Abstract
Based on the conformationally constrained d-Trp-Phe-d-Trp (wFw) core of the prototype inverse agonist [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P, a series of novel, small, peptide-mimetic agonists for the ghrelin receptor were generated. By using various simple, ring-constrained spacers connecting the d-Trp-Phe-d-Trp motif with the important C-terminal carboxyamide group, 40 nm agonism potency was obtained and also in one case (wFw-Isn-NH2, where Isn is isonipecotic acid) ∼80% efficacy. However, in contrast to all previously reported ghrelin receptor agonists, the piperidine-constrained wFw-Isn-NH2 was found to be a functionally biased agonist. Thus, wFw-Isn-NH2 mediated potent and efficacious signaling through the Gαq and ERK1/2 signaling pathways, but in contrast to all previous ghrelin receptor agonists it did not signal through the serum response element, conceivably the Gα12/13 pathway. The recognition pattern of wFw-Isn-NH2 with the ghrelin receptor also differed significantly from that of all previously characterized unbiased agonists. Most importantly, wFw-Isn-NH2 was not dependent on GluIII:09 (Glu3.33), which otherwise is an obligatory TM III anchor point residue for ghrelin agonists. Molecular modeling and docking experiments indicated that wFw-Isn-NH2 binds in the classical agonist binding site between the extracellular segments of TMs III, VI, and VII, interacting closely with the aromatic cluster between TMs VI and VII, but that it does so in an opposite orientation as compared with, for example, the wFw peptide agonists. It is concluded that the novel peptide-mimetic ligand wFw-Isn-NH2 is a biased ghrelin receptor agonist and that the selective signaling pattern presumably is due to its unique receptor recognition pattern lacking interaction with key residues especially in TM III.
Keywords: G-protein-coupled Receptors (GPCR), Hormone Receptors, Neuropeptide, Peptide Hormones, Signal Transduction, 7TM Receptor, Ghrelin Receptor
Introduction
Ghrelin is a neuroendocrine hormone that differs from other peptide hormones by a fatty acid modification, which is crucial for both the binding and activation of its receptor (1). Ghrelin is synthesized mainly in the gastrointestinal tract, where the gene coding for the peptide sequence is expressed together with the enzyme responsible for the acylation of the fatty acid to the ghrelin peptide sequence (2, 3). Multiple functions have been described for ghrelin since it was discovered. Initially it was believed that growth hormone secretion induced by ghrelin receptors in the hypothalamus and the pituitary was the primary function of ghrelin (4). However, the function of ghrelin in the hypothalamus, and in particular in the arcuate nucleus, has become the focus of attention over the last decade. In the arcuate nucleus, ghrelin is responsible for increased activity in the NPY (neuropeptide Y) and AGRP (Agouti-related protein) neurones leading to increased appetite, decreased energy expenditure, and fat accumulation (5, 6). High receptor expression is also observed in the ventromedial nucleus of the hypothalamus, and the function in this area has been proposed to be orexigenic based on the regulation of fatty acid metabolism (7). More recently it has been demonstrated that ghrelin is also involved in reward-seeking behavior such as alcohol and cocaine abuse or intake of palatable food through interaction with the dopaminergic system in the ventral tegmental area and the substantia niagra (8–10).
The development of drugs that modulate the signaling of the ghrelin receptor system has been pursued by the pharmaceutical industry for the last three decades. Agonist compounds were developed as so-called growth hormone secretagogues even before it was realized that they worked through the ghrelin receptor and its cloning. Such compounds, for example MK-677, were used in clinical trials both for the treatment of growth hormone deficiency and for the treatment of the frail elderly. However, these compounds never reached the market, mainly because of a lack of efficacy (11, 12). Today non-peptide ghrelin receptor agonists and ghrelin analogues are being developed in attempts to treat, for example, various forms of cachexia and malnourishment in hospitalized patients (13). Antagonists or inverse agonists of the ghrelin receptor have been proposed as a potential treatment for obesity, diabetes, and also more recently alcohol abuse; but these are still in early development (14).
Interestingly, functionally biased agonists, i.e. agonists with the ability to induce selective receptor conformations responsible for interaction with only a limited selection of the downstream signaling pathways, have been described for many 7TM3 receptors (15, 16). One therapeutic potential of such biased ligands would be to avoid unwanted side effects mediated through a particular pathway, as described for the niacin receptor GPR109A agonists (17). In the case of the ghrelin receptor, a biased agonist could potentially act as a functionally specific agonist, i.e. able to modulate energy expenditure and food intake without affecting growth hormone secretion or vice versa. The structural understanding of biased signaling has been addressed only to a limited degree, and information is primarily based on mutations that selectively decouple one signaling pathway and not the other (18, 19).
Through structure-function analysis of the first inverse agonist for the ghrelin receptor, [d-Arg1,d-Phe5,d-Trp7,9,Leu11] substance P (20), we have previously identified the carboxyamidated, C-terminal pentapeptide (d-Trp-Phe-d-Trp-Leu-Leu) as the essential core peptide, which by itself displays a characteristic biphasic dose-response curve, i.e. combined agonism and inverse agonism (Fig. 1) (21). By the addition of a single amino acid at its N terminus this pentapeptide could, depending on the type of N-terminal residue, be converted into either a pure agonist or a pure inverse agonist (21, 22). Importantly, the characteristic essential d-Trp-Phe-d-Trp (wFw) motif strongly restricts the conformational freedom of this type of peptide ligand because essentially the wFw motif is found in only two different dominating conformations (22). The structure-activity relationship analysis indicated that the more flexible C-terminal Leu-Leu dipeptide functions mainly as a spacer or linker between the wFw core and the functionally highly important C-terminal carboxyamide moiety. Thus, one aim of the present study was to try to exchange the Leu-Leu part of wFw ligands with a non-peptide linker structure (Fig. 1). In this way novel, potent, ghrelin receptor ligands could in fact be generated. However, in contrast to all previously characterized peptide and non-peptide ligands, these novel wFw peptide-mimetic ligands were found to be biased agonists in respect to their ability to activate signaling through the classical Gαq pathway but not through the Gα12/13 SRE signaling pathway. Importantly, mutational mapping of important receptor residues for the function of the most efficacious biased agonists, wFw-Isn-amide, demonstrated a novel receptor interaction mode. This lacked a crucial, charged anchor point in TM III, GluIII:09, which is shared by all of the unbiased ghrelin receptor agonists.
FIGURE 1.
Overview of ligands containing the core peptide and their properties on ghrelin receptor activity. A, schematic drawings of the amino acid sequence of the mother peptide, [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P, the core peptide, wFwLL, the inverse agonist, KwFwLL, and the agonist, AwFwLL, which form the basis for the present study. Finally, the novel agonists are indicated as wFwXxx. The colors of the boxes indicate the chemical properties of the amino acids in each peptide: green, aromatic amino acids; gray, hydrophobic amino acids; pink, hydrophilic amino acids; blue, positively charged amino acids. For the names of the peptides, small single-letter amino acid abbreviations denote d-amino acids, whereas capital letters denote the l-amino acids. B, dose-response curves are shown for AwFwLL (triangles), KwFwLL (circles), wFwLL (squares), [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P (dashed line), and ghrelin (dotted line). C, chemical structure of the core peptide, wFwLL, and of the novel agonists, wFw-Isn-NH2. Each bond in the linker between the d-Trp-Phe-d-Trp motif and the C-terminal functionally important carboxyamide moiety is marked with a number.
EXPERIMENTAL PROCEDURES
Materials
Ghrelin was purchased from Bachem (Bubendorf, Swicherland). The non-peptide compound MK-677 (L-163,191) was kindly provided by Andrew Howard (Merck Research Laboratories). γ-Amino butyric acid (Abu), 3-aminobenzoic acid (3Abz), 4-aminobenzoic acid (4Abz), (1R,4S)-(+)-4-amino-2-cyclopentene-1-carboxylic acid (Acp), 4-(2-aminoethyl)piperazine-1-ylacetic acid (Aep), β-alanine (βAla), 3-aminomethylbenzoic acid (3Amb), 4-aminomethylbenzoic acid (4Amb), isonipecotic acid (Isn), and aminohexanoic acid (Ahx) were purchased from Fluka or Sigma-Aldrich.
Peptide Synthesis
The peptides were synthesized by solid-phase technique on an automated multiple peptide synthesizer (Syro; MultiSynTech, Bochum, Germany) by using Rink amide resin (30 mg, resin loading 0.6 mmol/g) as described recently (23). The non-natural amino acids Abu, 3Abz, 4Abz, Acp, Aep, βAla, 3Amb, 4Amb, Isn, and Ahx were coupled manually directly at the Rink amide resin (30 mg, resin loading 0.6 mmol/g) after removal of the Fmoc group with 30% piperidine in N,N-dimethylformamide twice for 20 min. The coupling reaction was performed twice with four equivalents of the Fmoc-protected un-natural amino acids, which were activated by 4 equivalents of 2-(1H-7-Azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate methanaminium and 8 equivalents of N,N-diisopropyl-ethylamin for 1 h. Completeness of the reaction was analyzed by a ninhydrin assay (24). The core segment wFw was synthesized as described previously (23). The peptide mimetics were cleaved from the resin in one step using trifluoroacetic acid, precipitated from ice-cold diethyl ether, washed, and finally lyophilized. Purification of the peptides was achieved by preparative HPLC on an RP C18 column (Vydac, 250 × 25 mm, 10 μm) with a gradient of 20–60% B in A (A = 0.1% trifluoroacetic acid in water; B = 0.08% trifluoroacetic acid in acetonitrile) over a 60-min time span at a flow rate of 10 ml/min (λ = 220 nm).
The peptides were analyzed by MALDI mass spectrometry on an Voyager-DE RP work station (Applied Biosystems, Darmstadt, Germany) and by analytical reversed-phase HPLC on a Vydac RP-18 column (4.6 × 250 mm, 5 μm, 300 Å) using linear gradients of 10–60% B in A over 30 min and a flow rate of 0.6 ml/min (λ = 220 nm). The observed masses were in full agreement with the calculated masses, and the purity of all peptides was >95% accordingly to analytical HPLC.
