Abstract
Gephyrin is a scaffold protein essential for stabilizing glycine and GABAA receptors at inhibitory synapses. Here, recombinant intrabodies against gephyrin (scFv-gephyrin) were used to assess whether this protein exerts a transynaptic action on GABA and glutamate release. Pair recordings from interconnected hippocampal cells in culture revealed a reduced probability of GABA release in scFv-gephyrin-transfected neurons compared with controls. This effect was associated with a significant decrease in VGAT, the vesicular GABA transporter, and in neuroligin 2 (NLG2), a protein that, interacting with neurexins, ensures the cross-talk between the post- and presynaptic sites. Interestingly, hampering gephyrin function also produced a significant reduction in VGLUT, the vesicular glutamate transporter, an effect accompanied by a significant decrease in frequency of miniature excitatory postsynaptic currents. Overexpressing NLG2 in gephyrin-deprived neurons rescued GABAergic but not glutamatergic innervation, suggesting that the observed changes in the latter were not due to a homeostatic compensatory mechanism. Pulldown experiments demonstrated that gephyrin interacts not only with NLG2 but also with NLG1, the isoform enriched at excitatory synapses. These results suggest a key role of gephyrin in regulating transynaptic signaling at both inhibitory and excitatory synapses.
Keywords: GABA Receptors; Glutamate; Glutamate Receptors Ionotropic (AMPA, NMDA); Neurobiology; Synapses; GABAergic Transmission; Gephyrin; Glutamatergic Transmission; Innervation; Neuroligin
Introduction
Speed and reliability of synaptic transmission are essential for information coding and require the presence of clustered neurotransmitter receptors at the plasma membrane in precise apposition to presynaptic release sites. The postsynaptic organization comprises a large number of proteins that ensure the correct targeting, clustering, and stabilization of neurotransmitter receptors. Among them, the tubulin-binding protein gephyrin plays a crucial role in the functional organization of inhibitory synapses (1). Through its self-oligomerizing properties, gephyrin can form a hexagonal lattice that traps glycine (2) and GABAA receptors in the right place at postsynaptic sites (3, 4) by linking them to the cytoskeleton. Disruption of endogenous gephyrin leads to reduced GABAA receptor clusters (3), an effect that has been shown to be accompanied by a loss of GABAergic innervation (5, 6). This observation suggests the existence of cross-talk between the post- and presynaptic sites. The retrograde control of presynaptic signaling may occur via neuroligins (NLGs),3 postsynaptic cell adhesion molecules known to transynaptically interact with presynaptic neurexins (7). NLG1 is enriched at glutamatergic synapses (8, 9), whereas NLG2 is preferentially associated with GABAergic connections (10). Overexpression of NLGs has been shown to increase the number of GABAergic and glutamatergic synaptic contacts (11). Interestingly, increasing the expression level of PSD-95, the scaffold molecule that directly binds NLG1, caused an enhancement of the glutamatergic innervation at the expense of the GABAergic one. This effect was accompanied by the recruitment of NLG2 to glutamatergic synapses (11–13). Moreover, the recent demonstration of a direct interaction between NLG2 and gephyrin (14) suggests a role for this protein in regulating transynaptic signaling at inhibitory connections. Altogether, these findings have led to the hypothesis that scaffolding molecules can establish and maintain the proper excitatory (E)/inhibitory (I) balance necessary for the correct functioning of neuronal networks, by modulating neuroligin localization and function at particular synapses (15–17). Understanding the molecular mechanisms involved in the maintenance of a proper E/I balance is a challenge as an alteration of this parameter underlies several devastating forms of neurological diseases including autism spectrum disorders (18). Previous studies on cultured hippocampal neurons have demonstrated that removal of gephyrin with single chain antibody fragments (scFv-gephyrin) (19) produces changes in the gating properties of GABAA receptors associated with a decrease in GABAergic innervation (6).
In the present study, scFv-gephyrin fragments were used to characterize further the transynaptic contribution of gephyrin to maintaining and stabilizing GABAergic synapses. Double patch experiments from monosynaptically connected cells revealed a reduction in the probability of GABA release to scFv-gephyrin-transfected cells. Moreover, transfection with scFv-gephyrin affected not only GABA but also glutamate release as demonstrated by the reduction in frequency of spontaneous and miniature glutamatergic synaptic events. Immunocytochemical data revealed a significant reduction in the number of NLG2 clusters together with a decrease of VGAT and VGLUT, the vesicular GABA and glutamate transporters, respectively. Finally, biochemical experiments demonstrated that gephyrin can form a complex not only with NLG2 but also with NLG1 in the brain, suggesting a role of this scaffold protein in regulating both excitatory and inhibitory synaptic transmission.
EXPERIMENTAL PROCEDURES
Neuronal and Cell Cultures
All experiments were carried out in accordance with the European Community Council Directive of 24 November 1986 (86/609 EEC) and were approved by the local authority veterinary service. Primary cell cultures were prepared as described previously (20). Briefly, 2–4-day-old (P2–P4) Wistar rats were decapitated after being anesthetized with an intraperitoneal injection of urethane (2 mg/kg). Hippocampi were dissected free, sliced, and digested with trypsin, mechanically triturated, centrifuged twice at 40 × g, plated in Petri dishes, and cultured for up to 14 days. Experiments were performed on cells cultured for at least 7 days. For paired recording experiments, neurons were plated at low density (∼40,000 cells/ml).
HEK-293 cells were maintained in DMEM supplemented with 10% fetal calf serum, penicillin (100 units/ml), and streptomycin (100 mg/ml) and transiently transfected with various plasmid constructs using the standard calcium phosphate method. Cells were collected 24–48 h after transfection.
Construction of Plasmid Vectors, scFv-Gephyrin
Complementary DNAs encoding full-length FLAG-tagged gephyrin have been described previously (21). The N-terminal truncated gephyrin polypeptide (amino acids 2–188) fused to GFP is described by Maas et al. (22). It acts as a dominant negative protein due to its lack of dimerization motif and is able to deplete endogenous gephyrin clusters in neurites within 24 h of expression. The murine HA-tagged NLG1 and HA-tagged NLG2 were constructed as reported elsewhere (23, 24). HA-tagged NLG2Y770A point mutant was kindly provided by Dr. Varoqueaux (14). NLG2-GFP was constructed by using PCR-based mutagenesis. A PvuI restriction site was introduced 10 amino acids downstream of the sequence encoding for the transmembrane domain of NLG2-HA. This restriction site was then used to clone the EGFP coding sequence amplified using oligonucleotides containing PvuI consensus sites.
The last 94 amino acids of the cytoplasmic domains of both NLGs were inserted into pGEX4T1 vector for bacterial expressions as glutathione S-transferase (GST)-NLGs 94-amino acid fusion proteins. All PCR-amplified products were fully sequenced to exclude the possibility of second site mutations. The technique for isolating scFv-gephyrin has already been reported (19).
Neuronal Transfection and Immunocytochemistry
Hippocampal neurons in culture were transfected with EGFP alone or co-transfected with EGFP and scFv-gephyrin using the calcium phosphate transfection method. For each Petri dish, 3 μg of DNA was transfected in total. Reliable co-transfection was ensured by routinely transfecting 0.9 μg of EGFP and 2.1 μg of scFv-gephyrin and identified by the increased EGFP signal around the nucleus. For the rescue experiments, scFv-gephyrin and the full-length HA-tagged NLG2 (NLG2-HA), NLG2Y770A (NLG2Y770A-HA), and NLG1 (NLG1-HA) were co-transfected at a ratio of 1:2.
