Abstract
This is a review of the research during the past 25 years on cortical processing of color signals. At the beginning of the period the modular view of cortical processing predominated. However, at present an alternative view, that color and form are linked inextricably in visual cortical processing, is more persuasive than it seemed in 1985. Also, the role of the primary visual cortex, V1, in color processing now seems much larger than it did in 1985. The re-evaluation of the important role of V1 in color vision was caused in part by investigations of human V1 responses to color, measured with functional magnetic resonance imaging, fMRI, and in part by the results of numerous studies of single-unit neurophysiology in non-human primates. The neurophysiological results have highlighted the importance of double-opponent cells in V1. Another new concept is population coding of hue, saturation, and brightness in cortical neuronal population activity.
INTRODUCTION
Using common sense or introspection, we feel that color is a visual property of objects and surfaces that we perceive separately from shape or direction of motion or depth order in the visual scene. Most of us have experienced seeing black and white movies, and we know that we can understand the action of a film, like a Charlie Chaplin film in black and white, without requiring that it be in true color. Furthermore, vision scientists have studied color perception often under conditions where form is minimal. It is certainly possible to perceive colors under such reduced conditions and such colors have even been given a name, "aperture colors" (Katz, 1935). The idea that color is analyzed separately by the brain is so ingrained that when the editors of Vision Research organized the 50th anniversary issue, they asked us to write a review about color in the cortex as distinct from other review papers about, for instance, motion or face perception in the visual cortex. It is just natural for us as scientists and also as human beings to think of color as separate and apart.
The main point of our review of visual neuroscience over the past 25 years is that color is not separate and apart, but rather that color and form and motion are inextricably linked as properties of objects in visual perception and in the visual cortex (Wallach, 1935, transl. Wuerger Shapley and Rubin 1996; Lennie 1999). The famous psychologist Gaetano Kanizsa was an eloquent advocate of this viewpoint; he wrote
"…space and color are not distinct elements but, rather, are interdependent aspects of a unitary process of perceptual organization." (Kanizsa 1979)
The reason for the linkage of color and form is that the brain needs to construct a color signal to recover, as well as it can, the reflective properties of a surface, independent of illumination (Brainard, 2004; Shevell and Kingdom, 2008). To accomplish this task, the neural mechanisms of color perception must make computations that take into account the spatial layout of the scene as well as the spectral reflectances of different uniformly colored surfaces (Brainard 2004). Perhaps even more fundamental is the neural computation of the spatial variation, or flow, of color across a surface or surfaces, and this kind of computation also requires the integration of signals about color and form (Ben-Shahar and Zucker 2004, 2010). Based on results obtained over the past 25 years about orientation and spatial selectivity for color patterns, reviewed below, we support the suggestion that the primary visual cortex, V1, may play an important role in neural computations for color integrated with form perception (Wachtler et al 2003; Hurlbert and Wolf, 2004).
Psychophysical and perceptual studies have established reciprocal links between form and color in human vision. Color has been found to influence the perception of form. One example is a psychophysical result on cross-masking between equiluminant red-green sine grating patterns and black-white luminance gratings, suggesting that the neural mechanisms of color detection are selective for spatial frequency and orientation (Switkes et al 1988; Losada and Mullen 1994). Analogous experiments on spatial-frequency adaptation revealed spatial-frequency-tuned, adaptable detection mechanisms for both luminance and color patterns (Bradley et al 1988). Thus even though the contrast sensitivity function for equiluminant colored patterns was found to be spatially low-pass (Mullen 1985), the adaptation and masking results suggested that the low-pass function was an envelope encompassing many narrow-band, tuned, spatial-chromatic mechanisms. Additional compelling evidence for chromatic signals carrying spatial information comes from results on orientation discrimination, which was found to be almost as fine with a red-green equiluminant pattern as with a luminance pattern (Webster et al 1990; Beaudot and Mullen 2005). Moreover, the tilt illusion was found to be a strong effect for equiluminant as well as luminance patterns (Clifford et al 2003). Even the geometric illusions, such as the Ebbinghaus, Mueller-Lyer, Poggendorff, Ponzo, and Zoellner illusions, were present with red-green equiluminant stimuli as long as the thickness of the colored lines in the illusory figures was large enough (Hamburger et al. 2007).
There is extensive evidence for form's influence on color perception. One example is filling-in of color in stabilized images across long distances from an unstabilized boundary (Yarbus, 1967). Color filling-in in the periphery of the visual field can be seen even with voluntary fixation (Krauskopf 1963). The fading of a target's color with voluntary fixation happens faster if the boundary between colored target and background is weakened by smoothing or blur. More evidence for the importance of visual edges in color perception comes from the phenomenon of Gauzkontrast: the enhancement of apparent color contrast in a visual pattern when it is viewed through gauze or coarse cloth such that brightness differences at visual edges are effectively masked (Berliner 1949). These and many other perceptual results indicate that the color appearance of a region sometimes is even more dependent on color contrast at the boundary of the region than it is on the spectral reflectance of the region.
Many different neuroscientists in many different laboratories around the world, using different ways of measuring brain activity, have found that the visual cortex's response to color is strongly linked to other visual properties like lightness contrast, shape, texture, orientation. The neuroscientific investigations were inspired by the perceptual work on color and form. When one finally realizes the full implications of the linkage between color and form, the realization helps clarify what cortical neurons are doing and how they work together in a cortical network to produce the perception of color. Also, getting on the right side of the data will allow us to move forward with investigations of how the cerebral cortex uses color in constructing visual perception.
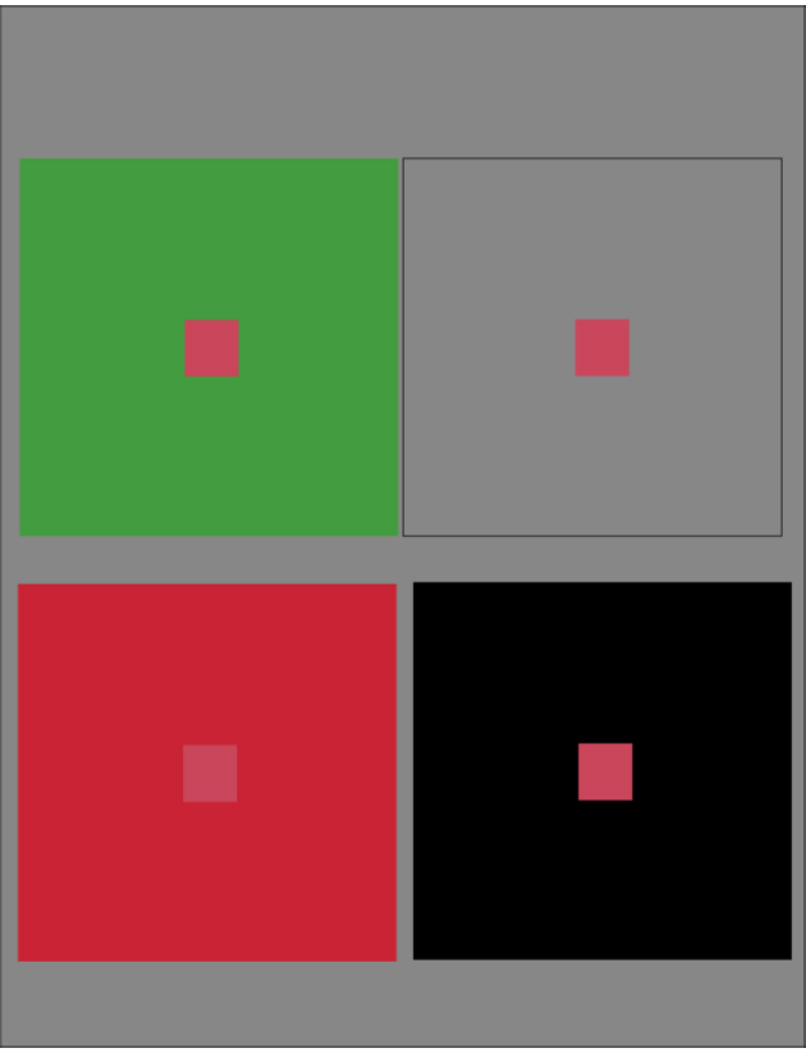
A familiar and very important property of color perception is its sensitivity to context. The strong influence of surrounding regions on the color of a target region has been known for centuries (Mollon, 2006). An illustration of the surprisingly strong context effect is shown in Figure 1, where four smaller squares of identical wavelength spectra are placed on four different surrounds. The two upper square targets appear intensely red, while the two lower square targets are pink on a black background and pinkish-white (to us it appears almost white) on a red background. Therefore the appearance of these physically identical targets is very strongly affected by the color and brightness of the surrounding area. Figure 1 illustrates that either color contrast or brightness contrast can have a major effect on perceived color. Color and form interact through the spatial layout of surrounding forms and their colors and brightnesses relative to a target region, and the influence by contrast that the surrounding forms exert on the target. Color contrast effects are assumed to be involved in the important subject of color constancy (Brainard, 2004). It has been suggested that the neural substrate for color constancy is the population of orientation-selective double-opponent neurons (see the review by Gegenfurtner, 2003). In our review we discuss the double-opponent neurons extensively. A lot of work has gone into exploring the neural mechanisms of color contrast. It is worth noting that color contrast and other contextual effects on color perception are one kind of evidence for linkage between color and form. It is the presence of other colors (including black and white) in a spatial pattern that provides the contextual influence that is so powerful in color perception.
Figure 1.
Color contrast, brightness contrast and color appearance. All four central squares have identical wavelength spectra. Yet the color appearance of each central square can be strongly influenced by the surrounding region. A: the red disk surrounded by an equiluminant green or B: an equiluminant grey results in a saturated red disk. C: when the surround is red but reduced in luminance compared to the central patch then the central red square appears a desaturated pink and almost white. D: The red square on a black background results in the perception of reduced saturation and increased apparent brightness, a bright pink square.
Modules and Streams
In 1985, the beginning of the period we are reviewing, the most widely-publicized view was that color, motion, and form were analyzed in parallel by separate visual cortical modules fed by parallel streams emanating from the retina. This “segregated-color” view was in part a legacy of the ideas of Hering transmitted by Hurvich and Jameson (1957). The “segregated-color” or modular view was also influenced by the work of Krauskopf and colleagues in their work on cardinal directions in color space (Krauskopf et al, 1982; Derrington et al., 1984). We will review briefly the neurophysiological results on the visual cortex from the late 1980s that were interpreted in terms of the modular view (after Hubel and Livingstone, 1987 and Zeki and Shipp, 1988) and then move on to reviewing more recent research on color signals in the cortex that either still reflects the modular view or that supports a more integrated view of color and form (reviewed also in Gegenfurtner and Kiper, 2003; Gegenfurtner, 2003). Since most studies on color in the cortex are related to trichromatic processing in primates, including humans, most of this review concerns studies that investigate cortical processing in the monkey or human brain.