Molecular Biology
The human ghrelin/receptor cDNA was cloned by PCR from a human brain cDNA library. The cDNA was cloned into the eukaryotic expression vector pCMV-Tag2B made by Stratagene (La Jolla, CA) for epitope tagging of proteins. Mutations were constructed by PCR using the overlap expression method (25). The PCR products were digested with the appropriate restriction endonucleases (BamHI and EcoRI), purified, and cloned into the pCMV-Tag2B vector. All PCR experiments were performed using Pfu polymerase (Stratagene) according to the instructions of the manufacturer. All mutations were verified by restriction endonuclease mapping and subsequent DNA sequence analysis using an automated sequencer (ABI PRISM 310; Applied Biosystems, Foster City, CA).
Transfection and Tissue Culture
COS-7 cells were grown in Dulbecco's modified Eagle's medium 1885 supplemented with 10% fetal calf serum, 2 mm glutamine, 180 units/ml penicillin, and 45 μg/ml streptomycin. Cells were transfected using the calcium phosphate precipitation method with chloroquine added. The amount of cDNA (20 μg/75 cm2) resulting in maximal basal signaling was used for the dose-response curves. HEK-293 cells were grown in Dulbecco's modified Eagle's medium adjusted to contain 4500 mg/liter glucose (Invitrogen), 10% fetal bovine serum, 180 units/ml penicillin, and 45 μg/ml streptomycin at 10% CO2 and 37 °C. Stably transfected HEK-293 cells were grown in the same medium.
Phosphatidyl Turnover Assay
One day after transfection, COS-7 cells were incubated for 24 h with 5 μCi of myo-[3H]inositol (Amersham Biosciences) in 0.3 ml of medium supplemented with 10% fetal calf serum, 2 mm glutamine, 180 units/ml penicillin, and 45 μg/ml streptomycin/well. Cells were washed twice in buffer (20 mm HEPES, pH 7.4, supplemented with 140 mm NaCl, 5 mm KCl, 1 mm MgSO4, 1 mm CaCl2, 10 mm glucose, and 0.05% (w/v) bovine serum) and incubated in 0.5 ml of buffer supplemented with 10 mm LiCl at 37 °C for 30 min. After stimulation with various concentrations of peptide and/or non-peptides for 45 min at 37 °C, cells were extracted with 10 mm formic acid followed by incubation on ice for 30 min. The resulting supernatant was purified on anion exchange resin (AG 1-X8; Bio-Rad) to isolate the negatively charged inositol phosphates. After application of the cell extract to the column, the content was washed twice with washing buffer (60 mm sodium formate and 5 mm sodium tetraborate decahydrate) to remove glycerophosphoinositol. Inositol phosphates were eluded by the addition of elution buffer (1 m ammonium formate and 100 mm formic acid), and eluates were added to 10 ml of Wallac Optiphase HiSafe 3 (PerkinElmer Life Sciences). Determinations were made in duplicates. The columns containing AG 1-X8 anion exchange resin were regenerated by the addition of 3 ml of regeneration buffer (3 m ammonium formate and 100 mm formic acid) and 10 ml of water.
Cell Surface Expression Measurement (ELISA)
Cells were transfected and seeded out in parallel with those used for inositol phosphate accumulation assay. The cells were washed twice, fixed, and incubated in blocking solution (phosphate-buffered saline and 3% dry milk) for 60 min at room temperature. Cells were kept at room temperature for all subsequent steps. Cells were incubated for 2 h with anti-FLAG (M2) antibody (Sigma) at a 1:300 dilution. After three washes, cells were incubated for 2 h with anti-mouse horseradish peroxidase (Amersham Biosciences)-conjugated antibody at a dilution of 1:4000. After extensive washing, the immunoreactivity was revealed by the addition of horseradish peroxidase substrate according to the manufacturer's instructions.
Calculations
EC50 values were determined by nonlinear regression using Prism version 3.0 software (GraphPad Software, San Diego, CA). The basal constitutive activity is expressed as a percentage of the ghrelin-induced activation for each mutant construct of the ghrelin receptor. In Tables 1 and 2, Fmut indicates the -fold shift in potency induced by the structural change in the mutated receptor compared with the wild-type receptor.
TABLE 1.
Characterization of nine small peptide compounds with their respective structures
The potency (EC50) and efficacy (Emax) of the compounds with respect to stimulating inositol phosphate (IP) accumulation were determined in COS-7 cells expressing the wild-type form of the ghrelin receptor. The affinity of the compounds was also determined by a competition binding assay measuring the displacement of 3H-MK-677 bound to the wild-type receptor.
TABLE 2.
Characterization of a library of 23 mutant versions of the ghrelin receptor with substitutions systematically placed throughout the main ligand-binding crevice and in the extracellular part of the receptor
The constructs were expressed in transiently transfected COS-7 cells. Under the “Ghrelin” column, expression of each mutation as assessed by cell surface ELISA is stated as a fraction of wild-type receptor expression. In the next column, the constitutive activity of the mutant receptors is shown as percent basal signaling activity of the maximal ghrelin-stimulated activity (37). The potency (EC50) of wFw-Isn-NH2 with respect to stimulating inositol phosphate accumulation was determined in cells expressing either the wild-type or mutant form of the ghrelin receptor. Fmut indicates the -fold shift in potency induced by the structural change in the receptor compared with the wild-type receptor.
| Mutation | Ballesteros-Weinstein numbering | Ghrelin |
Constitutive activity |
wFw-Isn-NH2 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Expression level | n | %a | n | % Emax | EC50 | n | Fmut | ||
| nm | |||||||||
| WT | 1 | 3 | 46 ± 1 | 52 | 100 | 42 ± 5 | 21 | 1,0 | |
| AspII:20Asn | 2.60 | 0,62 ± 0,15 | 3 | 69 ± 2 | 8 | 57 ± 11 | 36 ± 5 | 4 | 0,9 |
| PheIII:04Ser | 3.28 | 1,20 ± 0,21 | 3 | 39 ± 2 | 21 | 77 ± 18 | 28 ± 5 | 4 | 0,7 |
| GlnIII:05Ala | 3.29 | 0,37 ± 0,11 | 3 | 49 ± 3 | 11 | 33 ± 9 | 26 ± 6 | 3 | 0,6 |
| SerIII:08Ala | 3.32 | 1,20 ± 0,08 | 3 | 36 ± 2 | 16 | 47 ± 10 | 100 ± 30 | 4 | 2,4 |
| GluIII:09Gln | 3.33 | 0,75 ± 0,18 | 3 | 44 ± 2 | 10 | 30 ± 2 | 54 ± 17 | 4 | 1,3 |
| ThrIII:12Ala | 3.36 | 0,73 ± 0,17 | 3 | 64 ± 5 | 4 | 110 ± 9 | 8,3 ± 3 | 5 | 0,2 |
| SerIV:16Ala | 4.56 | 0,78 ± 0,12 | 3 | 41 ± 3 | 10 | 72 ± 15 | 120 ± 10 | 3 | 2,9 |
| IleIV:20Ala | 4.60 | 1,00 ± 0,3 | 3 | 46 ± 2 | 10 | 72 ± 18 | 350 ± 13 | 4 | 8,3 |
| R198L | 0,94 ± 0,08 | 3 | 31 ± 3 | 7 | 190 ± 42 | 140 ± 17 | 3 | 3,3 | |
| E196Q | 0,99 ± 0,04 | 3 | 49 ± 2 | 8 | 130 ± 28 | 47 ± 14 | 4 | 1,1 | |
| MetV:05Ala | 5.36 | 1,30 ± 0,4 | 3 | 49 ± 2 | 13 | 81 ± 19 | 74 ± 16 | 4 | 1,8 |
| VaIV:08Ala | 5.42 | 0,53 ± 0,06 | 3 | 53 ± 1 | 11 | 51 ± 15 | 110 ± 10 | 3 | 2,6 |
| SerV:09Ala | 5.43 | 0,73 ± 0,12 | 3 | 41 ± 2 | 7 | 54 ± 12 | 83 ± 15 | 3 | 2,0 |
| PheV:12Ala | 5.46 | 0,68 ± 0,07 | 3 | 22 ± 2 | 12 | 60 ± 19 | 110 ± 20 | 3 | 2,6 |
| PheVI:16Ala | 6.51 | 0,72 ± 0,23 | 3 | 0 ± 1 | 13 | 53 ± 5 | 260 ± 50 | 4 | 6,2 |
| ArgVI20:Gln | 6.55 | 0,67 ± 0,26 | 3 | 20 ± 2 | 12 | 33 ± 15 | 110 ± 80 | 4 | 2,6 |
| SerVI:24Ala | 6.58 | 1,00 ± 0,20 | 3 | 45 ± 2 | 7 | 89 ± 26 | 62 ± 24 | 4 | 1,5 |
| PheVI:23Ala | 6.59 | 1,10 ± 0,20 | 3 | 50 ± 2 | 7 | NA | >1000 | 3 | >24 |
| GlnVII: −02Ala | 7.32 | 0,49 ± 0,06 | 3 | 43 ± 4 | 6 | 92 ± 30 | 62 ± 23 | 4 | 1,5 |
| AsnVII:02Ala | 7.35 | 1,10 ± 0,2 | 3 | 26 ± 2 | 14 | 67 ± 11 | 75 ± 28 | 3 | 1,8 |
| PheVII:06Leu | 7.39 | 1,20 ± 0,5 | 3 | 38 ± 3 | 8 | 87 ± 17 | 1000 ± 200 | 5 | 24 |
| PheVII:09Ala | 7.42 | 0,64 ± 0,14 | 3 | 24 ± 5 | 8 | 17 ± 4 | 91 ± 10 | 3 | 2,2 |
a 100% = maximal ghrelin stimulation of WT receptor.
SRE Reporter Assay
HEK-293 cells (30,000 cells/well) seeded in 96-well plates were transiently transfected with a mixture of SRE-Luc (PathDetect SRE Cis reporting system; Stratagene) and the indicated amounts of receptor DNA. After transfection, cells were maintained in low serum (2.5%) throughout the experiments and treated with the respective inhibitor of the intracellular signaling pathways. One day after transfection, cells were treated with the respective ligands in an assay volume of 100 μl of medium for 5 h. The assay was terminated by washing the cells twice with PBS and adding 100 μl of luciferase assay reagent (LucLite; PerkinElmer Life Sciences). Luminescence was measured in a TopCount NXT (PerkinElmer Life Sciences) microplate scintillation and luminescence counter for 5 s. Luminescence values are given as relative light units.
