Abstract
The norepinephrine (NE) transporter (NET) regulates NE signaling by rapidly clearing synaptic NE. Cocaine binds NET and modulates NE transport. These actions contribute to rewarding effects and abuse liability of cocaine. Activation of mitogen-activated protein kinase (MAPK) cascades is implicated in cocaine-induced neuroadaptations. However, the role of MAPK and the mechanisms involved in cocaine modulation of NET are not clear. Acute intra-peritoneal injections of cocaine (20 mg/kg body weight) to rats resulted in increased NE uptake by prefrontal cortex (PFC) synaptosomes with a parallel increase in the surface expression of endogenous NET. Cocaine also enhanced the immunoreactivity of phospho-p38 MAPK in the PFC synaptosomes without affecting the total p38 MAPK. In vitro cocaine (30–50 μm) treatment of rat PFC synaptosomes increased native NET function, surface expression, and phosphorylation in a manner sensitive to p38 MAPK inhibition by PD169316. We next examined cocaine-elicited effects on wild-type human NET (hNET) expressed heterologously in human placental trophoblast cells to gain more insights into the mechanisms involved. Cocaine treatment of hNET expressing human placental trophoblast cells up-regulated the function, surface expression, and phosphorylation of hNET in a PD169316-sensitive manner. In addition, cocaine inhibited constitutive endocytosis of hNET. Mutational analysis of serine and threonine residues revealed that substitution of threonine 30, located at the amino terminus of hNET with alanine (T30A-hNET), abolished cocaine-induced up-regulation of NET function, surface expression, and phosphorylation. Furthermore, cocaine did not alter T30A-hNET endocytosis. These studies identify a novel molecular mechanism that cocaine-activated p38 MAPK-mediated phosphorylation of NET-T30 dictates surface NET availability, and hence, NE transport.
Keywords: Endocytosis, Neurotransmitter Transport, p38 MAPK, Post-translational Modification, Trafficking, Catecholamine, Exocytosis, Membrane Protein, Norepinephrine, Phosphorylation
Introduction
The norepinephrine transporter (NET)2 is the primary determinant of synaptic norepinephrine (NE) levels (1–3). Indeed, mice lacking the gene encoding NET exhibit diminished NE clearance, tissue NE concentrations, and NE signaling as well as elevated extracellular NE levels (4, 5). NET contains 12 transmembrane domains with NH2 and COOH termini located intracellularly and shares significant homology with the recently reported high-resolution structure of the prokaryotic sodium-dependent leucine transporter (6). NET is expressed both in the CNS and periphery and is an important target for tricyclic antidepressants, and psychostimulant drugs such as cocaine (7).
Cocaine binds to monoamine transporters and inhibits amine transport. These actions are implicated in the rewarding effects of cocaine and its abuse liability (8, 9). NET binding sites are increased in the stria terminalis of non-human primates self-administering cocaine (10) and selective NET inhibitor reinstates compulsive cocaine seeking (11). In addition to its abuse potential resulting from monoaminergic dysregulation, the use of cocaine during pregnancy has been associated with an increased risk for adverse intrauterine effects including growth restriction and placental abruption. NET binding sites are also increased in the placenta of pregnant dams following chronic cocaine exposure (12). Placental NET is thought to shield the fetus from maternal catecholamines (13, 14), and fetomaternal complications associated with cocaine addiction have been attributed to alterations in placental NET function (15). Although it is clear that cocaine can alter NET function, knowledge of the molecular and cellular mechanisms mediating cocaine regulation of NET is limited.
The amino acid sequence of NET contains potential phosphorylation sites for several kinases. Previously, we demonstrated that PKC activation stimulates NET internalization (16). Threonine (Thr) 258 and serine (Ser) 259 phosphorylation contributes to this effect because their mutation to nonphosphorylatable alanines abolishes PKC-induced NET internalization (17). Moreover, PKC-induced phosphorylation is blunted in the hNET T258A,S259A mutation indicating that Thr258/Ser259 is a trafficking motif linked to PKC-induced phosphorylation (17). It is also apparent that other kinases, including members of the mitogen-activated protein kinase (MAPK) family can regulate NET function (18–23). Although cocaine and other psychostimulants induce MAPK signaling in the brain (24–27), fundamental questions exist as to the role of these kinase cascades in mediating cocaine-evoked alterations in NE transport. Here, we demonstrate that acute in vivo cocaine administration results in NET up-regulation as well as p38 MAPK activation in rat PFC. Using PFC synaptosomes and HTR-hNET cells, we find that in vitro application of cocaine stimulates NET function, surface expression, and phosphorylation in a manner sensitive to p38 MAPK inhibition. We identify that cocaine up-regulates NET function by inhibiting transporter endocytosis. Furthermore, we provide evidence for cocaine-induced p38 MAPK-dependent phosphorylation of Thr30 dictating NET endocytosis and hence NE transport. These data demonstrate a novel molecular mechanism by which cocaine controls NE transport and provide new insights into the cocaine regulation of NET.
EXPERIMENTAL PROCEDURES
Materials
The HTR cell line was provided by Dr. Charles H. Graham, Queen's University, Ontario, Canada. Monoclonal antibodies specific to hNET and mouse/rodent NET were from Mab Technologies (Stone Mountain, Atlanta, GA). Mouse anti-transferrin receptor (TfR) antibody was from Zymed Laboratories Inc. (South San Francisco, CA). Anti-calnexin antibody was from Stressgen Biotechnologies (Victoria, BC, Canada). Anti-phospho-ATF2 and anti-phospho-p38 MAPK antibodies were from Cell Signaling Technology Inc. (Beverly, MA). Anti-total p38 MAPK antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). FuGENE 6 transfection reagent was from Roche Applied Science. l-[7,8-3H]Norepinephrine ([3H]NE) and [32P]orthophosphate were from GE Healthcare. PD169316 (4-(4-fluorophenyl)-2-(4-nitrophenyl)-5-(4-pyridyl)-1H-imidazole) and SB202190 (4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole) were from Calbiochem. Sulfo-NHS-acetate, sulfo-NHS-biotin, sulfo-NHS-SS-biotin, and NeutrAvidin beads were from Pierce. HRP-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Cocaine, pargyline, protease inhibitors, and sodium 2-mercaptoethanesulfonate were obtained from Sigma. Other reagents were of the highest grade possible from standard sources such as Sigma, Fisher Scientific (Pittsburgh, PA), and VWR (Suwanee, GA).
In Vivo Cocaine Administration and Rat Brain Synaptosome Preparations
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee. In some experiments, male Sprague-Dawley rats (150–200 g) received intraperitoneal injections of vehicle (saline) or cocaine (20 mg/kg body weight), sacrificed 1 h later, and brains were collected. In other experiments, rats were decapitated, and the brains were collected in ice-cooled dishes. Brain tissues from prefrontal cortex (PFC) and ventral striatum were dissected and collected in 10 volumes (w/v) of cold 0.32 m sucrose. The tissue was immediately homogenized using a Teflon-glass homogenizer and centrifuged at 1,000 × g for 10 min at 4 °C. The resulting supernatant was centrifuged at 12,000 × g for 20 min and the pellet was washed by resuspending in 0.32 m sucrose (28). The synaptosomes were suspended in 0.32 m sucrose saturated with 95% O2, 5% CO2. Protein concentration was determined by DC protein assay (Bio-Rad) using bovine serum albumin as standard. Tissue was pooled from 2 to 4 rats based on the experiment conducted and all experiments were replicated at least three times.
NE Uptake Measurements in Synaptosomes
The synaptosomes suspended in 300 μl of KRH buffer, pH 7.4 (120 mm NaCl, 4.7 mm KCl, 2.2 mm CaCl2, 10 mm HEPES, 1.2 mm MgSO4, 1.2 mm KH2PO4, 5 mm Tris, and 10 mm d-glucose), were used for the uptake assays as described previously (28) using [3H]NE. Briefly, 50 μg of synaptosomes (50 μl suspension) were incubated in 250 μl of KRH buffer, pH 7.4, containing 0.1 mm ascorbic acid and 0.1 mm pargyline and 40 nm [3H]NE for 5 min. Synaptosomes were preincubated with the NET inhibitor desipramine (DMI) (100 μm) at 37 °C for 10 min followed by the addition of [3H]NE to determine the nonspecific NE uptake. Uptake was terminated by addition of 1 ml of ice-cold KRH buffer containing 100 μm DMI followed by rapid filtration over 0.3% polyethylenimine-coated GF-B filters on a Brandel Cell Harvester (Gaithersburg, MD). Filters were washed rapidly with 15 ml of cold PBS and radioactivity bound to filters was quantified by liquid scintillation counting using MicroBeta2 LumiJet (PerkinElmer Health Sciences). Nonspecific uptake was defined as uptake in the presence of 100 μm DMI and subtracted from total accumulation to yield specific NET-mediated NE uptake. Mean values of specific uptake ± S.E. of at least three separate experiments were determined.
