Abstract
RH-RhoGEFs are a family of guanine nucleotide exchange factors that contain a regulator of G protein signaling homology (RH) domain. The heterotrimeric G protein Gα13 stimulates the guanine nucleotide exchange factor (GEF) activity of RH-RhoGEFs, leading to activation of RhoA. The mechanism by which Gα13 stimulates the GEF activity of RH-RhoGEFs, such as p115RhoGEF, has not yet been fully elucidated. Here, specific residues in Gα13 that mediate activation of p115RhoGEF are identified. Mutation of these residues significantly impairs binding of Gα13 to p115RhoGEF as well as stimulation of GEF activity. These data suggest that the exchange activity of p115RhoGEF is stimulated allosterically by Gα13 and not through its interaction with a secondary binding site. A crystal structure of Gα13 bound to the RH domain of p115RhoGEF is also presented, which differs from a previously crystallized complex with a Gα13-Gαi1 chimera. Taken together, these data provide new insight into the mechanism by which p115RhoGEF is activated by Gα13.
Keywords: Crystal Structure, G Proteins, Protein Conformation, Rho, Signal Transduction, Guanine Nucleotide Exchange Factor, RGS Domain
Introduction
Heterotrimeric guanine nucleotide binding proteins (G proteins), composed of α, β, and γ subunits, act as molecular switches that cycle between an inactive, GDP-bound state and an active, GTP-bound state upon stimulation of G protein-coupled receptors (1). Once activated, the GTP-bound Gα subunit dissociates from the Gβγ dimer, both of which regulate the activity of multiple intracellular effectors to elicit cellular responses. The duration of signaling mediated by Gα is dictated by the lifetime of bound GTP. All Gα subunits have intrinsic GTPase activity that hydrolyzes GTP to GDP, and this rate of hydrolysis is enhanced by GAPs,2 such as RGS proteins (2), which bind the switch regions of activated Gα subunits, stabilize the transition state for GTP hydrolysis, and thus accelerate the hydrolysis of GTP to GDP (3, 4). Once in the GDP-bound state, Gα subunits reassociate with Gβγ and are capable of initiating another round of signaling.
Gα13 is one of two members of the G12 family of G proteins (5) and has been implicated in regulating multiple cellular processes that depend on activation of the monomeric GTPase RhoA, including gene transcription, embryogenesis, and rearrangement of the actin cytoskeleton (6–8). A direct link between Gα13 and RhoA was established with the discovery of p115RhoGEF, the founding member of a family of RhoA-specific GEFs containing an RH domain in their N termini (RH-RhoGEFs) (9). Together with p115RhoGEF, PDZ-RhoGEF and LARG constitute the RH-RhoGEF family in mammals (10, 11). The RH domain of p115RhoGEF has low sequence similarity to canonical RGS proteins but nevertheless functions as a GAP for Gα13 (12). Although the structure of the RH domain of p115RhoGEF is similar to that of other RGS proteins (13), additional elements flanking the RGS box are required for GAP activity (14) and stability of the isolated domain (15). In addition to its RH domain, p115RhoGEF also contains the tandem DH/PH domains characteristic of Dbl family RhoGEFs. The nucleotide exchange activity of p115RhoGEF can be directly stimulated by Gα13 in vitro (9), and Gα13 acts synergistically with p115RhoGEF to activate Rho-dependent signaling in cells (16). Thus, p115RhoGEF functions as both an effector and a GAP for Gα13. Although the relationship between p115RhoGEF and Gα13 has been well established, the precise mechanism by which Gα13 regulates the activity of p115RhoGEF remains to be fully elucidated.
The molecular mechanisms regulating p115RhoGEF activity are likely to be complex and may involve multiple intermolecular interfaces with Gα13. It has been clearly demonstrated that Gα13 binds directly to the RH domain of p115RhoGEF (12, 15). Furthermore, this domain is required for both basal and Gα13-stimulated nucleotide exchange activity, suggesting that it plays a critical role in the activation mechanism (15). A structure of the RH domain of p115RhoGEF bound to an AlF4−-activated Gα13-Gαi1 chimera has been solved by x-ray crystallography and revealed that the α subunit engages this domain through two distinct interfaces (17). In addition to interacting with switch regions I and II of Gα13/i1 through an N-terminal extension of the RGS box, the RH domain also docks into the hydrophobic groove between the α2 and α3 helices of Gα13/i1, a highly conserved effector interface among other Gα-effector pairs (18–21). However, many of the residues in the α3 helix of this chimera are derived from Gαi1, and its ability to activate p115RhoGEF has not been clearly demonstrated. Thus, the role of residues in the α3 helix in regulating p115RhoGEF activity is unknown. Gα13 has also been reported to bind to the isolated DH/PH domains of p115RhoGEF in vitro (15); however, it has no capacity to directly stimulate the GEF activity of this fragment. Therefore, the role of additional binding sites outside of the RH domain is also unclear.
Here, specific residues in Gα13 that mediate activation of p115RhoGEF are identified. We demonstrate that mutation of these residues, which bind to the RH domain of p115RhoGEF, significantly impairs binding of Gα13 to p115RhoGEF as well as stimulation of GEF activity in cells and in vitro. Our results suggest that the initial step in stimulation of exchange activity by Gα13 involves allosteric regulation through interaction with the RH domain of p115RhoGEF. We also present a crystal structure of Gα13 bound to the RH domain of p115RhoGEF, which differs in several respects from the previously crystallized complex using the Gα13/i1 chimera (17) and reinforces the results of our biochemical analysis. This structure provides a more accurate picture of the Gα13-p115RhoGEF RH interface, whereas the biochemical data provide new insight into the mechanism by which p115RhoGEF is activated by Gα13.
EXPERIMENTAL PROCEDURES
Generation of Constructs
Gα13 Mutants
Single point mutations were introduced into the mammalian expression vector pCMV5 harboring murine Gα13 Q226L for use in SRE-luciferase assays or the baculovirus transfer vector pFastBac HTa (Invitrogen) encoding Gαi/13 for protein expression using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. The T274E/N278A double mutant was generated using either pCMV5-Gα13 Q226L T274E or pFastBac HTa-Gαi/13 T274E as the template. Sense and antisense primer pairs used to introduce each mutation were as follows: N270A, 5′-CGCCTTACAGAATCTCTGGCCATTTTTGAAACAATTG and 5′-CAATTGTTTCAAAAATGGCCAGAGATTCTGTAAGGCG; T274E, 5′-CTGAACATTTTTGAAGAGATTGTCAACAATCGGGTTTTCAGC and 5′-GCTGAAAACCCGATTGTTGACAATCTCTTCAAAAATGTTCAG; N278A, 5′-GAAACAATTGTCAACGCTCGGGTTTTCAGCAACG and 5′-CGTTGCTGAAAACCCGAGCGTTGACAATTGTTTC; T274E/N278A, 5′-GAAGAGATTGTCAACGCTCGGGTTTTCAGCAACG and 5′-CGTTGCTGAAAACCCGAGCGTTGACAATCTCTTC. Mutated bases are underlined. Introduction of the desired mutation was verified by automated dideoxy sequencing of each construct.
Full-length Gα13
Full-length Gα13 (Gα13 FL) was generated by first introducing a silent mutation into pCMV5-Gα13 by QuikChange site-directed mutagenesis that eliminates an internal HindIII site using the primers 5′-GCCCGAGAGAAGCTCCATATTCCCTGGGG and 5′-CCCCAGGGAATATGGAGCTTCTCTCGGGC. Mutated bases are underlined. KpnI and HindIII sites were then introduced at the 5′- and 3′-ends, respectively, of Gα13 by PCR using pCMV5-Gα13 lacking the internal HindIII site as the template and the primers 5′-GGTACCGCGGACTTCCTGCCGTC and 5′-GACCAAGCTTTCACTGCAGCATGAGCTG. The PCR products were digested with KpnI and HindIII and ligated into the pFastBac HT(−) vector containing the N-terminal α1 helix of Gαi1 such that the construct contains, from the N terminus, a His6 tag, residues 1–28 of Gαi1, a TEV protease site, and residues 2–377 of Gα13.
p115RhoGEFΔC
Tyr763 of p115RhoGEF was mutated to a stop codon using QuikChange site-directed mutagenesis using pFastBac HTc-p115RhoGEF as the template and the primers 5′-CACTGAGACTGCCGGATAACTGAAAGTCCCTGCCC and 5′-GGGCAGGGACTTTCAGTTATCCGGCAGTCTCAGTG. Mutated bases are underlined.