MAP Kinase Assay
COS-7 cells (seeding density, 150,000 cells/well) were transfected in the assay plates. Two days after transfection, the indicated concentrations of ligand were added to the assay medium without any serum and incubated for 10 min at 37 °C. The reactions were stopped by removal of the medium and two washing steps with ice-cold PBS. The cells were lysed in sample buffer and separated on SDS-10% PAGE according to the method of Laemmli (26). Proteins were transferred onto nitrocellulose, and Western blot analysis was carried out using a 1:5000 dilution of mouse monoclonal anti-phospho-ERK1/2 antibody (Santa Cruz Biotechnology). Total ERK protein was determined using a 1:10,000 dilution of anti-ERK antibody (Santa Cruz Biotechnology). Blots were probed using anti-mouse horseradish peroxidase-conjugated secondary antibodies, visualized using enhanced chemiluminescence reagent (Amersham Biosciences), and quantified by densitometric analysis. ERK1/2 phosphorylation was normalized according to the loading of protein by expressing the data as a ratio of phospho-ERK1/2 over total ERK1/2. Results were expressed as a percentage of the value obtained in nonstimulated, mock-transfected cells.
Competition Binding Assay
Transfected COS-7 cells were transferred to culture plates 1 day after transfection at a density of ∼5000 cells/well, aiming at 5–8% binding of the radioactive ligand. Two days after transfection competition binding experiments were performed for 3 h at 4 °C using ∼25 pm 35S-labeled MK-677 (provided by Andrew Howard, Merck). Binding assays were performed in 0.1 ml of a 50 mm HEPES buffer, pH 7.4, supplemented with 1 mm CaCl2, 5 mm MgCl2, 0.1% (w/v) bovine serum albumin, and 40 μg/ml bacitracin. Nonspecific binding was determined as the binding in the presence of 1 μm unlabeled ghrelin. Cells were washed twice in 0.1 ml of ice-cold buffer; 50 μl of lysis buffer/scintillation fluid (30% ethoxylated alkylphenol and 70% diisopropylnaphthalene isomers) was added, and the bound radioactivity was counted. Determinations were made in triplicate. Initial experiments showed that steady state binding was reached with the radioactive ligand under these conditions.
Internalization Study
Stably transfected HEK-293TR cells (Invitrogen) overexpressed the ghrelin receptor cDNA modified with an N-terminal SNAP tag (New England Biolabs) and under the control of a tetracycline-inducible promoter. Cells were seeded into poly-d-lysine-coated 96-well imaging plates (Greiner 655090; Greiner Bio-One, Gloucester, UK), and receptor expression was initiated by tetracycline treatment (100 ng/ml) for 18–21 h. Cell surface ghrelin receptors were first labeled with membrane-impermeant SNAP-Surface AF488 (0.1 μm in DMEM, New England Biolabs) for 30 min at 37 °C, washed, and treated with ligands at 37 °C in Hanks' balanced salt solution containing 0.1% BSA and 5 μg/ml Alexa Fluor 633-conjugated transferrin (Invitrogen). Incubations were terminated by fixation (3% paraformaldehyde), and cell nuclei were also labeled (H33342, 1 μg/ml in phosphate-buffered saline). Images at four sites/well were then acquired using the IX Ultra confocal plate reader (Molecular Devices, Sunnyvale, CA; 40× ELWD objective) with the appropriate excitation and emission filters for nuclei labeling (405 nm excitation), SNAP-Surface AF488-labeled ghrelin receptors (488 nm), and transferrin (633 nm).
Automated translocation analysis of plate reader images (MetaXpress 2.0, Molecular Devices) quantified the fluorescence intensity of labeled ghrelin receptors within 3-μm-diameter internal compartments identified by transferrin labeling, which the predominant destination of internalized ghrelin receptors. Individual concentration response curves performed in triplicate were normalized to vehicle (0%) and 1 μm ghrelin (100%) controls. Pooled data were used to obtain EC50 values with GraphPad Prism (sigmoidal fit, nH 0.9–1.0).
Rho Activation Assay
GTP-Rho and activated Rho of RC-4B/C cell lysates were assessed by a pulldown assay according to the manufacturer's description (catalog No. BK036, Cytoskeleton, Inc., Denver, CO). In short, cells were grown to 60–80% confluency and incubated in serum-free media. The cells were subjected to 10 min of stimulation by ghrelin, Isn-wFw-NH2, or solute. The cells were washed in PBS and lysed. After protein quantification, 500–800 μg of total protein in the lysates was precipitated by rhotekin beads. Precipitates were loaded onto a NuPAGE 10% bis-tris gel (Invitrogen), transferred to a PVDF membrane (Invitrogen) in a transfer buffer (40 mm glycine, 50 mm Trizma base, 1.3 mm SDS, and 20% ethanol (v/v)), blocked with TBST (150 mm NaCl, 50 mm Trizma base, and 0.1% Tween 20) supplemented with 5% BSA, and immunoblotted using anti-RhoA monoclonal antibody (Cytoskeleton, Inc., primary) and secondary antibody (goat anti-mouse IgG horseradish peroxidase-conjugated antibody, Thermo Scientific), both in TBST. The membranes were washed, and SuperSignal (Thermo Scientific) was added for visualization. The PVDF membranes were analyzed on a FluorChem HD2 (Alpha Innotech).
Food Intake Study
12 Sprague-Dawley rats (Taconic, Ejby, Denmark) were stereotaxically implanted with a stainless steel cannula (Holm Finmekanik AS, Copenhagen, Denmark) aimed at the right lateral ventricle (1 mm caudal, 1.5 mm lateral to the bregma, and 4 mm ventral to the cranium externa). The cannula and supporting bolts were secured with dental cement (Poly-F Plus, Dentsply). The animals were anesthetized with Hypnorm/Dormicum, 0.2 ml/kg body weight (fentanyl citrate, 0.07875 mg/ml; fluanisone, 2.5 mg/ml; and midazolam, 1.25 mg/ml). Pre- and post-surgery rats received analgesic treatment (Rimadyl (Pfizer), 5 mg/kg). Rats were handled daily during the recovery week and were housed in feeding cages for adaptation. After recovery, cannula placement was confirmed by measuring the drinking response to administration of angiotensin II (100 nmol/rat in 4 μl of saline; data not shown). Rats that showed a positive drinking response were used in the study. Injection with ghrelin, Isn-wFw-NH2, or vehicle (1% dimethyl sulfoxide and 0.09% saline) was done during the light phase, and food intake was subsequently measured in MANI FeedWin cases (Ellegaard Systems). The study was performed in a crossover fashion on separate days.
Conformational Analysis
Molecular dynamics simulations were performed by CHARM using the CHARM22 force field as described previously (22). Briefly, the system was minimized, heated to 310 K followed by equilibration, and simulated using Langevin dynamics for 0.1 μs. The molecular dynamic trajectory was analyzed, and structures of wFw-Isn-NH2 were clustered based on the backbone, phi and psi; and the side chain χ1 dihedral angle. The average cluster energy (kcal/mol), number of cluster members, and estimated probabilities were calculated.
Docking of the Ligand to the Ghrelin Receptor
A total set of 400 preliminary homology models including the extracellular loops of the human ghrelin receptor (Q92847) was generated using the homology modeling and ab initio structure prediction software suite Rosetta 3.1, which originally was developed to address the protein folding problem, i.e. to predict the three-dimensional shapes of proteins and focus on the design of protein structures, protein folding mechanisms, protein-protein interactions, and docking (27, 28). The models consisted of 4 × 100 models produced for each of the x-ray template structures, rhodopsin (PDB entry 1F88 (29)) and the b2-adrenergic (PDB entry 2RH1 (30)), b1-adrenergic (PDB entry 2VT4 (31)), and adenosine A2A receptors (PDB entry 3EML (32)). Pairwise sequence alignment between the human ghrelin receptor and the template structures was obtained using the biopolymer modules in SYBYL-X, and a position-specific matrix of alignment weights (class A profile) was used along the default substitution matrix to enforce alignment of conserved class A 7TM receptor sequence motifs and conserved generic fingerprints in the transmembrane helices. Manual adjustment was necessary to eliminate gaps in the TM regions. During the model construction, a disulfide bridge between Cys116 (CysIII:01) and Cys198 in the second extracellular loop, together with an imposed helical structure of Arg199-Gly208 in extracellular loop IIb, was applied as a structural constraint. Otherwise, loops were modeled ab initio. Secondly, a set of 60 representative ghrelin receptor models (composed of 15 models for each template) was selected based on energy and structural diversity. Finally, fully flexible ligand docking to each of the 60 receptor models was performed by an ICM-biased probability Monte Carlo docking routine under softened van der Waals conditions using four-dimensional grids represented by six grid potentials of 0.5 Å spacing, including three van der Waals grid potentials for a carbon probe, large atom probe, or hydrogen probe, a hydrogen bonding grid potential, an electrostatic grid potential, and a hydrophobic grid potential ICM (33). The docking grids were defined to encompass a binding pocket described by all corresponding receptor residues within 4.5 Å of the ligands in the template crystal structure of bovine rhodopsin and the β2-adrenergic, β1-adrenergic, and adenosine A2A receptors when superimposed onto the stack of generated ghrelin receptor models. The final docking grid was extended ∼8 Å toward TMs I and II to allow the longer ligands to occupy and interact with minor subpockets located between TMs II, III, and VII.
Individual best scored docking poses were subsequently optimized using a combined Monte Carlo and minimization procedure (using the MMFF94 force field), keeping the ligand and surrounding protein residues (in an 8 Å radius from the starting position) flexible. All backbone coordinates were held fixed. Two rounds of optimization were performed: an initial refinement under a softened van der Waals potential and a second refinement with the full van der Waals potential. A final stack of 50 conformations was generated, which were scored and analyzed manually to identify the complexes between the wFw ligands and the ghrelin receptor in agreement with the experimental data.