In in vitro/ex vivo experiments, synaptosomes (300 μg) were preincubated with vehicle or 20 μm PD169316 at 37 °C for 15 min and then incubations were continued in the presence or absence of 50 μm cocaine for 60 min in a total volume of 1 ml. This cocaine concentration was used based on previous reports (29–31) and our initial concentration effect studies (10–100 μm) of NE uptake and NET surface expression measurements, which showed reproducible NET up-regulation in response to 30–50 μm cocaine. The concentration of PD169316 used produces a selective inhibition of p38 MAPK (18, 32–34). Following drug treatments, the reaction mixture was centrifuged at 12,000 × g for 20 min and the pellet was resuspended in 300 μl of KRH buffer. The resuspended synaptosomes were washed three times using 1 ml of KRH buffer followed by centrifugations at 12,000 × g for 10 min and each time transferring the resuspended synaptosomes into fresh tubes. The pellet from the final wash was suspended in 300 μl of KRH buffer and the uptake assay was performed as described previously (28) and above using [3H]NE.
Surface Biotinylation of Synaptosomes
Synaptosomes (300 μg) obtained from saline- or cocaine-injected rats or synaptosomes treated in vitro with drugs as described above (in the uptake assay) were subjected to surface biotinylation and isolation of avidin-bound and unbound fractions as described previously (28). Aliquots from total extracts (50 μl) and the entire eluted fractions were separated by SDS-PAGE (10%), transferred to membrane, and probed with mouse NET antibody. This rodent NET-specific monoclonal antibody has been characterized for its suitability to identify rat and mouse NET protein by Western blotting, immunoprecipitations, and immunocytochemistry (35). Rat NET proteins were visualized using ECL Plus reagent followed by exposure to Hyperfilm-ECL. Multiple exposures of immunoblots were taken to ensure that the band development on the film was within the linear range. Band densities were quantified by scanning and analyzed using NIH ImageJ (version 1.62) software. Subsequently, the blots were stripped and reprobed with anti-calnexin antibody to validate the surface biotinylation of plasma membrane proteins. NET band densities from total and biotinylated (representing the surface pool) fractions were normalized using levels of calnexin in the total extract (28).
Measurement of NET Phosphorylation in Rat PFC Synaptosomes
Synaptosomes (300 μg) were incubated with 5.0 mCi of carrier-free orthophosphate/mg of protein for 30 min before the addition of modulators. Following metabolic labeling with 32P, synaptosomes were treated with drugs as described above (in the uptake assay). At the end of the incubation, samples were centrifuged, and the pellet was resuspended in RIPA buffer containing protease and phosphatase inhibitors (10 mm NaF, 50 mm sodium pyrophosphate, 1 mm sodium orthovanadate, and 1 μm okadaic acid) (36) by passing 10 times through a 25-gauge needle, and solubilized by gentle shaking on a nutator for 1 h at 4 °C. The clear supernatant obtained after centrifuging the solubilized synaptosomes at 25,000 × g for 30 min at 4 °C was subjected to immunoprecipitation with NET-specific antibody as given below. The supernatants were first precleared using Protein A-Sepharose (3 mg in 100 μl). NET protein was immunoprecipitated overnight at 4 °C by the addition of NET-specific polyclonal antibody (NET-82) on end-over-end continuous mixing, followed by a 2-h incubation with Protein A-Sepharose (4 mg in 100 μl in RIPA buffer) at 22 °C (room temperature). NET-82 antibody has been previously characterized for its specificity in immunoprecipitating NET from rat placenta (16) and we have immunoprecipitated NET from transfected HTR cells and native NETs expressed in rat and mouse brain synaptosomes.3 The immunoadsorbents captured by Protein A-Sepharose beads were washed with RIPA buffer and eluted by adding 50 μl of Laemmli buffer. The eluates were subjected to SDS-PAGE (10%), and the radiolabeled proteins were detected on autoradiograms. Quantitation from digitized autoradiograms was evaluated on multiple exposures to ensure quantitation within the linear range of the film using NIH Image software.
Cell Culture, Transfections, and Development of Stable Cell Lines
HTR-hNET cell lines expressing hNET were generated following transfection of HTR cells with WT-hNET and selecting stable transformants in the presence of 5 μg/ml of blasticidin (17). Stable cells were cultured in a mixture of RPMI 1640 (Mediatech-Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum and penicillin (100 units/ml)- streptomycin (100 μg/ml) containing (1.0 μg/ml of blasticidin). Cells seeded in 24-well cell culture plates (100,000 cells/well) or 12-well plates (200,000 cells/dish or well) were grown in an atmosphere of 95% air, 5% CO2 for 48 h and used for experiments.
Site-directed Mutagenesis
The cDNA encoding the wild-type His-tagged human NET (WT-hNET) was kindly provided by Dr. Randy Blakely (Vanderbilt University school of Medicine, Nashville, TN). The hNET cDNA was subcloned into the mammalian vector pIRES containing the blasticidin resistance gene. Phosphosite mutants of hNET, which include single site mutants harboring T19A, T30A, T58A (in the N terminus), the double mutant harboring T258A and S259A in intracellular loop 2 (ICL2), N-tail triple mutant harboring T19A, T30A, T58A (in the N terminus), and C-tail triple + S502A mutant harboring S579A, T580A, S583A (in the C terminus) along with S502A (in ICL5) were generated by PCR-based mutagenesis in this background using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and the mutations were confirmed by restriction enzyme mapping and automated sequencing of the entire DNA sequences on both strands.
Cell Culture and Transient Transfections
HTR cells seeded in 24- or 12-well plates were transfected with 0.5 or 1 μg of WT-hNET or N-tail triple mutant or C-tail triple mutant + S502A or N-tail single mutants T19A, T30A, or T58A using FuGENE 6 transfection reagent. Cells were used for NE uptake or biotinylation or phosphorylation studies 48 h post-transfection.
Drug Treatments and NE Uptake Measurements Using Cells
HTR-hNET stable cells or HTR cells transiently transfected with WT-hNET or T30A-hNET were treated with vehicle or cocaine (50 μm) for 60 min at 37 °C in Krebs-Ringer-HEPES (KRH) buffer, pH 7.4, containing 100 μm ascorbic acid and 100 μm pargyline. In some experiments, cells were pretreated with 20 μm PD169316 for 15 min at 37 °C and then incubated in the presence or absence of cocaine (50 μm) for a further 60 min at 37 °C. Following treatments, drugs were removed and cells were washed three times at 15-min intervals with KRH buffer at 4 °C. NE uptake measurements were then performed as described previously using [3H]NE (16, 17). Specific NE uptake was measured by subtracting NE uptake measured in the presence of 10 μm DMI from total uptake measured in the absence of DMI. NE uptake in cells transfected with WT-hNET or T30A-hNET was measured in the presence of cocaine concentrations from 10 nm to 1 mm to calculate Ki values by nonlinear least-squares fits, using two-parameter logistic equations (Kaleidagraph, Synergy Software, Reading, PA): the percentage of specific NE transport remaining = 100/[1 + (IC50/[I])n]. The IC50 is the concentration of cocaine giving 50% inhibition, [I] is the cocaine concentration, and n is the slope (Hill coefficient). IC50 values were converted to Ki values using the Cheng and Prusoff (37) correction for substrate concentration. Data are expressed as the mean ± S.E. of six independent measurements in three separate batches of cells.
Cell Surface NET Measurements by Surface Biotinylation Using Cells
HTR-hNET stable cells or HTR cells transiently transfected with WT, N-tail triple mutant, C-tail triple + S502A mutant, or the N-tail single mutants T19A, T30A, or T58A or the T258A,S259A double mutant of hNET were treated with vehicle or 50 μm cocaine for 60 min at 37 °C. In some experiments, HTR cells transiently transfected with WT-hNET or T30A-hNET were treated with vehicle or 50 μm cocaine for 5, 15, and 30 min at 37 °C. Treated cells were subjected to surface biotinylations and isolations were subjected to surface biotinylation followed by isolation of avidin-bound and unbound fractions as described previously (16, 17). An aliquot (40 μl) of total cell lysate from each sample and all (50 μl) avidin-bound samples were analyzed by immunoblotting with NET-specific antibody. To visualize intracellular NET, equal amounts (40 μl) of unbound fractions were analyzed by immunoblotting with NET antibody. To validate equal loading and surface localization of biotinylated NET protein, corresponding total and bound blots were stripped and reprobed with anticalnexin antibody. Band intensities were quantified using NIH Image J (version 1.62). Multiple exposures were taken to validate linearity of quantitation. Values of total, nonbiotinylated, and surface NET proteins were normalized using levels of calnexin immunoreactivity in total cell extract and values were averaged across three experiments.