"Segregated-color" or Modular view
The modular view was emphasized in the work of Semir Zeki on the functions of extra-striate visual cortex (Zeki 1973,1978a,b). Zeki reported that different extra-striate regions in the macaque monkey cortex (Figure 2A) were specialized for different visual features. For instance, V5 (also called MT) cortex was reported to be comprised of neurons almost all of which were directionally-selective. Thus V5 was viewed as the motion area, the region of the visual cortex designed to respond to motion in the visual scene. On the other hand, cortical area V4 was reported to contain mainly neurons that were color-responsive.
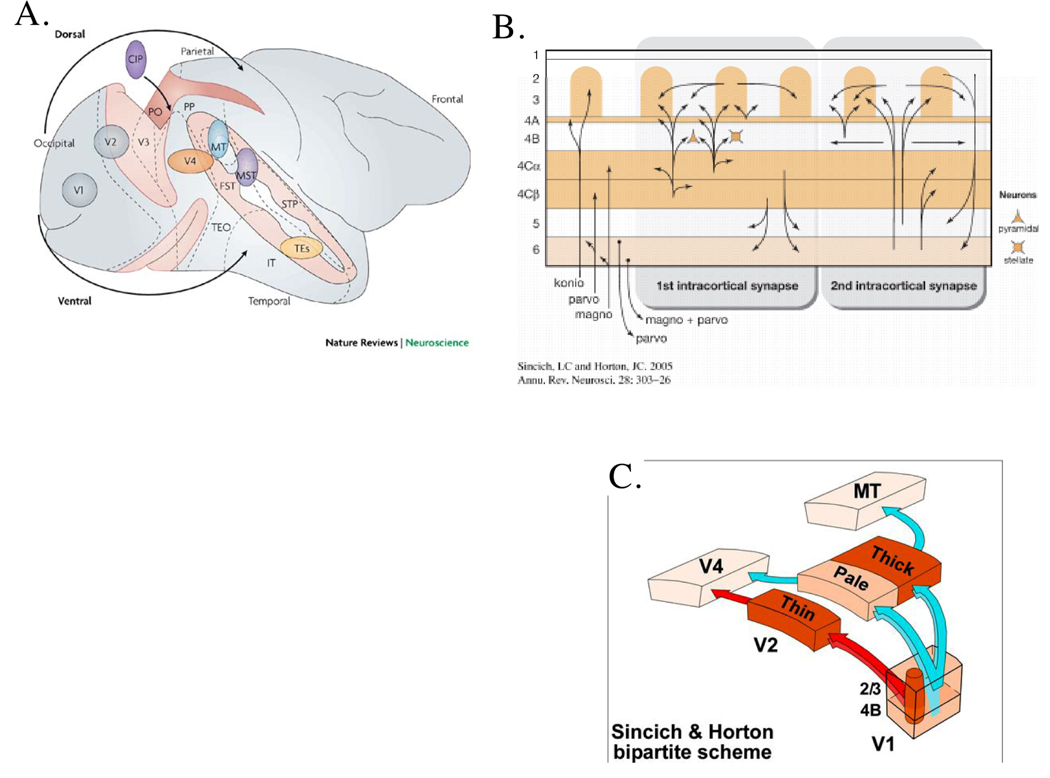
Figure 2.
A: Lateral view of the Old-World monkey brain. The striate (V1) and extrastriate regions are shown on a surface where the sulci have been “opened” to allow viewing of the borders between regions that are otherwise embedded in a sulcus. The regions in the upper parietal region of extrastriate cortex form the dorsal stream whereas those in the lower temporal region form the ventral stream. B: A schematic view of the processing in V1. The darker brown regions indicate the cytochrome oxidase (CO) rich regions of V1 that correspond to layers receiving the stronger input from the lateral geniculate nucleus (LGN), the light brown shows an intermediate level of CO staining. The input streams from the different divisions of the LGN magnocellular (magno or M), parvocellular (parvo or P) and koniocellular (konio or K) are shown at the lower left of the figure. The main axonal projections within the cortex (intracortical projections) are shown as the 1st and 2nd intracortical projections in the center and right of the figure. The cytochrome rich regions of V1 in layer 3 and lower 2 are referred to as “CO blobs”. The regions between blobs are the interblobs. C: The axonal projections to the first extrastriate visual area V2 from neurons in the CO-rich blobs (dark brown barrels in V1) project preferentially to the thin CO-rich stripes. The axonal projections from the interblob regions in V1 (turquoise) preferentially project to the CO poor (pale) and thick CO-rich stripes in V2. The further projections from the V2 regions are shown to visual area V4 and MT (V5). These two extrastriate areas are those that were identified by Zeki as the ‘Color’ area (V4) and the ‘Motion’ area (MT or V5).
A comprehensive analysis of motion perception is beyond the scope of this review, but it is worth considering briefly the modular view of motion processing in comparison with modular color processing. The modular view of the brain’s separate and independent analysis of color and motion has been transformed by later research (see reviews by Cropper and Wuerger, 2005; Shevell and Kingdom, 2008). Many of the results from studies in the last 25 years indicate that motion perception can be accomplished with chromatic stimuli indicating links between color and motion. However, the relative contribution of different classes of motion detectors – luminance or chromatic – to direction and speed judgments is still unresolved. Two very different kinds of experimental result also indicate that there is more interaction between color and motion than the modular view implies. First is the classical result of Wallach on colored plaid patterns (Wallach, 1935; English transl. Wueger et al 1996). Wallach introduced the notion of motion transparency in plaid patterns among his many studies of the interdependence of pattern and motion perception. He investigated color and form and motion when he used plaid patterns composed of overlapping gratings of red and green stripes. The grating stripes were not equiluminant and therefore provided both chromatic and luminance signals. When the color plaids had just begun to move, the plaid motion appeared coherent and the regions of overlap of the red and green stripes appeared gray because of color mixture. After observers looked at the plaids for a longer time, motion coherency disappeared and the stripe patterns appeared to slide with respect to one another, a phenomenon that Wallach (1935) called motion transparency. During motion transparency, the color appearance of the intersections of the green and red stripes no longer looked gray but instead took on the color of the stripe pattern that appeared in front, sometimes red and sometimes green. Wallach (1935) concluded that the motion percept, either coherent or transparent, was linked, respectively, with the color percept, either color mixture or color transparency. This was part of Wallach’s overall thesis (Wallach, 1935 was his PhD thesis) that motion perception and form perception were linked not parallel and independent. The second result that indicates a departure from modularity of color and motion is the finding that there are strong reciprocal synaptic connections between areas V4 and V5 (MT) (Ungerleider et al, 2008), putatively the independent color and motion areas in the modular view.
Returning to the arguments in favor of modularity, we consider Zeki’s later work that analyzed the differences between V4 and V1 (Zeki, 1983a,b). In the 1983 papers, Zeki compared color responses in the monkey cortex with human color perception with experiments in which wavelength distribution and perceived color were dissociated by surrounding context. Zeki reported that while a fraction of V1 cells responded to the wavelength distribution of a target in their receptive fields, only V4 cells responded to the perceived color of the target in experiments in which context was manipulated. Based on these results and his earlier work on the functional specificity of anatomical areas, Zeki proposed that V4 was a color center in the monkey brain.
The results on a color center in the monkey brain were extended during the last quarter century by Zeki and his colleagues in studies of human brain responses to color, utilizing imaging of brain activity with PET (Lueck et al, 1989) and fMRI (Bartels and Zeki, 2000). The studies of a color center in the human brain were motivated by the phenomenon of cerebral achromatopsia (reviewed in Zeki, 1990). Cerebral achromatopsia is the name given to the condition caused by lesions in ventral occipital cortex that cause a loss of the ability to recognize colors without the loss of the perception of form and motion. Achromatopsia has been interpreted as strong evidence for the modular view of color vision because the brain areas where lesions cause achromatopsia are near if not identical to the area or areas identified as the color center of the human brain. We will return to consider achromatopsia and the color center of the brain later when we review the recent literature on color processing in human cortex.
The famous studies of V1 and V2 cortex in monkeys by Livingstone and Hubel (1984, 1988; Hubel and Livingstone, 1987) supported the modular concept and linked it to parallel processing in the retina and the lateral geniculate nucleus (LGN). The landmark studies of DeValois (1965) first suggested that color-opponent neurons existed in the primate visual system and could be important for color vision. De Valois described single-opponent neurons that have opponent inputs from two or more cone photoreptors. There are two main categories of single-opponent cells. First, the L-M or M-L cells that receive input from long wavelength (L) cones opposed by signals from middle wavelength (M) cones: the L/M opponent cells are sometimes called red/green opponent. Second are the category of cells that receive signals from the short wavelength (S) cone opposed by a neural signal that is the sum (L+M) of L and M cone signals. The S/(L+M) opponent cells are sometimes referred to as blue/yellow opponent cells. Wiesel and Hubel (1966) found that color opponent LGN cells were found in the Parvocellular layers of the monkey LGN while Magnocellular layer neurons were largely color-blind. Later work on the LGN supported the idea that the Parvocellular stream from retina to LGN carried red-green opponent signals while the Magnocellular stream conveyed signals about low luminance contrast (Kaplan and Shapley, 1982, 1986; Derrington et al., 1984; Lennie and D'Zmura 1988; Reid and Shapley 1992, 2002; Benardete and Kaplan, 1999; Chatterjee and Callaway, 2003). Subsequent work revealed that there was a third retina-->cortex stream, the Koniocellular pathway (Casagrande, 1994; Hendry and Reid, 2000) that innervated V1 directly and in parallel to the Parvocellular and Magnocellular pathways (Figure 2B; Hendry and Yoshioka, 1994; Casagrande et al, 2007).
Livingstone and Hubel (1984, 1988) hypothesized that Parvocellular signals were further subdivided in V1 cortex into two separate streams, one for color processing, localized in the CO patches (Wong-Riley, 1979; Horton and Hubel, 1981) or blobs, and one for form processing in the inter-blob regions of layers 2/3 – layers that provide a substantial part of the output of V1 to extrastriate visual areas (Figure 2C). The Livingstone-Hubel hypothesis projected the parallel Parvo and Magno streams out of V1 into the CO stripe compartments in V2 cortex (see Sincich and Horton, 2005; Federer et al, 2009; Sincich et al, 2010 for recent anatomical studies on compartmentalized V1 to V2 projections). Our concerns in this review are about color signals and color processing so we will not delve into the anatomical details of the Livingstone-Hubel hypothesis but we do want to underline that it was a specific version of a modular hypothesis.