Protein Purification
His6-Gαi/13 (Wild Type and Mutant)
His6-Gαi/13 and His6-Gαi/13 harboring the N270A, T274E, N278A, or T274E/N278A mutation(s) were purified from the soluble fraction of Sf9 cells as described previously (22). The His6 tag was removed for crystallography by incubating His6-Gαi/13 with 2% (w/w) His6-TEV protease overnight at 4 °C. Uncut His6-Gαi/13 and His6-TEV protease were removed using a HisTrap HP column (GE Healthcare Life Sciences). Gαi/13 was subjected to gel filtration chromatography on a Superdex 200 10/300 size exclusion column (GE Healthcare) and eluted in buffer containing 20 mm HEPES, pH 8.0, 100 mm NaCl, 10 mm 2-mercaptoethanol, 1 mm MgCl2, 10 μm GDP, and 10% glycerol. The protein was aliquoted, snap-frozen in liquid nitrogen, and stored at −80 °C until use.
Full-length Gα13
His6-Gαi/13 FL was purified from Sf9 cells in the same fashion as His6-Gαi/13. After elution from nickel-NTA resin (Qiagen), fractions containing His6-Gαi/13 FL were pooled and incubated with 2% (w/w) His6-TEV protease overnight at 4 °C to remove the His6 tag and α1 helix of Gαi1. The protein was concentrated and buffer-exchanged to imidazole-free buffer using an Amicon Ultra 30k centrifugal filter device (Millipore Corp.). The protein was aliquoted, snap-frozen in liquid nitrogen, and stored at −80 °C until use.
p115RhoGEF
Sf9 cells expressing His6-p115RhoGEF were resuspended in lysis buffer (20 mm HEPES, pH 8.0, 100 mm NaCl, 10 mm imidazole, 10 mm 2-mercaptoethanol, 16 μg/ml TPCK, TLCK, and PMSF, and 3.2 μg/ml leupeptin and lima bean trypsin inhibitor) and lysed by nitrogen cavitation. The lysate was clarified by centrifugation at 100,000 × g for 30 min at 4 °C. The soluble portion of the lysate was diluted 2-fold with lysis buffer and applied to a nickel-NTA column equilibrated with lysis buffer. The column was washed with 20 bed volumes of wash buffer (20 mm HEPES, pH 8.0, 400 mm NaCl, 20 mm imidazole, 10 mm 2-mercaptoethanol, 16 μg/ml TPCK, TLCK, and PMSF, and 3.2 μg/ml leupeptin and lima bean trypsin inhibitor), and bound protein was eluted from the column using elution buffer (20 mm HEPES, pH 8.0, 400 mm NaCl, 300 mm imidazole, 10 mm 2-mercaptoethanol, 16 μg/ml TPCK, TLCK, and PMSF, and 3.2 μg/ml leupeptin and lima bean trypsin inhibitor). Peak fractions containing His6-p115RhoGEF were pooled and dialyzed overnight at 4 °C against dialysis buffer (20 mm HEPES, pH 8.0, 400 mm NaCl, 10 mm 2-mercaptoethanol) in the presence of 2% (w/w) His6-TEV protease. The solution was supplemented with 10% glycerol and applied to a HisTrap HP column to remove remaining His6-p115RhoGEF and His6-TEV protease. Flow-through fractions were collected and applied to a HiPrep 16/60 Sephacryl S-300 HR column (GE Healthcare) equilibrated in gel filtration buffer (20 mm HEPES, pH 8.0, 150 mm NaCl, 10 mm 2-mercaptoethanol, 10% glycerol). Fractions containing p115RhoGEF were pooled and subjected to further purification on a Superdex 200 HR 10/30 size exclusion column (GE Healthcare) equilibrated in gel filtration buffer. Fractions containing p115RhoGEF were pooled and concentrated using an Amicon Ultra 30k centrifugal filter device. The protein was aliquoted, snap-frozen in liquid nitrogen, and stored at −80 °C until use.
p115RhoGEFΔC
His6-p115RhoGEFΔC(1–762) was purified from Sf9 cells in the same fashion as full-length p115RhoGEF except that the final round of size exclusion chromatography utilized a HiLoad Superdex 200 16/60 size exclusion column (GE Healthcare), and the protein was eluted in buffer containing 20 mm HEPES, pH 8.0, 100 mm NaCl, 1 mm EDTA, 10% glycerol, and 2 mm DTT. The protein was aliquoted, snap-frozen in liquid nitrogen, and stored at −80 °C until use.
GST-p115RhoGEF RH Domain
The GST-tagged RH domain of p115RhoGEF was purified from Escherichia coli as described previously (22).
RhoA
GST-RhoA lacking the C-terminal CAAX motif (RhoA(1–181)) was expressed from the plasmid pGEX-6P (GE Healthcare) in the E. coli strain BL21-CodonPlus (DE3)-RP (Stratagene). Cells were grown at 30 °C to an A600 of 0.6–0.8, and protein expression was induced with 200 μm isopropyl β-d-1-thiogalactopyranoside for 6 h. Cells were resuspended in lysis buffer (50 mm HEPES, pH 7.5, 1 mm EDTA, 1 mm DTT, 200 mm NaCl, 5 mm MgCl2, 10 μm GDP, 10% glycerol) and lysed with lysozyme and sonication. The lysate was clarified by centrifugation at 100,000 × g for 30 min at 4 °C. The soluble portion of the lysate was applied to a column of glutathione-Sepharose 4B beads (GE Healthcare) equilibrated with lysis buffer. The column was washed with 10 bed volumes of lysis buffer, followed by 20 bed volumes of cleavage buffer (50 mm Tris, pH 7.5, 1 mm EDTA, 1 mm DTT, 5 mm MgCl2, 10 μm GDP). On-column cleavage of the GST tag was achieved using PreScission protease according to the manufacturer's protocol (GE Healthcare). Cleaved RhoA was eluted from the column using lysis buffer. Fractions containing RhoA were pooled and concentrated using an Amicon Ultra 15k centrifugal filter device. The protein was aliquoted, snap-frozen in liquid nitrogen, and stored at −80 °C until use. To form nucleotide-free RhoA for crystallography, the protein was incubated with an excess of EDTA on ice.
Biochemical Experiments
SRE-luciferase Assays
Assays were performed as described (23), with the following modification. Each well was co-transfected with 200 ng of pGL3-SRE.L, 100 ng of pCMV5-LacZ, and 10 ng of empty pCMV5 vector or the indicated Gα13 construct using Lipofectamine2000 (Invitrogen). For immunoblotting of cell lysates, samples were harvested directly into SDS-PAGE sample buffer and boiled. Cell lysate was separated by SDS-PAGE, and Gα13 was detected by Western blotting with the B860 antibody (24). GAPDH was detected using a monoclonal antibody (clone 6C5, Ambion).
In Vitro Trypsin Protection Assays
3 μg of Gαi/13 protein (wild type or mutant) was diluted into reaction buffer (20 mm HEPES, pH 8.0, 5 mm MgCl2, 100 μm GDP) and incubated on ice for 45 min in the presence or absence of AlF4− (10 mm NaF, 30 μm AlCl3). Trypsin was added to a final concentration of 13.3% (w/w), and the samples were incubated at 30 °C for 15 min. The reaction was terminated by the addition of SDS-PAGE sample buffer and boiling.
GST Pull-down Assays
Assays were preformed as described previously (22), with the exception that bound proteins were released from the beads by the addition of SDS-PAGE sample buffer and boiling.
Single Turnover GAP Assays
GTPase assays were performed as described previously (12), except that reactions were incubated on ice. [γ-32P]GTP (specific activity = 6,000 Ci/mmol) was obtained from PerkinElmer Life Sciences.
Gel Filtration of Full-length p115RhoGEF with His6-Gαi/13 or His6-Gαi/13 T274E/N278A
2.8 nmol of full-length p115RhoGEF were incubated with 5.6 nmol of His6-Gαi/13 (wild type or mutant) for 30 min on ice in gel filtration buffer (20 mm HEPES, pH 8.0, 1 mm EDTA, 2 mm DTT, 150 mm NaCl, 5 mm MgCl2, 10 μm GDP) in the presence or absence of AlF4− (10 mm NaF, 20 μm AlCl3). The total volume of each sample was 200 μl. The proteins were fractionated at 4 °C on a Superdex 200 HR 10/30 size exclusion column equilibrated in the same buffer as the sample.
In Vitro RhoGEF Assays
Assays were performed as described previously (25). [35S]GTPγS (specific activity = 1,250 Ci/mmol) was obtained from PerkinElmer Life Sciences.
Crystallization and Data Collection
Crystallization
To form the Gαi/13-p115ΔC-RhoA complex, Gαi/13 was activated with 8× AlF4− (80 mm NaF, 160 μm AlCl3) on ice for 15 min and mixed with an equimolar amount of p115RhoGEFΔC and nucleotide-free RhoA. The complex was subjected to size exclusion chromatography on a HiLoad Superdex 200 10/16 gel filtration column, and fractions containing the stoichiometric complex were pooled and concentrated using an Amicon4 10k centrifugal filter device. Screening of the Gαi/13-p115RhoGEFΔC-RhoA complex was carried out using vapor diffusion at 20 °C. Diffraction quality crystals of the Gαi/13-p115RhoGEF RH domain complex were grown against a reservoir solution containing 15% PEG 4000 and 0.1 m HEPES (pH 7.0). Single crystals were coated with the reservoir solution containing 20% PEG 400 as a cryoprotectant, mounted using a nylon loop, and flash-cooled in the cold stream of the goniometer.