Measurement of NET Plasma Membrane Recycling Using Cells
NET insertion into the plasma membrane was measured as previously described (17). HTR-hNET stable cells or HTR cells transiently transfected with WT-hNET or T30A-hNET were washed with PBS/Ca-Mg and incubated twice with 1 mg/ml of sulfo-NHS acetate in PBS/Ca-Mg for 1 h at 4 °C (trafficking nonpermissive condition) to block all free amino groups (38). After washing away the sulfo-NHS acetate with cold PBS/Ca-Mg, the cell membrane-impermeable sulfo-NHS-biotin (1 mg/ml) in PBS/Ca-Mg containing cocaine (50 μm) or the vehicle (pre-warmed at 37 °C) was added to the cells and incubated further for the indicated time periods at 37 °C (trafficking permissive condition). Biotinylated NETs inserted into the plasma membrane (surface) and nonbiotinylated (intracellular) NETs were analyzed as described above. Biotinylated TfR was analyzed by stripping and reprobing the blot with TfR antibody. The accumulation of biotinylated NET or TfR at each time point was measured by quantifying the band densities using NIH ImageJ (version 1.62).
Measurement of NET Endocytosis Using Cells
Reversible biotinylation was performed as described (17). HTR-hNET stable cells or cells transiently transfected with WT-hNET or T30A-hNET were cooled rapidly to 4 °C to inhibit endocytosis by washing with cold PBS/Ca-Mg and surface biotinylated with a disulfide-cleavable biotin (sulfo-NHS-SS-biotin). Free biotinylating reagent was removed by quenching with glycine. NET endocytosis was initiated by incubating cells with prewarmed PBS/Ca-Mg containing cocaine (50 μm) or vehicle for the indicated time periods at 37 °C. At the end of incubations, the reagents were removed, and fresh pre-chilled PBS/Ca-Mg was added to stop the endocytosis. The cells were then washed and incubated twice with 250 μm sodium 2-mercaptoethanesulfonate (MesNa), a reducing agent in PBS/Ca-Mg for 20 min to dissociate the biotin from the cell surface-resident proteins via disulfide exchange. To define total biotinylated NETs, one dish of biotinylated cells was not subjected to reduction with MesNa, and directly processed for extraction followed by isolation by avidin beads. To define MesNa-accessible NETs, another dish of cells was treated with MesNa immediately (at 0 time) following biotinylation at 4 °C to reveal the quantity of surface NET biotinylation that MesNa can reverse efficiently. Following treatments, cells were solubilized in RIPA and biotinylated NET protein was separated from non-biotinylated proteins using NeutrAvidin beads. Biotinylated proteins were eluted from beads and resolved by SDS-PAGE. NET proteins in the fractions were visualized with a NET-specific antibody as described under the “Surface Biotinylation.” Band densities were quantified by NIH ImageJ (version 1.62) software.
Measurement of NET Phosphorylation in Cells
HTR-hNET or HTR cells transiently transfected with WT-hNET or T30A-hNET were incubated at 37 °C in phosphate-free DMEM for 1 h and then with 1 mCi/ml of carrier-free [32P]orthophosphate (17). Labeled cells were treated with the reagents as described elsewhere in the continued presence of 32P. Cells were washed with cold PBS and lysed in 400 μl of RIPA buffer containing protease inhibitors and phosphatase inhibitors (10 mm NaF, 50 mm sodium pyrophosphate, 1 mm sodium orthovanadate, and 1 μm okadaic acid) (36). Extracts were centrifuged at 20,000 × g for 30 min at 4 °C and the supernatants were subjected to immunoprecipitation by NET-82 antibody followed by SDS-PAGE analysis and autoradiography. Quantitation from digitized autoradiograms was evaluated on multiple exposures to ensure quantitation within the linear range of the film using NIH Image software.
Immunoblot Analysis to Examine p38 MAPK Activation
PFC synaptosomes obtained from cocaine- or saline-injected rats, or rat PFC synaptosomes treated in vitro with cocaine or vehicle as described elsewhere were subjected to SDS-PAGE and immunoblotting first with phospho-p38 MAPK antibody and then reprobed with total p38 MAPK antibody. HTR-hNET cells treated as described elsewhere were extracted with RIPA buffer and the extracts were subjected to SDS-PAGE and sequential immunoblot analysis using phospho-p38 MAPK, phospho-ATF2, total p38 MAPK, and calnexin antibodies.
RESULTS
Acute in Vivo Cocaine Induces NET Up-regulation in Rat PFC Synaptosomes
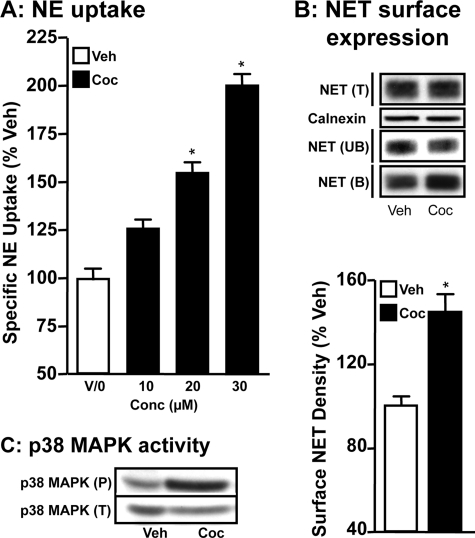
NE uptake by PFC synaptosomes of cocaine-injected rats was higher compared with NE uptake by synaptosomes prepared from saline-injected rats (Fig. 1A). Cocaine produced concentration-dependent increases in NE uptake, where 20 or 30 mg/kg of cocaine produced significantly higher (1.5-fold to 2.0-fold increase) NE uptake compared with saline-injected rats (Fig. 1A). Surface biotinylation experiments using synaptosomes from rats injected with saline or 20 mg/kg of cocaine revealed significant increases in surface NET levels in synaptosomes of cocaine-injected rats compared with control saline-injected rats (Fig. 1B). Total NET protein levels did not differ between vehicle and cocaine groups. The increases in NET surface expression following cocaine injections (Fig. 1B) were similar to increases in NE uptake (Fig. 1A). To determine whether in vivo cocaine injections induce p38 MAPK activity, immunoreactivity of phospho-p38 MAPK was examined using synaptosomes from rats injected with saline or 20 mg/kg of cocaine. Although there was no difference in the total p38 MAPK level between cocaine or saline-injected rats, the phospho-p38 MAPK level in cocaine-injected rats was substantially higher compared with that found in saline-injected rats (Fig. 1C).
FIGURE 1.
Acute intraperitoneal injection of cocaine stimulates NE uptake and increases NET surface expression in rat PFC synaptosomes. Rats were injected intraperitoneally with 10, 20, or 30 mg of cocaine/kg body weight and brains were collected 1-h post-injection for PFC synaptosomal preparations. A, NE uptake: synaptosomes were used for NE uptake assays as described under “Experimental Procedures.” NE uptake data derived from three separate experiments, each in triplicate are given as mean ± S.E. *, indicates significant change (p < 0.01) in NE transport (one-way analysis of variance; Dunnett's test: F(4,10) = 34.68; p < 0.01). B, surface biotinylation: synaptosomes obtained from cocaine-injected or saline-injected rats (20 mg/kg body weight) were biotinylated and biotinylated NETs were isolated by avidin binding. Equal aliquots from total (T) and avidin unbound fractions (UB) and entire eluates from avidin beads representing bound fractions (B) were loaded onto gels and the blots were probed with commercially available mouse NET monoclonal antibody as described under “Experimental Procedures.” Representative blots show NET-specific band at ∼85 kDa. Bar graph shows biotinylated NET band densities as % of vehicle. Data derived from three separate experiments are given as mean ± S.E. *, indicates significant change (p < 0.05) in surface NET immunoreactivity (Student's t test: t = 6.1, df = 4). Calnexin immunoblots corresponding to total are shown for equal protein loading. C, phospho and total p38 MAPK immunoblotting: PFC synaptosomes from cocaine- or saline-injected rats were subjected to SDS-PAGE and immunoblotted with phospho-p38 MAPK antibody and reprobed with total p38 MAPK antibody. The phospho- p38 MAPK and total p38 MAPK immunoreactive bands are shown. The experiment was repeated twice essentially with similar results.