Neurons were transfected at 7 days in vitro and used for immunostaining 48 h later. All steps were carried out at room temperature. After fixation with 4% paraformaldehyde in PBS for 10 min, neurons were quenched in 0.1 m glycine in PBS for 5 min and permeabilized with 0.1% Triton X-100 in PBS for 2 min. For the rescue experiments, cells were fixed with precooled 4% paraformaldehyde in PBS for 5 min at 4 °C then 5 min at room temperature. They were then blocked in 0.2% BSA/1% FCS or 10% FCS in PBS for 30 min. After incubation with primary antibodies for 1 h, cells were incubated with Alexa Fluorophore-conjugated secondary antibodies (1:400) for 45 min. In the case of double-immunostaining, cells were incubated with biotinylated secondary antibodies (1:100, 45 min) followed by streptavidin-conjugated fluorophores (1:100, 30 min). The coverslips were washed in PBS, rinsed in water, and mounted with VectaShield (Vector Laboratories).
The antibodies used were as follows: mouse monoclonal anti-VGAT (1:200, Synaptic Systems), mouse monoclonal anti-VGLUT1 (1:200, Synaptic Systems), rabbit polyclonal anti-NGL2 (1:200, Synaptic Systems), biotinylated goat anti-mouse IgG (Vector Laboratories). All secondary antibodies were obtained from Invitrogen.
In Vitro Binding, Immunoprecipitation, and Western Blot Analysis
Transfections were performed with the calcium phosphate method. GST pulldown assays were performed as described previously (21). For NLGs and gephyrin co-immunoprecipitation, HEK 293 cells overexpressing NLG1-HA/NLG2-HA and gephyrin-FLAG were lysed in 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 0.1% Tween 20, 10% glycerol, 10 mm EDTA, 2 mm MgCl2, and protease inhibitor mixture and immunoprecipitated by the anti-FLAG antibody. Analysis of NLG1/NLG2-gephyrin interactions was performed on postnuclear homogenates from neonatal rat brains using the following lysis buffer: 50 mm-Tris HCl, pH 7.5, 150 mm NaCl, 0.5% CHAPS, 1 mm EDTA, 10% glycerol, and protease inhibitor mixture. After a 2-h incubation with monoclonal anti-gephyrin antibody, an immunoprecipitation experiment was performed according to standard procedures. Primary antibodies were revealed by HRP-conjugated secondary antibodies (Sigma) followed by ECL (Amersham Biosciences). The following primary antibodies were used: mouse monoclonal anti-FLAG M2 (Sigma), mouse monoclonal anti-gephyrin 3B11 (Synaptic Systems), high affinity rat monoclonal anti-HA 3F10 (Roche), rabbit polyclonal anti-NLG2 (Synaptic Systems), and rabbit polyclonal anti-NLG1 (Synaptic Systems).
Confocal Microscopy and Image Analysis
Fluorescence images were acquired on a TCS-SP confocal laser scanning microscope (Leica, Bensheim, Germany) with a 40× 1.4 NA oil immersion objective, additionally magnified 2-fold with the pinhole set at 1 Airy unit. Stacks of z-sections with an interval of 0.4 μm were sequentially scanned twice for each emission line to improve the signal/noise ratio. Cluster analysis was carried out using MetaMorph Imaging System (Universal Imaging, Westchester, PA). First a binary template was created using the EGFP staining to identify transfected neurons, then cluster intensities in regions overlapping with the binary template were analyzed. Images were segmented to select immunofluorescent puncta over background labeling, and clusters were defined as >3 pixels as determined by visual inspection. Integrated Morphometry Analysis function of MetaMorph was used to quantify the number and size of clusters (four or five cells from at least four different experiments). For the rescue experiments, NLG2 staining was used to create the binary template for the NLG2-HA/scFv-gephyrin and NLG2Y770A-HA/scFv-gephyrin co-transfected cells. As excessive NLG2-HA expression masks the rescuing effect and results in an overall increase in synaptic staining (similar to NLG2-HA overexpression alone), cells with a moderate amount of NLG2-HA/NLG2Y770A-HA expression (as identified by the unsaturated NLG2 fluorescence signal) was selected for the analysis of the rescue effect. Representative figures were prepared using ImageJ software.
Electrophysiological Recordings
Spontaneous excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs) were recorded at 9 days in vitro from cultured hippocampal neurons (transfected at 7 days in vitro with different constructs) at 22–24 °C using a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA). In the case of transfected cells, mIPSCs or mEPSCs were recorded from single transfected cells surrounded by nontransfected ones. Patched cells were identified as putative principal cells on the basis of their passive membrane properties (Vrest and Rinput) which were similar to those described in identified pyramidal neurons in culture at the same days in vitro (25). No differences were found in these parameters between control and transfected neurons. Pooled data gave a mean Vrest value of −51 ± 1 mV and a mean Rinput value of 610 ± 43 megohms (n = 47). Patch electrodes pulled from borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany) had a resistance of 3–4 megohms when filled with an intracellular solution containing 137 mm CsCl, 1 mm CaCl2, 2 mm MgCl2, 11 mm BAPTA, 2 mm ATP, and 10 mm HEPES (the pH was adjusted to 7.3–7.4 with CsOH). IPSCs were recorded at a holding potential of −70 mV in the presence of 20 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 50 μm d-2-amino-5-phosphonopentanoic acid (d-AP5) to block AMPA and NMDA receptors, respectively. EPSCs were recorded in the presence of 10 μm bicuculline and 50 μm d-AP5 to block GABAA and NMDA receptors, respectively. Miniature PSCs were recorded in the presence of tetrodotoxin (TTX, 1 μm) to block sodium currents and propagated action potentials and the respective GABAA or AMPA/NMDA receptor antagonists.
For double patch recordings, pairs of action potentials (at 50-ms interval) were evoked in nontransfected presynaptic neurons (in current clamp mode) by injecting depolarizing current pulses at a frequency of 0.1 Hz. IPSCs were detected from postsynaptic transfected (scFv-gephyrin) and nontransfected (controls) neurons in voltage clamp mode at a holding potential of 0 mV (near the reversal potential for glutamate). In this case, the intracellular solutions contained 135 mm KMeSO4, 10 mm KCl, 10 mm HEPES, 1 mm MgCl2, 2 mm Na2ATP, and 0.4 mm Na2GTP (the pH was adjusted to 7.3 with KOH). It is worth noting that the probability of finding interconnected cells was 10–20%. Only ∼6% of all neurons in a culture dish are GABAergic, and usually these cells are morphologically distinguishable (26). In all experiments, the cells were perfused with an external solution containing 137 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 20 mm glucose, and 10 mm HEPES, pH 7.4, with NaOH. Data were sampled at 10 kHz and low pass-filtered at 3 kHz. The stability of the patch was checked by repetitively monitoring the input and series resistances during the experiments. Cells exhibiting 15–20% changes were excluded from the analysis. The series resistance was 10–15 megohms. All drugs (except TTX, which was purchased from Latoxan, Valence, France) were obtained from Tocris (Cookson Ltd., Bristol, UK). All drugs were dissolved in external solution, except DNQX, which was dissolved in dimethyl sulfoxide. The final concentration of dimethyl sulfoxide in the bathing solution was 0.1%. At this concentration, dimethyl sulfoxide alone did not modify the shape or the kinetics of synaptic currents.