Data Collection
Diffraction data were collected at 100 K with a wavelength of 1.0 Å at SPring-8 beam line BL41XU (Harima, Japan). Data were processed with the HKL2000 program (26). The structure of the Gαi/13-p115RhoGEF RH domain complex was determined by molecular replacement with the program MOLREP (CCP4), using the RH domain of p115RhoGEF (Protein Data Bank entry 1IAP, chain A) and Gαi/13 (Protein Data Bank entry 1ZCB) as search models. The model was corrected iteratively using O (27), and structure refinement was carried out using CNS (Crystallography and NMR System) (28). The final refinement statistics are shown in Table 1. The quality of the model was inspected by the program PROCHECK in the CCP4 suite (29). Structural similarities were calculated with DALI (30). Solvent-accessible surface area was calculated with the program AREALMOL in the CCP4 suite (29). Graphic figures were created with the program PyMOL (31).
TABLE 1.
Crystallography statistics
All numbers in parentheses refer to the highest resolution shell statistics.
| Parameters | Values |
|---|---|
| Data collection | |
| Space group | P1 |
| Unit cell parameters | a = 50.5 Å, b = 70.6Å, c = 88.1 Å, α = 77.8°, β = 84.5°, γ = 80.1° |
| Wavelength (Å) | 1.0 |
| Resolution range (Å) | 50–2.4 |
| Redundancy | 1.7 |
| Unique reflections | 37,777 |
| Completeness (%) | 81.8 (82.1) |
| I/σ(I) | 9.0 (2.3) |
| Rsyma | 0.074 (0.296) |
| Refinement | |
| Resolution range (Å) | 48.88–2.40 (2.49–2.40) |
| No. of reflections | 37,316 |
| R-Factor/Free R-factorb | 0.205/0.280 |
| No. of protein atoms | 8,091 |
| No. of magnesium ion atoms | 2 |
| No. of ligand atoms | 66 |
| No. of water molecules | 87 |
| Root mean square deviations | |
| Bond lengths (Å) | 0.009 |
| Bond angles (degrees) | 1.4 |
| Average B-value (Å2) | 51.6 |
a Rsym = Σ|Iavg − Ii|/ΣIi, where Ii is the observed intensity and Iavg is the average intensity.
b Free R-factor is calculated for 10% of randomly selected reflections excluded from refinement.
RESULTS
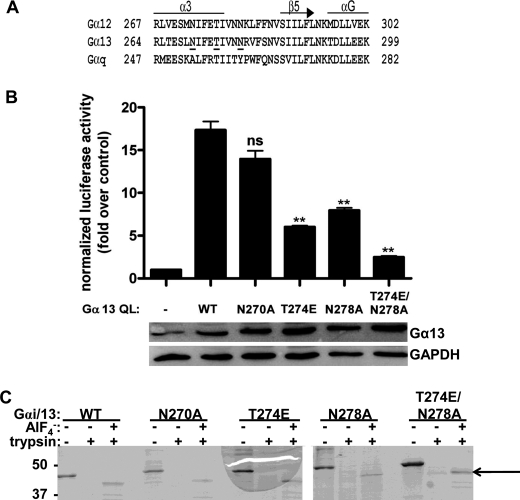
Previous studies have suggested that the region of Gα13 responsible for stimulating the guanine nucleotide exchange activity of p115RhoGEF is located C-terminal to the three switch regions (23, 32). These results are supported by crystallographic data, which demonstrate that the α3 helix and α3-β5 loop in Gα13 form a putative effector binding site with the RH domain of p115RhoGEF (17). However, the capacity of residues in this region to stimulate GEF activity has not been directly demonstrated. We utilized these data along with the interaction between Gαq and its effectors PLC-β and GRK2 as models in order to identify specific amino acid residues responsible for activating p115RhoGEF. Recent work has identified several residues that are critical for the interaction between Gαq and these effectors, namely Ala253, Thr257, and Tyr261 (20, 33).3 The Gαq-PLC-β interaction was selected as a model because, like RH-RhoGEFs, PLC-β functions both as a GAP (34) and effector (35) for Gαq. Based on these data, Asn270, Thr274, and Asn278 in Gα13, which correspond to Ala253, Thr257, and Tyr261 in Gαq (Fig. 1A), were targeted for site-directed mutagenesis, and the effects of these mutations on the ability of Gα13 to regulate p115RhoGEF activity were examined.
FIGURE 1.
Characterization of Gαi/13 mutants. A, a primary sequence alignment of murine Gα12, Gα13, and Gαq was generated using the program T-Coffee. Residues in Gα13 analyzed by site-directed mutagenesis in this study are underlined. Secondary structure was assigned based on the crystal structure of the Gα13/i1-p115 RH domain complex (Protein Data Bank entry 1SHZ) (17). B, the T274E and N278A mutations in Gα13 impair Rho activation in cells. HeLa cells were transiently transfected with empty vector or the indicated Gα13 QL construct. The luciferase activity of cell lysates was determined as described under “Experimental Procedures.” Total cell lysate was immunoblotted for either Gα13 or GAPDH. Data are presented as the mean ± S.E. (error bars) of triplicate determinations from a single experiment, representative of three independent experiments with similar results. Data were analyzed by one-way analysis of variance followed by Dunnett's post-test. Statistically significant difference from Gα13 QL is shown as follows. ns, not significant; **, p < 0.01. C, Gαi/13 mutants can undergo activation-dependent conformational changes. Gαi/13 (wild type or mutant) was subjected to limited trypsin digestion in the presence or absence of AlF4−. After proteolysis, proteins were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. The primary protected species is indicated with an arrow. The positions of molecular mass standards, in kDa, are shown on the left.
The T274E and N278A Mutations Impair Rho Activation in Cells
A constitutively active mutant of Gα13, Gα13 Q226L (hereafter referred to as Gα13 QL), has been shown to stimulate transcription from the SRE in a Rho-dependent manner when overexpressed in cells (6). Therefore, to test the ability of the N270A, T274E, N278A, and T274E/N278A mutants to stimulate Rho activation, HeLa cells, which endogenously express RH-RhoGEFs and RhoA, were transiently co-transfected with plasmids encoding each mutant in the background of Gα13 QL and a construct encoding the luciferase reporter gene under the control of the SRE. The activation status of Rho in cells was indirectly measured as luciferase activity of cell lysates. In this system, the N270A mutation in Gα13 QL did not affect Rho activation compared with Gα13 QL (Fig. 1B). However, both the T274E and N278A mutations decreased Rho activation by ∼50%, and Rho activity could be reduced to nearly basal levels by the T274E/N278A double mutation. Immunoblotting of cell lysates demonstrated equal expression levels of the constructs. These data suggested that residues Thr274 and Asn278, but not Asn270, are important for the ability of Gα13 to stimulate Rho activation in cells.
Gαi/13 N270A, T274E, N278A, and T274E/N278A Are Functional Gα Subunits
To analyze these mutants in more detail, each mutation was introduced into a construct harboring amino acid residues 1–28 of Gαi1 and 47–377 of Gα13 (hereafter referred to as Gαi/13). The resulting protein was expressed and purified from Sf9 cells for biochemical reconstitution assays. The use of this Gαi/13 chimera facilitates the production of recombinant protein by increasing the yield from Sf9 cells, and it retains all of the biochemical properties characteristic of native Gα13, including the ability to regulate RH-RhoGEFs (22). In order to confirm that the mutants were functional Gα subunits, limited trypsin digestion assays were performed. In their inactive, or GDP-bound, form, Gα subunits adopt a conformation that renders them susceptible to digestion by the serine protease trypsin (36). However, in the active form, which can be induced by binding to the reversible activator AlF4−, the conformation changes such that the Gα subunit is protected from digestion, largely due to ordering of the α2 helix in switch II, although trypsin still cleaves the N terminus of the Gα subunit. Thus, the protected species runs at a smaller molecular weight than the native protein. Trypsin digestion assays can therefore be used to determine if a Gα subunit is capable of adopting an activated conformation. As shown in Fig. 1C, each Gαi/13 mutant was protected from tryptic digestion in the presence of AlF4−, as evidenced by the existence of the lower molecular weight species, indicating that these mutants were capable of undergoing the activation-dependent conformational changes indicative of a functional Gα subunit.