Cocaine-induced NET Up-regulation and Phosphorylation Are p38 MAPK-dependent in Rat PFC Synaptosomes
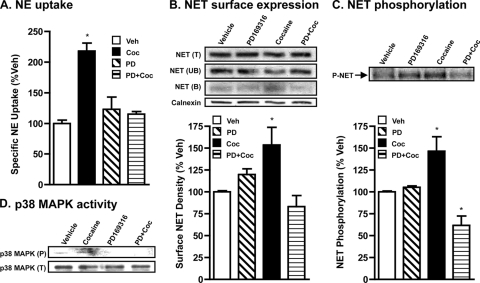
Given our findings from rats receiving acute cocaine injections, we next examined whether cocaine up-regulation of NET in vivo occurs due to effects from altered neuronal circuitry or due to direct effects of cocaine on native NET by studying the effect of in vitro application of cocaine on PFC synaptosomes. Because cocaine is known to induce p38 MAPK activity, we also sought to examine whether p38 MAPK plays a role in cocaine up-regulation of NET. In initial studies, varying concentrations of cocaine (1–100 μm) were tested to establish the dose of cocaine that produces a consistently significant increase in NE uptake following 60 min treatment. We found that 30 to 50 μm cocaine produced a significant increase in NE uptake and chose to use 50 μm cocaine in all our experiments. Incubation of rat PFC synaptosomes with cocaine (50 μm) for 1 h produced a 2-fold increase in NE transport capacity compared with vehicle controls (Fig. 2A). Surface biotinylation experiments revealed a similar increase in surface NET levels (Fig. 2B). As shown in Fig. 2C, cocaine treatment significantly increased (1.5–2.0-fold) basal NET phosphorylation. Furthermore, pretreatment of PFC synaptosomes with the p38 MAPK-specific inhibitor PD169316 abolished the stimulatory effect of cocaine on NE transport (Fig. 2A), surface NET expression (Fig. 2B), and NET phosphorylation (Fig. 2C). Importantly, treatment with PD169316 alone did not significantly affect NET phosphorylation. PD169316 when present together with (in the presence of) cocaine, not only blocked cocaine-induced NET phosphorylation in rat PFC synaptosomes but also reduced the transporter phosphorylation to below basal level. To determine whether cocaine exposure alters p38 MAPK activity, immunoreactivity of phospho-p38 MAPK was quantified following treatment of synaptosomes with cocaine (50 μm). Exposure of synaptosomes to cocaine produced an increase in immunoreactivity of phospho-p38 MAPK. This effect was p38 MAPK dependent because PD169316 pretreatment completely abolished the cocaine-induced increase in phospho-p38 MAPK (Fig. 2D). Treatment with cocaine or PD169316 alone or together did not alter total p38 MAPK levels (Fig. 2D). Analogous results (all of the above) were obtained in ventral striatal synaptosomes (data not shown).
FIGURE 2.
In vitro application of cocaine stimulates NE uptake, NET surface expression, and phosphorylation in rat PFC synaptosomes via p38 MAPK. Rat PFC synaptosomes were preincubated with vehicle or 20 μm PD169316 at 37 °C for 15 min. Incubations were continued in the presence or absence of 50 μm cocaine for 60 min. A, NE uptake: drug-treated synaptosomes were used for NE uptake assays as described under “Experimental Procedures.” Data were derived from three separate experiments, each in triplicate are given as mean ± S.E. *, indicates significant change (p < 0.01) in NE transport (one-way analysis of variance; Dunnett's test: F(4,10) = 20.48; p < 0.01). B, surface biotinylation: synaptosomes treated with drugs as above were biotinylated and biotinylated NETs were isolated and analyzed as described under “Experimental Procedures” and as described in the legend to Fig. 1. Bar graph shows biotinylated NET band densities as % of vehicle. Data derived from three separate experiments are given as mean ± S.E. *, indicates a significant change (p < 0.05) in surface NET immunoreactivity following cocaine treatment compared with vehicle control (one-way analysis of variance; Dunnett's test: F(4,8) = 5.911, p = 0.02). Calnexin immunoblots corresponding to the total are shown for equal protein loading. C, phosphorylation: following metabolic labeling with 32P and drug treatments, synaptosomes were solubilized and the extract was subjected to immunoprecipitation with NET-82 antibody and autoradiography as described under “Experimental Procedures.” Representative autoradiogram is shown. Bar graph shows phospho-NET levels as % of vehicle. *, indicates significant changes (p < 0.01) in phospho-NET (one-way analysis of variance; Dunnett's test: F(4,8) = 12.16, p = 0.002). D, phospho-p38 MAPK and total p38 MAPK levels: synaptosomes treated as above were subjected to SDS-PAGE and immunoblotting with phospho-p38 MAPK antibody and reprobing with total p38 MAPK antibody. The phospho-p38 MAPK and total p38 MAPK immunoreactive bands are shown. The experiment was repeated twice essentially with similar results.
Cocaine Up-regulates WT-hNET Function and Surface Expression
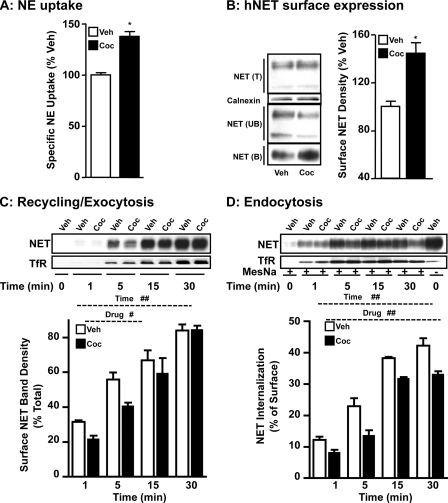
To identify the molecular mechanisms underlying NET up-regulation by cocaine, we sought to explore cocaine regulation using HTR cells heterologously expressing the NET protein. As seen with synaptosomes (Fig. 2), exposure of HTR cells stably expressing WT-hNET to 50 μm cocaine for 1 h significantly increased NE uptake compared with vehicle treatment (Fig. 3A). Because NE transport reflects functional NET expressed on the cell surface, biotinylation and immunoblotting experiments were performed to assess changes in surface NET following cocaine treatment. Fig. 3B shows that cocaine treatment increased cell surface expression of WT-hNET. Quantification of surface NET band densities revealed a significant (30–40%) increase in the amount of cell surface expression of WT-hNET (Fig. 3B). The magnitude of this effect was similar to that observed in uptake studies. Corresponding decreases in the amount of nonbiotinylated intracellular transporter were observed. Total NET level did not change following cocaine treatment. No differences between treatments in calnexin levels were observed in the total fractions indicating equal protein loading and transfer (Fig. 3B). Importantly, calnexin was not detected in the bound fractions suggesting no contamination with intracellular proteins (data not shown).
FIGURE 3.
Cocaine up-regulates hNET expressed in HTR cells and the up-regulation results from a net effect on NET exocytosis and endocytosis. A, NE uptake: HTR-hNET cells were treated with cocaine (50 μm) for 60 min at 37 °C. NE uptake assays were performed as described under “Experimental Procedures.” Specific NE uptake data derived from three separate experiments, each in triplicate are given as mean ± S.E. *, indicates significant difference (p < 0.01) compared with vehicle treatment (Student's t test; WT: t = 8.0, df = 20; DM: t = 4.2, df = 22). B, surface biotinylation: cells treated as above were subjected to surface biotinylation as described under “Experimental Procedures.” Total (T), nonbiotinylated (UB), and biotinylated (B) NETs were analyzed using hNET monoclonal antibody. Representative blot shows hNET-specific bands at ∼85 and ∼48 kDa (upper panel). Densities of biotinylated NETs (∼85 kDa) from three separate experiments are given as mean ± S.E. (bar graphs). *, indicates significant change (p < 0.01) in cell surface NET (Student's t test; WT, t = 5.1 df = 6). Calnexin immunoblots corresponding to total are shown for equal protein loading. C, plasma membrane recycling: sulfo-NHS acetate-treated cells were subjected to biotinylation in the presence of vehicle or 50 μm cocaine for the indicated time periods as described under “Experimental Procedures.” Representative NET and TfR immunoblots are shown. Biotinylated NET band densities expressed as % of total from three different experiments are given as mean ± S.E. (bar graphs). # and ## indicate significant changes in NET recycling (#, p < 0.05 for drug and ##, p < 0.001 for time: two-factor analysis of variance; Bonferroni's test. WT: drug, F(1,16) = 6.6, p = 0.02; time: F(3,16) = 65, p = 0.0001; interaction: F(3,16) = 1.2, p = 0.35). D, endocytosis: cells were subjected to internalization assay as described under “Experimental Procedures.” Representative NET and TfR immunoblots are shown. Biotinylated NET band densities from three separate experiments are given as mean ± S.E. (bar graphs). ##, indicates significant changes in NET internalization (##, p < 0.001 for drug, and time, two-factor analysis of variance; Bonferroni's test. WT: drug, F(1,16) = 49, p = 0.0001; time: F(1,16) = 170.9, p = 0.0001; interaction: F(3,16) = 1.5, p = 0.25).