There is one specific component of the Livingstone-Hubel hypothesis that we focus on in our review: the cortical mechanism(s) that make color and brightness contrast such strong determinants of perceived color. Foremost among all mechanisms is the proposed “double-opponent” cell. Such a hypothesized neuron is double-opponent because it is affected by opposite-signed inputs from different cones (cone opponency) and also opposite-signed inputs from cone-opponent inputs at different locations in the cell’s receptive field (spatial opponency). Thus, at one place in the double-opponent cell’s receptive field there might be +L-M cone inputs while at another location there might be −L+M inputs. The defining characteristic of a double-opponent cell is that it is strongly responsive to color patterns but weakly or non-responsive to full-field color stimuli (or color stimuli of low spatial frequency, or shallow gradients of color). Livingstone and Hubel (1984, 1988) reported that roughly half of cells in the CO blobs in layer 2/3 of V1 cortex were color-responsive, and that most of the color-responsive cells in the blobs were double-opponent cells. The double-opponent cells were described as strongly responsive to color bars but insensitive to full-field color stimuli. The segregated-color channel from CO blobs to V2 thin stripes was therefore conceived as carrying color-contrast signals from the double-opponent cells in CO blobs.
Besides their sensitivity to color contrast, another feature of the double-opponent cells in the CO blobs described by Livingstone and Hubel (1984; Hubel and Livingstone, 1987) was that the cells’ receptive fields were roughly circularly-symmetric and that the cells responded at all orientations of a (colored) bar. But in the same studies, color-responsive neurons that were orientation-tuned were found in the inter-blob regions of layer 2/3 in V1 cortex. Therefore, Livingstone and Hubel hypothesized in their tripartite model that signals about color that affected the neural computation of form were carried by the interblob cells' projections (mainly to the CO pale stripes in V2) while signals about color appearance were carried via the double-opponent blob cell projections to the thin CO stripes in V2. The lack of orientation tuning of the double-opponent color cells in the CO blobs was an important part of the role they were supposed to play in the tripartite model (cf. Sincich and Horton, 2005). In the mid 1980s it was thought that the neural pathways and networks that computed color appearance were spatially-isotropic systems (e.g. Grossberg and Mingolla, 1985). Orientation-untuned color signals made sense in that conceptual framework.
Now that we have set the stage for a review of the last 25 years, we will list the topics covered in our review as open questions that were discussed and debated and studied over the last quarter century.
OPEN QUESTIONS FOR THE QUARTER CENTURY OF RESEARCH 1985–2010
In the period 1985–2010, responding to what was known about color in the cortex in 1985, visual neuroscientists have focused on answering the following questions:
What is the relative responsiveness of V1 cortex to color vs black-white stimuli?
How specialized are the color and non-color cells in V1 cortex?
What are the spatial receptive field properties of color-responsive neurons in primary visual cortex?
How segregated within the V1/V2 network are neurons that respond to color from those that respond to form?
How are blue-yellow color signals processed in the visual cortex?
Are there signs of color contrast in the responses of neurons in early visual cortex?
Is V4 the center of color signal processing in the macaque monkey cortex?
Is there a color center in human cortex?
Answering all the above questions has led to a very different view of cortical color processing in 2010 from what formed the conceptual framework of visual neuroscience in 1985. We will consider the answers to all these questions in sequence below, but it is important to state at the outset of the conceptual journey major new ideas that guided our thinking: namely (1) the new understanding that V1 plays an important role in color perception through the combined activity of two kinds of color-sensitive neurons, single-opponent and double-opponent cells, and (2) the possible importance for color perception of population coding of color in cortical neuronal populations. About point 1, single- and double-opponent V1 neurons are part of a cortical organization that extends from V1 all the way to inferotemporal cortex. Single-opponent and double-opponent cells have different functions. The single-opponent cells respond to large areas of color, and to the interiors of large patches. Double-opponent cells respond to color patterns, textures, and color boundaries. For perceiving the color of objects and for understanding color pictures, the double-opponent cells are probably most important. For responding to scenes and color atmospheres, single-opponent cells have an advantage. Color contrast would be an aspect of perception that depends on double-opponent cells. Color assimilation and color-spreading (Jameson and Hurvich, 1975; De Valois and DeValois, 1988) are perceptions that may derive from the activity of single-opponent cells. It will be a challenge for the future to find out what is the relative magnitude of the contribution of single- and double-opponent cells to the perception of color in visual scenes.
Point 2 about population coding is more of a suggestion for future work than a completed project. As discussed below, Wachtler et al (2003) and Lehky and Sejnowski (1999) have suggested that color should be understood as encoded in the distribution of activity across the visual cortical population of neurons. This is a suggestion worth further investigation. In direction coding and motor control the idea of population coding has been fruitful. Similar ideas might enable vision scientists to comprehend the remaining mysteries of neural encoding of hue and saturation by moving away from the single neuron point of view to the population's.
Now we embark on the consideration of the specific answers to the specific questions scientists have posed during the preceding quarter century.
What is the relative responsiveness of V1 cortex to color vs black/white stimuli?
It was not clear in 1985 how much of the primary visual cortex was devoted to color vision and so the first question to ask was, "What is the relative responsiveness of V1 cortex to color vs black/white stimuli?" DeValois (1965) and Wiesel and Hubel (1966) among others had established that a large signal about color was coming into V1 via the Parvocellular pathway. Derrington et al. (1984) had studied Parvocellular LGN cells in detail and found that almost all Parvocellular cells were cone-opponent and color-responsive. However, it was known also that Parvocellular cells could respond to black and white, especially to patterned black and white stimuli such as bars, edges, and grating patterns, as shown by among others DeValois and Pease (1971). Some early studies of V1 indicated that most V1 cells responded better to black and white stimuli rather than to color. For example Hubel and Wiesel (1968) reported over 80% of V1 cells responded to spatial patterns independent of color. However, other early studies revealed a substantial fraction of V1 cells were sensitive to the color of the stimulus (Dow and Gouras, 1973; Vautin and Dow, 1985). Thorell et al (1984) stated that almost 80% of macaque V1 cells were color-selective.
Over the last 25 years opinion has changed and now seems to be settling on the conclusion that there are many color-responsive and color-selective cells in macaque V1. The paper by Lennie et al (1990) sent a complicated message about the issue of how many cells in V1 were color-responsive. They reported that most V1 cells they studied preferred achromatic stimuli, in agreement with Hubel and Wiesel (1968). However, Lennie et al (1990) calculated that most cells they studied received cone-opponent inputs and that there were few non-opponent cells in V1. In retrospect these apparently contradictory statements may be reconcilable at least in part if one takes into account the relative cone contrasts of the stimuli of equivalent length in the stimulus space used by Lennie et al (1990). The cone contrast of an achromatic stimulus of unit length was approximately 20X greater than the unit of red-green contrast along what was called the “constant-Blue” axis in the Lennie et al (1990) study. If a cortical neuron had equal sensitivity for red-green and achromatic stimuli, in terms of cone contrast, the direction of its preferred stimulus in the DKL color space used by Lennie and colleagues (Lennie et al, 1990; Derrington et al., 1984) would have been very close to the achromatic axis. Therefore, such a neuron might appear to prefer achromatic stimuli.
Other studies were clearer about there being a majority of color-responsive neurons in V1 cortex. Victor et al. (1994), recording local field potentials (LFPs) in macaque V1 with multi-electrode probes, found LFP responses to equiluminant red-green modulation both in upper and lower cortical layers at a majority of recording sites. Leventhal et al (1995) found that a large fraction of cells in the upper layers of macaque V1 were color-sensitive. Single-unit studies undertaken in the 21st century benefited from the insights derived from fMRI studies of human V1 that indicated that human V1 responded very strongly to red-green color contrast (Engel et al, 1997; reviewed below). From our own observations (with Elizabeth Johnson) we estimated that about 40% of all macaque V1 cells we recorded were color-selective but this percentage rose in layer 2/3 where 60% of the cells were sensitive to the color of the stimulus (Johnson et al, 2001, 2004, 2008). Friedman et al. (2003) found a very similar percentage, 64%, of color-selective cells in layer 2/3 of macaque V1.
Many fMRI results comparing color and luminance responses in human V1 indicated that color responses were larger. For instance, Engel et al (1997) found that the strongest fMRI modulation in human V1 was caused by red-green modulation that evoked opposite-signed L and M cone responses (Figure 3). The data were plotted as criterion response contours in cone contrast space. V1 response contours were approximately elliptical with the minor axis of the ellipse oriented in the M-L direction (Figure 3) meaning response/cone contrast was greatest in the L-M direction and least in the L+M direction. These results supported the concept that human V1 was strongly tilted for color, a result that has been replicated over and over again in many different laboratories (Kleinschmidt et al, 1996; McKeefry and Zeki, 1997; Hadjikhani et al 1998; Beauchamp et al 1999; Brewer et al., 2005; Mullen et al, 2007; Wade et al., 2008). One might question what the fMRI signal is measuring because the relation between fMRI and neuronal activity is still a controversial topic. But there is a good argument for taking these fMRI results seriously because it has been established that the magnitudes of the fMRI signals are highly correlated with human behavioral performance in pattern detection (Engel et al, 1997) and adaptation to color contrast (Engel and Furmanski, 2001).
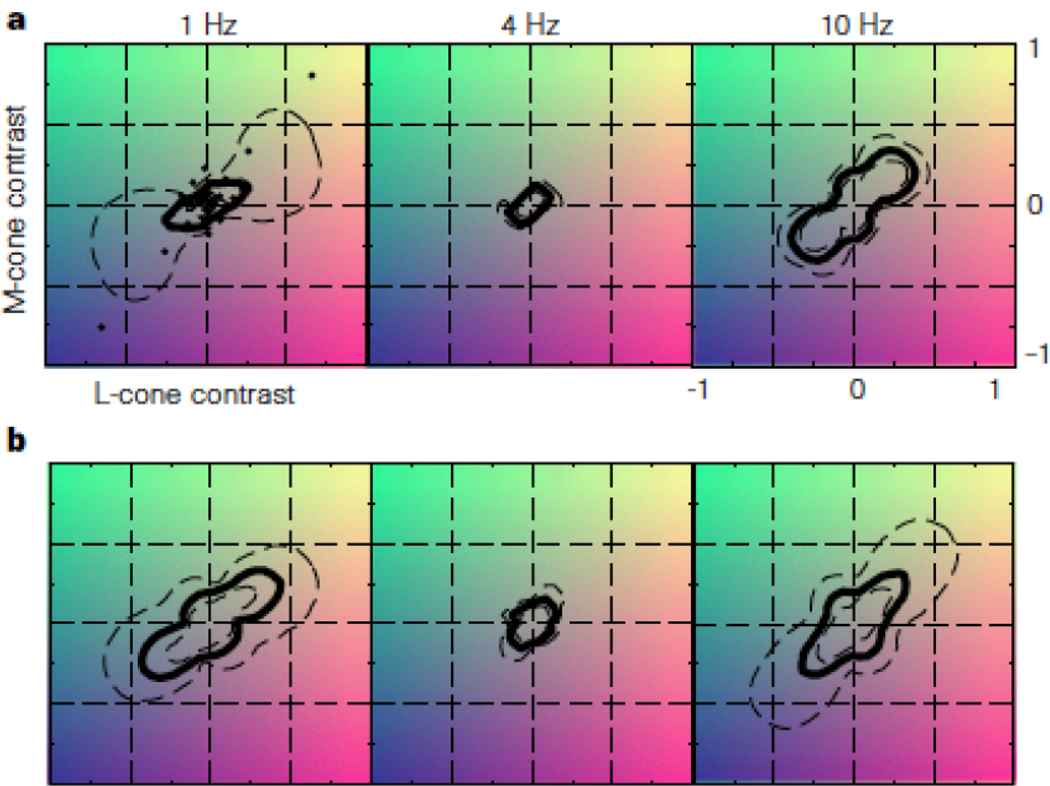
Figure 3.