The T274E and N278A Mutations Disrupt Binding to the RH Domain of p115RhoGEF and Impair GAP Activity
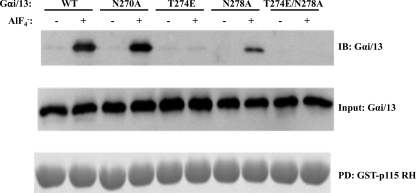
Because these mutations were known to be in the Gα13-p115RhoGEF RH domain interface (17), the ability of these mutants to interact with the RH domain was assessed using GST pull-down assays, and their capacity to respond to the GAP activity of this domain was determined using single turnover GTPase assays. The activation-dependent interaction between each mutant and the RH domain of p115RhoGEF is shown in Fig. 2. Although the N270A mutation had no apparent effect on the ability of Gαi/13 to interact with GST-p115 RH, the Gαi/13 T274E mutant had significantly reduced binding, whereas the N278A mutation produced an intermediate effect. The Gαi/13 T274E/N278A double mutant had no detectable binding to the RH domain under these conditions.
FIGURE 2.
The T274E and N278A mutations impair binding of Gαi/13 to the RH domain of p115RhoGEF. Gαi/13 (wild type or mutant) was incubated with a 50-fold molar excess of GST-p115 RH in the presence or absence of AlF4−. GST-p115 RH was pulled down (PD) with glutathione-Sepharose beads. Bound proteins were released by boiling and separated by SDS-PAGE. Gαi/13 was detected by immunoblotting (IB). GST-p115 RH was stained with Coomassie Brilliant Blue. Data presented are from one experiment, representative of three independent experiments with similar results.
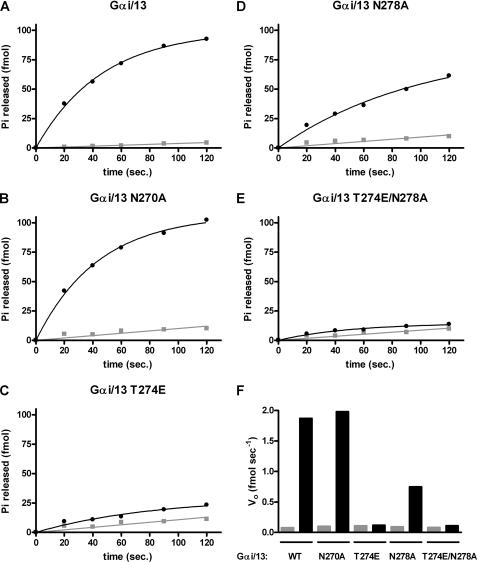
Previous work has established that the isolated RH domain (amino acids 1–252) of p115RhoGEF is as effective a GAP for Gα13 as the full-length protein (15). Therefore, GST-p115 RH was also used to examine the effects of these mutations on p115 RH-stimulated GAP activity in vitro (Fig. 3). Wild type Gαi/13 has a measurable rate of basal GTP hydrolysis, which can be markedly increased by the presence of GST-p115 RH (Fig. 3A). At 0 °C, 10 nm GST-p115 RH increased the initial velocity of the hydrolysis reaction (Vo) from ∼0.08 to ∼1.8 fmol/s, or ∼22-fold (Fig. 3F). Gαi/13 N270A displayed a very similar rate of RH domain-stimulated hydrolysis (Fig. 3F). Consistent with the fact that Gαi/13 T274E binds the RH domain of p115RhoGEF poorly (Fig. 2), its response to the GAP activity of the RH domain was also significantly impaired. In the presence of the RH domain, GTP hydrolysis by Gαi/13 T274E was reduced to nearly basal levels (Fig. 3, C and F). In the case of Gαi/13 N278A, the reduction in GAP activity in response to the RH domain was ∼50% (Fig. 3, D and F). Like Gαi/13 T274E, the double mutant, Gαi/13 T274E/N278A, had a rate of RH-stimulated hydrolysis that was reduced to essentially basal levels (Fig. 3, E and F). Therefore, the T274E mutation strongly affects the ability of Gαi/13 to bind the RH domain of p115RhoGEF and respond to its GAP activity, whereas the effect of the N278A mutation was also clear but less pronounced. The N270A mutation had no detectable effect on RH domain binding or GAP activity under the assay conditions used in Figs. 2 and 3.
FIGURE 3.
The T274E and N278A mutations in Gαi/13 impair the GAP response to the RH domain of p115RhoGEF. A–E, Gαi/13 or the indicated mutant was preloaded with [γ-32P]GTP (final Gα concentration 8–10 nm), and hydrolysis of bound GTP was initiated by mixing with buffer containing 8 mm MgSO4 and 1 mm GTP in the presence (●) or absence (■) of 10 nm GST-p115 RH. Reactions were incubated on ice, and aliquots were removed and quenched in activated charcoal slurry (pH 3) at the indicated times. F, the apparent initial rate of GTP hydrolysis by Gαi/13 or the indicated mutant is shown in the presence (black bar) or absence (gray bar) of 10 nm GST-p115 RH. Data presented are from one experiment representative of three independent experiments with similar results.
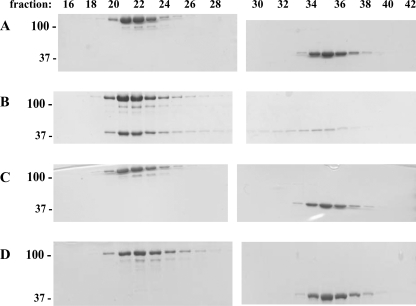
Gαi/13 T274E/N278A Cannot Bind to Full-length p115RhoGEF
Next, we assessed the ability of the T274E/N278A double mutant to bind to full-length p115RhoGEF by size exclusion chromatography. As shown in Fig. 4, upon activation with AlF4−, Gαi/13 forms a stable complex with p115RhoGEF, as evidenced by its elution in earlier, p115RhoGEF-containing fractions. In contrast, binding of the double mutant to full-length p115RhoGEF in the presence of AlF4− was not detectable at the level of Coomassie Brilliant Blue staining. Given that micromolar concentrations of p115RhoGEF and Gαi/13 T274E/N278A were insufficient to form a stable complex during the course of gel filtration, the affinity of Gαi/13 T274E/N278A for full-length p115RhoGEF is likely to be quite low, suggesting that the primary binding site for Gα13 is in the RH domain.
FIGURE 4.
The T274E/N278A double mutation in Gαi/13 impairs binding to full-length p115RhoGEF. p115RhoGEF was incubated with Gαi/13 and GDP (A), Gαi/13 and AlF4− (B), Gαi/13 T274E/N278A and GDP (C), or Gαi/13 T274E/N278A and AlF4− (D). The molar ratio of Gαi/13 (wild type or mutant) to p115RhoGEF was 2:1. Proteins were fractionated on a Superdex 200 size exclusion column, separated by SDS-PAGE, and stained with Coomassie Brilliant Blue. Fraction numbers are indicated. The positions of molecular mass standards, in kDa, are shown on the left.
Gα13 T274E/N278A Fails to Activate p115RhoGEF in Vitro
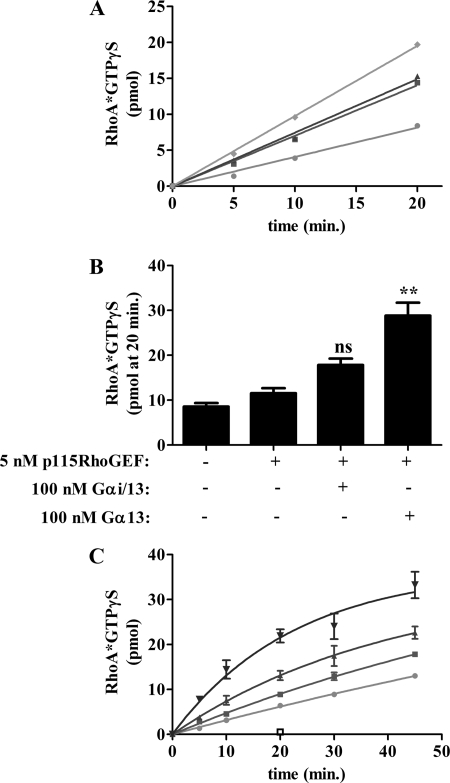
We next examined whether Gαi/13 T274E/N278A could regulate p115RhoGEF activity directly by measuring GTPγS binding to RhoA in vitro. Although Gαi/13 was able to stimulate RhoA activation through p115RhoGEF (Fig. 5A), Gαi/13 T274E/N278A failed to increase GTPγS binding to RhoA over the p115RhoGEF only condition. These results demonstrate that the T274E/N278A double mutation inhibits the ability to Gαi/13 to stimulate the nucleotide exchange activity of p115RhoGEF.
FIGURE 5.