Cocaine-mediated NET Up-regulation Results from Its Net Effect on NET Recycling/Exocytosis to Plasma Membrane and Endocytosis
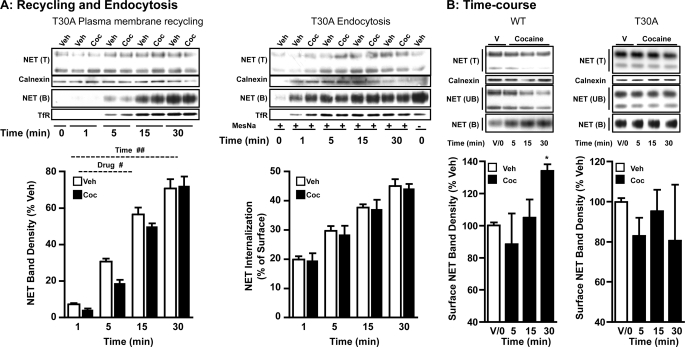
Cocaine-induced increases in NET cell surface expression could arise from enhanced plasma membrane insertion, reduced endocytosis of NET, or a combination of both. Fig. 3C shows the results of biotinylation experiments in which plasma membrane insertion of WT-hNET was measured in HTR-hNET cells following cocaine treatment. No biotinylated NET or TfR was present at the zero time point in sulfo-NHS acetate-treated cells suggesting that all pre-existing surface NETs or TfRs are blocked (from modification by biotinylation). Therefore, biotinylated NET observed at subsequent time points after warming the cells to 37 °C represents newly delivered NET. Vehicle treatment produced a gradual, time-dependent increase in NET plasma membrane insertion in HTR-hNET cells, which was maximal at the 30-min time point. Our previous study in HTR-hNET cells revealed no further increase at later times indicating a plateau in effect at the 30-min time point (17). This increase represents constitutive transporter delivery to the plasma membrane. Cocaine treatment significantly decreased constitutive plasma membrane recycling of NET in HTR-hNET cells as evidenced by the significant 20–30% decrease 1 and 5 min after cocaine. Time-dependent increases in plasma membrane TfR levels were observed under similar conditions indicating that under trafficking permissive conditions, the majority of NET protein reaches the plasma membrane in 30 min. Cocaine had no effect on TfR insertion (Fig. 3C).
As shown above, exposure of WT-hNET cells to cocaine for 1 h stimulated NE uptake and NET surface expression (Fig. 2, A and B). However, only a brief exposure to cocaine inhibited plasma membrane recycling of WT-hNET (Fig. 3C). Therefore, we examined internalization of WT-hNET HTR-hNET cells under basal/constitutive conditions, and following treatment with cocaine using reversible biotinylation strategies and by quantifying the fraction of surface NET that moves in a time-dependent manner to an intracellular compartment. Biotin from biotinylated proteins remaining on the surface at the end of a particular treatment protocol was removed by treatment with MesNa, a non-permeant reducing agent that reduces disulfide bonds and liberates biotin from biotinylated proteins at the cell surface. The amount of biotinylated proteins resistant (inaccessible) to MesNa treatment or reversal of biotinylation is defined as “the amount of protein endocytosed or internalized.” Incubation of the cells at 37 °C in the absence or presence of cocaine before MesNa reversal of surface biotinylation permitted evaluation of NET internalization occurring in the absence of drugs (basal endocytosis) versus cocaine-mediated changes in NET internalization. The amount of NET biotinylated in the absence of MesNa represents the total biotinylated transporter. MesNa treatment immediately after biotinylation revealed less than 2–3% of total biotinylated NET indicating very little internalization at 4 °C. Following vehicle treatment, a gradual, time-related increase in biotinylated WT-hNET (Fig. 3D) was seen. This increase represents constitutive internalization (e.g. basal endocytosis). Cocaine significantly reduced constitutive WT-hNET endocytosis when compared with vehicle treatment at all time points (Fig. 3D). Under basal (un-stimulated) conditions, a maximum of ∼50% of surface-biotinylated NET is internalized in HTR-hNET cells, after 30 min. After cocaine treatment, a 30–40% reduction in basal endocytosis is observed. By contrast, time-dependent internalization of TfR is unaffected by cocaine treatment. These results collectively suggest that inhibition of transporter endocytosis contributes to cocaine-mediated up-regulation of WT-hNET.
Cocaine-induced hNET Up-regulation Requires Threonine 30
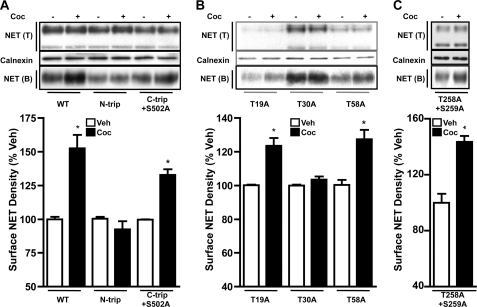
Using HTR cells transfected with the WT-hNET or N-tail triple mutant NET or C-tail triple + Ser502 mutant (S579A,T580A,S583A,S502A) NET or the hNET-T258A, S259A double mutant, we examined the amino acid residues required for cocaine-evoked increases in cell surface expression. As shown in Fig. 4A, cocaine significantly increased (30–50%) cell surface expression of either the WT-hNET or the C-tail triple + Ser502 mutant, but failed to increase surface expression of the N-tail triple mutant. Analysis of T19A, T30A, and T58A single mutants revealed that cocaine significantly increased (∼30%) surface expression of the T19A and T58A mutants, but not the T30A mutant (Fig. 4B). Cocaine significantly increased surface expression of the hNET-T258A,S259A double mutant expressing in HTR cells (Fig. 4C). These results collectively suggested that Thr30 is the site of action for cocaine to up-regulate NET.
FIGURE 4.
Identification of T30A as the site of cocaine action. HTR cells transiently transfected with WT-hNET, N-tail triple mutant, C-tail triple + S502 mutant (A), or T19A, T30A, or T58A single mutants (B) or the T258A,S259A double mutant (C) were treated with vehicle or 50 μm cocaine and surface biotinylation experiments were performed as described under “Experimental Procedures” and in the legend Fig. 3. Representative blots are shown in the upper panels. Biotinylated NET band densities expressed as % of vehicle are shown in the bar graphs. Data derived from three separate experiments are given as mean ± S.E. *, indicate significant changes (p < 0.01) in surface NET immunoreactivity following cocaine treatment compared with respective vehicle-control (Student's t test: WT, t = 5.2, df = 8; N-trip; t = 1.3, df = 6; C-triple + S502A, t = 8.2, df = 6; T19A: t = 4.9, df = 6; T30A, t = 1.7, df = 6; T58A, t = 4.4, df = 4; and T258A,S259A, t = 5.7, df = 4). Calnexin immunoblots corresponding to total are shown for equal protein loading.
Cocaine Up-regulation of NET Is Mediated by Cocaine-induced p38 MAPK-dependent Phosphorylation of Thr30 in the hNET
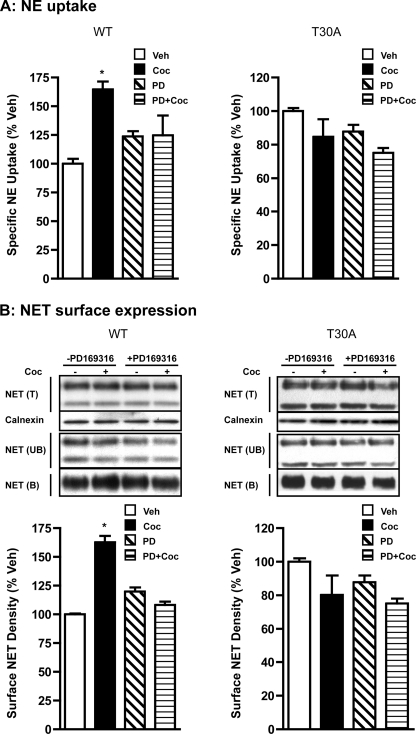
Having known that the T30A mutant is resistant to cocaine-mediated up-regulation, next we conducted parallel experiments to examine the changes in NE uptake, transporter surface expression, and phosphorylation of WT-hNET and T30A-hNET in response to cocaine. Cocaine treatment produced a significant, PD169316-sensitive increase (30–35%) in NE uptake in HTR cells transiently transfected with WT-hNET (Fig. 5A). However, in contrast, cocaine did not alter NE uptake in HTR cells transfected with T30A-hNET (Fig. 5A). Cocaine treatment also produced a significant (50–60%) increase in cell surface NET levels in HTR cells transfected with WT-hNET, but not in cells transfected with T30A-hNET (Fig. 5B). Pretreatment with PD169316 completely blocked the cocaine-induced up-regulation of surface NET levels in HTR cells transfected with WT-hNET (Fig. 5B). Analogous effects were observed with another p38 MAPK inhibitor, SB202190 (data not shown). PD169316 alone had no significant effect on cell surface NET levels in HTR cells transfected with WT-hNET or T30A-hNET (Fig. 5B). NE uptake capacity, as well as total and plasma membrane expression levels of the T30A-hNET mutant were comparable with WT levels.
FIGURE 5.
T30A-hNET mutant is resistant to cocaine-induced up-regulation. A, NE uptake: HTR cells transiently transfected with WT-hNET or T30A-hNET mutant were pretreated with vehicle or PD169316 and incubations were continued in the presence or absence of 50 μm cocaine for 60 min. Drug-treated cells were used for NE uptake assays as described under “Experimental Procedures” and as described in the legend to Fig. 3. Data derived from three separate NE uptake experiments, each in triplicate are given as mean ± S.E. *, indicate significant changes (p < 0.01) in NE transport (one-way analysis of variance; Dunnett's test: WT, F(4,8) = 7.745, p = 0.0094; T30A, F(4,14) = 1.679, p = 0.2169). B, surface biotinylation: cells transfected with WT-hNET or T30A-hNET mutant were treated with drugs as above and subjected to surface biotinylation as described under “Experimental Procedures” and in the legend to Fig. 3. Representative blots are shown in the upper panels. Bar graph shows biotinylated NET band densities as % of vehicle. Data derived from three separate experiments are given as mean ± S.E. *, indicates significant changes (p < 0.01) in surface NET immunoreactivity (one-way analysis of variance; Dunnett's test: WT, F(4,8) = 24.01, p < 0.002; T30A, F(4,12) = 1.86, p = 0.1888). Calnexin immunoblots corresponding to total are shown.