Human fMRI responses in cone contrast space from Engel et al, 1997. The amount of contrast required to reach a criterion response is plotted for 18 different stimulus color directions – shown by the small dots in the panel labeled 1Hz in a. Each of the panels shows the responses modulated at three different temporal frequencies: 1, 4 and 10 Hz for two subjects. The data for BW is shown in the row marked a, for SE in the row marked b. The shape of the threshold contour is reciprocal to the relative strength of the response in different color directions. When the contour is close to the origin it means that the amount of contrast required to evoke a criterion response was low. If the points on the negative diagonal are closer to the origin than the points on the positive diagonal it means that the opponent L-M mechanism is more sensitive than the luminance L+M mechanism. The L-M opponent mechanism is more sensitive than the luminance mechanism for 1 and 4 Hz. Similar results were obtained in psychophysical experiments.
How specialized are the color and non-color cells in V1 cortex?
Another question that persisted and that led to substantial new research is, How specialized are V1 neurons for color? The modular viewpoint would predict that the only cells that contribute to color perception are cells highly specialized for color detection and highly selective for different colors (reviewed in Gegenfurtner and Kiper, 2003). An exemplar of this approach is the study of color responses in V1 and V4 neurons by Zeki (1983a,b). In that study, Zeki selected from the population of V1 neurons only those cells that gave a bigger response to color than to black-white stimuli. Then he found that these cells were not really color-selective but rather wavelength-selective, as described earlier. This is the line of reasoning that led Zeki to conclude that V1 was not really responding to color.
Others have adopted a somewhat different viewpoint, for instance Livingstone and Hubel (1984, 1987, 1988) who hypothesized there were V1 cells that contributed to color perception: the double-opponent cells in cytochrome oxidase (CO) blobs. Livingstone and Hubel proposed that V1 had a definite role as the initial stage of cortical color processing through the signals provided by the circularly-symmetric, color-selective double-opponent cells. However, they did not offer evidence that the cells in the blobs were more sensitive to, or more selective for, color than cells located elsewhere in layer 2/3 or in other V1 layers.
Some more recent studies have also focused on V1 cells that respond much more to color than to achromatic stimuli (Conway 2001; Conway et al. 2002; Conway and Livingstone 2006). Here as in Zeki's (1983a,b) studies, neurons were selected for investigation if and only if they responded best to color. The criterion used to screen cells for color selectivity in Conway (2001) and Conway and Livingstone (2006) was opposite signs of response to the same type of cone-isolating stimuli used in the main experiments: so-called sparse noise (Reid et al, 1997) i.e. spots flashed briefly at random locations in the visual field. The only comparison between “color” cells and non-color cells was at the screening stage. Data about the “non-color” cells were not presented. The studies of Conway (2001), Conway et al (2002), and Conway and Livingstone (2006) were motivated by the modular point of view. Their idea was that the only cells that contribute to color perception were those that were highly specific in responding to color and not to achromatic visual stimuli.
Other investigators found many color-responsive neurons that were also selective for spatial patterns. For example, Lennie et al (1990) reported the existence of many neurons that were responsive to equiluminant color stimuli and also tuned for spatial frequency, as indeed had Thorell et al, (1984) earlier. Lennie et al. (1990) studied all neurons they encountered in V1 with sinusoidal grating patterns of optimal spatial frequency that varied over a range of directions in DKL color space (Derrington et al., 1984). Although their experimental approach was very different from Zeki's, like Zeki (1983a,b) Lennie et al (1990) hypothesized that the only V1 neurons that were important for color perception were the small percentage of neurons they found that responded much more strongly to equiluminant color than to black-white grating patterns and that were most responsive at low spatial frequencies. We would now call this class of cells the cortical single-opponent cells. The idea that color perception depends on this small group of strongly color-preferring neurons is inconsistent with the integrated-color viewpoint articulated later by Lennie (1999) though the old idea resurfaced in the review by Lennie and Movshon (2005).
The integrated-color viewpoint was represented unambiguously by Leventhal et al (1995). They tested all neurons they encountered in the upper layers (2–4) of macaque V1 with a battery of visual tests of sensitivity for color, direction of motion, orientation, and also with different spatial patterns: gratings, bars, and spots. Then they compared orientation selectivity and direction selectivity between neurons that were more or less color selective. In retrospect this seems a logical requirement for understanding how specialized the “color” neurons were. Leventhal et al.’s well-known result was that the neurons they studied were selective on many dimensions. Cells sensitive to color were orientation-selective approximately as much as the cells that were unselective for color (Figures 9, 11 in Leventhal et al., 1995).
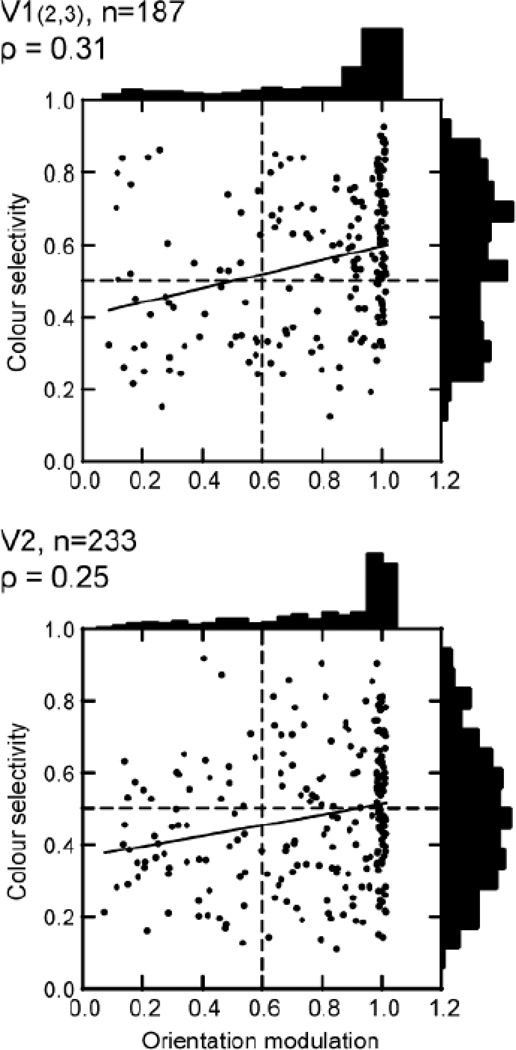
Friedman and colleagues (2003) used flashed, uniformly-colored, geometric figures as stimuli to study color coding in awake macaque monkeys. They concluded that "contrary to the idea of feature maps, colour, orientation and edge polarity are multiplexed in cortical signals". They studied the response of cells in V1 upper layers and V2 to squares or bars of color (including neutral gray, white and black) flashed in different positions with respect to cell receptive fields, on neutral gray backgrounds. Using several different indices of color selectivity, Friedman et al (2003) found a substantial proportion (64%) of color-selective neurons in the upper layers of V1, and most of these were edge-sensitive. The proportions of color-selective, edge-sensitive cells were very similar to the estimates of color-luminance cells in the upper layers of V1 measured in our laboratory (Johnson et al 2001). Friedman et al (2003) also found a smaller proportion of what they termed color-surface-responsive cells, so-called because these cells were not especially sensitive to edges. They found that the edge-responsive color cells were mostly orientation selective, while the surface-responding cells were not selective for orientation. They stated," the vast majority of colour-coded cells are orientation tuned." This parallels Leventhal et al’s (1995) findings about orientation selectivity in color-responsive cells in the upper layers of V1. Friedman et al’s findings seem quite congruent with Leventhal's about the large numbers of color responsive cells in layers 2/3 of striate cortex. Also like Leventhal et al (1995), Friedman et al (2003) specifically analyzed the correlation between orientation selectivity and sensitivity to color and found little correlation in upper layers of V1 or in V2 cortex as illustrated in Figure 4 (from Friedman et al, 2003). In fact, as the authors note, the weak correlation they found (Fig.4 top) was slightly positive, meaning that high selectivity for orientation was slightly more likely when there was high sensitivity for color, a result opposite from that expected from the modular hypothesis.
Figure 4.
Color and orientation selectivity in V1 and V2 from Friedman et al (2003). Selectivity for stimulus orientation (x-axis) and for color (y-axis) is shown for neurons recorded in V1 and V2 of the awake monkey (from Friedman et al 2003). The orientation modulation was measured with bars of the optimal color oscillating across the receptive field at 1 Hz. The Orientation Modulation index was calculated as [Rmax − Rmin]/[Rmax + Rmin]. An Orientation Modulation index of 1 indicates that there was no response at the orientation 90 degrees to the optimal orientation, and an index close to zero means that the neuron was untuned for orientation. The Color Selectivity index was calculated as the relative response to 15 flashed bars of different colors. A Color Selectivity index of 0.93 indicates a response to only one of the colored bars, whereas an index close to zero indicates an equal response across all colors. There are many orientation-selective neurons that are also color-selective in both V1 and V2 shown by points in the upper right quadrant of both the panels.
In our own work (with Elizabeth Johnson; Johnson et al, 2001) we studied all visually responsive single neurons that we recorded in macaque monkey V1 and compared color-sensitive and color-insensitive neurons' responses to spatial patterns of color and luminance. We attempted to equate colored and black-white stimuli for average cone contrast so that their effectiveness in driving V1 neurons could be compared quantitatively (in this way following a procedure similar to Thorell et al, 1984). In order to compare relative color sensitivity across the population of neurons, we assigned to each neuron a single number, its sensitivity index, defined as a ratio: I=max{equilum response}/max{lum response}. The index I was distributed broadly ranging from 0–64. High values indicated preference for colored stimuli compared to achromatic. We divided the population somewhat arbitrarily into three groups: luminance-preferring (I<0.5); color-luminance cells (0.5<I<2); and color-preferring (I>2). A majority (60%=100/167) of V1 cells sampled were luminance-preferring, while color-luminance cells were about 29% of the total. The color-preferring cells were only 11% of the cells recorded, in agreement with previous studies. The percentage of color-luminance cells was higher in layer 2/3 where more than 50% of the cells we recorded were color-luminance cells. Solomon and Lennie (2005) replicated the parcellation of V1 cells into these three groups.