Data Analysis
The analysis of spontaneous events was performed with Clampfit 10.1 software (Axon Instruments, Foster City, CA). This program uses a detection algorithm based on a sliding template. The template did not induce any bias in the sampling of events because it was moved along the data trace one point at a time and was optimally scaled to fit the data at each position. The detection criterion was calculated from the template-scaling factor and from how closely the scaled template fitted the data.
For evoked IPSCs, transmission failures were identified visually. Mean IPSC amplitude was obtained by averaging successes and failures. The paired-pulse ratio (PPR), known to be inversely correlated to the initial release probability (27), was calculated as the ratio between the mean amplitudes of IPSC2 over IPSC1. The coefficient of variation (CV−2) was calculated as the square root of the ratio between the standard deviation of IPSC1 and the mean amplitude of IPSC1 (28).
Values are given as mean ± S.E. Unless otherwise stated, significance of differences was assessed by Student's t test. The differences were considered significant when p < 0.05.
RESULTS
Impairing Gephyrin Function with scFv-Gephyrin Reduces the Probability of GABA Release
As recently reported (6), transfecting cultured hippocampal neurons with scFv-gephyrin reduced the number of gephyrin and synaptic γ2 subunit-containing GABAA receptor clusters. These effects were associated with a severe impairment of both phasic and tonic GABAA receptor-mediated inhibition. The mechanisms underlying these effects relied on changes in GABAergic innervation as suggested by the concomitant reduction in the number and size of presynaptic VGAT clusters.
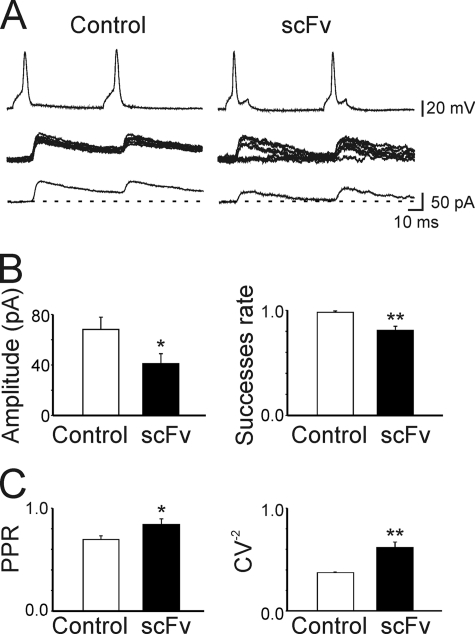
According to the quantal theory, the synaptic efficacy E, the mean amplitude of unitary IPSCs, can be defined as E = mQ, where m is the quantal content or mean number of quanta released per presynaptic action potential and Q is the quantal size or amplitude of the unitary IPSC (29). Whereas Q depends on both pre- and postsynaptic mechanisms, m depends on presynaptic factors, namely the number of release sites N and the probability of release (P). To see whether a decrease in quantal content could account for the observed effects, simultaneous recordings were obtained from pairs of interconnected neurons (the postsynaptic one expressing or not expressing scFv-gephyrin; see “Experimental Procedures”). As shown in Fig. 1, IPSCs evoked in nontransfected cells by pairs of presynaptic action potentials (50 ms apart, delivered at a frequency of 0.1 Hz, Control) were highly reliable and usually did not exhibit synaptic failures. In contrast, with respect to control, IPSCs from scFv-gephyrin-transfected cells (n = 6) exhibited a significant reduction in amplitude (from 68.2 ± 9.7 pA to 41.1 ± 7.8 pA; p < 0.05, Mann-Whitney Rank test) and in successes rate (from 0.98 ± 0.01 to 0.80 ± 0.03; p < 0.01, Mann-Whitney Rank test; Fig. 1, A and B). These effects were associated with a significant increase in the PPR (from 0.69 ± 0.03 to 0.84 ± 0.05; p < 0.05; Fig. 1C), which is considered an index of presynaptic release probability (27, 28). Furthermore, the coefficient of variation (CV−2) was significantly increased (from 0.37 ± 0.007 to 0.61 ± 0.05; p < 0.01; Fig. 1C), indicating changes in quantal content (28).
FIGURE 1.
Hampering gephyrin function with scFv-gephyrin reduces the probability of GABA release. A, pair recordings obtained from two interconnected neurons. The postsynaptic cell was transfected with scFv-gephyrin (right). As control a neighboring nontransfected cell was used (left). Top traces are pairs of action potentials evoked in presynaptic cells at a 50-ms interval by depolarizing current steps of variable amplitude every 10 s. Middle traces are monosynaptic IPSCs (successes and failures) evoked at 0 mV (EGABA −70 mV) by presynaptic action potentials. Bottom traces are averaged responses. B, mean amplitude and successes rate obtained in monosynaptically connected cells in control (white columns; n = 7) and in scFv-transfected neurons (black columns). C, PPR and CV−2 of monosynaptically connected neurons (n = 6) recorded from control and scFv-transfected cells. *, p < 0.05; **, p < 0.01. Error bars, S.E.
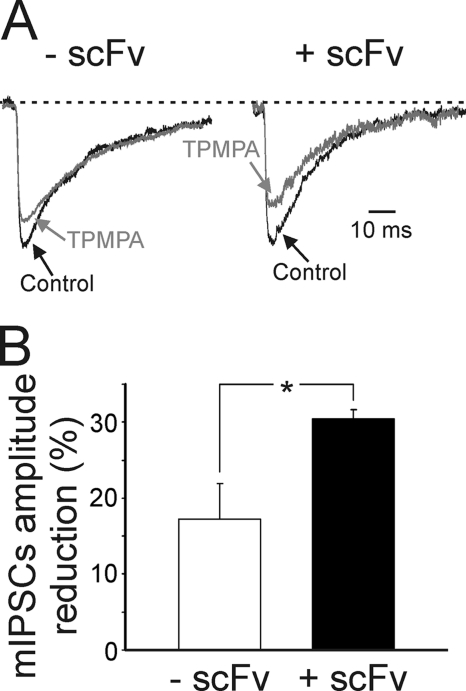
To assess further whether gephyrin depletion affects presynaptic GABA release, as an additional approach we used 100 μm TPMPA, a weak competitive GABAA receptor antagonist that has a very fast dissociation constant and competes with synaptically released GABA for the ligand binding site on GABAA receptors (30, 31). The reduction of mIPSC amplitude by TPMPA would therefore be influenced by relative changes in synaptic GABA transient in the cleft (31). As shown in Fig. 2, in scFv-gephyrin-transfected cells, the block of mIPSCs by TPMPA was significantly (p < 0.05) larger than controls (30.4 ± 1.3% versus 17.2 ± 4.7%; n = 6 for scFv-gephyrin and 7 for controls; p < 0.5; Fig. 2, A and B), indicating that for scFv-gephyrin-transfected cells, GABA concentration in the cleft was lower than control. Overall, these data strongly suggest that hampering gephyrin function with scFv-gephyrin reduces the release of GABA from presynaptic terminals.
FIGURE 2.