Analysis of RhoA activation in vitro. GTPγS binding to RhoA (final concentration 500 nm) was measured in the presence of buffer, 5 nm p115RhoGEF, or 5 nm p115RhoGEF and 100 nm of the indicated AlF4−-activated Gα subunit. Samples were incubated at 30 °C and quenched in ice-cold buffer containing 10 mm MgSO4 at the indicated time. A, Gαi/13 T274E/N278A fails to stimulate p115RhoGEF activity in vitro. Samples are as follows: RhoA only (●), p115RhoGEF (■), p115RhoGEF plus Gαi/13 T274E/N278A (▴), and p115RhoGEF plus Gαi/13 (♦). Data are presented as the mean of single determinations pooled from two independent experiments. B, full-length Gα13 is a more efficacious activator of p115RhoGEF in vitro than Gαi/13. GTPγS binding to RhoA was determined after a 20-min incubation as described above. Data are presented as the mean ± S.E. (error bars) of single determinations pooled from three independent experiments. Data were analyzed by one-way analysis of variance, followed by Dunnett's post-test. Statistically significant difference from the p115RhoGEF condition is shown as follows. ns, not significant; **, p < 0.01. C, the T274E/N278A double mutation significantly reduces p115RhoGEF activation by full-length Gα13. Samples are as follows: RhoA only (●), p115RhoGEF (■), p115 RhoGEF plus Gα13 T274E/N278A (▾), p115RhoGEF plus Gα13 (▴), and Gα13 alone (□). Data are presented as the mean ± S.E. of single determinations pooled from three independent experiments.
Recently, we successfully generated recombinant, full-length Gα13 comprising amino acid residues 2–377. In the course of analyzing this protein, we found that it is a more efficient activator of RhoA in vitro than a functionally equivalent amount of Gαi/13 (Fig. 5B). This suggests that the N-terminal region of Gα13 may play a role in efficient stimulation of RhoGEF activity. Due to the higher activity of Gα13 in the in vitro RhoGEF assay, we chose to confirm the effect of the T274E/N278A mutations in the background of this full-length protein and examined its ability to stimulate RhoGEF activity. 100 nm full-length Gα13 was able to significantly increase the amount of GTPγS bound to RhoA compared with the p115RhoGEF only condition (Fig. 5, B and C). In contrast, RhoA activation stimulated by a functionally equivalent amount of Gα13 T274E/N278A was significantly reduced compared with wild type Gα13 (Fig. 5C). The effects of the T274E and N278A mutations on the ability of Gα13 to stimulate p115RhoGEF activity in vitro are shown in supplemental Fig. 1. We note that in contrast to the SRE assay, in which the activity of both Gα13 QL T274E and Gα13 QL N278A was reduced ∼50% (Fig. 1B), Gα13 T274E failed to stimulate RhoA activation in vitro, whereas Gα13 N278A had no apparent defect. However, in vitro, the functional concentration of Gα13 and its mutants was quantitated by [35S]GTPγS binding, which allows for a comparison between functionally equivalent amounts of protein. In contrast, the SRE assay is a cell-based overexpression system that precludes the tight control over the concentration of Gα13 afforded by our reconstitution assay. Thus, it is difficult to make a direct comparison between the behavior of the mutants in vitro and in cell-based experiments. Taken together, the results using both Gαi/13 and Gα13 are consistent and in agreement with the results of the cell-based assays, thus providing strong evidence that residues in the α3 helix of Gα13 regulate the GEF activity of p115RhoGEF.
X-ray Crystallography of the Gαi/13-p115RhoGEF RH Domain Complex
To gain more insight into the interaction between Gαi/13 and p115RhoGEF, we purified the ternary complex of Gαi/13, a deletion mutant of p115RhoGEF lacking the region C-terminal to the PH domain (p115RhoGEFΔC), and RhoA by size exclusion chromatography in the presence of AlF4− and subjected the complex to crystallization screening. Platelike crystals formed at 20 °C after ∼6 weeks and contained a complex of Gαi/13 and the RH domain of p115RhoGEF. However, the region C-terminal to the RH domain, including the DH and PH domains and RhoA, was not present in the crystal. The x-ray crystal structure of the p115RhoGEF RH domain in complex with Gαi/13, GDP, Mg2+, and AlF4− was determined at 2.4 Å resolution (Table 1). The structure contained two pairs of the Gαi/13-p115RhoGEF RH domain complex in the asymmetric unit (A-B and C-D; molecules A and C designate Gαi/13, and molecules C and D designate the p115RhoGEF RH domain (see supplemental Tables 1 and 2)). The final model contains residues 47–336 and 341–372 of molecule A; residues 22–34, 46–85, 93–121, and 134–233 of molecule B; residues 47–336 and 341–368 of molecule C; residues 22–32, 46–85, 92–115, 138–176, and 183–233 of molecule D; 2 GDP; 2 Mg2+; 2 AlF4−; and 87 water molecules. For the purpose of discussing the crystal structure, we hereafter refer to the complex as Gα13-p115 RH because the N terminus of Gαi/13 containing the residues from Gαi1 was disordered, and thus, only residues native to Gα13 were present.
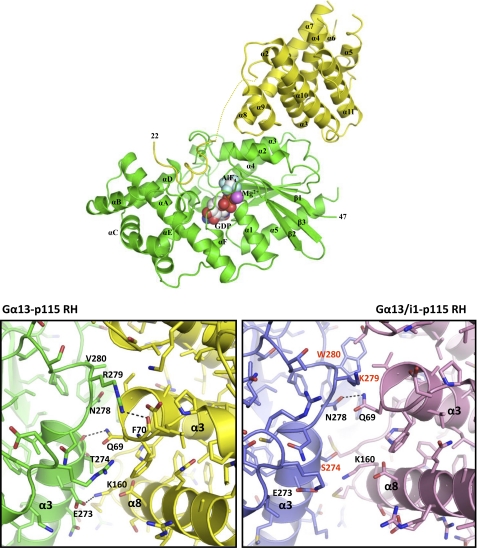
Overall Structure
In general, the structure of the Gα13-p115 RH complex is similar to the previously reported crystal structure of the Gα13/i1 chimera-p115RhoGEF RH domain complex (17). Both Gα13/i1 and Gα13 interact with the RH domain of p115RhoGEF through two distinct surfaces (Fig. 6). The N-terminal extension of the RH domain, the βN-αN hairpin, makes extensive contacts with the helical domain and switch regions I and II of Gα13/i1. This interface is also present in the current structure; however, the βN strand is disordered (Fig. 6). Additionally, the RGS box binds to an effector-like interaction surface consisting of the α2 and α3 helices and α3-β5 loop of both Gα13/i1 and Gαi/13 (Fig. 6). However, unlike the Gαi/13 used in the current study, Gα13/i1 contains several residues from Gαi1 in its effector interface, and its ability to stimulate RhoGEF activity has not been clearly demonstrated. The result of these substitutions is a significant difference in the effector interfaces between the two structures at the atomic level.
FIGURE 6.
Structure of the Gα13-p115 RH complex. Top, the Gα13-p115RhoGEF RH domain complex. The complex is shown as a ribbon diagram, with Gα13 colored in green and the RH domain of p115RhoGEF in yellow. GDP, Mg2+, and AlF4− are shown as space-filling spheres. Carbon, oxygen, nitrogen, phosphate, magnesium, aluminum, and fluoride atoms are colored white, red, dark blue, orange, purple, light gray, and light blue, respectively. The disordered region in p115RhoGEF between the GAP interface and the RGS box is shown as a dashed yellow line. Bottom, a detailed view of the Gα13-p115 RH domain effector interface. The Gα13-p115 RH complex is shown on the left, and for comparison, the Gα13/i1-p115 RH complex (Protein Data Bank entry 1SHZ) is shown on the right (17). The former is colored as in the top, whereas in the latter structure, Gα13/i1 is colored blue, and the p115 RH domain is purple. Residues that contribute to the Gα-p115 RH interface are labeled. In Gα13/i1, residues that are not native to Gα13 are indicated in red. Hydrogen bonds are depicted as dashed lines. Oxygen and nitrogen atoms are colored red and blue, respectively.
The Effector Interface
Compared with Gα13/i1, Gα13 is shifted 3 Å closer to the RH domain of p115RhoGEF. This is probably a reflection of the higher affinity of this domain for Gα13 versus Gα13/i1. Additionally, the bulky side chain of Trp280 in Gα13/i1, which corresponds to Val280 in Gα13, may sterically inhibit the formation of a tight complex. Gα13 forms more hydrogen bonds with p115 RH as compared with Gα13/i1 (9 versus 3 possible hydrogen bonds) (Fig. 6). In Gα13, Glu273 forms an ion pair with Lys160 in the RH domain of p115RhoGEF. In contrast, Glu273 in Gα13/i1 makes no contact with the RH domain, whereas Lys160 participates in a van der Waals interaction with Ser274 of Gα13/i1. The backbone of Thr274 in Gα13 (Ser274 in Gα13/i1) engages the side chain of Gln69 of the RH domain through hydrogen bonding, whereas Gα13/i1 interacts with Gln69 via a hydrogen bond with the side chain of Asn278. Given that this is one of the three possible hydrogen bonds between Gα13/i1 and the RH domain of p115RhoGEF, perturbing this interaction might be expected to result in a large decrease in binding. However, the results of our experiments with the N278A mutant of Gα13 suggest that this residue is not as critical for the interaction with the RH domain of p115RhoGEF as Thr274. Consistent with this notion, in Gα13, Asn278 does not participate in hydrogen bonding to p115 RH but rather is in hydrophobic contact with Leu68. Arg279 of Gα13 makes extensive contacts with p115 RH, including a hydrogen bond to the backbone carboxyl group of Phe70, a residue that has previously been implicated in binding to Gα13 (14). In contrast, Phe70 makes no contact with Gα13/i1. Additionally, Arg279 hydrogen-bonds to two additional residues in the RH domain, Ala67 and Leu68, through a main chain-side chain bond and a main chain-main chain bond, respectively. As a result of these amino acid differences, the calculated surface area buried in the effector interface of the Gα13-p115 RH complex (1,600 Å2) is significantly larger than that of the Gα13/i1-p115 RH complex (1,200 Å2).