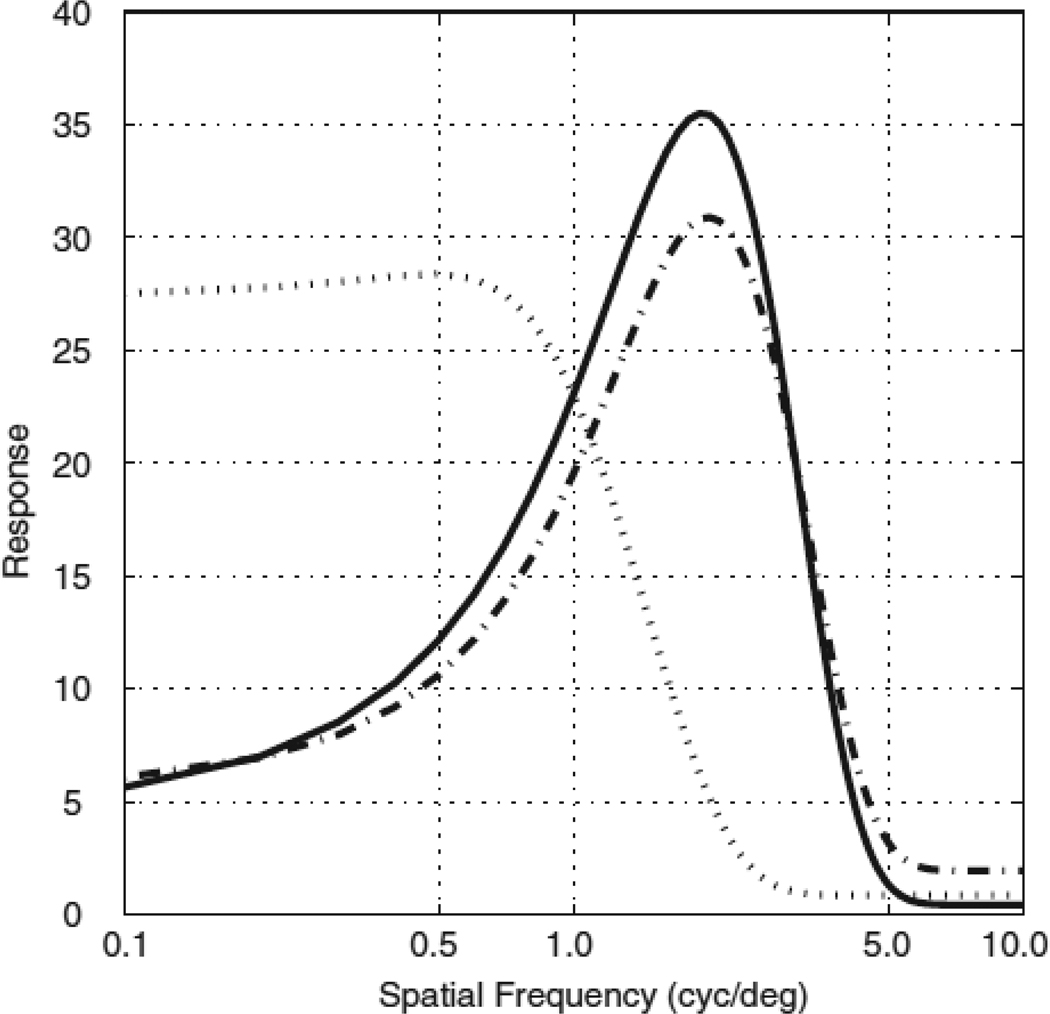
Color-luminance cells were spatially tuned for equiluminant and also for black-white grating patterns (Johnson et al, 2001). In fact, the spatial frequency preference and bandwidth for a color-luminance cell was approximately the same for black-white or red-green equiluminant patterns (cf. Thorell et al, 1984). Most color-preferring cells were not spatially tuned for equiluminant grating patterns; they preferred the lowest spatial frequency (Lennie et al, 1990; Solomon and Lennie, 2005). The population-averaged spatial frequency tuning curves for luminance-preferring, color-preferring, and color-luminance cells are drawn in Figure 5 (from Schluppeck and Engel, 2002). There are (at least) three quantitative observations one can glean from Fig.5: 1) Color-preferring cells did not respond to red-green grating patterns at higher spatial frequencies (> 3 cy/deg); 2) Spatial frequency tuning of luminance-preferring cells was similar to that of color-luminance cells in preference and bandwidth; 3) Color-luminance cells responded poorly to color (and luminance) patterns at low spatial frequencies (<0.5 cy/deg). Later (Johnson et al., 2004) we realized that most color-luminance cells were in fact double-opponent cells. We will discuss that topic in the following section about receptive field properties.
Figure 5.
The average spatial frequency tuning for three populations of V1 neurons. The tuning functions were estimated by Schluppeck and Engel (2002) from 230 neurons recorded by Johnson, Hawken, and Shapley. The dotted line represents the responses of the color-preferring neurons. It shows the characteristic low-pass spatial frequency tuning reported in most studies (Thorell et al, 1984; Lennie et al, 1990; Johnson et al, 2001, 2004; 2008; Solomon et al, 2004; 2005). The dashed line shows the responses of color-luminance neurons: cells classified as having robust responses to equiluminant color and to black/white luminance when the stimuli are matched for cone-contrast. Most of the chromatically opponent color-luminance simple cells are double opponent in that they have spatially separated chromatically opponent responses to L- and M-cones (Johnson et al, 2008; Figure 6). The solid line is the average spatial frequency tuning of the luminance-preferring neurons. The maximum responses of luminance cells to luminance patterns are more than twice the amplitude of the best response to equiluminance. The tuning of the color-luminance and luminance cells are bandpass and similar in both preferred spatial frequency (2.56 ± 1.26 cyc/° and 2.09 ± 1.00 cyc/° respectively) and in bandwidth (2.05 ± 0.70 octaves (full width, half height) and 2.96 ± 0.69 octaves respectively).
A proponent of the modular color view might believe that color-luminance cells could not be important for color perception because they send mixed signals about color and brightness contrast (Conway et al, 2002; Lennie and Movshon, 2005). Our own view is that color and brightness are not so segregated in perception as they are in the modular hypothesis. We base our view on the following data: demonstrations like Fig. 1 (the two right-hand panels) where brightness has a strong effect on perceived color; measurements of perceived saturation of chromatic induction reach a peak at minimal brightness contrast (Kirschmann, 1891; Gordon and Shapley, 2006); there is strong masking of color patterns by brightness patterns of the same spatial frequency and orientation (Switkes et al, 1988). The results on brightness-color interactions do not prove the hypothesis that color-luminance cells are involved in color perception, but the data do not rule out the hypothesis.
Another line of work entirely, the study of human visual evoked potentials (VEPs) also implied that color responses in human V1 cortex were produced by color-sensitive neural mechanisms that were spatially tuned. The biggest VEP evoked by a contrast-reversed, equiluminant, grating pattern was evoked by a 3–4 c/deg grating rather than full-field or low spatial frequency (Rabin et al 1994; Tobimatsu et al 1995). These results were consistent with the psychophysical results of, for instance, Switkes et al (1988) and Losada and Mullen (1994) on the existence of spatial frequency-tuned color mechanisms from masking experiments, and the neurophysiology (Thorell et al, 1984; Johnson et al, 2001, 2004) about how most color-responsive cells in early visual cortex, the color-luminance cells, were tuned for spatial frequency. The VEP tuning results also suggested that the low-pass color-preferring cells did not contribute a larger VEP signal than the color-luminance cells because if they had, the VEP would have had it maximal response at the lowest spatial frequency (see Fig. 5). Another VEP experiment on the spatial symmetry of chromatic and achromatic neurons yielded the important result that color-responsive neurons should have the same diversity of spatial symmetry in their receptive fields as non-color responsive cells, and in particular that there ought to be odd-symmetric color-responsive cells (Girard and Morrone, 1995). This prediction from VEP experiments on human observers was completely confirmed in studies of the receptive field properties of single- and double-opponent cells in macaque V1, as discussed next.
What are the spatial receptive field properties of color-responsive neurons in primary visual cortex?
It is an article of faith among neurophysiologists who study the visual cortex that the receptive fields of visual cortical neurons can explain the visual performance of those neurons (Hubel and Wiesel, 1962; DeAngelis et al 1995; among many others). This belief is based on the tremendous success of receptive field analysis in explaining the visual properties of retinal ganglion cells (Hartline, 1940; Barlow, 1953; Rodieck and Stone, 1965; Enroth-Cugell and Robson, 1966). During the period of review (1985–2010) but especially since the year 2000, there was a burst of effort in measuring and analyzing the receptive field properties of macaque V1 neurons responsive to color. The main motivation was that receptive field analysis would solve the problems of understanding how neurons contribute to color signal processing.
Before discussing data and data analyses, we need to consider receptive field models of color-responsive neurons that can be compared to the data (see also Shapley and Hawken, 2002). The spatio-chromatic sensitivity function of the receptive field can be written as
| (1) |
where L(λ), M(λ), S(λ) are the spectral response functions of the L.M,S cones and rL(x,y), rM(x,y), rS(x,y) are the spatial sensitivity distributions for each cone input. In the simplest linear model of vision, the response of a neuron would be the convolution of a stimulus pattern I(x,y,λ) with the spatio-chromatic sensitivity function R(x,y,λ). It may seem obvious but it is important enough to mention that for a neuron that is described by eq. 1, the relative contribution to the total response from signals from the three cones L, M, S, will depend on the spatio-chromatic stimulus I(x,y,λ). In other words, the spatio-chromatic sensitivity function is not in general separable into the product of spatial and chromatic factors (cf. Horwitz et al, 2007).
The simplest color receptive field model is the single-opponent cell model (De Valois 1965; Wiesel and Hubel, 1966) that has been used to explain the properties of LGN cells and retinal ganglion cells that respond to color. The single-opponent model is a special case of Eq. 1 with the following properties.
For single-opponent red-green cells, aS=0, and aL = − aM. approximately.
For single-opponent blue-yellow cells aS= − [aL + aM] approximately.
For single-opponent cells in the LGN, rL(x,y), rM(x,y), rS(x,y) can be approximated by circular 2-D Gaussian functions (Rodieck and Stone, 1965; Enroth-Cugell and Robson, 1966; Reid and Shapley, 1992).