RESULTS
C-terminal Modification of the d-Trp-Phe-d-Trp Motif
In previous studies we identified the C-terminal carboxyamidated pentapeptide, d-Trp-Phe-d-Trp-Leu-Leu-CONH2 (wFwLL) to be the active core of the prototype ghrelin receptor inverse agonist, [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P (21). This pentapeptide displayed a characteristic molecular pharmacological phenotype by being a partial agonist at low concentrations and a partial inverse agonist at higher concentrations (Fig. 1). Conformational analysis revealed that the N-terminal wFw peptide favored two closely related, constrained, L-shaped, low energy conformations populated with 34 and 30% of the population, respectively (22). In contrast, the two C-terminal leucine residues were highly flexible (22), and in the structure-activity relation analysis performed on the full-length [d-Arg1,d-Phe5,d-Trp7,9,Leu11] substance P inverse agonist, the Leu-Leu dipeptide appeared to function mainly as a linker between the d-Trp-Phe-d-Trp motif and the C-terminal functionally important carboxyamide moiety (21). Accordingly, in the present study we replaced the C-terminal Leu-Leu dipeptide of the wFwLL pentapeptide with various non-natural amino acid linkers or spacers of variable length and flexibility (Table 1). Nine compounds that contain all of the N-terminal d-Trp-Phe-d-Trp motif but different peptide-mimetic spacers at the C terminus were characterized in COS-7 cells transiently transfected with the ghrelin receptor using both signal transduction assays to measure inositol triphosphate accumulation and competition binding assays against the 3H-labeled non-peptide agonist MK-677 (Table 1 and Fig. 2).
FIGURE 2.
Signaling properties of wFw-based peptide mimetic. Effect of the partial agonists wFw-Isn-NH2 and wFw-4Abz- NH2 on ghrelin receptor Gαq-mediated signaling measured as inositol phosphate accumulation. The dose-response curve of ghrelin (open squares) is compared with that of wFw-4Abz-NH2 (triangles in A) and wFw-Isn-NH2 (triangles in B) (n = 5–15). All experiments were performed in duplicates.
C-terminal Aliphatic Spacers
In the peptide backbone “spacer” of the wFwLL there are five bonds (Fig. 1C; the bonds are marked with numbers) between the α-nitrogen of the N-terminal Leu residue and the α-carbon of the carboxyamide group. In the first three compounds, the dileucinyl motif has been replaced by an aliphatic CH2 carbon chain of three, four, and six bonds, respectively, using β-alanine, γ-amino butyric acid, or amino hexanoic acid initially coupled to the Rink amide resin to yield the respective C-terminal amides (Table 1). As opposed to the original wFwLL pentapeptide amide, these three wFw peptide mimetics were all agonists with relatively low potencies and efficacies only 20–30% of that of ghrelin; but they did not display any inverse agonist properties. Thus, the agonist potency was 3700 nm for the shortest peptide (wFw-βAla-NH2) but improved stepwise to 1450 nm (wFw-Abu-NH2) and 756 nm (wFw-Ahx-NH2) by extension of the spacer with one or three bonds, respectively (Table 1).
Ring-constrained C-terminal Spacers
Six wFw analogues were synthesized in which the spacer to the C-terminal carboxyamide group was constrained by an aromatic or a non-aromatic ring system. Like the peptide-mimetic compounds with aliphatic spacers, all of these ring-constrained compounds were pure agonists but with highly improved potency and in most cases also improved efficacy as compared with the peptide mimetics with aliphatic spacers (Table 1). Among the ring-constrained derivatives, wFw-Aep-NH2, which has a linker consisting of eight bonds including a piperazine ring, showed the lowest efficacy (34%) and potency (83 nm).
wFw-Acp-NH2 contains 4-amino-2-cyclopentene-1-carboxylic acid as a four-bond spacer, similar to the γ-amino butyric acid in wFw-Abu-NH2; however, the cyclopentene constraint increased the potency from an EC50 value of 1450 nm for wFw-Abu-NH2 to 29 nm for wFw-Acp-NH2 and increased the agonist efficacy from 21 to 41% of that of ghrelin (Table 1). Constraining the two or three middle bonds of a four- or five-bond linker by a benzene ring resulted in peptide mimetics (wFw-3Amb- NH2, wFw-4Abz- NH2, and wFw-3Abz-NH2) displaying rather similar potencies between 36 and 52 nm, i.e. similar to that of wFw-Acp-NH2 but with efficacies between 51 and 63% of that of ghrelin (Table 1). The dose-response curve of wFw-3Abz-NH2 is shown in Fig. 2A. However, almost full efficacy, i.e. 79% of ghrelin, was obtained by building the three N-terminal bonds of a four-bond linker into a piperidine ring as shown in the structure of wFw-Isn-NH2, which had a potency of 42 nm (Fig. 2B and Table 1). None of the wFw derivatives was able to compete well against the non-peptide agonist 3H-MK-677, and the apparent affinity as judged from the competition binding experiment was in most cases 15–40-fold lower than the potency observed in functional analysis of inositol accumulation (Table 1).
Conformational Analysis of the Piperidine-constrained wFw-Isn-NH2
Molecular dynamic analysis of wFw-Isn-NH2 revealed that, as expected, it is highly conformationally constrained. As shown previously for the wFwLL pentapeptide, ∼65% of the low energy structures of the wFw part of the molecule populated only two almost equally large clusters, which is very unusual for such a small peptide (22). Importantly, in contrast to the highly flexible Leu-Leu sequence of the original pentapeptide, the piperidine spacer of wFw-Isn-NH2 could adopt only a few conformations as determined by its simple ring structure (Fig. 3). Thus, the piperidine-constrained wFw peptide mimetic preferentially adopts an L-shaped low energy conformation in which the side chains of the characteristic, aromatic wFw sequence all radiate away from the convex long arm of the L, whereas the C-terminal carboxyamide moiety is positioned at the end of the short arm of the L (Fig. 3). We concluded that potent and efficacious wFw peptide-mimetic agonists for the ghrelin receptor can be generated through insertion of a short, conformationally constraining non-peptide spacer between the wFw motif and the C-terminal carboxyamide moiety.
FIGURE 3.
Conformation of the most highly potent and efficacious wFw-based peptide mimetic. Shown are random conformations from the most populated cluster of conformations of the wFw-Ins-NH2 peptide mimetic sampled after molecular dynamics simulations (aligned according to the backbone of the wFw sequence).
Biased Signaling Property of the Piperidine-constrained wFw-Isn-NH2
It has been demonstrated previously that the ghrelin receptor in addition to classical Gαq coupling, as measured for example by inositol phosphate accumulation, also signals through other pathways and that the endogenous agonist ghrelin and a number of prototype non-peptide and short peptide agonists all act as unbiased agonists (34). Thus, all of the previously described ghrelin receptor agonists stimulate signaling through the different pathways relatively similarly, albeit with slightly different potencies (34). To determine whether this was also the case for the new class of ring-constrained wFw peptide-mimetic agonists, we tested the ability of wFw-Isn-NH2 to stimulate ghrelin receptor signaling through the ERK1/2 and SRE pathways.
As shown in Fig. 4A, wFw-Isn-NH2 was a potent partial agonist in respect to stimulating ERK1/2 phosphorylation, which is the only ghrelin receptor-induced signaling pathway that does not reveal high constitutive activity (35). Interestingly, the potency of wFw-Isn-NH2 in stimulating ERK1/2 phosphorylation was ∼10-fold higher than the potency observed in inositol accumulation assays, with the EC50 value being 3.9 and 40 nm, respectively (Figs. 2B and 4A). The novel peptide mimetic wFw-Ins-NH2 was also able to induce internalization with a potency (540 nm) only 22-fold lower than that observed for ghrelin (24 nm) (Fig. 4B). The internalization experiment was performed in an inducible HEK cell line transiently overexpressing the SNAP-tagged ghrelin receptor, which resulted in higher cell surface expression than observed previously (35) while retaining a level of basal internalization driven by constitutive receptor activity. Administration of the agonists further increased the ghrelin receptor endocytosis (supplemental Fig. 1).
FIGURE 4.
Biased signaling properties of wFw-Isn-NH2. A, dose-response curve for ghrelin (empty triangles) and wFw-Isn-NH2 (filled triangles) in ERK1/2 phosphorylation as measured by Western blot experiments. Representative Western blots for three independent experiments are shown below for each treatment. Data are shown as the mean ± S.E. of three independent experiments performed. B, dose-response curves for ghrelin (empty circles)-induced and wFw-Isn-NH2 (filled circles)-induced ghrelin receptor internalization measured as average ghrelin receptor label intensity in transferrin-labeled compartments. The data from 5–16 independent experiments are merged and normalized in comparison with vehicle controls. C, dose-response curve for ghrelin (empty squares)-induced and wFw-Isn-NH2 (filled squares)-induced SRE-induced transcriptional activity measured as luciferase activity in gene reporter assay. Data are shown as the mean ± S.E. of 3–5 independent experiments performed in quadruplicates for the SRE reporter assay and in duplicates for the ERK phosphorylation assay.
In contrast, wFw-Isn-NH2 was completely unable to stimulate SRE-mediated transcriptional activity, a signal transduction pathway through which the ghrelin receptor reveals its strong constitutive activity and in which ghrelin is a potent (1.1 nm) and highly efficacious agonist (Fig. 4C). In principle, SRE activation can occur through either the Gαi or the Gα12/13 pathway. As shown in Fig. 5, neither the high basal level of SRE signaling nor the ghrelin-induced SRE signaling was affected by treatment with pertussis toxin (Fig. 5A), indicating that for the ghrelin receptor, Gαi is not involved in this pathway, which is the case for example for chemokine receptors (36). Another well described pathway responsible for SRE activation is Rho kinase activation mediated by Gα12/13 activation (37). As shown in Fig. 5B, treatment with the specific inhibitor of Rho kinase, Y27632, strongly decreased both constitutive and ghrelin-induced SRE activity. In addition the dominant negative mutant of Gα13 (DN-G13) suppressed ghrelin-induced SRE signaling to the same level as observed for the RhoA kinase inhibitor (Fig. 5C).
FIGURE 5.
Delineation of G-protein coupling responsible for SRE-mediated transcriptional activity. A, effect of pertussis toxin (100 ng/ml) on SRE-induced transcription: empty circles, vehicle-treated; filled circles, pertussis toxin-treated. B, effect of Rho kinase inhibitor Y27632 (20 nm) on SRE-induced transcription: empty squares, vehicle-treated; filled squares, Y27632. Data are shown as mean ± S.E. of 4–6 independent experiments performed in quadruplicates. C, effect of dominant negative mutant of the Gα13 subunit DN-G13 (2 ng of plasmid) on SRE-induced transcription: empty triangles, control plasmid; filled triangles, DN-G13. Data are shown as mean ± S.E. of 3–5 independent experiments performed in quadruplicates.