The GAP Interface
Although there are significant differences in the effector interface between Gα13 and Gα13/i1, both interact with the GAP surface of the RH domain in an essentially identical manner (supplemental Fig. 2), which is consistent with the fact that the α helical domain and switch regions of Gα13/i1 are largely derived from Gα13. The interaction between the catalytic residue Arg200 of Gα13 and Glu27 of p115 RH is found in both structures. Arg260 in Gα13/i1, which hydrogen-bonds with the main chain carbonyl of Ile23 and forms an ion pair with Asp28 in p115 RH, participates in the same bonding interactions in the Gα13-p115 RH structure. Likewise, the interaction between Met257 from Gα13 and Phe31 of p115 RH, which in the Gα13/i1-p115 RH domain structure is a van der Waals contact, is also present in the current structure (supplemental Table 1).
Structural Aspects of the N270A, T274E, and N278A Mutations
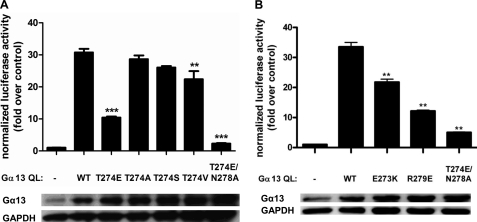
In the Gα13-p115 RH domain complex, Asn270 of Gα13 interacts with p115 RH primarily via hydrophobic contact with Met163. However, this interaction is well outside of the effector interface, supporting the results of cell-based experiments demonstrating that the N270A mutation does not impact GEF activity. In contrast, the T274E mutation resulted in reduced Rho activation in cells and severely disrupted binding to p115 RH and the GAP response. These observations are consistent with the fact that Thr274 hydrogen-bonds, via its backbone carbonyl, to Gln69 in the p115 RH domain, a residue known to be important for Gα13 binding (14). It is likely that the introduction of a longer, charged side chain in place of Thr274, as is the case with the T274E mutation, disrupts the interaction with Gln69 and that this reduced binding results in impaired GAP and GEF activity. Additionally, in molecules C and D, Thr274 in Gα13 makes additional contacts with p115 RH via hydrogen bonding of its side chain to the side chain of Lys160 and hydrophobic interaction with Met165. Thus, the effects of the T274E mutation may also be manifest in the form of steric clashing and perturbation of hydrophobic contacts. To probe the role of the side chain of Thr274 in the interaction with the RH domain of p115RhoGEF, we made several additional point mutations at this position in Gα13 and assessed the activity of these mutants in the SRE assay. As shown in Fig. 7A, neither the T274A nor the T274S mutation affected Rho activation by Gα13 QL in this system. Although the T274V mutant had slightly reduced activity, the effect was not nearly as severe as the T274E mutation. Thus, these data strongly suggest that Thr274 interacts with the RH domain primarily through its backbone. Asn278 interacts with p115 RH solely through a hydrophobic interaction with residue Leu68. Thus, the modest decreases in binding to the RH domain and GAP activity caused by the N278A mutation are consistent with the fact that Asn278 does not contribute substantially to the interface with p115 RH. In addition, other residues in Gα13 that we did not initially target for site-directed mutagenesis also contribute to the interface with the RH domain of p115RhoGEF, namely Glu273 and Arg279. In order to determine if these residues contribute to the ability of Gα13 to stimulate GEF activity, the E273K and R279E mutants were generated and analyzed by an SRE assay. As shown in Fig. 7B, mutation of these residues impairs the ability of Gα13 to stimulate Rho activation in cells, consistent with the notion that the Gα13-p115 RH interface has the capacity to regulate effector activity. Thus, the results of the current biochemical analysis as well as previously published mutational analysis of p115RhoGEF (14) are more consistent with the Gα13-p115 RH interface presented here than with the interface between the RH domain and Gα13/i1.
FIGURE 7.
Mutational analysis of the effector interface between Gα13 and p115 RH. A, the T274A and T274S mutations in Gα13 fail to impair Rho activation in cells. HeLa cells were transiently transfected with empty vector or the indicated Gα13 QL construct. The luciferase activity of cell lysates was determined as described under “Experimental Procedures.” Total cell lysate was immunoblotted for either Gα13 or GAPDH. Data are presented as the mean ± S.E. (error bars) of triplicate determinations from a single experiment, representative of three independent experiments with similar results. Data were analyzed by one-way analysis of variance followed by Dunnett's post-test. Statistically significant difference from Gα13 QL is shown as follows. **, p < 0.01; ***, p < 0.001. B, the E273K and R279E mutations in Gα13 impair Rho activation in cells. Assays were performed as in A. Statistically significant difference from Gα13 QL is shown as follows. **, p < 0.01.
Comparison with PDZ-RhoGEF
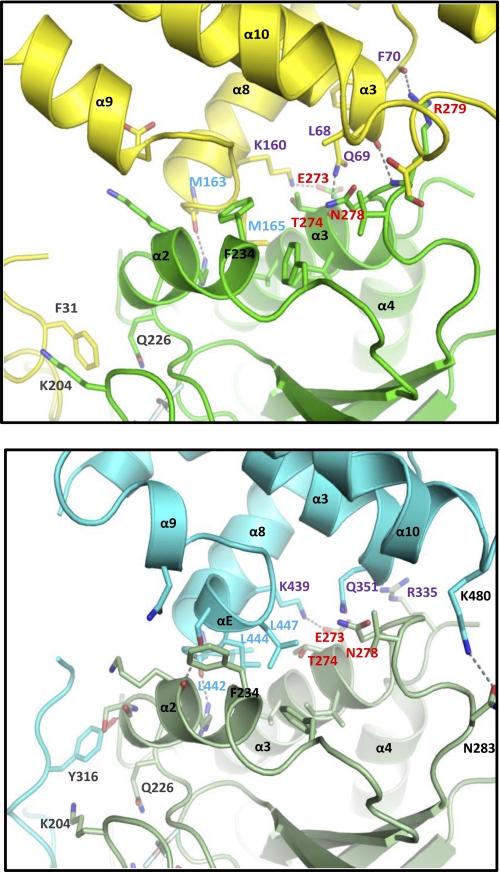
Interestingly, the effector interface between Gα13 and the RH domain of p115RhoGEF is largely conserved in PDZ-RhoGEF (Fig. 8 and supplemental Table 3). Although Asn270 of Gα13 participates in hydrophobic interaction with p115 RH, mutation of this residue has no apparent effect on binding, GAP, or GEF activity in cells. There is no interaction between this residue and PDZ-RhoGEF. In contrast, residue Thr274 of Gα13, due to rotation of the side chain, makes more extensive contact with PDZ-RhoGEF RH than with p115RhoGEF RH. In addition to the interaction with Gln351, which is analogous to Gln69 in p115RhoGEF, via its backbone carbonyl group, Thr274 is also involved in hydrophobic interactions with Lys439 and Leu444. Residue Asn278 in Gα13 also interacts with Gln351 of PDZ-RhoGEF via hydrophobic contact. Arg279 of Gα13, which has extensive interaction with the RH domain of p115RhoGEF, makes fewer contacts with PDZ-RhoGEF. However, the main chain-main chain interaction between Arg279 and Leu68 of p115RhoGEF is conserved in the analogous residue of PDZ-RhoGEF, Ser350. Thus, the prominent features of the effector interface between Gα13 and p115RhoGEF RH are conserved in the Gα13-PDZ-RhoGEF RH crystal structure as well, suggesting that this interface may also play a role in regulating the GEF activity of PDZ-RhoGEF.
FIGURE 8.