The removal of gephyrin with scFv reduces synaptic GABA transient in the cleft. A, representative traces of mIPSCs recorded at −70 mV (dashed lines) from a control (left) and from a scFv-gephyrin-transfected cell (right) in the absence (black) or in the presence of TPMPA 100 μm (gray). In both cases, mIPSC amplitudes are normalized to those obtained in pre-drug conditions. Each trace is the average of 20–30 individual traces. B, each column representing the mean TPMPA-induced reduction in amplitude of mIPSCs in control (white, n = 7) and scFv-gephyrin-transfected cells (black, n = 6; *, p < 0.05). Note the significantly larger TPMPA inhibition in transfected cells. *, p < 0.05. Error bars, S.E.
Gephyrin 2–188, a Dominant Negative Form of Gephyrin, Mimics the Effect of scFv-Gephyrin on GABAergic Function
To validate the results obtained with scFv-gephyrin, a truncated gephyrin polypeptide comprising the N-terminal (amino acids 2–188) of gephyrin fused with EGFP, known to act as a dominant negative protein, was used (22). Due to the lack of dimerization motif, this polypeptide interferes with the endogenous gephyrin lattice formation and depletes gephyrin clusters in neurites within 24 h of expression on cultured neurons.
Immunocytochemical experiments on hippocampal neurons transfected with gephyrin 2–188 revealed a significant reduction in the number of VGAT clusters (without an effect on their size), indicating an effect on GABAergic innervation similar to that observed for scFv-gephyrin (supplemental Fig. 1, A and B). As with scFv-gephyrin, this effect was accompanied by a significant reduction in amplitude and frequency of spontaneous and miniature IPSCs (in cells transfected with gephyrin 2–188 the reduction in amplitude of sIPSCs and mIPSCs was 54 ± 9% and 79 ± 9% of controls, respectively; the reduction in frequency of sIPSCs and mIPSCs was 32 ± 7% and 37 ± 1% of controls, respectively; supplemental Fig. 1, C–E). These data further support the hypothesis that gephyrin not only regulates postsynaptic organization of synaptic GABAA receptors but also GABAergic innervation.
Gephyrin Removal Reduces the Density and Size of Neuroligin 2 Clusters
How can gephyrin interfere with GABA release? One possibility is that this protein interacts with cell adhesion molecules such as neuroligins which, by binding neurexins, ensure the cross-talk between the pre- and postsynaptic sites (7). Of particular interest is NLG2, because this protein is known to play a pivotal role in the organization of GABAergic synapses (14). To verify whether disrupting gephyrin affects NLG2 distribution, hippocampal neurons transfected with scFv-gephyrin were immunostained for NLG2.
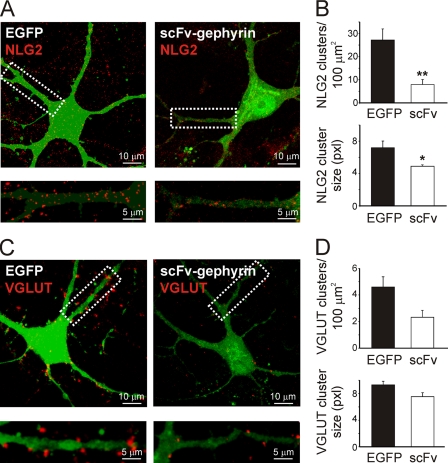
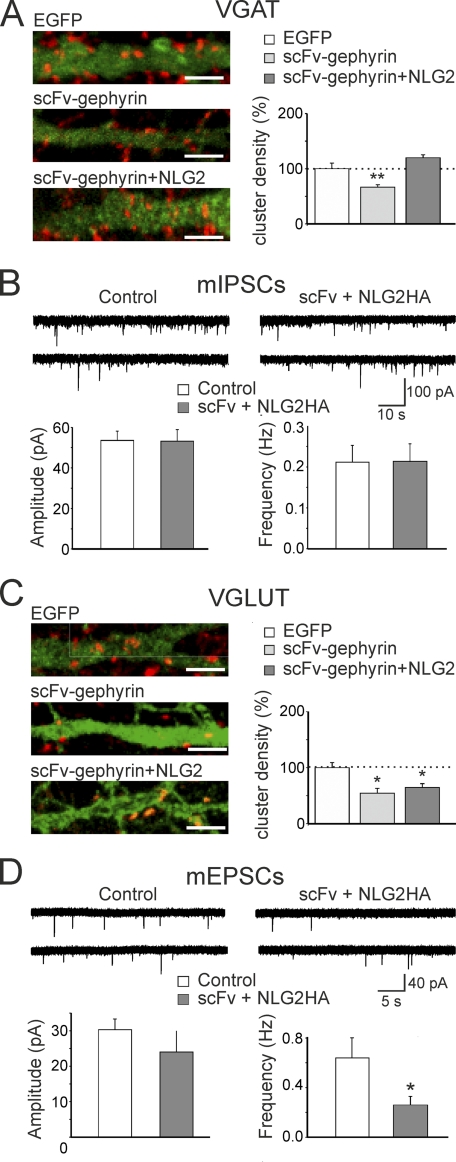
As shown in Fig. 3, A and B, scFv-gephyrin-transfected neurons exhibited a significant reduction in the density of NLG2-positive clusters compared with EGFP-transfected controls (7.9 ± 2.1 clusters/100 μm2 for scFv-gephyrin versus 27.2 ± 4.8 clusters/100 μm2 for EGFP; p < 0.01; n = 9). In addition, the average size of these clusters was smaller for scFv-gephyrin- than for EGFP-transfected neurons (4.9 ± 0.2 μm2 for scFv-gephyrin versus 7.2 ± 0.8 μm2 for EGFP; p < 0.05; n = 9). NLG2 did not relocalize to glutamatergic synapses because the synaptic fraction co-localized with VGLUT was barely detectable (4.1 ± 0.01% in control and 5.3 ± 0.02% in scFv-gephyrin-transfected cells, respectively; these values were not significantly different; p > 0.05; data not shown).
FIGURE 3.
scFv-gephyrin reduces the number and size of NLG2 and VGLUT clusters. A, neurons transfected (green) with EGFP (left) or EGFP/scFv-gephyrin (right) and immunostained for NLG2 (red). Bottom panels are magnifications of the white boxes marked on top. B, quantification of NLG2 clusters density (top) and cluster size (bottom). C and D, as in A and B, but neurons were immunostained for the presynaptic glutamatergic marker VGLUT (red). Note the significant reduction in the density and size of NLG2 and VGLUT clusters. *, p < 0.05; **, p < 0.01. Error bars, S.E.
Impairing Gephyrin Function with scFv-Gephyrin Reduces Glutamatergic Innervation
The interaction of NLGs with scaffolding proteins is crucial for ensuring the correct excitatory/inhibitory balance, critical for the proper functioning of neuronal networks. Therefore, the following experiments were performed to assess whether disrupting gephyrin function with scFv-gephyrin can affect not only GABAergic but also glutamatergic transmission.