The single-opponent model was tested and found to explain LGN data very well. The spatial parameters that characterize the spatial spread of the cone spatial sensitivity distributions in the single-opponent model are σL,σM, σS. In the LGN, usually the spatial spreads were not identical, that is, usually it was not true that σL= σM. If σL= σM, an LGN cell would be designated as Type II (Wiesel and Hubel, 1966) but when one or the other sigma were larger, the cell would be called Type I. Consistent with Eq. 1, DeValois and Pease (1971) found that Type I cells in macaque LGN had a bandpass spatial frequency tuning function for achromatic (black-white) patterns but were low-pass spatially for equiluminant (red-green) patterns. This result on spatial frequency tuning was a consequence of the spatial receptive field analysis, as in Eq. 1, and it also was a consequence of the fact we noted above, that for any neuron described by eq. 1, its receptive field will change with the color pattern. To a black-white pattern the single-opponent receptive field from Eq. 1 will be a Difference-of-Gaussians, but its spatial sensitivity function in response to a red-green equiluminant pattern will be a sum-of-Gaussians. A Difference-of-Gaussians receptive field will generate a bandpass tuning function for spatial frequency, where the degree of attenuation at low spatial frequency will depend on the strength of the center relative to the surround. A sum-of-Gaussians will generate a low-pass spatial frequency response. Thus, the shape of the spatial frequency response was diagnostic for the receptive field structure of a single-opponent cell. Spatio-temporal mapping of macaque LGN cells with stimuli that were cone-isolating also confirmed the single-opponent model (Reid and Shapley 1992, 2002).
One question is, are there cells in V1 cortex that are consistent with the single-opponent model? The orientation-selectivity of a single opponent cell in the cortex will depend on the 2-D structure of the receptive field. It depends on whether or not the rL(x,y), rM(x,y), rS(x,y) functions of single-opponent cells in V1 are circularly symmetric as in macaque LGN cells, which are not orientation-selective. The answer to this question would be significant because departures from circular symmetry would be expected if a neuron is orientation-tuned, and conversely if the spatial sensitivity distributions were circularly symmetric one would expect the cell to be untuned for orientation (Johnson et al, 2008).
As in the LGN, the first tests of the single- and double-opponent receptive field models in V1 cortex came from experiments with grating patterns (Thorell et al, 1984; Johnson et al, 2001). There were single-opponent cells in V1 and they were the color-preferring cells described earlier (Fig. 5). These cells had low-pass spatial frequency responses to equiluminant red-green grating patterns—like the single-opponent LGN cells studied by De Valois and Pease (1971). However, unlike the LGN cells, they responded very poorly to achromatic patterns of higher spatial frequency. The V1 single opponent cells, like LGN single opponent cells, had nearly equal but opposite inputs from L and M cones, and some received S cone input (Johnson et al, 2004). When the receptive fields of V1 single-opponent cells were mapped with cone-isolating stimuli, the result was that their receptive fields appeared to be Type II and roughly circularly symmetric consistent with their weak to nonexistent orientation selectivity (Johnson et al, 2008).
For double-opponent cells, Eq. 1 still applies but the spatial sensitivity distributions, rL(x,y), rM(x,y), rS(x,y) would not be simply Gaussian functions but rather each of them would be approximated as a Difference-of-Gaussian function. That is, each cone input could be positive or negative at different visual field positions, at different values of (x,y). Furthermore, it was a separate question whether or not the rL(x,y), rM(x,y), rS(x,y) functions of double-opponent cells in V1 were circularly symmetric. If they were circularly symmetric, one would expect that the double-opponent cells would be untuned for orientation but if the spatial sensitivities were elliptical one would expect the double opponent cells would be orientation selective.
The double-opponent cells in V1 cortex were almost all color-luminance cells. Spatial frequency analysis with cone-isolating stimuli, and with equiluminant stimuli, revealed that (most) color-luminance cells were tuned for spatial frequency in the 1–3 cy/deg range (Johnson et al, 2001) and this could only be accounted for in the linear model of Eq. 1 with double-opponent receptive fields. Furthermore, when the receptive fields of V1 double-opponent cells were mapped with cone-isolating stimuli, we obtained results like those in Figure 6 B,C (redrawn from Johnson et al, 2008). The L and M cone inputs had plus and minus regions indicating that there was spatial opponency for each cone. Furthermore, the cone inputs were of opposite sign at each location—therefore cone opponent everywhere. A cell with such a receptive field would be expected to respond strongly to color patterns and edges—but would respond poorly to extended areas of color, or to color patterns of low spatial frequency. This was precisely how the color-luminance, double-opponent cells responded. Most double-opponent cells were orientation-selective for both achromatic and chromatic stimuli (Johnson et al, 2008). There were a few color-preferring double-opponent cells that responded weakly to achromatic stimuli (Johnson et al 2004, 2008).
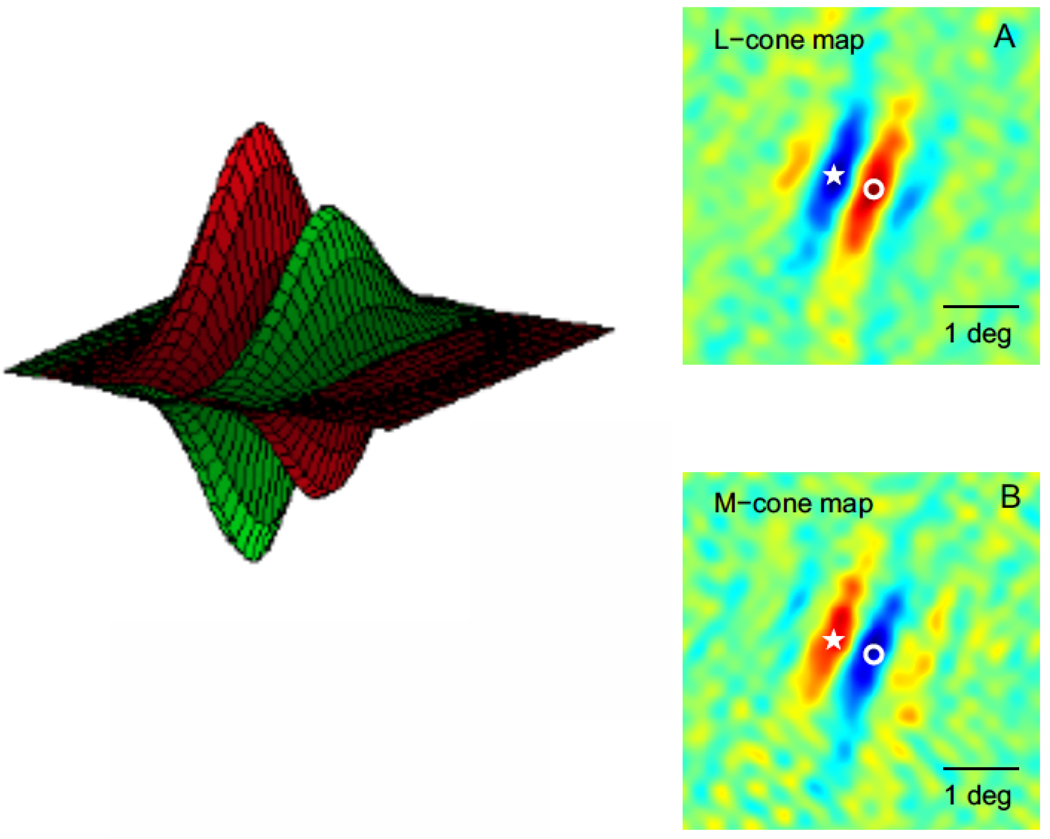
Figure 6.
Double-opponent cells in V1 from Johnson et al (2008). The spatial organization of an orientation-selective, spatial-frequency-bandpass, double-opponent neuron’s receptive field (after Johnson et al 2008). A: shows a schematic receptive field with side-by-side spatially antagonistic regions with opponent cone-weights. The weighting above the horizontal plane is “ON”, where an increment of light will evoke an increase in response, whereas the weighting below the line is “OFF” where a decrement will result in a response. B: shows the two-dimensional spatial map obtained from a neuron in V1 by means of the subspace reverse correlation technique (Ringach et al, 1997) with L-cone isolating grating stimuli. C: the map obtained with M-cone isolating stimuli. At the starred location in B, the L-cone map is decrement excitatory, whereas in C, at the same location the M-cone map is increment excitatory, and vice versa for the location marked by the open circles. The schematic in A is a three dimensional representation of the overlay of the two cone maps to give an overall profile. A is not to scale with respect to B and C.
It is interesting that the spatial maps of the double-opponent cells were not only elongated, but also departed from even symmetry. The example in Fig.6 appears odd-symmetric but other examples appeared neither even nor odd but of mixed spatial symmetry. The spatial symmetries of double-opponent cells resemble those observed for the V1 population as a whole (Ringach, 2002). This was a departure from the classical concept (Daw 1967) of an even-symmetric double-opponent receptive field model. However, odd symmetry or mixed symmetry fits better with the role of double-opponent cells’ responding to color edges. It also fits with the spatial phase analysis of Johnson et al (2004) of the responses of double opponent cells to drifting gratings. The result on the departure from even symmetry also was more consistent with the aforementioned results on the spatial phase dependence of human VEPs in response to equiluminant red-green grating patterns (Girard and Morrone, 1995).
Conway and Livingstone (2006) used a different approach and arrived at substantially different conclusions. They reported roughly circularly-symmetric, roughly even-symmetric, double-opponent cells in macaque cortex. Their experiments involve mapping receptive fields by reverse correlation with cone-isolating stimuli that were flashed, colored squares on a gray background. They selected neurons for study that responded well to the flashed, colored squares and did not report the results on any of the other cells in their large sample. They stated that some of their double-opponent cells were "weakly orientation-selective." Johnson et al (2008) reported the orientation selectivity distributions of double-opponent, single-opponent, and non-opponent cells in V1; the double-opponent cells as a population were orientation-selective but not as selective as the non-opponent cells. Most cells that Conway and Livingstone (2006) called double-opponent had very weak surround effects (see their Fig. 7A). One possible explanation for the weak surrounds is that most of the cells Conway and Livingstone studied were what Johnson et al (2001, 2004, 2008) would classify as color-preferring, single-opponent cells. This conjecture is supported by another piece of evidence: the relative cone weights of L and M cone input. Conway and Livingstone (2006) reported that the relative cone weights, L:M, were roughly 1:−1. This is the cone weight ratio we reported for single-opponent cells (Johnson et al, 2004). The double-opponent cells we studied had a broad distribution of L: M ratios for optimal spatial stimuli ranging from 0.1: −1 to 1:− 0.1, though always negative (Johnson et al, 2004; see also Solomon and Lennie, 2005 – group B cells).