In the rat pituitary adenoma cell line RC-4B/C, which endogenously expresses ghrelin receptors (38), the signaling properties of wFw-Isn-NH2 were studied to verify the physiological relevance of the biased signaling. In this cell line the potency for ghrelin was 3.6 nm, and as observed in the heterologous expression system, the potency of wFw-Isn-NH2 was 100-fold lower (400 nm). The efficacy of wFw-Isn-NH2 was surprisingly good in this cell line, reaching almost 100% (Fig. 6A). To study coupling to Gα13, we measured the level of the GTP-bound active form of RhoA (39). The concentration of ghrelin (10 nm) that induced maximal inositol phosphate accumulation in this cell line increased the GTP-bound RhoA level 3-fold above basal level (Fig. 6B, gray column). In contrast, wFw-Isn-NH2 (1000 nm) did not increase GTP-bound RhoA (Fig. 6B, black column).
FIGURE 6.
Data from RC-4B/C cells that endogenously express the Ghrelin receptor. A, inositol phosphate accumulation of RC-4B/C cells treated with ghrelin (filled squares) and wFw-Isn-NH2 (filled triangles) is shown. Data are shown as mean ± S.E. of three independent experiments performed in duplicates. 100% equals the maximal ghrelin-induced stimulation, and 0 is defined as the level of constitutive activity. B, RhoA activation assay data from Western blots of RC-4B/C that were analyzed for the intensity of the bands after treatment with vehicle (white column), wFw-Isn-NH2 (1000 nm; black column), and ghrelin (10 nm; gray column). Data are shown as mean ± S.E. of three independent experiments. Directly below the graph is a representative Western blot (upper gel) of the active GTP-bound RhoA form when RC-4B/C cells were treated with ghrelin (rightmost band), wFw-Isn-NH2 (middle band), and vehicle (leftmost band). Lower gel, shown as a control, the total RhoA of each lysate used in the upper gel. C, effect of ghrelin and wFw-ISN-NH2 on feeding. Rats where injected intracerebroventricularly in the light phase with 10 nmol of wFw-ISN-NH2, 0.1 nmol of ghrelin, or vehicle (1% dimethyl sulfoxide in saline) (n = 11). Data are shown as mean ± S.E. Statistical analysis was done by one-way analysis of variance followed by Tukey's multiple comparison test; *, p ≤ 0.05.
To study the in vivo physiological relevance of the biased signaling property of wFw-Isn-NH2, we compared its ability to stimulate food intake after intracerebroventricular administration in rats (40). As expected, the accumulated food intake after 30 min was increased by ∼3-fold in rats treated with 0.1 nmol of ghrelin, whereas rats treated with a 100-fold higher dose of wFw-Isn-NH2 (10 nmol) showed no increase in food intake (Fig. 6C).
It is concluded that piperidine-constrained wFw-Isn-NH2 is a functionally biased ghrelin receptor agonist that activates classical Gαq pathways, internalization, and ERK1/2 phosphorylation; but in contrast to all previously reported ghrelin receptor agonists, it is unable to stimulate SRE-mediated transcriptional activity, which conceivably is mediated through the Gα13 pathway. The biased signaling of wFw-Isn-NH2 is also observed in cell lines naturally expressing the ghrelin receptor, suggesting a physiological relevance and that it is not an artifact due to receptor expression levels. The fact that neither ghrelin nor wFw-Isn-NH2 is dependent on the receptor expression level is illustrated in supplemental Fig. 2.
Interaction of the Piperidine-constrained wFw-Isn-NH2 Agonist with the Receptor
Single amino acid substitutions at 22 positions, located in and above the supposed main ligand-binding pockets between TMs II, III, IV, V, VI, and VII in the ghrelin receptor, were used to map the binding sites of wFw-Isn-NH2 (Table 2 and Fig. 7A). The substitutions were selected from a library of mutants based on their properties in both introducing significant structural change and being expressed at a reasonable level at the cell surface compared with the wild-type receptor, as published previously (41). Thus, as judged by cell surface ELISA, the expression level of the mutants was between 0.37- and 1.3-fold of the expression level of the wild-type receptor (Table 2). The mutants were transiently transfected into COS-7 cells, and the signaling property of the agonist was evaluated by full dose-response curves of stimulation of inositol phosphate accumulation. For most of the mutants, the high constitutive signaling activity of the ghrelin receptor was preserved, which provides another certification of the functionality of the mutant receptors (Table 2). Although mutants of PheVI:16 and ArgVI:20 displayed low constitutive activity, they were included in the analysis because these positions often are ligand interaction sites and are important for the function of other ghrelin receptor agonists (41).
FIGURE 7.
Substitutions in the ghrelin receptor that affect the potency of wFw-Isn-NH2. A, helical wheel of the ghrelin receptor where residues that affect the potency of the partial agonist wFw-Isn-NH2 are indicated with the following color code: red, >100-fold; orange, 5–100-fold; yellow, 2–5-fold. Residues that are substituted and tested but do not affect the signaling properties are indicated in white on black. B—E, graphs depicting the dose-response curves for wFw-Isn-NH2 on the ghrelin receptor (empty circles) compared with four substitutions (filled triangles): Phe(VI:23)Ala (B), Phe(VII:06)Leu (C), Phe(VII:09)Ala (D), and Thr(III:12)Ala (E). Data are given as mean ± S.E. of 3–24 independent experiments performed in duplicates.
Receptor Interaction Sites Shared with wFw Peptide Ligands
Based on mutational analysis of N-terminally extended wFw peptide agonists (AwFwLL) and inverse agonists (fQwFwLL and K-wFwLL), it has been proposed previously that the aromatic wFw motif interacts with a central aromatic cluster at the interface of TM VI and TM VII of the receptor. The wFw-Isn-NH2 was also highly dependent on this aromatic cluster, as the Ala substitution of PheVI:23 basically eliminated its action, and mutation of PheVII:06 shifted its dose-response curve to the far right (Fig. 7, B and C, and Table 2). Interestingly, Ala substitution of the deeply located PheVII:09, which is very important for the constitutive activity of the receptor (35), affected the efficacy more than the potency of wFw-Isn-NH2 (Fig. 7C). Substitution of PheVI:16 was also a clear hit for wFw-Isn-NH2, although it shifted its potency only 6.2-fold to the right (Table 2).
Substitution of ThrIII:12 with an alanine, which basically removes the side chain, improved the potency of wFw-Isn-NH2 5-fold (Fig. 7E). Such improved potency could either be because of a specific interaction that is improved by the substitution or be interpreted as an indirect effect due to a generally improved propensity to obtain the active conformation. The latter theory is supported by the fact that this mutation showed increased constitutive activity, although the surface expression was slightly decreased (Table 2).
Residues Important for Ghrelin Receptor Agonists in General but Not for wFw-Isn-NH2
Mutations of residues located in the main ligand-binding pocket of the ghrelin receptor at the faces of TMs III, IV, and V (i.e. “opposite” from the aromatic cluster on TMs VI and VII) can be divided into two classes: (a) those that are classical “hits” that destroy binding and action of the ligands, e.g. GluIII:09 and GlnIII:05; and (b) those that swap the efficacy of the wFw peptide ligands (21, 22).
Previously described agonists for the ghrelin receptor (peptides and non-peptide ligands) are all strongly dependent on GluIII:09 as an anchor point (21, 41). For example, the potency of ghrelin itself is decreased 250-fold, and the prototype small-molecule agonist MK-677 is affected more than 10.000-fold by substitution at this position (Fig. 8, A and B) (21, 41). GluIII:09 is thought to play a similar role for agonist ligands in the ghrelin receptor as the neighboring classical AspIII:08 does for ligands in general in the monoamine receptor system (42). However, as shown in Fig. 8, highly surprisingly, the wFw-Isn-NH2 compound was not dependent upon GluIII:09 as opposed to ghrelin tested in parallel. GlnIII:05, located one helical turn above GluIII:09, is also an important interaction site for the previously described peptide agonists and for most non-peptide agonists of the ghrelin receptor. Also in this case, wFw-Isn-NH2 was not affected by the Ala substitution of GlnIII:05 (Table 2).
FIGURE 8.
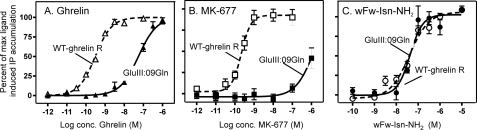
Residues important for ghrelin receptor agonists in general but not for wFw-Isn-NH2. Shown are dose-response curves of ghrelin (A), MK-677 (B), and wFw-Isn-NH2 (C) on wild-type ghrelin receptor (empty symbols) and GluIII:09Gln substitution (filled symbols). Data are expressed as mean ± S.E. of 5–20 independent experiments performed in duplicates.
“Efficacy Swapping” Residues
The efficacy of N-terminally extended wFw peptides such as A-wFwLL (agonist) and K-wFwLL (inverse agonist) can be swapped to the opposite type without affecting the potency by mutations, especially PheIII:04, SerIII:08, and IleIV:20 (22). This was not the case for wFw-Isn-NH2. Only minimal changes in potency were observed upon mutation of PheIII:04 and SerIII:08, and Ala substitution of IleIV:20 even impaired the potency of the piperidine-constrained wFw peptide agonist 8.3-fold (Table 2).
Similarly, mutations of ValV:08 and PheV:12, which from TM V point toward TM IV, have previously been demonstrated to improve the potency of the originally N-terminally extended wFw inverse agonist ligand [d-Arg1,d-Phe5,d-Trp7,9, Leu11] substance P (21) and to severely abolish the function of the N-terminally extended agonist AwKwLL (22). However, these mutations had only minor effects on wFw-Isn-NH2 (Table 2).
Thus the mutational analysis for the novel, biased, peptide-mimetic agonist wFw-Isn-NH2 indicates that although the aromatic cluster between TMs VI and VII is essential for its action, it is surprisingly, and in contrast to all previously described ghrelin receptor agonists, not dependent upon key anchor point residues in TM III. Also, mutations that previously have been shown to swap the efficacy of N-terminally extended wFw peptide agonists to inverse agonism do not affect this wFw peptidomimetic agonist.