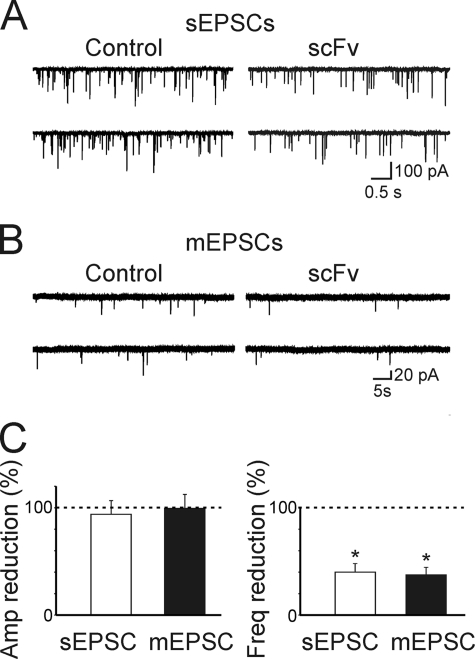
To this aim, cultured hippocampal neurons transfected with scFv-gephyrin were immunostained for VGLUT, a widely used marker for presynaptic glutamatergic terminals (32). Compared with controls (EGFP-transfected cells) in scFv-gephyrin-transfected cells VGLUT-immunopositive clusters were significantly reduced in density and size (Fig. 3, C and D). In particular, the density of VGLUT clusters was reduced from 4.6 ± 0.8 clusters/100 μm2 in EGFP to 2.3 ± 0.5 clusters/100 μm2 in scFv-gephryin (p < 0.05; n = 12). The size of these clusters was reduced from 9.3 ± 0.5 μm2 to 7.5 ± 0.6 μm2 (p < 0.05). Furthermore, whole cell voltage clamp recordings performed in the presence of bicuculline (10 μm) and d-AP5 (50 μm), to block GABAA and NMDA receptors, respectively, revealed a significant reduction in frequency (but not in amplitude) of spontaneous EPSCs (the frequency reached 40 ± 8%; p < 0.05; n = 12; the amplitude 95 ± 13%; p > 0.05; n = 12) recorded from scFv-gephyrin-transfected neurons compared with controls (Fig. 4, A and C). Similarly, in scFv-gephyrin-transfected cells, the frequency of miniature EPSCs recorded in the presence of TTX was significantly reduced with respect to controls (to 37 ± 7%; p < 0.05; from 0.78 ± 0.14 Hz to 0.32 ± 0.05 Hz; n = 12) whereas the amplitude was unchanged (to 100 ± 13%; p > 0.05; from 34 ± 6 pA to 34 ± 5 pA; n = 7; Fig. 4, B and C). Altogether, these results strongly support the involvement of gephyrin in regulating not only GABAergic but also glutamatergic synaptic transmission.
FIGURE 4.
scFv-gephyrin reduces the frequency but not the amplitude of sEPSCs and mEPSCs. A, samples of spontaneous EPSCs recorded from controls and scFv-gephyrin-transfected neurons at a holding potential of −70 mV in the presence of 10 μm bicuculline and 50 μm d-AP5. B, samples of miniature EPSCs recorded from controls and scFv-gephyrin-transfected neurons at a holding potential of −70 mV in the presence of 1 μm TTX. C, each column reprsents the reduction in amplitude (left) and in frequency (right) of sIPSC (white) and mIPSCs (black) obtained from scFv-gephyrin-transfected neurons (n = 12) and expressed as percentage of controls (n = 12; dashed lines). *, p < 0.05. Error bars, S.E.
The Loss of GABAergic but Not Glutamatergic Innervation in Gephyrin-deprived Neurons Can Be Rescued by Overexpressing NLG2
To assess further the possibility that the reduced GABAergic innervation in scFv-gephyrin-transfected cells is mediated by NLG2 which may convey information in a retrograde way from post- to presynaptic sites, NLG2 was co-expressed with scFv-gephyrin. In immunocytochemical experiments, co-expression of NLG2 with scFv-gephyrin induced a significant increase in the density of VGAT-positive clusters compared with cells transfected with scFv-gephyrin alone (180 ± 8%; from 10.6 ± 0.7 clusters/100 μm2 to 19.1 ± 0.9 clusters/100 μm2; p < 0.01; n = 11 and 8 for scFv and scFv/NLG2, respectively), restoring VGAT cluster density to control levels (Fig. 5, A and B). In line with previous studies (9), overexpression of NLG2 alone led to a 2-fold increase in the density of VGAT clusters compared with EGFP-transfected controls (data not shown).
FIGURE 5.
Co-expression of NLG2 with scFv-gephyrin restores the loss of GABAergic but not glutamatergic innervation. A, left, representative images of neurons transfected with EGFP (top), scFv-gephyrin (middle) or co-transfected with scFv-gephyrin and NLG2-HA (bottom). Dendrites were visualized by EGFP signal or NLG2 staining (green). Neurons were immunostained for VGAT (red). Scale bars, 5 μm. Right, quantification of VGAT cluster densities relative to the mean value obtained from EGFP-transfected neurons (dashed line). **, p < 0.01. C and D, as in A and B but for neurons immunostained for VGLUT (red). Scale bars, 5 μm. B, samples of spontaneous mIPSCs recorded from cells co-transfected with scFv-gephyrin plus NLG2-HA and from neighboring nontransfected cells (Control) at a holding potential of −70 mV in the presence of 1 μm TTX, 20 μm DNQX, and 50 μm d-AP5. The columns below the traces represent the mean amplitude (left) and frequency (right) of mIPSC from control (white; n = 7) and from scFv-gephyrin-transfected cells (gray; n = 8). C, as in A but for cells immunostained for VGLUT. D, as in B but for mEPSCs. These were recorded from cells co-transfected with scFv-gephyrin plus NLG2-HA (n = 9) and from neighboring nontransfected cells (Control, n = 8) in the presence of 1 μm TTX and 10 μm bicuculline. Error bars, S.E.
Parallel electrophysiological experiments from cultured neurons revealed no changes in amplitude and frequency of spontaneous mIPSCs between cells co-transfected with scFv-gephyrin/NLG2 and controls (neighboring nontransfected cells). On average, the frequency of mIPSCs was 0.211 ± 0.040 Hz and 0.213 ± 0.044 Hz (p = 0.97) whereas the amplitude was −55 ± 4 pA and −53 ± 6 pA (p = 0.7) in control (n = 7) and in co-transfected neurons (n = 8), respectively (Fig. 5B).
To see whether the observed rescue was gephyrin-dependent, similar experiments were performed using the NLG2 mutant (NLG2Y770A-HA) which lacks gephyrin binding (14). In this case, the number of VGAT-positive clusters/100 μm2 was 19.8 ± 1.7 (n = 9), a value not significantly different from that obtained with NLG2 (p > 0.5; supplemental Fig. 2A).
In electrophysiological recordings, no changes in amplitude or frequency of mIPSCs were detected between scFv-gephyrin/NLG2Y770A-transfected cells and controls. The frequency of mIPSCs was 0.22 ± 0.07 Hz and 0.16 ± 0.06 Hz in control (n = 7) and in scFv-gephyrin/NLG2Y770A-transfected cells (n = 6), respectively (p > 0.05; supplemental Fig. 2, B and C). The amplitude of mIPSCs was 42 ± 5 pA and 32 ± 5 pA in the absence or in the presence of scFv-gephyrin/NLG2Y770A, respectively (supplemental Fig. 2, B and C).
Altogether, these experiments indicate that overexpression of NLG2 is able to rescue the loss of GABAergic innervation induced by scFv-gephyrin. However, our data with NLG2Y770A suggest that this effect only partially relies on the direct recruitment of gephyrin by NLG2 at inhibitory synapses (see “Discussion”).