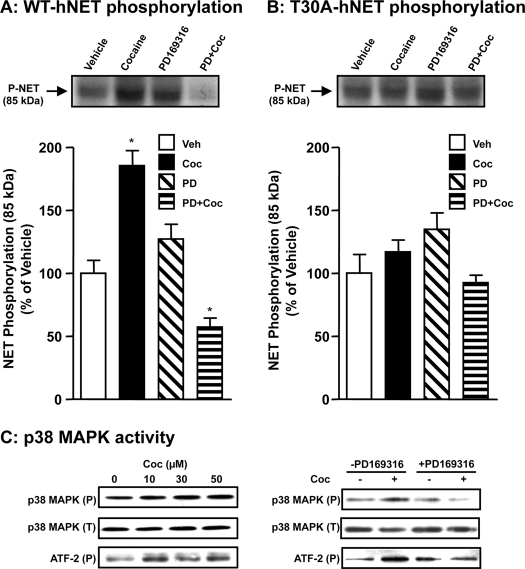
Parallel phosphorylation assays showed that cocaine-induced NET phosphorylation occurs in WT-hNET-transfected HTR cells (Fig. 6A), but not in cells transfected with the T30A-hNET mutant (Fig. 6B). Cocaine produced a 2-fold increase in WT-hNET phosphorylation (Fig. 6A) but did not affect T30A-hNET phosphorylation (Fig. 6B). Treatment with PD169316, alone, produced a small non-significant (15–20%) increase in basal phosphorylation of WT-hNET (Fig. 6A) and T30A-hNET (Fig. 6B). However, PD169316 pretreatment effectively blocked cocaine-induced phosphorylation of hNET in HTR cells transfected with WT-hNET (Fig. 6A). In addition, PD169316 when present together with (in the presence of) cocaine, not only blocked cocaine-induced WT-hNET phosphorylation but also reduced the transporter phosphorylation to below the basal level (Fig. 6A). Interestingly this later effect was not seen with T30A-hNET (Fig. 6B). Cocaine at 10, 30, and 50 μm concentrations produced an increase in the immunoreactivity of both phospho-p38 MAPK and phospho-ATF2 without affecting the total p38 MAPK levels (Fig. 6C). Pretreatment with 20 μm PD169316 completely abolished the increase in phospho-p38 MAPK immunoreactivity induced by 50 μm cocaine (Fig. 6C). Collectively, these results indicate that p38 MAPK signaling is required for cocaine-induced NET up-regulation and phosphorylation of the Thr30 moiety.
FIGURE 6.
T30A-hNET is resistant to cocaine-induced phosphorylation. HTR cells transiently transfected with WT-hNET (A) or T30A-hNET mutant (B) were metabolically labeled with 32P and incubated with vehicle or 20 μm PD169316 for 15 min at 37 °C and incubations were continued in the presence or absence of 50 μm cocaine for 60 min. Cell extracts were subjected to immunoprecipitation with NET-82 antibody and autoradiography as described under “Experimental Procedures.” Representative autoradiograms show phospho-NET bands in the upper panels. Bar graph shows phospho-NET levels as % of vehicle. Data derived from three separate experiments are given as mean ± S.E. *, indicate significant changes (p < 0.01) in phospho-NET (one-way analysis of variance; Dunnett's test: WT, F(4,10) = 7.349, p = 0.0069; T30A, F(4,14) = 1.679, p = 0.7173). C, total and phospho-p38 MAPK and phospho-ATF2 levels: HTR-hNET cells were treated with 10, 30, or 50 μm cocaine for 1 h at 37 °C in one set of experiments. In another set, cells were preincubated with vehicle or 20 μm PD169316 and incubations were continued in the presence or absence of 50 μm cocaine for a further 60 min. Equal aliquots of cell extracts were subjected to SDS-PAGE and sequential immunoblotting with phospho-p38 MAPK, phospho-ATF2, and total p38 MAPK antibodies. The phospho-p38 MAPK, phospho-ATF2, and total p38 MAPK bands are shown. The experiment was repeated twice with essentially similar results.
Cocaine Inhibits Plasma Membrane Recycling of T30A-hNET but Fails to Alter (Inhibit) T30A-hNET Endocytosis
To determine whether cocaine-induced NET up-regulation results from a net inhibitory effect of cocaine on exocytosis and endocytosis, we examined the effects of cocaine on exocytosis and endocytosis of the T30A mutant. Interestingly, although cocaine retained its inhibitory effect on T30A exocytosis/recycling, it failed to alter T30A endocytosis (Fig. 7A). Similar to observations made with WT-hNET (Fig. 2A), cocaine-induced inhibition of T30A-hNET exocytosis/recycling was evident following 1- and 5-min cocaine treatment and beyond 5 min there was no significant effect (Fig. 7A). In contrast, to WT-hNET (Fig. 2B), endocytosis of T30A-hNET was unaltered following cocaine treatment at all time points tested (Fig. 7A).
FIGURE 7.
Although plasma membrane recycling of T30A-hNET is inhibited by cocaine following short time treatments similar to WT-hNET, T30A-hNET endocytosis is unaltered by cocaine. A, plasma membrane recycling and endocytosis: recycling, HTR cells transiently transfected with T30A-hNET mutant were treated with sulfo-NHS acetate prior to biotinylation in the presence of vehicle or 50 μm cocaine for the indicated time periods. Representative total (T) and avidin bound (B) NET and TfR immunoblots as well as calnexin blot corresponding to total are shown. Biotinylated NET band densities expressed as % of total from three different experiments are given as mean ± S.E. Bar graphs, # and ## indicate significant changes in the recycling of plasma membrane T30A-hNET (#, p < 0.05 for drug and ##, p < 0.001 for time; two-factor analysis of variance; Bonferroni's test: drug, F(1,16) = 5.1, p = 0.04; time, F(3,16) = 176.3, p = 0.0001; interaction: F(3,16) = 1.6, p = 0.22). Endocytosis: cells transfected with T30A-hNET mutant were subjected to internalization assay as described under “Experimental Procedures.” Representative NET (T&B) and TfR immunoblots as well as calnexin blot corresponding to total are shown. Biotinylated NET band densities from three separate experiments are given as mean ± S.E. Bar graphs, # and ## indicate significant changes in T30-hNET internalization (#, p < 0.05 for drug and ##, p < 0.001 for time; two-factor analysis of variance; Bonferroni's test: drug, F(1,16) = 0.21, p = 0.65; time, F(3,16) = 48.8, p = 0.0001; interaction, F(3,16) = 0.02, p = 0.99). B, steady-state levels of cell surface WT-hNET and hNET-T30A: cells transfected with WT-hNET or T30A-hNET were treated with 50 μm cocaine for the indicated time periods and steady-state levels of cell surface WT-hNET or T30A-hNET were measured by surface biotinylation as described under “Experimental Procedures” and in the legend to Fig. 3. Representative total (T), avidin bound (B), and unbound (UB) NET immunoblots and calnexin immunoblots corresponding to total are shown. Bar graphs show biotinylated NET band densities as % of vehicle. Data derived from three separate experiments are given as mean ± S.E. *, indicate significant changes (p < 0.05) in surface NET (one-way analysis of variance; Dunnett's test: WT, F(4,11) = 4.01, p < 0.05; T30A, F(4,11) = 0.64, p = 0.60).
Although cocaine failed to inhibit T30A endocytosis, it retained its inhibitory effect on T30A recycling. Therefore, next we examined the steady-state levels of WT-hNET and T30A-hNET in parallel experiments. Surface biotinylation experiments examining the time-dependent effects of cocaine on steady-state levels of WT-hNET and T30A-hNET are shown Fig. 7B. Exposure of WT-hNET cells to cocaine for either 5 or 15 min did not affect cell surface expression. However, a significant increase (∼35%) in cell surface WT-hNET was evident following 30 min incubation with cocaine. In contrast, and regardless of the duration of exposure, we failed to observe a significant effect of cocaine on cell surface levels of T30A-hNET (Fig. 7B).
DISCUSSION
The rewarding and powerfully addictive effects of psychostimulants such as cocaine are attributed to their capacity to increase DA levels in terminal regions of mesolimbic neurons (39, 40). Studies using knock-out mice and/or pharmacological transporter blockade have provided strong evidence that extracellular DA clearance in the PFC is largely controlled by NET (41–45). In addition, a functional coupling exists between DA and NE transmission, and DA-related behavior at the transport level is linked to NET function (46). Cocaine up-regulates both dopamine transporter and NET (10, 12, 29, 47, 48). However, the underlying cellular and molecular mechanisms are unknown. The present study for the first time, documents acute cocaine-induced up-regulation of native NET in the PFC and provides new insights as to the motif and trafficking mechanisms as well as kinase/signaling pathway(s) involved in cocaine up-regulation of NET function.