Receptor Modeling and Docking of the Biased Agonist wFw-Isn-NH2
A multi-conformational docking setup was employed in which different conformations of the ghrelin receptor were generated by a large set of comparative homology models based on not only different template structures but also different packing of side chains and different loop conformations. In brief, initially a total set of 400 preliminary models of the ghrelin receptor was generated with Rosetta-based homology modeling and ab initio structure prediction, employing as templates each of the four x-ray structures, bovine rhodopsin (29) and the β2-adrenergic (30), β1-adrenergic (31), and adenosine A2A receptors (32). A disulfide bridge between Cys116 (CysIII:01) and Cys198 in the second extracellular loop, together with an imposed helical structure, Arg199-Gly208, in extracellular loop IIb, was applied as the structural constraint. Sixty representative receptor models (15 from each template) were selected based on energy and structural diversity considerations, and fully flexible docking of the wFw-Isn-NH2 ligand was performed for each of the 60 receptor models. The individual best scored docking poses were subsequently optimized using a combined Monte Carlo and minimization procedure, keeping the ligand and surrounding receptor side chains flexible. A final stack of 50 conformations was generated, each of which were manually analyzed.
The resulting top scoring docking poses revealed two main clusters of very different or opposite docking modes in which the L-shaped low energy conformation of the piperidine-constrained wFw-Isn-NH2 ligand fitted nicely into the complementary binding pocket of the ghrelin receptor. In one mode, the long arm of the “L” corresponding to the extended N-terminal wFw sequence extends down into the binding pocket to make aromatic hydrophobic interactions with the aromatic cluster of residues located at the interface of TMs VI and VII (Fig. 9, A and B). In particular, the indole side chain of the N-terminal d-Trp is positioned in a lower aromatic pocket, where it makes aromatic-aromatic edge-to-face and aromatic-aromatic stacking interactions with PheVI:16, PheVII:06, and PheVII:09 as well as hydrophobic interactions with LeuII:21, whereas the Phe side chain of the wFw motif interacts with PheVI:23. In this mode the backbone amides are positioned to make potential hydrogen bond interactions with ArgVI:20 and GlnIII:05, and with Glu201 in ECL-2b, which all point into the binding pocket. In this binding mode, which we call “C-out,” the short arm of the L, i.e. the piperidine moiety, extends along with ECL-2b to position the C-terminal carboxyamide moiety to make hydrogen bond interactions with Arg198 in the loop (Fig. 9, A and C). Importantly, the anchor point for all other agonists, GluIII:09, is not in direct contact with the wFw-Isn-NH2 ligand in this binding mode.
FIGURE 9.
Preferred binding mode for the functionally biased agonist wFw-Isn-NH2 in the main ligand-binding pocket of the ghrelin receptor compared with the wFwLL compound. Rosetta-based molecular modeling and docking was performed as described under “Experimental Procedures.” A, the preferred C-out docking mode for the L-shaped wFw-Isn-NH2 ligand (in green) in the main ligand-binding pocket between TMs III, VI, and VII of the ghrelin receptor as viewed from the side (from TM VII). B, the same docking mode as in A but viewed from TM IV. C, the same docking mode as in A but viewed from the extracellular space. Note the interaction of the important C-terminal carboxyamide group, especially with Arg198 in ECL-2b; the aromatic-aromatic interaction of the wFw motif with the aromatic cluster, for example PheVII:06, PheVI:16, and PheVI:23; and the lack of interaction with GluIII:09 in this docking mode. D and E, the C-in docking mode of the structurally closely related and also L-shaped wFwLL ligand in the same binding pocket between TMs III, VI, and VII. Note that the ligand docks in the opposite mode with the C-terminal carboxyamide closely interacting with GluIII:09, the free N-terminal NH2 group at the receptor surface leaving free space for various N-terminal extensions, and the wFw aromatic motif interacting with the aromatic cluster between TMs VI and VII but in a different mode than the wFw-Isn-NH2 ligand.
In the other, “C-in” binding mode, the ligand is basically turned around, and the C-terminal piperidine with the carboxyamide moiety is instead located deep in the binding pocket where the C-terminal carboxyamide moiety makes hydrogen bond interactions with GluIII:09 and ThrIII:12 (this binding mode is shown for the original wFwLL-NH2 core peptide in Fig. 9, D and E). However, in this binding mode especially the second d-Trp of the wFw motif of the ligand makes good aromatic-aromatic edge-to-face and aromatic-aromatic stacking as well as hydrophobic interactions with the cluster of aromatic residues in TMs VI and VII of the receptor, i.e. PheVI:16, PheVII:06, PheVII:09, and LeuII:21, whereas the central phenylalanine makes aromatic interactions with PheVI:23. In addition, the indole nitrogen of the second d-Trp is involved in a hydrogen bond network with SerIII:08 and TyrVII:10. In the C-in binding mode the backbone amides are also in the correct position to make potential hydrogen bond interactions with ArgVI:20, GlnIII:05, and Glu201, in ECL-2b, just as they are in the C-out binding mode.
As indicated schematically previously (Ref. 22, Fig. 4 therein), we believe that this C-in pose, with the N terminus pointing outward, is the preferred binding mode, for example for N-terminally extended wFw ligands, which have been shown to be crucially dependent upon the presence of GluIII:09. This binding mode also allows various N-terminal extensions to interact with residues at the extracellular face of the receptor, especially in the region of the extracellular end of TM III and the loops around it. Although wFw-Isn-NH2 according to the computational chemical analysis can bind in a C-in binding mode (data not shown), the mutational analysis clearly demonstrates that this piperidine-constrained wFw peptide-mimetic compound, in contrast to all other previously characterized ghrelin receptor agonists, is not dependent upon GluIII:09 (see above). This indicates that the C-in binding mode is not the preferred mode for this compound. Thus we propose that the piperidine-constrained wFw-Isn-NH2 binds in a C-out binding mode, as indicated in Fig. 9, A–C.
DISCUSSION
In the present study we have generated a series of novel peptide-mimetic agonists for the ghrelin receptor based on the conformationally constrained wFw scaffold derived from the active core of the prototype inverse agonist [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P. Surprisingly, the most efficacious of these novel wFw peptide mimetics was found to be a biased agonist that mediates potent and efficacious signaling through Gαq internalization and the ERK1/2 signaling pathway but not through the SRE (conceivably the Gα12/13 pathway), i.e. in contrast to all previous reports for both peptide and non-peptide ghrelin receptor agonists. Importantly, the molecular interaction pattern of this wFw peptide mimetic also differed significantly from that of all previously characterized ghrelin receptor agonists, for example in not being dependent upon the major agonist anchor point in TM III, GluIII:09. Interestingly, the preliminary in vivo data suggest that Gα12/13 coupling is required to increase the acute food intake.
Novel wFw Peptide-mimetic Ghrelin Receptor Agonists
In previous studies (21), we delineated the essential core of the prototype 11-amino acid inverse agonist peptide [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P as the C-terminally carboxyamidated wFwLL pentapeptide (Fig. 1). In itself, the wFwLL core peptide displays a characteristic bell-shaped dose-response curve, indicating the potential to both activate and inhibit the function of the ghrelin receptor (21) (Fig. 1). Accordingly, N-terminal extension of the wFwLL pentapeptide with a single, positively charged residue, for example a lysine residue, rescued the pure inverse agonist function observed for the full-length inverse agonist peptide (22). Conversely, when the pentapeptide instead was extended N-terminally with a small nonpolar residue such as an alanine, the resulting A-wFwLL peptide behaved as an equally potent partial agonist (22). The notion that the wFw peptides are balanced between agonism and inverse agonism was substantiated by the finding that the efficacy of the N-terminally extended wFw peptides could be swapped from agonism to inverse agonism, and the other way around, by a series of mutations located at key positions in the ligand-binding pocket of the ghrelin receptor (22).
In the present study we exchanged the Leu-Leu sequence of the core wFwLL peptide with a linker or spacer to connect the important C-terminal carboxyamide group with the wFw motif. In all cases the resulting wFw-based peptide mimetics behaved like pure agonists, as observed previously with the glycine-substituted peptide wFwGG (22). Double digit nanomolar potency was obtained for the wFw peptide mimetics by use of ring-constrained spacer groups, of which a piperidine-constrained spacer also provided close to full (79%) agonist efficacy (wFw-Isn-NH2).
Unique Receptor Recognition of wFw Peptide-mimetic Agonists
The mutational map for the piperidine-modified wFw-Isn-NH2 on the ghrelin receptor was found to be surprisingly different from that observed with all previously characterized agonists, including the N-terminally extended wFw peptide agonists. The interaction site for ghrelin itself is restricted to a narrow part in the middle of the binding crevice between TMs III, VI, and VII (21) as often observed for 7TM agonists (43). Small-molecule ghrelin receptor agonists, both non-peptides and classical oligopeptides such as GHRP-6, also interact with this central part of the binding pocket (30). However, in addition, these non-ghrelin agonists are dependent upon different residues located throughout the entire binding pocket (30). Importantly, all previously characterized ghrelin receptor agonists are crucially dependent upon GluIII:09 (Glu124) as a key, supposedly charge-charge anchor point in TM III, which is located next to the classical AspIII:08 anchor site for monoamine ligands in their receptors. This interaction with GluIII:09 was described very early for MK-677 and later also for ghrelin (21, 32) just as the corresponding GluIII:09 (Glu119) in the closely related motilin receptor has been shown to be a key charge-charge interaction point for small-molecule agonists such as erythromycin (33).
It was therefore highly surprising that mutation of GluIII:09 did not affect the novel wFw peptide-mimetic agonist wFw-Isn-NH2 and that it also was not dependent upon GlnIII:05, which is another commonly used interaction site for ghrelin receptor agonists in TM III (30). However, on the opposing face of the main ligand-binding pocket, wFw-Isn-NH2 joins all of the previously characterized ghrelin receptor agonists in being highly dependent upon different members of the aromatic cluster located between TMs VI and VII, i.e. PheVI:16, PheVI:23, PheVII:06, and PheVII:09 (41). This aromatic cluster, which also is very important for the high constitutive activity of the ghrelin receptor, has previously been suggested to be the binding site for the characteristic aromatic wFw motif of this class of peptide ligands (21, 22). The most likely explanation would be that the wFw motif of the peptide-mimetic agonists also interacts with the aromatic cluster between TMs VI and VII but that it does so in a significantly different way, making the rest of the molecule independent upon interaction with GluIII:09 and other parts of TM III.