Comparison of the interaction between Gα13 and the RH domains of p115RhoGEF and PDZ-RhoGEF. Top, the effector interface formed by Gα13 and the RH domain of p115RhoGEF. The interface is depicted as a ribbon diagram, with Gα13 colored in green and the RH domain in yellow. Oxygen and nitrogen atoms are colored red and blue, respectively. Hydrogen bonds are depicted as dashed lines. Residues that contribute to the GAP interface are labeled in gray, whereas hydrophobic residues that contribute to the effector interface by the RH domain are labeled in light blue. Residues in Gα13 that affect its capacity to stimulate the GEF activity of p115RhoGEF are labeled in red, and their bonding partners in the RH domain are labeled in purple. The conformationally flexible residue Phe234 in Gα13 is labeled in black. Bottom, the effector interface formed by Gα13 and the RH domain of PDZ-RhoGEF (Protein Data Bank entry 3CX7) (45). The interface is depicted as a ribbon diagram, with Gα13 colored in olive green and the RH domain in light blue. Atoms and amino acid residues are labeled as in the top.
DISCUSSION
The RH domains found in RH-RhoGEFs, such as p115RhoGEF, are characterized by low sequence identity to classical RGS domain-containing proteins, such as RGS2 and RGS4 (12, 37). Additionally, unlike these RGS proteins, the RGS box of p115RhoGEF does not contain the residues necessary to accelerate GTP hydrolysis by Gα13; these catalytic residues are found in the acidic βN-αN hairpin N-terminal to the RGS box (14, 17). The RH domain of p115RhoGEF has also been implicated in the regulation of nucleotide exchange activity because deletion of the first 288 amino acids of p115RhoGEF impairs both basal and Gα13-stimulated GEF activity (15). Given that removal of the first 42 amino acids of p115RhoGEF, which are required for GAP activity, has no effect on the ability of Gα13 to stimulate GEF activity (15), the GAP and GEF functions are clearly distinct properties of the RH domain. However, specific residues in Gα13 responsible for stimulating p115RhoGEF activity have not been identified. Although x-ray crystallography has demonstrated that a Gα13/i1 chimera forms a putative effector-like interface with the RH domain of p115RhoGEF (17), the capacity of residues in this interface, many of which are derived from Gαi1, to regulate GEF activity is unknown.
Our results demonstrate that Gα13 regulates p115RhoGEF activity via the classical effector interface it forms with the RH domain. Specifically, mutation of Thr274 and Asn278 in the α3 helix of Gα13 impairs the ability of Gα13 to stimulate Rho activation in cells, impairs binding to p115RhoGEF, and reduces the capacity of Gα13 to stimulate the guanine nucleotide exchange activity of p115RhoGEF in vitro. We also determined the structure of the Gα13-p115RhoGEF RH complex and found that this effector interface differs in several important respects from the interface formed with Gα13/i1. Importantly, these new structural data are strongly supported by the results of our biochemical experiments.
In the context of previously reported data, our results are consistent with earlier studies that broadly defined the RhoGEF-activating surface of Gα13 as the region C-terminal to the Ras-like domain (32), more specifically the last 100 amino acid residues (23). However, given that we have demonstrated that the interaction between the RH domain and Gα13 also regulates the effector activity of p115RhoGEF, this suggests that the previously described interface between Gα13 and the DH/PH domains of p115RhoGEF may not contribute significantly to stimulation of GEF activity (15). If the RH domain represents the sole binding site for Gα13, how might this interaction regulate the activity of the DH/PH domains? One study using PDZ-RhoGEF has suggested that the RH domain may work in concert with elements found in the linker region between the RH and DH domains to regulate GEF activity (38). Specifically, an acidic cluster of residues located in the linker may interact directly with residues in the DH domain to autoinhibit PDZ-RhoGEF activity. Mutation of these residues enhances GEF activity in vitro but only in the absence of the RH domain. Recently, the linker between the RH and DH domains of p115RhoGEF has also been implicated in regulating basal GEF activity, although the mechanism of autoinhibition appears to be distinct from that employed by PDZ-RhoGEF (39). A stretch of 40 amino acids located in the linker region of p115RhoGEF has been proposed to disrupt the interaction between the DH domain and residues in the “GEF switch” immediately N-terminal to it, an interaction that is critical for basal GEF activity. Thus, one consequence of Gα13 binding to the RH domain may be to reorient elements within the linker region of these RH-RhoGEFs, allowing for enhanced exchange activity.
A comparison of the structures of Gα13 bound to the RH domains of p115RhoGEF and PDZ-RhoGEF reveals that it binds this domain in a nearly identical manner. Additionally, the T274E and N278A mutations in Gα13 also impair the interaction with the RH domain of LARG in vitro (data not shown). Thus, it is likely that Gα13 engages the RH domains of all three RH-RhoGEFs in the same manner and stimulates RhoGEF activity through a similar mechanism. Although LARG is clearly regulated directly by Gα13 in vitro (40), stimulation of the RhoGEF activity of PDZ-RhoGEF by Gα13 in vitro has not been detectable under the conditions tested (15). However, PDZ-RhoGEF has been linked to Gα13-mediated Rho activation in cells (10). Thus, although the effector interface formed between Gα13 and the RH domain of p115RhoGEF bears striking resemblance to the Gα13-PDZ-RhoGEF RH interface, in the latter case, this interaction is not sufficient to regulate RhoGEF activity in vitro. Post-translational modification, such as phosphorylation, or interaction with a co-activating protein may be required to render PDZ-RhoGEF susceptible to regulation by Gα13.
It is also possible that activation of p115RhoGEF by Gα13 requires multiple intermolecular interfaces. Binding of Gα13 to the RH domain may result in a conformational change that brings additional regions of p115RhoGEF, perhaps the catalytic DH/PH domains, into contact with Gα13, allowing for efficient activation of exchange activity. Although activated Gα13 clearly fails to stimulate the GEF activity of p115RhoGEF fragments lacking the RH domain, it has been reported to bind to the isolated DH/PH domains of p115RhoGEF in vitro (15). Additionally, the N terminus of Gα13, which is replaced by the αN helix of Gαi1 in Gαi/13 and is disordered in the present crystal structure, may contribute to the binding interface with p115RhoGEF. This hypothesis is supported by the fact that Gα13 exhibits more robust activity in the RhoGEF assay compared with Gαi/13 in vitro (Fig. 5B). The results of our gel filtration analysis suggest that Gαi/13 T274E/N278A cannot bind to p115RhoGEF and are consistent with the in vitro RhoGEF assays demonstrating that Gαi/13 T274E/N278A fails to stimulate RhoA activation over the p115RhoGEF condition. These data argue in favor of a model in which Gα13 binds exclusively to the RH domain; however, it is difficult to rule out the possibility of additional low affinity binding sites outside of the RH domain. Given that the RH domain occupies the classical effector interface on Gα13, any additional interface would probably be a novel surface that has not been observed in other Gα-effector pairs. Aside from the residues reported here, however, screening of additional Gα13 mutants has so far failed to identify other residues that affect its capacity to stimulate Rho activation in cells.4
Little is known about the mechanism by which the other G12 family member, Gα12, stimulates RH-RhoGEF activity. p115RhoGEF also functions as a GAP for Gα12 and serves as an effector in cells; however, Gα12 is incapable of activating p115RhoGEF in vitro (9). Because Gα12 and Gα13 have a high degree of identity at the primary sequence level (∼69%) and have nearly identical α3 helices and α3-β5 loops, it is worthwhile to consider biochemically whether Gα12 and Gα13 bind to the RH domain of p115RhoGEF using the same interface. When mutations corresponding to the Gα13 mutants described here were introduced into Gα12, the pattern of Rho activation in SRE assays and binding to the RH domain of p115RhoGEF was similar to that observed with the Gα13 mutants (supplemental Fig. 3). This suggests that the binding site for Gα12 overlaps with that of Gα13 in the RH domain of p115RhoGEF and probably explains the observation that activated Gα12 can inhibit Gα13-mediated activation of p115RhoGEF despite the fact that Gα12 cannot directly activate p115RhoGEF in vitro (9). However, these data imply that the inability of Gα12 to stimulate p115RhoGEF is not due to a difference in primary sequence, as had previously been suggested (23). Thus, the reasons behind this lack of activity remain unknown. However, as another RH-RhoGEF, LARG, needs to be tyrosine-phosphorylated in order to be responsive to Gα12 in vitro (40), this may be the case with p115RhoGEF as well.
Although the precise activation mechanism of p115RhoGEF remains to be fully elucidated, we have identified residues in Gα13 that play a role in regulating the nucleotide exchange activity of p115RhoGEF. However, given that p115RhoGEF activity is also reported to be regulated by subcellular localization (41–43) and post-translational modification (44), the activation mechanism in cells is likely to be complex. Ongoing efforts to crystallize Gα13 and Gα12 with p115RhoGEF constructs containing the RH domain and DH/PH domains will aid in directly addressing the presence of additional binding sites and will help to clarify the molecular mechanism by which heterotrimeric G proteins stimulate activation of this RhoGEF.