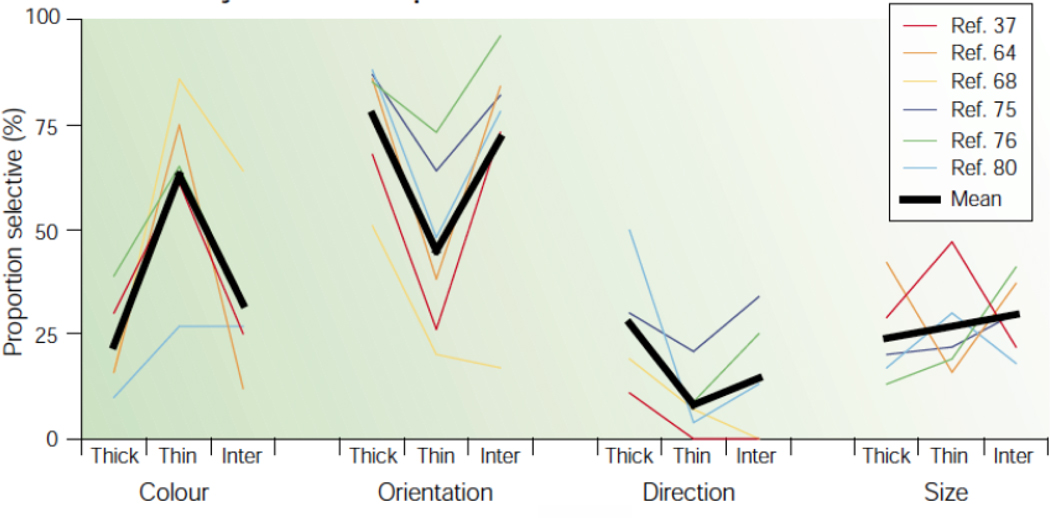
Figure 7.
V2 stripe selectivity from Gegenfurtner (2003). The proportions of cells selective for color, orientation, direction of motion and size in different cytochrome oxidate (CO) compartments (thick stripes, thin stripes and interstripes) of macaque monkey area V2. The data are from six studies 37(DeYoe and Van Essen, 1985), 64(Peterhans and von der Heydt, 1993), 68 (Levitt et al, 1994), 75 (Roe and Ts’o, 1995), 76(Gegenfurtner et al, 1996), 80(Shipp and Zeki, 2002). The heavy black lines represent the means across all six studies. Despite the different methods used in these studies, the results show remarkable agreement.
It is especially important to compare the results of Conway and Livingstone (2006) with the results of Livingstone and Hubel (1984) to mark an inconsistency. As we pointed out in the Introduction, an important property of what Livingstone and Hubel (1984) called double-opponent cells was that they did not respond to large fields of color. The spatial maps of the cells studied by Conway and Livingstone (2006) would be expected to support large responses to full-field color because the receptive field surrounds were so weak compared to the receptive field centers. The cells of 2006 do not explain the result of 1984.
The double-opponent receptive field drawn in Figure 6 would be consistent with the properties of the orientation-selective, edge-responsive color neurons described by Friedman et al (2003). Within the interior of a large color figure, the response of a neuron with the spatial sensitivity distribution drawn in Fig. 6 would be small. But at the edge of such a figure, if properly oriented within the cell’s receptive field, the double-opponent neurons would produce a relatively large response. Thus, it is reasonable to hypothesize that the edge-responsive, color-responsive neurons studied by Friedman et al (2003) are identical with the double-opponent neurons we mapped in Johnson et al (2008). Both groups of neurons were responsive to color and brightness, were edge-sensitive, and orientation-selective. They formed the majority of color-responsive neurons in the upper layers of V1. Furthermore, it is also a reasonable hypothesis that the area-responsive neurons of Friedman et al (2003) correspond to single-opponent cells. Neither single-opponent cells nor area-responsive neurons were edge-sensitive, and neither group was orientation-selective. The area-responsive and the single-opponent cells would produce large responses to the interior regions of large color figures. Both groups, area-responsive and single-opponent, were a small minority of the color-responsive neurons in layer 2/3. While the hypotheses connecting edge-responsive with double-opponent, and area-responsive with single-opponent, seem reasonable, they need to be proved by additional experiments. We believe it would be important to confirm or disconfirm these hypotheses.
The receptive field models we have been discussing only apply to neurons that act in a quasi-linear manner. Nonlinearities could cause edge responses and grating responses to fail to correspond to predictions from receptive field maps. While people who work with color often have favored linear models of cortical function (reviewed in Gegenfurtner, 2003), cortical neurophysiologists have been cautious about assuming linearity without proof (Friedman et al, 2003). It would be desirable in future research to compare responses to multiple stimulus sets to check for the linearity of the cortical network in experiments on color in the cortex because very large nonlinear effects have been observed in other cortical experiments (Yeh et al, 2009).
Cone weights and color spaces
There are many papers in the color-neuroscience literature where responses are plotted in cone contrast space, as in Fig. 3, or in a space whose coordinate axes are the activations of color-opponent mechanisms, DKL space (Derrington et al, 1984; Gegenfurtner, 2003). The concept behind DKL space is that the LGN input to V1 carries signals in three discrete channels that have different color properties that can be characterized by their weighting of cone inputs. Thus an achromatic channel can be conceived of as summing the cone inputs L+M+S. A red-green channel can be conceived as taking the difference L-M or M-L. A blue-yellow channel can be conceived of as combining S-(L+M). The activity of these mechanisms can be used to define directions in color space, the so-called cardinal directions (Derrington et al, 1984). The main conceptual utility of DKL space is to represent stimuli and cell responses in coordinates defined by the LGN input to V1.
But there is a problem with the use of DKL space in this way. Derrington et al (1984) demonstrated that the null planes in DKL space for Parvocellular and Magnocellular LGN neurons were not invariant with the spatial pattern of the stimulus. They found systematic shifts in elevation when they compared responses to 4 cy/deg grating patterns with those to full field. The reason is the receptive field organization of LGN cells. Type I Parvocellular cells of Wiesel and Hubel (1966), where the spatial extent of the receptive field center mechanism is smaller than that of the surround, only responded with the weighting L-M to full field stimuli; the weighting changed significantly for a small spot in the center of the receptive field where the cone weighting was in some cases L-0.1*M. Reid and Shapley (2002) measured Parvocellular cell-cone weights for responses to spots in the receptive field center and for responses to stimuli that fill the receptive field surround, and for full field stimulation; they were all different from each other--as one would expect from the single-opponent model for LGN cells. While there was a fairly narrow distribution of LGN cell cone weights around the 1:−1 ratio for L:M when full-field stimuli were used (Derrington et al, 1984; Reid and Shapley, 2002), the L:M distributions for other spatial stimuli should be much broader because of differences in center-surround ratios and weightings (Reid and Shapley, 2002).
Our conclusion from these well-known, non-controversial facts is that DKL space only characterizes the LGN input to the cortex for full-field stimuli. If spatial patterned stimuli are used, the interpretation of the axes of DKL space as the directions of stimuli that isolate the LGN cell classes is not correct and can lead to misunderstandings. Another way of looking at the problem is that for one given spatial pattern that is not full-field, there will be a very large number of possible color spaces that characterize the LGN input to the cortex, one color space for each different LGN neuron. The ensemble of color spaces will shift for each different spatial pattern. In other words, color scientists, in using a concept like DKL space, sought a low-dimension reduction of the neural input to cortex from the LGN, implicitly assuming that Eq. 1 was separable, which it isn't. One prediction from this line of reasoning is that the distribution of directions of maximal response in DKL space for the V1 population in response to spatial patterns should be a broad distribution, because with a spatially patterned stimulus, V1 will receive LGN input that is broadly distributed in DKL space. This expected result was reported by Lennie et al (1990) and later by Johnson et al (2004).
The change in color directions of the LGN input to cortex with change in spatial pattern content is not such a problem in cone-contrast spaces like those used by Stromeyer et al (1985) and Poirson et al (1990). Spatio-chromatic inseparability of P retinal ganglion cells and Parvocellular neurons affects response contours of LGN cells in cone contrast space but this leads to no confusion because the axes of the space are only meant to represent cone activation not the opponent color mechanisms, as in DKL space.
How segregated within the V1/V2 network are neurons that respond to color from those that respond to form?
One component of the modular view of cortical color processing was the idea that color-responsive and/or color selective cells would be clustered in groups within V1 and V2 cortex, within CO blobs in V1 and within thin CO stripes in V2 (Livingstone and Hubel, 1984, 1988). This is an area of research that has produced controversial results. Studies that have been done with optical imaging of cortical activity have found clusters of red-green color-selective and color-responsive cells in early visual cortex. Optical imaging results on the clustering of blue-yellow cells in V1 and V2 were negative. Studies employing electrophysiological techniques have yielded mixed results on cortical clustering of red-green neurons. As yet, there has been no definitive resolution of the apparent contradictions.
Results in favor of clustering were obtained in a number of optical imaging studies. Landisman and Ts'o (2002a,b) used optical imaging and microelectrode recording combined in a study of macaque V1. They found a significant degree of overlap between CO blobs and regions of heightened color sensitivity but there were many regions of color response outside CO blobs. Moreover, Landisman and Ts’o reported that color domains were usually larger than the CO blobs, sometimes covering two neighboring blobs and the interblob region between. Their electrophysiological recordings were targeted to the color domains identified in optical imaging experiments, and confirmed that there tended to be more color-selective neurons near CO blobs. Lu and Roe (2008) used similar optical imaging techniques and confirmed the presence of patches of color-selective neurons in V1, but their results implied the color patches were more localized to CO blobs than Landisman and Ts’o had found. Lu and Roe (2008)) argued that differences between their study and the earlier results of Landisman and Ts’o might have been caused by signal/noise of the optical signal and by how optical signals were averaged. Lu and Roe (2008) also concluded that color selectivity and orientation selectivity were not localized together in macaque V1. However, this conclusion needs to be reconsidered because of the possibility that orientation preference might be changing more rapidly near CO blobs than far from them, an idea proposed by Edwards et al (1992). The low spatial resolution of the optical imaging technique could confound regions of rapid orientation-preference change with regions of low orientation selectivity. As reviewed below, single-unit studies, which have high spatial resolution, usually have not confirmed that neurons in CO blob regions have poor orientation selectivity (Lennie et al, 1990; Leventhal et al 1995).
Two related papers by Xiao and colleagues reinforce the idea of segregation and localization of color in V1 and V2 (Xiao et al, 2003; Xiao et al, 2007). In their experiments Xiao and colleagues studied the dependence on the hue of the color stimulus of the peak of the evoked response measured by optical imaging. They found systematic hue maps, first in V2 and then in V1 cortex, using differential optical imaging of responses to large squares or full fields of color vs the responses to achromatic grating patterns.
Two points about the optical imaging studies are worth considering. First, the investigators usually used low-spatial-frequency grating patterns to evoke color responses. They calculated optical images as the difference signal between the response to a low-spatial-frequency color pattern and the response to a high contrast black/white grating pattern. The choice of spatial frequency probably meant the color stimuli activated the single-opponent color cells selectively, much more than the double-opponent cells. Second, the image contrast in the optical image could have been a thresholded, nonlinear function of neuronal activity. It is possible that the differences in neuronal response between color and achromatic patterns could have been quite small and the imaging may have exaggerated small differences.