It is possible that the observed reduction in glutamatergic innervation following gephyrin depletion with scFv-gephyrin represents a homeostatic compensatory mechanism to prevent hyperexcitability and to maintain the right E/I balance within the neuronal network (33). If this is the case, rescuing GABAergic innervation should lead to a concomitant change in glutamatergic transmission. However, this was not the case because overexpressing NLG2 in gephyrin-depleted neurons failed to restore VGLUT immunoreactive puncta (0.5 ± 0.1 and 0.6 ± 0.1 clusters/100 μm2 for scFv and scFv/NLG2, respectively; p > 0.5; Fig. 5C) as well as the frequency of mEPSCs to control levels. The frequency of mEPSCs was 0.64 ± 0.16 Hz and 0.26 ± 0.07 Hz in the absence or presence of NLG2 overexpression; p < 0.05; the amplitude of mEPSCs was 29 ± 3 pA and 25 ± 7 pA in the absence (n = 8) or in the presence (n = 9) of NLG2 overexpression (p > 0.05; Fig. 5D).
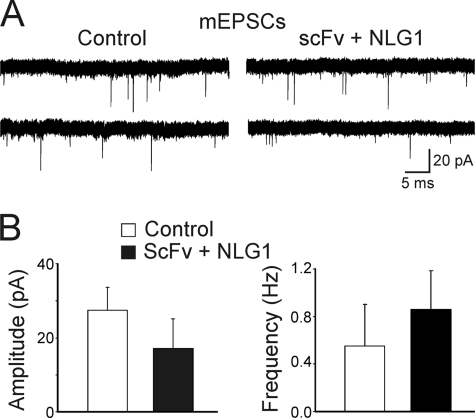
However, the co-expression of NLG1 with scFv-gephyrin was able to rescue the frequency of mEPSCs to control levels. The frequency of mEPSCs was 0.55 ± 0.35 Hz and 0.86 ± 0.33 Hz in control (n = 7) and in scFv-gephyrin/NLG1-transfected cells (n = 5), respectively (p > 0.05; Fig. 6). No changes in mEPSC amplitude were detected between control and scFv-gephyrin/NLG1-transfected cells (27 ± 6 pA in the absence and 17 ± 8 pA in the presence of NLG1 overexpression; p > 0.05; Fig. 6). Overall, these data indicate that the observed NLG1-induced rescue of glutamatergic function in gephyrin-deprived neurons is at least partially dependent on gephyrin activity.
FIGURE 6.
Co-expression of NLG1 with scFv-gephyrin restores glutamatergic innervation. A, samples of spontaneous mIPSCs recorded from cells co-transfected with scFv-gephyrin plus NLG1 and from neighboring nontransfected cells (Control) at a holding potential of −70 mV in the presence of 1 μm TTX and 10 μm bicuculline are shown. B, each column represents the mean amplitude (left) and frequency (right) of mIPSC from control (white, n = 7) and from scFv-gephyrin/NLG1-transfected cells (black, n = 5).
Gephyrin Interacts Directly with NLG1
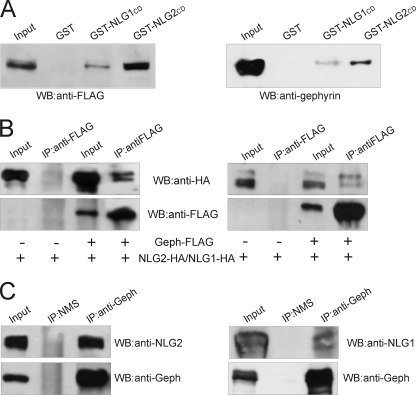
It has been recently reported that at inhibitory synaptic contacts gephyrin binds NLG2 directly (14). The amino acid sequence identified as gephyrin-binding motif on NLG2 is highly conserved in all NLGs, and indeed gephyrin binds to all four NLGs in yeast two-hybrid assays (14). To test whether gephyrin can form a complex with NLG1 in mammalian cells, lysates of HEK-293 cells transfected with gephyrin-FLAG were subjected to a pulldown assay with beads loaded with GST-NLG1 cytoplasmic domain (NLG1CD), GST-NLG2CD, or with GST alone as a negative control. In agreement with previous observations (14), NLG2CD was able to precipitate a consistent amount of gephyrin-FLAG (Fig. 7A, left). Interestingly, a small but significant fraction of gephyrin-FLAG was also found in complex with GST-NLG1CD (Fig. 7). Similar pulldown experiments were then performed to assay the ability of endogenous gephyrin present on neonatal rat brain homogenates to interact with NLG1 and NLG2. Also in this case gephyrin was not only associated with GST-NLG2CD fusion protein but also with GST-NLG1CD (Fig. 7A, right). Here, the immunoblot analysis was performed using a monoclonal antibody raised against the C-terminal domain of gephyrin.
FIGURE 7.
Gephyrin interacts with NLG2 and NLG1. A, GST-NLG1/2CD pulldown assay using lysates of HEK-293 cells transfected with gephyrin-FLAG (left) and rat brain lysates (right). B, lysates of HEK-293 cells transfected with either NLG2-HA (left) or NLG1-HA (right) in the presence of gephyrin-FLAG or with the vector alone (as a negative control) immunoprecipitated with monoclonal anti-FLAG antibodies. Immunoprecipitates were analyzed by Western blotting using anti-HA and anti-FLAG monoclonal antibodies. C, co-immunoprecipitation experiments on rat brain lysates using a monoclonal anti-gephyrin antibody and normal mouse serum (NMS) as negative control. Immunoprecipitates were analyzed by Western blotting using a monoclonal anti-gephyrin antibody and a polyclonal antibody against NLG2 and NLG1.
We then performed immunoprecipitation experiments to investigate the presence of NLG1-HA/gephyrin-FLAG complexes in vitro. HEK-293 cells were co-transfected with plasmids encoding for NLG1/2-HA and gephyrin-FLAG, or NLG1/2-HA alone, and cell lysates were immunoprecipitated with the anti-FLAG monoclonal antibody. The bound protein complexes were analyzed by Western blotting using anti-HA and anti-FLAG for NLG1 and gephyrin detection, respectively. As shown in Fig. 7B (right), NLG1-HA was immunoprecipitated only from cells co-expressing gephyrin-FLAG. The same experimental conditions were also applied to detect the expected presence of NLG2-HA/gephyrin-FLAG complexes in mammalian cells. Indeed, we found that a lower amount of gephyrin-FLAG was able to precipitate a higher amount of NLG2-HA compared with NLG1-HA, thus supporting previous in vitro observations. Finally, endogenous NLG1 and NLG2 were found in native complexes with gephyrin upon co-immunoprecipitation from mouse brain homogenates (Fig. 7C). These data suggest that gephyrin, by interacting directly with NLG2 and to a lesser extent with NLG1, may affect not only GABAergic but also glutamatergic synaptic transmission.