Supplementary Material
Acknowledgments
We are grateful to Dr. John Tesmer for critical reading of the manuscript. We thank Hideko Wakasugi-Masuho, Ami Vora, Terry Phonxanasinh, Dara Chanthavong, Dr. Takashi Umehara, Dr. Kazushige Katsura, Yumiko Terazawa, Takako Fujimoto, and Dr. Motoaki Wakiyama for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants GM61454 and GM074001 (to T. K.). This work was also supported by a grant-in-aid from the Greater Midwest Affiliate of the American Heart Association (to T. K.), the Targeted Proteins Research Program (TPRP) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Funding Program for World-Leading Innovative R&D on Science and Technology from the Japan Society for the Promotion of Science; a predoctoral fellowship from the Greater Midwest Affiliate of the American Heart Association (to N. H.); and the Japan Society for the Promotion of Science (to N. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Tables 1–3.
The atomic coordinates and structure factors (code 3AB3) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
K. Tsuboi and T. Kozasa, unpublished data.
N. Hajicek and T. Kozasa, unpublished data.
- GAP
- GTPase-activating protein
- DH
- Dbl homology
- GEF
- guanine nucleotide exchange factor
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- LARG
- leukemia-associated RhoGEF
- PDZ
- post-synaptic density protein 95, discs large, zonula occludens
- PH
- pleckstrin homology
- RGS
- regulator of G protein signaling
- RH
- RGS homology
- Sf9
- Spodoptera frugiperda
- SRE
- serum response element
- TEV
- tobacco etch virus
- TLCK
- Nα-tosyl-l-lysine chloromethyl ketone
- TPCK
- N-p-tosyl-l-phenylalanine chloromethyl ketone.
REFERENCES
- 1. Oldham W. M., Hamm H. E. (2006) Q. Rev. Biophys. 39, 117–166 [DOI] [PubMed] [Google Scholar]
- 2. Hollinger S., Hepler J. R. (2002) Pharmacol. Rev. 54, 527–559 [DOI] [PubMed] [Google Scholar]
- 3. Berman D. M., Kozasa T., Gilman A. G. (1996) J. Biol. Chem. 271, 27209–27212 [DOI] [PubMed] [Google Scholar]
- 4. Tesmer J. J., Berman D. M., Gilman A. G., Sprang S. R. (1997) Cell 89, 251–261 [DOI] [PubMed] [Google Scholar]
- 5. Strathmann M. P., Simon M. I. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 5582–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao J., Yuan H., Xie W., Simon M. I., Wu D. (1998) J. Biol. Chem. 273, 27118–27123 [DOI] [PubMed] [Google Scholar]
- 7. Lin F., Chen S., Sepich D. S., Panizzi J. R., Clendenon S. G., Marrs J. A., Hamm H. E., Solnica-Krezel L. (2009) J. Cell Biol. 184, 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buhl A. M., Johnson N. L., Dhanasekaran N., Johnson G. L. (1995) J. Biol. Chem. 270, 24631–24634 [DOI] [PubMed] [Google Scholar]
- 9. Hart M. J., Jiang X., Kozasa T., Roscoe W., Singer W. D., Gilman A. G., Sternweis P. C., Bollag G. (1998) Science 280, 2112–2114 [DOI] [PubMed] [Google Scholar]
- 10. Fukuhara S., Murga C., Zohar M., Igishi T., Gutkind J. S. (1999) J. Biol. Chem. 274, 5868–5879 [DOI] [PubMed] [Google Scholar]
- 11. Kourlas P. J., Strout M. P., Becknell B., Veronese M. L., Croce C. M., Theil K. S., Krahe R., Ruutu T., Knuutila S., Bloomfield C. D., Caligiuri M. A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2145–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., Gilman A. G., Bollag G., Sternweis P. C. (1998) Science 280, 2109–2111 [DOI] [PubMed] [Google Scholar]
- 13. Chen Z., Wells C. D., Sternweis P. C., Sprang S. R. (2001) Nat. Struct. Biol. 8, 805–809 [DOI] [PubMed] [Google Scholar]
- 14. Chen Z., Singer W. D., Wells C. D., Sprang S. R., Sternweis P. C. (2003) J. Biol. Chem. 278, 9912–9919 [DOI] [PubMed] [Google Scholar]
- 15. Wells C. D., Liu M. Y., Jackson M., Gutowski S., Sternweis P. M., Rothstein J. D., Kozasa T., Sternweis P. C. (2002) J. Biol. Chem. 277, 1174–1181 [DOI] [PubMed] [Google Scholar]
- 16. Mao J., Yuan H., Xie W., Wu D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12973–12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Z., Singer W. D., Sternweis P. C., Sprang S. R. (2005) Nat. Struct. Mol. Biol. 12, 191–197 [DOI] [PubMed] [Google Scholar]
- 18. Tesmer J. J., Sunahara R. K., Gilman A. G., Sprang S. R. (1997) Science 278, 1907–1916 [DOI] [PubMed] [Google Scholar]
- 19. Slep K. C., Kercher M. A., He W., Cowan C. W., Wensel T. G., Sigler P. B. (2001) Nature 409, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 20. Tesmer V. M., Kawano T., Shankaranarayanan A., Kozasa T., Tesmer J. J. (2005) Science 310, 1686–1690 [DOI] [PubMed] [Google Scholar]
- 21. Lutz S., Shankaranarayanan A., Coco C., Ridilla M., Nance M. R., Vettel C., Baltus D., Evelyn C. R., Neubig R. R., Wieland T., Tesmer J. J. (2007) Science 318, 1923–1927 [DOI] [PubMed] [Google Scholar]
- 22. Kreutz B., Yau D. M., Nance M. R., Tanabe S., Tesmer J. J., Kozasa T. (2006) Biochemistry 45, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreutz B., Hajicek N., Yau D. M., Nakamura S., Kozasa T. (2007) Cell. Signal. 19, 1681–1689 [DOI] [PubMed] [Google Scholar]
- 24. Singer W. D., Miller R. T., Sternweis P. C. (1994) J. Biol. Chem. 269, 19796–19802 [PubMed] [Google Scholar]
- 25. Tanabe S., Kreutz B., Suzuki N., Kozasa T. (2004) Methods Enzymol. 390, 285–294 [DOI] [PubMed] [Google Scholar]
- 26. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 27. Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. (1991) Acta Crystallogr. A 47, 110–119 [DOI] [PubMed] [Google Scholar]
- 28. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 29. Collaborative Computational Project No. 4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–76315299374 [Google Scholar]
- 30. Holm L., Sander C. (1993) J. Mol. Biol. 233, 123–138 [DOI] [PubMed] [Google Scholar]
- 31. DeLano W. L. (2010) The PyMOL Molecular Graphics System, Version 1.3r1, Schrodinger, LLC, New York [Google Scholar]
- 32. Vázquez-Prado J., Miyazaki H., Castellone M. D., Teramoto H., Gutkind J. S. (2004) J. Biol. Chem. 279, 54283–54290 [DOI] [PubMed] [Google Scholar]
- 33. Waldo G. L., Ricks T. K., Hicks S. N., Cheever M. L., Kawano T., Tsuboi K., Wang X., Montell C., Kozasa T., Sondek J., Harden T. K. (2010) Science 330, 974–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berstein G., Blank J. L., Jhon D. Y., Exton J. H., Rhee S. G., Ross E. M. (1992) Cell 70, 411–418 [DOI] [PubMed] [Google Scholar]
- 35. Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. (1991) Science 251, 804–807 [DOI] [PubMed] [Google Scholar]
- 36. Fung B. K., Nash C. R. (1983) J. Biol. Chem. 258, 10503–10510 [PubMed] [Google Scholar]
- 37. Tesmer J. J. (2009) Prog. Mol. Biol. Transl. Sci. 86, 75–113 [DOI] [PubMed] [Google Scholar]
- 38. Zheng M., Cierpicki T., Momotani K., Artamonov M. V., Derewenda U., Bushweller J. H., Somlyo A. V., Derewenda Z. S. (2009) BMC Struct. Biol. 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Z., Guo L., Sprang S. R., Sternweis P. C. (2011) Protein Sci. 20, 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki N., Nakamura S., Mano H., Kozasa T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wells C. D., Gutowski S., Bollag G., Sternweis P. C. (2001) J. Biol. Chem. 276, 28897–28905 [DOI] [PubMed] [Google Scholar]
- 42. Bhattacharyya R., Wedegaertner P. B. (2003) Biochem. J. 371, 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhattacharyya R., Wedegaertner P. B. (2000) J. Biol. Chem. 275, 14992–14999 [DOI] [PubMed] [Google Scholar]
- 44. Holinstat M., Mehta D., Kozasa T., Minshall R. D., Malik A. B. (2003) J. Biol. Chem. 278, 28793–28798 [DOI] [PubMed] [Google Scholar]
- 45. Chen Z., Singer W. D., Danesh S. M., Sternweis P. C., Sprang S. R. (2008) Structure 16, 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.