
- 22. Standaert M. L., Galloway L., Karnam P., Bandyopadhyay G., Moscat J., Farese R. V. (1997) J. Biol. Chem. 272, 30075–30082 [DOI] [PubMed] [Google Scholar]
- 23. Shen W., Li B., Wetzel M., Rogers T. J., Henderson E. E., Su S. B., Gong W., Le Y., Sargeant R., Dimitrov D. S., Oppenheim J. J., Wang J. M. (2000) Blood 96, 2887–2894 [PubMed] [Google Scholar]
- 24. Hirai T., Chida K. (2003) J. Biochem. 133, 1–7 [DOI] [PubMed] [Google Scholar]
- 25. Chou M. M., Hou W., Johnson J., Graham L. K., Lee M. H., Chen C. S., Newton A. C., Schaffhausen B. S., Toker A. (1998) Curr. Biol. 8, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 26. Anderson K. E., Coadwell J., Stephens L. R., Hawkins P. T. (1998) Curr. Biol. 8, 684–691 [DOI] [PubMed] [Google Scholar]
- 27. Galve-Roperh I., Haro A., Díaz-Laviada I. (1997) FEBS Lett. 415, 271–274 [DOI] [PubMed] [Google Scholar]
- 28. Le Good J. A., Ziegler W. H., Parekh D. B., Alessi D. R., Cohen P., Parker P. J. (1998) Science 281, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 29. Standaert M. L., Bandyopadhyay G., Perez L., Price D., Galloway L., Poklepovic A., Sajan M. P., Cenni V., Sirri A., Moscat J., Toker A., Farese R. V. (1999) J. Biol. Chem. 274, 25308–25316 [DOI] [PubMed] [Google Scholar]
- 30. Le Y., Wetzel M. A., Shen W., Gong W., Rogers T. J., Henderson E. E., Wang J. M. (2001) Clin. Immunol. 99, 365–372 [DOI] [PubMed] [Google Scholar]
- 31. Nasser M. W., Marjoram R. J., Brown S. L., Richardson R. M. (2005) J. Immunol. 174, 6927–6933 [DOI] [PubMed] [Google Scholar]
- 32. Mandyam C. D., Thakker D. R., Standifer K. M. (2003) J. Pharmacol. Exp. Ther. 306, 965–972 [DOI] [PubMed] [Google Scholar]
- 33. Laudanna C., Mochly-Rosen D., Liron T., Constantin G., Butcher E. C. (1998) J. Biol. Chem. 273, 30306–30315 [DOI] [PubMed] [Google Scholar]
- 34. Etienne-Manneville S., Hall A. (2001) Cell 106, 489–498 [DOI] [PubMed] [Google Scholar]
- 35. Ueda Y., Hirai S., Osada S., Suzuki A., Mizuno K., Ohno S. (1996) J. Biol. Chem. 271, 23512–23519 [DOI] [PubMed] [Google Scholar]
- 36. Rubino T., Viganò D., Premoli F., Castiglioni C., Bianchessi S., Zippel R., Parolaro D. (2006) Mol. Neurobiol. 33, 199–213 [DOI] [PubMed] [Google Scholar]
- 37. Pollok-Kopp B., Schwarze K., Baradari V. K., Oppermann M. (2003) J. Biol. Chem. 278, 2190–2198 [DOI] [PubMed] [Google Scholar]
- 38. Martin P., Villares R., Rodriguez-Mascarenhas S., Zaballos A., Leitges M., Kovac J., Sizing I., Rennert P., Márquez G., Martinez A., Diaz-Meco M. T., Moscat J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9866–9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noda Y., Kohjima M., Izaki T., Ota K., Yoshinaga S., Inagaki F., Ito T., Sumimoto H. (2003) J. Biol. Chem. 278, 43516–43524 [DOI] [PubMed] [Google Scholar]
- 40. Diaz-Meco M. T., Moscat J. (2001) Mol. Cell Biol. 21, 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mamidipudi V., Wooten M. W. (2002) J. Neurosci. Res. 68, 373–384 [DOI] [PubMed] [Google Scholar]
- 42. Macey T. A., Lowe J. D., Chavkin C. (2006) J. Biol. Chem. 281, 34515–34524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McPherson J., Rivero G., Baptist M., Liorente J., Al-Sabah S., Krasel C., Dewey W. L., Bailey C. P., Rosethorne E. M., Charlton S. J., Henderson G., Kelley E. (2010) Mol. Pharmacol. 78, 756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whistler J. L., Chuang H. H., Chu P., Jan L. Y., von Zastrow M. (1999) Neuron 23, 737–746 [DOI] [PubMed] [Google Scholar]
- 45. Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Koblika B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.