DISCUSSION
The tubulin-binding protein gephyrin is a core protein of inhibitory postsynaptic densities that interacts with the cytoskeleton to stabilize inhibitory receptors in precise apposition to presynaptic active zones (1). In a previous study, we have demonstrated that disrupting endogenous gephyrin with selective scFv-gephyrin altered the gating properties of GABAA receptors, an effect that was found to be associated with modifications of GABAergic innervation (6). In the present study we hypothesized that hampering gephyrin function affects not only the number of release sites (as suggested by the reduction in VGAT clusters) but also the probability of GABA release. In support of this view, in double patch experiments from interconnected neurons, we found that, with respect to controls, scFv-gephyrin-expressing cells exhibited a significant decrease in the amplitude of individual synaptic currents accompanied by a clear increase in the number of transmitter failures and a reduction in the PPR. Changes in transmitter failures and in PPR are consistent with a decrease in release probability (27, 28). This would lead to a reduction in GABA concentration in the synaptic cleft as suggested by the TPMPA experiments.
The role of gephyrin in ensuring a correct communication between pre- and postsynaptic elements of synapses was further validated by the experiments in which a truncated form of gephyrin (gephyrin 2–188; 22) was used. This gephyrin mutant lacks the dimerization motif, but it can still interact with endogenous gephyrin molecules, producing dominant negative effects on postsynaptic gephyrin clusters. Similar to scFv-gephyrin, overexpression of gephyrin 2–188 caused a reduction in GABAergic innervation and a decrease in frequency of spontaneous and miniature IPSCs, further confirming a key role of gephyrin in maintaining the stability of GABAergic connections within the neuronal network. The ability of gephyrin to influence presynaptic innervation was already suggested by Yu et al., even though no mechanistic interpretation was provided (5).
The presynaptic action of gephyrin on GABA release implies the coordinated activity of other signaling molecules that interact directly or indirectly with gephyrin to ensure the corrected cross-talk between the post- and presynaptic elements of the synapse. Possible candidates are NLGs, specialized cell adhesion molecules that functionally couple the postsynaptic densities with the transmitter release machinery by forming transynaptic complexes with their presynaptic binding partners, neurexins (7). In particular, NLG2 is preferentially concentrated at inhibitory synapses (10) and binds gephyrin directly through a conserved cytoplasmic domain (14). Consistent with this finding, hampering gephyrin function with scFv-gephyrin promoted a significant decrease in the total number and size of NLG2 clusters upon scFv-induced gephyrin removal. It is interesting to note that in a recent study (34), knocking down gephyrin with siRNA led to a shift of endogenous NLG2 from inhibitory to excitatory synapses, in the absence of any change in the density of NLG2 clusters. In the present experiments instead we have observed a clear reduction in the density of NLG2 clusters without a detectable relocalization of this protein to glutamatergic synapses. Because scFv-mediated removal of gephyrin is associated with a significant reduction of synaptic γ2-containing GABAA receptors (6) and evidence has been provided for the reciprocal stabilization of NLG2 by GABAA receptors (35), the reduction of NLG2 staining could be a consequence of the loss of gephyrin-dependent GABAA receptor clustering. We cannot exclude the possibility that scFv-gephyrin may affect the function of additional gephyrin-bound factors important for the efficient localization of NLG2 to and from GABAergic terminals. Conventional kinesin (KIF5) and the dynein motor complex have been shown to be involved in microtubule-dependent transport of gephyrin, thus contributing to postsynaptic remodeling (22, 36). Because microtubule motors transport and remodel a variety of transmembrane and submembrane postsynaptic proteins (37, 38), similar mechanisms may account for NLG2 transport.
The role of NLG-neurexin complex as a coordinator between postsynaptic and presynaptic sites has been investigated at excitatory CA3-CA1 synapses in the hippocampus. This study has revealed a retrograde modulation of neurotransmitter release by PSD-95-NLG complex (39). The authors found that overexpression of the glutamatergic scaffold protein PSD-95 enhanced release probability via a mechanism involving the NLG-neurexin complex.
Along the same line, at GABAergic synapses, evidence has been provided that knocking down NLG2 produces a reduction in quantal content associated with a decrease in quantal size of unitary responses (40). In agreement with our electrophysiological data, these findings suggest a crucial role of gephyrin-NLG2 interaction on GABA release.
In our experiments, the reduction in the probability of GABA release after scFv-gephyrin transfection seems to involve a mechanism that only partially relies on the direct NLG2-gephyrin interaction. Hence, the NLG2 point mutant, NLG2Y770A, unable to bind gephyrin was as effective as the wild-type protein in rescuing GABAergic transmission in scFv-gephyrin-deprived neurons. Two possible hypotheses, which are not mutually exclusive, can be put forward to explain our results.
First, although the NLG2Y770A mutant is impaired in gephyrin binding activity it still maintains the ability to induce the activation of collybistin (14), a gephyrin partner known to promote its synaptic targeting in vivo (14). Interestingly, in collybistin knock-out mice, a reduction in frequency of mIPSCs similar to that detected in the present experiments was observed (41), thus suggesting a possible involvement of collybistin in transynaptic signaling.
Second, NLGs form homomultimers through the extracellular acetylcholinesterase-homologous domain (42). Therefore, it is possible that overexpressing NLG2Y770A in hippocampal neurons containing endogenous NLG2 allows the formation of multimers. As a consequence this mutant recruited at inhibitory synapses would act in concert with endogenous NLG2 to rescue GABAergic transmission.
Unexpectedly, hampering gephyrin function with scFv-gephyrin produced a significant reduction not only of GABAergic but also of glutamatergic innervation as assessed by the significant decrease in density of VGLUT-positive puncta associated with a significant reduction in frequency, but not in amplitude, of spontaneous and miniature glutamatergic events. This effect was not due to a homeostatic plasticity mechanism because overexpressing NLG2 in gephyrin-depleted neurons failed to reestablish glutamatergic innervation. However, a rescue was obtained by co-expressing NLG1 with scFv-gephyrin. Because the overexpression of NLG1 alone has been shown to enhance glutamatergic innervation by severalfold (9) the fact that this does not occur in gephyrin-deprived neurons suggests that the scaffold protein contributes to modulate NLG1-dependent transynaptic signaling. In support of this observation, co-immunoprecipitation experiments revealed the existence of native complexes not only between gephyrin and NLG2 but also with NLG1, which is localized primarily at excitatory synapses (8), thus confirming and extending previous data obtained with the yeast two-hybrid system (14). Moreover, in favor of the possible involvement of gephyrin in regulating transynaptic signaling at excitatory synapses is the observation that, at least in immature hippocampal neurons in vitro (as those used in the present study), this protein has been found localized opposite to glutamatergic release sites (43, 44). The presence of gephyrin at both GABAergic and glutamatergic synapses may be relevant for neuronal development. Altogether, these results show that gephyrin interacts, at least in immature neurons, with both NLG2 and NLG1 to regulate both excitatory and inhibitory inputs converging on the same neuron thus controlling the E/I balance at the network level.
Supplementary Material
Acknowledgments
We are grateful to P. Scheiffele (Biozentrum, Basel, Switzerland) for providing NLG1-HA and NLG2-HA constructs and F. Varoqueaux (Max Planck, Göttingen) for the NLG2Y770A-HA. We thank B. Pastore for excellent technical assistance on hippocampal neuronal cultures.
This work was supported by a grant from Ministero Istruzione, Università e Ricerca (MIUR) (to E. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- NLG
- neuroligin
- d-AP5
- d-2-amino-5-phosphonopentanoic acid
- DNQX
- 6,7-dinitroquinoxaline-2,3-dione
- EGFP
- enhanced green fluorescent protein
- E/I
- excitatory/inhibitory
- EPSC
- excitatory postsynaptic current
- IPSC
- inhibitory postsynaptic current
- PPR
- paired-pulse ratio
- scFv
- single chain antibody fragment
- TPMPA
- (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid
- TTX
- tetrodotoxin
- VGAT
- vesicular GABA transporter
- VGLUT
- vesicular glutamate transporter.