Two Potential Binding Modes for the wFw Peptides and Peptide Mimetics
Molecular modeling and docking experiments indicate that the wFw-based peptide mimetics, as well as peptides, can bind in two different, opposite orientations in the main ligand-binding pocket between TMs III, VI, and VII (Fig. 9). Both of these binding modes display excellent structural complementarities between ligand and receptor, and both are based on close interdigitations of the ligand with the aromatic cluster between TMs VI and VII. In one mode the C-terminal carboxyamide is buried deep in the pocket to interact with GluIII:09 (C-in), and in the other this group interacts with an Arg residue (Arg198) in extracellular loop 2b (C-out). It is suggested that the wFw peptide agonists, such as AwFwLL, bind in the C-in mode to interact with GluIII:09, in analogy with small-molecule agonists such as MK-677, SM-157,740, and L-692,429 and peptide agonists such as GHRP-6 and ghrelin itself (41) (shown for wFwLL in Fig. 9, D and E).
In contrast, it is suggested that wFw peptide-mimetic agonists such as wFw-Isn-NH2 bind in the C-out mode, where they do not interact with GluIII:09 (Fig. 9, A–C). It should be noted that there are several examples in which x-ray structures have revealed that a small change in the structure of a ligand can make it adopt a surprisingly different overall binding mode (44). It is generally assumed that a given ligand has only one binding mode to its biological target molecule; however, certain chemotypes such as the wFw-based peptides and peptide mimetics may be able to exploit more than one high affinity binding mode. This could be the basis for the phenomenon that wFwLL is both a high potency agonist and a slightly lower potency inverse agonist (Fig. 1) (21), that certain substitutions in the binding pocket of the receptor can turn the full inverse agonist KwFwLL into a combined agonist/inverse agonist just like wFwLL (21), and that other mutations are able to swap its efficacy to an equally high potency, pure agonist (22).
The wFw Peptide Mimetic Is a Biased Agonist
Biased signaling, the fact that some ligands may selectively affect certain signaling pathways as opposed to other pathways mediated by a given receptor, has attracted much attention recently (15, 16, 19). However, when a series of prototype peptide and non-peptide ghrelin receptor agonists were tested in parallel for their ability to activate various signal transduction pathways, they were all found to act as rather unbiased agonists (34). Thus, although some differences were observed in respect to efficacy and potency, each of these agonists appeared to activate the different signaling pathways of the ghrelin receptor rather similarly. That was not the case for the wFw peptide mimetic in the present study, as demonstrated for wFw-Isn-NH2, which behaved as a potent and efficacious agonist in respect to stimulation of inositol phosphate turnover, internalization, and ERK1/2 kinase activation but was unable to activate SRE-mediated transcriptional activity (Fig. 4). This selective or biased agonist property is in sharp contrast to the structurally rather similar, N-terminally extended wFw peptide agonist, A-wFwLL, which acts as a potent, full agonist also in respect to stimulation of the SRE pathway (22).
Inositol phosphate turnover is a classical Gαq-activated mechanism, whereas ERK1/2 phosphorylation is known to be induced through many different pathways, i.e. both through most G-protein pathways and through G-protein-independent pathways, of which β-arrestin-mediated ERK1/2 activation is the most well described mechanism (15, 19). It has been suggested that ghrelin-induced ERK1/2 phosphorylation involves phospholipase C and PKCϵ activation (45) but that it is independent of internalization and β-arrestin (46). The fact that the pharmacological profiles for ghrelin and wFw-Isn-NH2 are very similar in the signaling pathways analyzed in the present study would suggest that the ERK1/2 activation results from Gαq coupling. SRE activation can also occur through different pathways, such as Gαi and Rho kinase via Gα12/13 (37). However, the lack of effect of pertussis toxin, combined with the observation that almost all constitutive activity induced by the ghrelin receptor and more than 50% of the ligand-induced activity was eliminated by the addition of a specific inhibitor of Rho kinase and by a dominant negative mutant of Gα13, indicates that this signaling is mediated through Gα13 activation (37). Importantly, biased signaling was also observed in a cell line naturally expressing the ghrelin receptor. Thus, it is concluded that wFw-Isn-NH2, the piperidine-constrained wFw peptide mimetic, acts as a biased agonist for the ghrelin receptor, which selectively activates Gαq but is unable to activate Gα13-related pathways.
Interestingly, biased ligands have been reported previously for the ghrelin receptor, not for agonists but for inverse agonists. Thus, the N-terminally extended wFw peptide compound KwFwLL, which is a potent and efficacious inverse agonist in respect to inhibition of the high basal inositol phosphate turnover, does not affect SRE signaling, not even at micromolar concentrations (22). Importantly, the high spontaneous SRE activity mediated by the ghrelin receptor can potently and efficiently be inhibited by other ligands, for example the structurally similar prototype ghrelin receptor inverse agonist [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P. Thus, KwFwLL is a biased inverse agonist of the ghrelin receptor that selectively inhibits constitutive Gaq signaling but not the constitutive SRE signaling conceivably mediated through Gα12/13 (22).
Structural Basis for Biased Agonism
Notably, the wFw peptide mimetic wFw-Isn-NH2 differs from all previously characterized ghrelin receptor agonists, both in respect to being a biased agonist and in respect to not being dependent upon GluIII:09, i.e. having a different binding mode than all of the non-biased agonists. It is suggested that wFw-Isn-NH2 stabilizes an active receptor conformation that is different from the conformation stabilized by the classical non-biased ghrelin receptor agonists. Presumably the conformation stabilized by wFw-Isn-NH2 couples efficiently to Gαq but is unable to couple to the pathway responsible for signaling through SRE-induced transcriptional activity, presumably Gα12/13 (37).
The existence of multiple active conformations has been described previously for other 7TM receptors, where agonists that favor one signaling pathway over another have been characterized (15, 19, 47). The conformational diversity has been visualized by use of bioluminescence resonance energy transfer assay in combination with ligands of different functional profiles (48–50). For example, multiple different conformational rearrangements have been demonstrated between the probes inserted in the δ-opioid receptor and those inserted in the G-protein, dependent on the properties of the applied ligand (49). This phenomenon has been studies most intensively in receptors that couple to both a G-protein-dependent pathway and a G-protein-independent pathway such as MAP kinase activation (15, 19). In some cases it has been shown that the same ligand behaves as an agonist in one signaling pathway and as an inverse agonist in another pathway (51, 52).
Nevertheless, the molecular mechanism underlying the biased agonism is far from clear, although mutations that selectively decouple one of the possible signaling pathways have been described. Thus, in the NK1 receptor, mutation of PheIII:07 (Phe111) selectively decouples the receptor from Gs without affecting Gαq signaling (18). For the angiotensin receptor it has been shown that Ala substitution of ProII:18 in the AT1 receptor completely impairs angiotensin II signaling through Gαq without affecting high affinity binding of the peptide agonist and the ability of angiotensin II to stimulate ERK phosphorylation (53). Recently, a thorough study of the M2 receptor revealed that mutations in the orthosteric binding pocket selectively abolished signaling through ERK phosphorylation, whereas a mutation in the allosteric binding pocket had the opposite effect and increased the efficacy in this specific pathway (54).
Physiological Importance of Biased Agonists
In the GPR109A system a biased agonist, MK-0354, has been developed that selectively signals through Gαi. MK-0354 has a beneficial nicotinic acid-like effect on serum lipids but, importantly, does not affect the β-arrestin pathway causing the side effect of flushing (17, 55). For the ghrelin receptor, development of a biased agonist could have important therapeutic potential, as the receptor is responsible for both growth hormone secretion and induction of hunger and fat accumulation. One of the first therapeutic indications for ghrelin receptor agonists was to increase growth hormone secretion in patients with impaired healing of bone fractures (11). This group of patients, in most cases, does not benefit from an increase in fat mass, and a functionally biased agonist would be preferable.
The opposite scenario is also possible, i.e. the development of a biased antagonist or inverse agonists, which could decrease appetite and fat accumulation without affecting growth hormone secretion. In the present study we described that it is feasible to develop biased ligands for the ghrelin receptor that are functionally biased for only a limited selection of the possible signaling pathways. However we cannot conclude that Gα13 is required for appetite modulation, but it is suggested that the biased signaling properties of ghrelin receptor ligands should be considered in drug discovery processes. It is possible that compounds that only modulate Gαq signaling are not sufficient to modulate appetite and energy expenditure. Consideration of Gα13 coupling properties may be required in terms of both agonist and antagonist development. In addition we have demonstrated significant differences in the receptor interaction of these biased ligands as opposed to the unbiased ligand, which may guide future drug development of biased ligands for the ghrelin receptor.
Supplementary Material
This study was supported by grants from the Danish Medical Research Council, the NovoNordisk Foundation, the Lundbeck Foundation, the Alfred Benzon Foundation, and the German Science Foundation for Financial Support (KFO 152). This work was carried out as part of the “Food, Fitness & Pharma for Health and Disease” research program of the UNIK project, supported by the Danish Ministry of Science, Technology, and Innovation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- 7TM
- seven-transmembrane
- TM
- transmembrane
- SRE
- serum response element
- Abu
- γ-amino butyric acid
- Abz
- aminobenzoic acid
- Acp
- (1R,4S)-(+)-4-amino-2-cyclopentene-1-carboxylic acid
- Aep
- 4-(2-aminoethyl)piperazine-1-ylacetic acid
- Amb
- aminomethylbenzoic acid
- Isn
- isonipecotic acid
- Ahx
- aminohexanoic acid
- ECL-2b
- extracellular loop IIb
- Fmoc
- N-(9-fluorenyl)methoxycarbonyl
- bis-tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- PDB
- Protein Data Bank.