Acute intraperitoneal injection of cocaine stimulates NE transport and increases NET surface expression in rat PFC synaptosomes. Rat PFC and HTR cells express p38 MAPK as well as activated p38 MAPK (phospho-p38 MAPK). The MAPK pathway plays a key role in neuroadaptations induced by drugs of abuse (26, 49, 50). The present study demonstrates that cocaine-induced NET up-regulation (increased NE transport and surface NET level) is p38 MAPK dependent. Thus, pretreatment of PFC synaptosomes or cells expressing hNET with PD169316 prevented cocaine-evoked increases in NE transport and NET surface expression. Cocaine-induced NET phosphorylation is also sensitive to p38 MAPK inhibition. In addition, cocaine stimulated the levels of phospho-p38 MAPK and phospho-ATF2 in a PD169316-sensitive manner. These data collectively suggest that cocaine activates p38 MAPK, which in turn phosphorylates NET. Substrates and ligands for monoamine transporters regulate transporter phosphorylation and, thereby, transport function (31, 36, 51, 52). Previous reports have shown that cocaine by itself has no effect on the phosphorylation of serotonin transporter or dopamine transporter, although PKC-induced serotonin transporter phosphorylation and methamphetamine-induced dopamine transporter phosphorylation are blocked by cocaine (31, 51). Amphetamine, which is a substrate for serotonin transporter, increases serotonin transporter basal phosphorylation that is sensitive to p38 MAPK inhibition (18). These results suggest that psychostimulant drugs have distinctly different effects in regulating monoamine transporter expression and phosphorylation that are transporter-specific.
Transport function is determined by the number of transporters present on the cell surface, which in turn is dictated by transporter entry to the cell surface (exocytosis) and their exit from the surface (endocytosis). Here, we demonstrate that in HTR cells heterologously expressing hNET, cocaine-induced increases in NET function result from p38 MAPK-dependent phosphorylation of Thr30 and occur in concert with an increase in plasma membrane NET density. Our studies in heterologous cells demonstrate that cocaine-induced NET up-regulation is due to inhibition of NET endocytosis. Although cocaine inhibits both plasma membrane insertion/recycling and internalization of NET, inhibition of endocytosis is observed after both brief and more prolonged cocaine exposure, whereas inhibition of insertion is seen only after brief exposure (1 and 5 min). Cocaine failed to up-regulate NE transport function and surface expression of the T30A-hNET mutant. These data suggest that Thr30 is a potential phosphorylation site for cocaine-activated p38 MAPK. The analysis of inhibition constants for cocaine in HTR cells expressing WT-hNET or T30A-hNET revealed similar Ki values (WT, 0.25 ± 0.02 μm; T30A, 0.21 ± 0.04 μm). Thus, the inability of cocaine to up-regulate T30A-hNET cannot be attributed to altered affinity for cocaine. Although cocaine-induced inhibition of T30A-hNET plasma membrane recycling was intact, cocaine failed to alter T30A-hNET endocytosis and phosphorylation. Together these findings suggest that Thr30 phosphorylation by cocaine-linked/activated p38 MAPK dictates NET endocytosis and hence NET up-regulation. The N-terminal region of hNET has three threonine residues that can serve as potential phosphorylation sites for serine/threonine-directed kinases. Although these are not exact p38 MAPK motifs, the presence of several proline residues throughout the NET N-tail may render these sites suitable for proline-directed serine/threonine kinases such as p38 MAPK.
Data from time course studies of cocaine on steady-state cell surface NET levels suggest that cocaine-induced up-regulation of WT-hNET is primarily due to its effect on NET endocytosis and that T30A mutation renders NET resistant to cocaine-induced inhibition of NET endocytosis. Brief cocaine exposure was effective in inhibiting NET plasma membrane insertion and this action was retained in the T30A-hNET mutant. Interestingly, NE uptake and steady-state surface NET levels were unaltered following a 5-min cocaine treatment. This suggests that following brief cocaine exposure, the effect of decreased transporter recycling could nullify the effect of reduced endocytosis (WT) or it is too small to detect (WT or T30A). It is clear, however, that the greater effect of cocaine on WT-hNET endocytosis (inhibition) contributes to the overall up-regulation observed at longer periods. We observed non-significant decreases in T30A cell surface expression following cocaine treatment in all our experiments. This may indicate that the efficacy of cocaine in decreasing plasma membrane recycling is maintained in the T30A mutant. However, this could not be measured because surface NET levels plateau at 30 min (16). Nonetheless, the present study demonstrates the altered trafficking mechanisms mediate cocaine-mediated NET up-regulation and identify Thr30 as the trafficking motif.
Cocaine stimulated basal NET phosphorylation in a PD169316-sensitive manner both in a heterologous system and in native tissues. In cells, the ability of cocaine to phosphorylate hNET is prevented by T30A mutation. However, p38 MAPK inhibition alone had no significant effect on basal NET expression and phosphorylation. Although it is not known whether p38 MAPK directly phosphorylates NET, it is possible that when cocaine is bound to NET (cocaine bound NET), NET adopts a conformation that enables p38 MAPK itself or p38 MAPK-regulated kinases or phosphatases to act upon and alter NET phosphorylation, thereby, limiting NET endocytosis. Indeed, in the presence of cocaine, p38 MAPK inhibition not only blocked cocaine-induced up-regulation of NET surface expression, but also inhibited NET phosphorylation to a level lower than the basal level. This is not seen with the T30A-hNET mutant suggesting that when bound to cocaine, the T30A mutant may adopt a conformation not suitable for p38 MAPK to act upon.
NET function is regulated by several signaling pathways that involve associated proteins, including the SNARE protein, syntaxin 1A, protein phosphatase 2A catalytic subunit (PP2Ac), PICK1, Hic-5, and PP2A anchoring subunit (PP2AAr) (53–55). It is tempting to speculate that, cocaine, via MAPK signaling, may induce Thr30 phosphorylation and inhibit constitutive NET endocytosis and stabilize cell surface NET. However, further studies are needed to clarify whether other cellular mechanisms such as dephosphorylation, ubiquitinylation, and protein-protein interactions play a role in cocaine-induced NET up-regulation. Although the intermediary candidates or steps linking cocaine treatment to p38 MAPK activation await identification, it is clear that substrate/ligand modulation of transporter-mediated intracellular neurotransmitters can lead to direct activation of kinases (56, 57). Nonetheless, our findings that p38 MAPK mediates cocaine up-regulation of NET in the native neuronal model and heterologous system signify the role of endogenous NET regulation in cocaine modulation of catecholamine homeostasis with important physiological and therapeutic implications.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1-GM081054 (to L. D. J.) from the NIGMS, National Institute of Mental Health Grant RO1-MH62612 (to S. R.), and the National Institute on Drug Abuse Intramural Research Program (to T. S. S.).
O. Arapulisamy, P. Mannangatti, and L. D. Jayanthi, unpublished data.
- NET
- norepinephrine transporter
- NE
- norepinephrine
- PFC
- prefrontal cortex
- DMI
- desipramine
- TfR
- transferrin receptor
- MesNa
- sodium 2-mercaptoethanesulfonate.