REFERENCES
- 1. Fritschy J. M., Harvey R. J., Schwarz G. (2008) Trends Neurosci. 31, 257–264 [DOI] [PubMed] [Google Scholar]
- 2. Sola M., Bavro V. N., Timmins J., Franz T., Ricard-Blum S., Schoehn G., Ruigrok R. W., Paarmann I., Saiyed T., O'Sullivan G. A., Schmitt B., Betz H., Weissenhorn W. (2004) EMBO J. 23, 2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kneussel M., Brandstätter J. H., Laube B., Stahl S., Müller U., Betz H. (1999) J. Neurosci. 19, 9289–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tretter V., Jacob T. C., Mukherjee J., Fritschy J. M., Pangalos M. N., Moss S. J. (2008) J. Neurosci. 28, 1356–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu W., Jiang M., Miralles C. P., Li R. W., Chen G., de Blas A. L. (2007) Mol. Cell. Neurosci. 36, 484–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchionni I., Kasap Z., Mozrzymas J. W., Sieghart W., Cherubini E., Zacchi P. (2009) Neuroscience 164, 552–562 [DOI] [PubMed] [Google Scholar]
- 7. Südhof T. C. (2008) Nature 455, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song J. Y., Ichtchenko K., Südhof T. C., Brose N. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chih B., Engelman H., Scheiffele P. (2005) Science 307, 1324–1328 [DOI] [PubMed] [Google Scholar]
- 10. Varoqueaux F., Jamain S., Brose N. (2004) Eur J. Cell Biol. 83, 449–456 [DOI] [PubMed] [Google Scholar]
- 11. Levinson J. N., Chéry N., Huang K., Wong T. P., Gerrow K., Kang R., Prange O., Wang Y. T., El-Husseini A. (2005) J. Biol. Chem. 280, 17312–17319 [DOI] [PubMed] [Google Scholar]
- 12. Gerrow K., Romorini S., Nabi S. M., Colicos M. A., Sala C., El-Husseini A. (2006) Neuron 49, 547–562 [DOI] [PubMed] [Google Scholar]
- 13. Prange O., Wong T. P., Gerrow K., Wang Y. T., El-Husseini A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13915–13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poulopoulos A., Aramuni G., Meyer G., Soykan T., Hoon M., Papadopoulos T., Zhang M., Paarmann I., Fuchs C., Harvey K., Jedlicka P., Schwarzacher S. W., Betz H., Harvey R. J., Brose N., Zhang W., Varoqueaux F. (2009) Neuron 63, 628–642 [DOI] [PubMed] [Google Scholar]
- 15. Craig A. M., Kang Y. (2007) Curr. Opin. Neurobiol. 17, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalva M. B., McClelland A. C., Kayser M. S. (2007) Nat. Rev. Neurosci. 8, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerrow K., El-Husseini A. (2006) Front. Biosci. 11, 2400–2419 [DOI] [PubMed] [Google Scholar]
- 18. Rubenstein J. L., Merzenich M. M. (2003) Genes Brain Behav. 2, 255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zacchi P., Dreosti E., Visintin M., Moretto-Zita M., Marchionni I., Cannistraci I., Kasap Z., Betz H., Cattaneo A., Cherubini E. (2008) J. Mol. Neurosci. 34, 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andjus P. R., Stevic-Marinkovic Z., Cherubini E. (1997) J. Physiol. 504, 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zita M. M., Marchionni I., Bottos E., Righi M., Del Sal G., Cherubini E., Zacchi P. (2007) EMBO J. 26, 1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maas C., Tagnaouti N., Loebrich S., Behrend B., Lappe-Siefke C., Kneussel M. (2006) J. Cell Biol. 172, 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheiffele P., Fan J., Choih J., Fetter R., Serafini T. (2000) Cell 101, 657–669 [DOI] [PubMed] [Google Scholar]
- 24. Chih B., Gollan L., Scheiffele P. (2006) Neuron 51, 171–178 [DOI] [PubMed] [Google Scholar]
- 25. Yang J., Thio L. L., Clifford D. B., Zorumski C. F. (1993) Brain Res. Dev. Brain Res. 71, 19–26 [DOI] [PubMed] [Google Scholar]
- 26. Benson D. L., Watkins F. H., Steward O., Banker G. (1994) J. Neurocytol. 23, 279–295 [DOI] [PubMed] [Google Scholar]
- 27. Dobrunz L. E., Stevens C. F. (1997) Neuron 18, 995–1008 [DOI] [PubMed] [Google Scholar]
- 28. Korn H., Faber D. S. (1991) Trends Neurosci. 14, 439–445 [DOI] [PubMed] [Google Scholar]
- 29. Katz B. (1969) The Release of Neural Transmitter Substances, Liverpool University Press, Liverpool, UK [Google Scholar]
- 30. Jones M. V., Jonas P., Sahara Y., Westbrook G. L. (2001) Biophys. J. 81, 2660–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barberis A., Petrini E. M., Cherubini E. (2004) Eur. J. Neurosci. 20, 1803–1810 [DOI] [PubMed] [Google Scholar]
- 32. Yu W., De Blas A. L. (2008) J. Neurochem. 104, 830–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turrigiano G. G., Nelson S. B. (2004) Nat. Rev. Neurosci. 5, 97–107 [DOI] [PubMed] [Google Scholar]
- 34. Levinson J. N., Li R., Kang R., Moukhles H., El-Husseini A., Bamji S. X. (2010) Neuroscience 165, 782–793 [DOI] [PubMed] [Google Scholar]
- 35. Dong N., Qi J., Chen G. (2007) Mol. Cell. Neurosci. 35, 14–23 [DOI] [PubMed] [Google Scholar]
- 36. Maas C., Belgardt D., Lee H. K., Heisler F. F., Lappe-Siefke C., Magiera M. M., van Dijk J., Hausrat T. J., Janke C., Kneussel M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8731–8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirokawa N., Takemura R. (2005) Nat. Rev. Neurosci. 6, 201–214 [DOI] [PubMed] [Google Scholar]
- 38. Kneussel M. (2005) EMBO Rep. 6, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Futai K., Kim M. J., Hashikawa T., Scheiffele P., Sheng M., Hayashi Y. (2007) Nat. Neurosci. 10, 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gibson J. R., Huber K. M., Südhof T. C. (2009) J Neurosci 29, 13883–13897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papadopoulos T., Korte M., Eulenburg V., Kubota H., Retiounskaia M., Harvey R. J., Harvey K., O'Sullivan G. A., Laube B., Hülsmann S., Geiger J. R., Betz H. (2007) EMBO J. 26, 3888–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Comoletti D., Grishaev A., Whitten A. E., Tsigelny I., Taylor P., Trewhella J. (2007) Structure 15, 693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Studler B., Sidler C., Fritschy J. M. (2005) J. Comp. Neurol. 484, 344–355 [DOI] [PubMed] [Google Scholar]
- 44. Anderson T. R., Shah P. A., Benson D. L. (2004) Neuropharmacology 47, 694–705 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.