REFERENCES
- 1. Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. (1999) Nature 402, 656–660 [DOI] [PubMed] [Google Scholar]
- 2. Kirchner H., Gutierrez J. A., Solenberg P. J., Pfluger P. T., Czyzyk T. A., Willency J. A., Schürmann A., Joost H. G., Jandacek R. J., Hale J. E., Heiman M. L., Tschöp M. H. (2009) Nat. Med. 15, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang J., Zhao T. J., Goldstein J. L., Brown M. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10750–10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kojima M., Hosoda H., Matsuo H., Kangawa K. (2001) Trends Endocrinol. Metab. 12, 118–122 [DOI] [PubMed] [Google Scholar]
- 5. Tschöp M., Smiley D. L., Heiman M. L. (2000) Nature 407, 908–913 [DOI] [PubMed] [Google Scholar]
- 6. Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., Matsukura S. (2001) Nature 409, 194–198 [DOI] [PubMed] [Google Scholar]
- 7. López M., Lage R., Saha A. K., Pérez-Tilve D., Vázquez M. J., Varela L., Sangiao-Alvarellos S., Tovar S., Raghay K., Rodríguez-Cuenca S., Deoliveira R. M., Castañeda T., Datta R., Dong J. Z., Culler M., Sleeman M. W., Alvarez C. V., Gallego R., Lelliott C. J., Carling D., Tschöp M. H., Diéguez C., Vidal-Puig A. (2008) Cell Metab. 7, 389–399 [DOI] [PubMed] [Google Scholar]
- 8. Egecioglu E., Jerlhag E., Salome N., Skibicka K. P., Haage D., Bohlooly Y., Andersson D., Bjursell M., Perrissoud D., Engel J. A., Dickson S. L. (2010) Addict. Biol. 15, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jerlhag E., Egecioglu E., Landgren S., Salomé N., Heilig M., Moechars D., Datta R., Perrissoud D., Dickson S. L., Engel J. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 11318–11323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diano S., Farr S. A., Benoit S. C., McNay E. C., da Silva I., Horvath B., Gaskin F. S., Nonaka N., Jaeger L. B., Banks W. A., Morley J. E., Pinto S., Sherwin R. S., Xu L., Yamada K. A., Sleeman M. W., Tschöp M. H., Horvath T. L. (2006) Nat. Neurosci. 9, 381–388 [DOI] [PubMed] [Google Scholar]
- 11. Murphy M. G., Weiss S., McClung M., Schnitzer T., Cerchio K., Connor J., Krupa D., Gertz B. J. (2001) J. Clin. Endocrinol. Metab. 86, 1116–1125 [DOI] [PubMed] [Google Scholar]
- 12. Bach M. A., Rockwood K., Zetterberg C., Thamsborg G., Hébert R., Devogelaer J. P., Christiansen J. S., Rizzoli R., Ochsner J. L., Beisaw N., Gluck O., Yu L., Schwab T., Farrington J., Taylor A. M., Ng J., Fuh V. (2004) J. Am. Geriatr. Soc. 52, 516–523 [DOI] [PubMed] [Google Scholar]
- 13. Lundholm K., Gunnebo L., Körner U., Iresjö B. M., Engström C., Hyltander A., Smedh U., Bosaeus I. (2010) Cancer 116, 2044–2052 [DOI] [PubMed] [Google Scholar]
- 14. Holst B., Schwartz T. W. (2004) Trends Pharmacol. Sci. 25, 113–117 [DOI] [PubMed] [Google Scholar]
- 15. Galandrin S., Oligny-Longpré G., Bouvier M. (2007) Trends Pharmacol. Sci. 28, 423–430 [DOI] [PubMed] [Google Scholar]
- 16. Michel M. C., Alewijnse A. E. (2007) Mol. Pharmacol. 72, 1097–1099 [DOI] [PubMed] [Google Scholar]
- 17. Richman J. G., Kanemitsu-Parks M., Gaidarov I., Cameron J. S., Griffin P., Zheng H., Guerra N. C., Cham L., Maciejewski-Lenoir D., Behan D. P., Boatman D., Chen R., Skinner P., Ornelas P., Waters M. G., Wright S. D., Semple G., Connolly D. T. (2007) J. Biol. Chem. 282, 18028–18036 [DOI] [PubMed] [Google Scholar]
- 18. Holst B., Hastrup H., Raffetseder U., Martini L., Schwartz T. W. (2001) J. Biol. Chem. 276, 19793–19799 [DOI] [PubMed] [Google Scholar]
- 19. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Nat. Rev. Drug. Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holst B., Cygankiewicz A., Jensen T. H., Ankersen M., Schwartz T. W. (2003) Mol. Endocrinol. 17, 2201–2210 [DOI] [PubMed] [Google Scholar]
- 21. Holst B., Lang M., Brandt E., Bach A., Howard A., Frimurer T. M., Beck-Sickinger A., Schwartz T. W. (2006) Mol. Pharmacol. 70, 936–946 [DOI] [PubMed] [Google Scholar]
- 22. Holst B., Mokrosinski J., Lang M., Brandt E., Nygaard R., Frimurer T. M., Beck-Sickinger A. G., Schwartz T. W. (2007) J. Biol. Chem. 282, 15799–15811 [DOI] [PubMed] [Google Scholar]
- 23. Lang M., Söll R. M., Dürrenberger F., Dautzenberg F. M., Beck-Sickinger A. G. (2004) J. Med. Chem. 47, 1153–1160 [DOI] [PubMed] [Google Scholar]
- 24. Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. (1970) Anal. Biochem. 34, 595–598 [DOI] [PubMed] [Google Scholar]
- 25. Holst B., Zoffmann S., Elling C. E., Hjorth S. A., Schwartz T. W. (1998) Mol. Pharmacol. 53, 166–175 [DOI] [PubMed] [Google Scholar]
- 26. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 27. Barth P., Wallner B., Baker D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1409–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yarov-Yarovoy V., Schonbrun J., Baker D. (2006) Proteins 62, 1010–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 30. Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., Kobilka B. K. (2007) Science 318, 1266–1273 [DOI] [PubMed] [Google Scholar]
- 31. Warne T., Serrano-Vega M. J., Baker J. G., Moukhametzianov R., Edwards P. C., Henderson R., Leslie A. G., Tate C. G., Schertler G. F. (2008) Nature 454, 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaakola V. P., Griffith M. T., Hanson M. A., Cherezov V., Chien E. Y., Lane J. R., Ijzerman A. P., Stevens R. C. (2008) Science 322, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Totrov M., Abagyan R. (2008) Curr. Opin. Struct. Biol. 18, 178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holst B., Brandt E., Bach A., Heding A., Schwartz T. W. (2005) Mol. Endocrinol. 19, 2400–2411 [DOI] [PubMed] [Google Scholar]
- 35. Holst B., Holliday N. D., Bach A., Elling C. E., Cox H. M., Schwartz T. W. (2004) J. Biol. Chem. 279, 53806–53817 [DOI] [PubMed] [Google Scholar]
- 36. Rosenkilde M. M., Benned-Jensen T., Andersen H., Holst P. J., Kledal T. N., Lüttichau H. R., Larsen J. K., Christensen J. P., Schwartz T. W. (2006) J. Biol. Chem. 281, 13199–13208 [DOI] [PubMed] [Google Scholar]
- 37. Riobo N. A., Manning D. R. (2005) Trends Pharmacol. Sci. 26, 146–154 [DOI] [PubMed] [Google Scholar]
- 38. Falls H. D., Dayton B. D., Fry D. G., Ogiela C. A., Schaefer V. G., Brodjian S., Reilly R. M., Collins C. A., Kaszubska W. (2006) J. Mol. Endocrinol. 37, 51–62 [DOI] [PubMed] [Google Scholar]
- 39. Fukuhara S., Murga C., Zohar M., Igishi T., Gutkind J. S. (1999) J. Biol. Chem. 274, 5868–5879 [DOI] [PubMed] [Google Scholar]
- 40. Petersen P. S., Woldbye D. P., Madsen A. N., Egerod K. L., Jin C., Lang M., Rasmussen M., Beck-Sickinger A. G., Holst B. (2009) Endocrinology 150, 4920–4930 [DOI] [PubMed] [Google Scholar]
- 41. Holst B., Frimurer T. M., Mokrosinski J., Halkjaer T., Cullberg K. B., Underwood C. R., Schwartz T. W. (2009) Mol. Pharmacol. 75, 44–59 [DOI] [PubMed] [Google Scholar]
- 42. Strader C. D., Sigal I. S., Dixon R. A. (1989) FASEB J. 3, 1825–1832 [DOI] [PubMed] [Google Scholar]
- 43. Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 481–519 [DOI] [PubMed] [Google Scholar]
- 44. Mattos C., Rasmussen B., Ding X., Petsko G. A., Ringe D. (1995) Struct. Biol. 1, 55–58 [DOI] [PubMed] [Google Scholar]
- 45. Mousseaux D., Le Gallic L., Ryan J., Oiry C., Gagne D., Fehrentz J. A., Galleyrand J. C., Martinez J. (2006) Br. J. Pharmacol. 148, 350–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chu K. M., Chow K. B., Leung P. K., Lau P. N., Chan C. B., Cheng C. H., Wise H. (2007) Int. J. Biochem. Cell Biol. 39, 752–764 [DOI] [PubMed] [Google Scholar]
- 47. Zhang L., Brass L. F., Manning D. R. (2009) Mol. Pharmacol. 75, 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galandrin S., Oligny-Longpré G., Bonin H., Ogawa K., Galés C., Bouvier M. (2008) Mol. Pharmacol. 74, 162–172 [DOI] [PubMed] [Google Scholar]
- 49. Audet N., Galés C., Archer-Lahlou E., Vallières M., Schiller P. W., Bouvier M., Pineyro G. (2008) J. Biol. Chem. 283, 15078–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shukla A. K., Violin J. D., Whalen E. J., Gesty-Palmer D., Shenoy S. K., Lefkowitz R. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9988–9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gbahou F., Rouleau A., Morisset S., Parmentier R., Crochet S., Lin J. S., Ligneau X., Tardivel-Lacombe J., Stark H., Schunack W., Ganellin C. R., Schwartz J. C., Arrang J. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Azzi M., Charest P. G., Angers S., Rousseau G., Kohout T., Bouvier M., Piñeyro G. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reis R. I., Santos E. L., Pesquero J. B., Oliveira L., Schanstra J. P., Bascands J. L., Pecher C., Paiva A. C., Costa-Neto C. M. (2007) Regul. Pept. 140, 32–36 [DOI] [PubMed] [Google Scholar]
- 54. Gregory K. J., Hall N. E., Tobin A. B., Sexton P. M., Christopoulos A. (2010) J. Biol. Chem. 285, 7459–7474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walters R. W., Shukla A. K., Kovacs J. J., Violin J. D., DeWire S. M., Lam C. M., Chen J. R., Muehlbauer M. J., Whalen E. J., Lefkowitz R. J. (2009) J. Clin. Investig. 119, 1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.