REFERENCES
- 1. Iversen L. L. (1971) Br. J. Pharmacol. 41, 571–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schömig E., Fischer P., Schönfeld C. L., Trendelenburg U. (1989) Naunyn-Schmiedebergs Arch. Pharmacol. 340, 502–508 [DOI] [PubMed] [Google Scholar]
- 3. Trendelenburg U. (1991) Trends Pharmacol. Sci. 12, 334–337 [DOI] [PubMed] [Google Scholar]
- 4. Xu F., Gainetdinov R. R., Wetsel W. C., Jones S. R., Bohn L. M., Miller G. W., Wang Y. M., Caron M. G. (2000) Nat. Neurosci. 3, 465–471 [DOI] [PubMed] [Google Scholar]
- 5. Keller P. C., 2nd, Stephan M., Glomska H., Rudnick G. (2004) Biochemistry 43, 8510–8516 [DOI] [PubMed] [Google Scholar]
- 6. Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]
- 7. Tatsumi M., Groshan K., Blakely R. D., Richelson E. (1997) Eur. J. Pharmacol. 340, 249–258 [DOI] [PubMed] [Google Scholar]
- 8. Markou A., Kosten T. R., Koob G. F. (1998) Neuropsychopharmacology 18, 135–174 [DOI] [PubMed] [Google Scholar]
- 9. Robinson T. E., Berridge K. C. (1993) Brain Res. Brain Res. Rev. 18, 247–291 [DOI] [PubMed] [Google Scholar]
- 10. Macey D. J., Smith H. R., Nader M. A., Porrino L. J. (2003) J. Neurosci. 23, 12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Platt D. M., Rowlett J. K., Spealman R. D. (2007) J. Pharmacol. Exp. Ther. 322, 894–902 [DOI] [PubMed] [Google Scholar]
- 12. Shearman L. P., Meyer J. S. (1999) Eur. J. Pharmacol. 386, 1–6 [DOI] [PubMed] [Google Scholar]
- 13. Jayanthi L. D., Prasad P. D., Ramamoorthy S., Mahesh V. B., Leibach F. H., Ganapathy V. (1993) Biochemistry 32, 12178–12185 [DOI] [PubMed] [Google Scholar]
- 14. Ganapathy V., Prasad P. D. (2005) Toxicol. Appl. Pharmacol. 207, 381–387 [DOI] [PubMed] [Google Scholar]
- 15. Levitt P., Harvey J. A., Friedman E., Simansky K., Murphy E. H. (1997) Trends Neurosci. 20, 269–274 [DOI] [PubMed] [Google Scholar]
- 16. Jayanthi L. D., Samuvel D. J., Ramamoorthy S. (2004) J. Biol. Chem. 279, 19315–19326 [DOI] [PubMed] [Google Scholar]
- 17. Jayanthi L. D., Annamalai B., Samuvel D. J., Gether U., Ramamoorthy S. (2006) J. Biol. Chem. 281, 23326–23340 [DOI] [PubMed] [Google Scholar]
- 18. Samuvel D. J., Jayanthi L. D., Bhat N. R., Ramamoorthy S. (2005) J. Neurosci. 25, 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu C. B., Hewlett W. A., Feoktistov I., Biaggioni I., Blakely R. D. (2004) Mol. Pharmacol. 65, 1462–1474 [DOI] [PubMed] [Google Scholar]
- 20. Zhu C. B., Carneiro A. M., Dostmann W. R., Hewlett W. A., Blakely R. D. (2005) J. Biol. Chem. 280, 15649–15658 [DOI] [PubMed] [Google Scholar]
- 21. Morón J. A., Zakharova I., Ferrer J. V., Merrill G. A., Hope B., Lafer E. M., Lin Z. C., Wang J. B., Javitch J. A., Galli A., Shippenberg T. S. (2003) J. Neurosci. 23, 8480–8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolan E. A., Kivell B., Jaligam V., Oz M., Jayanthi L. D., Han Y., Sen N., Urizar E., Gomes I., Devi L. A., Ramamoorthy S., Javitch J. A., Zapata A., Shippenberg T. S. (2007) Mol. Pharmacol. 71, 1222–1232 [DOI] [PubMed] [Google Scholar]
- 23. Apparsundaram S., Sung U., Price R. D., Blakely R. D. (2001) J. Pharmacol. Exp. Ther. 299, 666–677 [PubMed] [Google Scholar]
- 24. Valjent E., Herve D., Girault J. A. (2005) Med. Sci. (Paris) 21, 453–454 [DOI] [PubMed] [Google Scholar]
- 25. Nestler E. J., Berhow M. T., Brodkin E. S. (1996) Mol. Psychiatry 1, 190–199 [PubMed] [Google Scholar]
- 26. Li G., Xiao Y., Zhang L. (2005) J. Pharmacol. Exp. Ther. 312, 112–119 [DOI] [PubMed] [Google Scholar]
- 27. Choe E. S., Wang J. Q. (2002) Neuroreport 13, 1013–1016 [DOI] [PubMed] [Google Scholar]
- 28. Samuvel D. J., Jayanthi L. D., Manohar S., Kaliyaperumal K., See R. E., Ramamoorthy S. (2008) J. Pharmacol. Exp. Ther. 325, 293–301 [DOI] [PubMed] [Google Scholar]
- 29. Daws L. C., Callaghan P. D., Morón J. A., Kahlig K. M., Shippenberg T. S., Javitch J. A., Galli A. (2002) Biochem. Biophys. Res. Commun. 290, 1545–1550 [DOI] [PubMed] [Google Scholar]
- 30. Chi L., Reith M. E. (2003) J. Pharmacol. Exp. Ther. 307, 729–736 [DOI] [PubMed] [Google Scholar]
- 31. Cervinski M. A., Foster J. D., Vaughan R. A. (2005) J. Biol. Chem. 280, 40442–40449 [DOI] [PubMed] [Google Scholar]
- 32. Aouadi M., Laurent K., Prot M., Le Marchand-Brustel Y., Binétruy B., Bost F. (2006) Diabetes 55, 281–289 [DOI] [PubMed] [Google Scholar]
- 33. Brooks A. C., Menzies-Gow N. J., Wheeler-Jones C., Bailey S. R., Cunningham F. M., Elliott J. (2007) Inflamm. Res. 56, 154–161 [DOI] [PubMed] [Google Scholar]
- 34. Chen L., Liu L., Yin J., Luo Y., Huang S. (2009) Int. J. Biochem. Cell Biol. 41, 1284–1295 [DOI] [PubMed] [Google Scholar]
- 35. Matthies H. J., Han Q., Shields A., Wright J., Moore J. L., Winder D. G., Galli A., Blakely R. D. (2009) BMC Neurosci. 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramamoorthy S., Giovanetti E., Qian Y., Blakely R. D. (1998) J. Biol. Chem. 273, 2458–2466 [DOI] [PubMed] [Google Scholar]
- 37. Cheng Y., Prusoff W. H. (1973) Biochem. Pharmacol. 22, 3099–3108 [DOI] [PubMed] [Google Scholar]
- 38. Lee-Kwon W., Kawano K., Choi J. W., Kim J. H., Donowitz M. (2003) J. Biol. Chem. 278, 16494–16501 [DOI] [PubMed] [Google Scholar]
- 39. Koob G. F. (1992) Ann. N.Y. Acad. Sci. 654, 171–191 [DOI] [PubMed] [Google Scholar]
- 40. Tzschentke T. M., Schmidt W. J. (2000) Crit. Rev. Neurobiol. 14, 131–142 [PubMed] [Google Scholar]
- 41. Carboni E., Tanda G. L., Frau R., Di Chiara G. (1990) J. Neurochem. 55, 1067–1070 [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto B. K., Novotney S. (1998) J. Neurochem. 71, 274–280 [DOI] [PubMed] [Google Scholar]
- 43. Morón J. A., Brockington A., Wise R. A., Rocha B. A., Hope B. T. (2002) J. Neurosci. 22, 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valentini V., Frau R., Di Chiara G. (2004) J. Neurochem. 88, 917–927 [DOI] [PubMed] [Google Scholar]
- 45. Carboni E., Silvagni A., Vacca C., Di Chiara G. (2006) J. Neurochem. 96, 473–481 [DOI] [PubMed] [Google Scholar]
- 46. Siuta M. A., Robertson S. D., Kocalis H., Saunders C., Gresch P. J., Khatri V., Shiota C., Kennedy J. P., Lindsley C. W., Daws L. C., Polley D. B., Veenstra-Vanderweele J., Stanwood G. D., Magnuson M. A., Niswender K. D., Galli A. (2010) PLoS Biol. 8, e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Little K. Y., Elmer L. W., Zhong H., Scheys J. O., Zhang L. (2002) Mol. Pharmacol. 61, 436–445 [DOI] [PubMed] [Google Scholar]
- 48. Mash D. C., Ouyang Q., Qin Y., Pablo J. (2005) J. Neurosci. Methods 143, 79–85 [DOI] [PubMed] [Google Scholar]
- 49. Gerdjikov T. V., Ross G. M., Beninger R. J. (2004) Behav. Neurosci. 118, 740–750 [DOI] [PubMed] [Google Scholar]
- 50. Fan L., Sawbridge D., George V., Teng L., Bailey A., Kitchen I., Li J. M. (2009) J. Pharmacol. Exp. Ther. 328, 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramamoorthy S., Blakely R. D. (1999) Science 285, 763–766 [DOI] [PubMed] [Google Scholar]
- 52. Vaughan R. A., Huff R. A., Uhl G. R., Kuhar M. J. (1997) J. Biol. Chem. 272, 15541–15546 [DOI] [PubMed] [Google Scholar]
- 53. Sung U., Jennings J. L., Link A. J., Blakely R. D. (2005) Biochem. Biophys. Res. Commun. 333, 671–678 [DOI] [PubMed] [Google Scholar]
- 54. Sung U., Blakely R. D. (2007) Mol. Cell. Neurosci. 34, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miner L. H., Jedema H. P., Moore F. W., Blakely R. D., Grace A. A., Sesack S. R. (2006) J. Neurosci. 26, 1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dipace C., Sung U., Binda F., Blakely R. D., Galli A. (2007) Mol. Pharmacol. 71, 230–239 [DOI] [PubMed] [Google Scholar]
- 57. Ahmed B. A., Bukhari I. A., Jeffus B. C., Harney J. T., Thyparambil S., Ziu E., Fraer M., Rusch N. J., Zimniak P., Lupashin V., Tang D., Kilic F. (2009) PLoS One 4, e4730. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]