Single-unit recording studies in V1 have provided evidence against segregation of color cells in different regions of V1 cortex. For instance, Lennie et al (1990) asserted that there was no correlation between color-preference and location of a cell in a CO blob. Leventhal et al (1995) stated there was no relationship between receptive field properties they studied and CO staining in upper layers of V1. Leventhal et al (1995) systematically explored CO compartments; they show records of electrode tracks through layer 2/3 of V1 and there was no evidence of clustering of color-sensitive cells in CO patches.
Findings about color and spatial coding are as complicated in studies of macaque V2 cortex as in studies of V1. According to Livingstone and Hubel (1984, 1988), in macaque V2 the thin CO-rich stripes had the most dedicated responses to color and they received direct excitatory inputs from the CO-rich blob modules in V1. The anatomical connections between V1 CO patches and V2 CO thin stripes have been confirmed in later studies (Sincich and Horton, 2005). But based on the reviewed lack of confirming evidence that CO blob cells in V1 are more dedicated to color than interblob cells, one might wonder about the specificity of color processing in V2 thin stripes. Several studies supported the modular function of V2 thin stripes as being specific for color, based on evidence derived from optical imaging (Roe and Ts’o, 1999; Ts’o et al, 2001; Xiao et al, 2003). Other studies found little functional segregation in V2 (Levitt et al, 1994; Gegenfurtner et al, 1996; Friedman et al, 2003). The possible issues with the V2 optical imaging studies have already been raised above in connection with optical imaging of color in V1: the low spatial frequencies of the color stimuli, and the data processing to obtain differential optical images. A possible critique of the single-units studies is that they may have failed to uncover the underlying neuronal organization because of inadequate population sampling. Nevertheless, a summary figure like Figure 7 from the review article by Gegenfurtner (2003) appears to support significant integration of color and other visual properties, and also some segregation of color processing in the thin CO stripes. In Figure 7, data are shown from six different studies of form and color and motion processing in V2 (DeYoe and Van Essen, 1985; Peterhans and von der Heydt, 1993; Levitt et al, 1994; Roe and Ts’o, 1995; Gegenfurtner et al, 1996; Shipp and Zeki, 2002). There is a reasonably good concordance of results and they imply that all three V2 CO compartments are most selective for color and orientation, and rather less specialized for motion direction and stimulus size.
The most convincing result about color and form in V2 came from Friedman et al (2003) who calculated the correlation of color and orientation selectivity on a cell by cell basis in V2 (Fig. 4 bottom), as they did also for V1 cells reviewed above (Fig.4 top). V2 selectivity in the two domains was slightly positively correlated, not negatively correlated as would be expected from the modular viewpoint.
How are blue-yellow color signals processed in the visual cortex?
Most research on color in the cortex has involved studies of red-green color vision that is very salient in monkeys and humans, but the color pathway that is more universal across mammalian species is the neuronal channel that carries blue-yellow signals from eye to cortex (see review by Jacobs, 2008). The koniocellular pathway in primates was proposed as the vehicle for blue-yellow signals to reach cortex (Hendry and Reid, 2000), and direct proof that S-(L+M) signals are carried by the koniocellular cells was provided for the marmoset LGN by Martin et al (1997) and for the macaque LGN by Roy et al (2009). Chatterjee and Callaway (2003) measured what we now know to be the koniocellular input to layer 4A/3B in their study of the laminar pattern of afferent LGN input to V1 in macaques. Buzas et al (2008) studied the laminar distribution of S-cone driven responses in marmoset V1 and found no evidence for clustering of S-cone driven cells in layer 3 blobs; rather the spatial distribution was uniform throughout layer 3.
There is no consensus on the relative contribution of S-cone driven color signals in V1. Some single-unit studies in macaque V1 report relatively weak S-cone input, commensurate with the relative frequency of recording S-cone single-opponent cells in the LGN (Johnson et al, 2004; Solomon and Lennie, 2005) but De Valois et al (2000) reported an enhanced S-cone input in V1. fMRI studies of human V1 also disagree on this point, with Liu and Wandell (2005) reporting relatively weak S-cone driven responses while Mullen et al (2007) report stronger S-cone activity that was approximately as strong as the L-M signal in V1.
A recent paper by Johnson et al (2010) reported studies of S-cone driven signals in the V1 cortex of tree shrews with the technique of intrinsic signal optical imaging and the higher resolution method of two-photon imaging. Tree shrews like many non-primate mammals have dichromatic vision mainly supported by signals from cells that compute S-L signals from the two cone photoreceptors available. Johnson et al (2010) found that tree shrew V1 cells that received S cone input could be color-opponent or not, and could be orientation-selective or not. The degree of color selectivity was not correlated with orientation selectivity, consistent with the results of Friedman et al (2003). The results of Johnson et al (2010) are consistent with the idea that S-cone opponent signals are combined with achromatic signals in cortical double-opponent cells. Support for this conclusion can also be found in the cone weight plots from macaque V1 in Johnson et al (2004).
Are there signs of color contrast in the responses of neurons in early visual cortex, or do signals about color contrast emerge only late in cortical processing?
Color contrast is what is demonstrated in Figure 1. The test square looks redder on a green or gray background than on a red background on which it looks almost white. If color appearance only depended on neurons that were "looking" at the interiors of the test squares, they would all look the same color. But what happens at the edge(s) between target and surroundings has a big influence on color appearance (Gordon and Shapley, 2006).
Zeki (1983a,b) compared V1 and V4 neurons and their sensitivity to color contrast. He concluded that V1 neurons were not responding to color but rather to wavelength. However, since we now have a better understanding of how color-sensitive neurons in V1 can be divided into single-opponent and double-opponent groups, it is worth reconsidering these conclusions. Zeki selected the V1 neurons on the basis that their response to color was stronger than their response to luminance. Probably these were what we term color-preferring, single-opponent neurons. Such a neuron will most likely have a single-opponent receptive field with a lowpass spatial frequency response like that shown in Fig. 5 (dotted line). Therefore, it would likely not be affected by color contrast. However, if Zeki had also examined the responses of orientation-selective, double-opponent cells, we conjecture he would have found the responses of some of them linked to the color of the illuminated targets as were the V4 cells he investigated. The edge-sensitive color cells studied by Friedman et al (2003) should also respond to color contrast at the edge of a target, based on the conjecture that most edge-sensitive color cells were from the same population that we termed double-opponent cells. In other words, it is plausible that the neural basis of color contrast perception (including color constancy) begins in V1 not in extrastriate cortex. This plausible prediction is testable.
Wachtler et al (2003) found that V1 neurons' responses could be affected by color contrast. They studied V1 in the awake monkey with single cell recording. They used as stimuli colored squares that were centered on a cell's receptive field but the squares were chosen to be at least two times wider and longer than the receptive field. The stimuli were stationary and flashed for 500 msec. They measured the effect of changing the surroundings of the stimulus square from mid-gray to different colors systematically arranged around the color circle. Wachtler et al (2003) found small but significant shifts in color preference of the cells that followed the shift in surrounding color, and these shifts were in the same direction as perceptual hue shifts caused by color induction from a surround. This was a very significant finding of color contrast in V1 but we don't yet know whether or not the chromatic shifts might have been much bigger if stimuli had been optimized. For instance, if instead of centering a big square on the receptive field, Wachtler et al had measured the response of a double-opponent cell near the boundary of the target square, perhaps the response would have been much more affected by color contrast. Solomon et al (2004) reported a negative result for a similar experiment where they measured tuning direction in DKL space as a function of the color of a surrounding region, and found no effect.
One innovation introduced by Wachtler et al (2003) may be important for future research. They calculated the neuronal basis for perceived hue as the response of a population of V1 neurons in analogy with the population vector approach of Georgopoulos et al (1986). This was a different way of thinking about what the cortex was doing in its representation of a hue, and moved us away from a strict single neuron viewpoint. In studying color representation in the cortex, many neuroscientists have adopted the single neuron viewpoint only, and that may have prevented us from seeing the forest for the trees. Visual perception could tap many populations of neurons in the visual cortex, and it could make use of many possible computations to extract percepts. The vector average computation is perhaps not the only way population activity can be used to reckon hue or saturation or brightness. For instance, Lehky and Sejnowski (1999) suggested that nonlinear combination of population signals might be utilized to compute the hue of a colored region. Further exploration of the possibilities of population coding for color perception may lead to a new understanding of color in the cortex, and of visual perception as an outcome of cortical population activity.
Is V4 the center of color signal processing in the macaque monkey cortex?
To quote from a classical paper, "Area V4 has been the subject of more interest and more controversy than probably any visual area in extrastriate cortex of the macaque. (Schein and Desimone, 1990)." The reason is that Zeki (1978b) implied that V4 was a color center in the macaque brain and on that conceptual foundation built the modular approach to the visual cortex that has had so much influence on how we think about cortical function. Among others, the work of Schein and Desimone (1990) called the whole modular approach into question. They showed that most V4 cells responded to shape cues as much as if not more than to color. Moreover, they reported that most V4 cells were color-luminance cells, responding almost equally as well to brightness as to color. Since the classical work of Schein and Desimone (1990), many others have studied the complicated and interesting visual properties of extrastriate area V4 in the monkey.
In the 21st century, V4 is viewed as an important extrastriate area for the analysis and synthesis of visual form. The idea of "centers" may be out of date, but if V4 is the center of anything, it is not color but form. There are many lines of evidence. Lesions in macaque V4 cause deficits in shape discrimination (Walsh et al, 1992). Single cell analysis in V4 was used to reconstruct a population code for visual shapes (Pasupathy and Conner, 2002). Many more experiments supporting the functional role of V4 in shape perception are reviewed in Orban (2008). There is even a very recent paper that found that V4 played an important role in the perception of shape from motion (Handa et al 2010). Therefore, though V4 probably does contribute to color perception, V4's activity is likely to integrate color and form information for perception, an argument against V4's role in a segregated-color processing scheme.
The idea of V4 as a color center seems to live on as an example of truthiness that is unaffected by accumulating factual counterevidence. Nevertheless, we are obliged to review one other kind of evidence against V4 as the color center in the macaque brain--the evidence of lesions of macaque V4 and their effect on color performance. The concept of V4 as a color center was strongly supported by the phenomenon of achromatopsia in humans, as reviewed earlier in this paper. If V4 in macaque was the color center, as hypothesized, then lesions of V4 should have produced in monkeys a condition like human achromatopsia. But they did not (Heywood et al, 1992; Cowey and Heywood, 1995). To quote: " ablation of cortical area V4, …, produces only mild impairments in colour discrimination" (Cowey and Heywood, 1995). Even more than that, lesions anterior to V4, in posterior inferotemporal cortex but sparing V4, produced a macaque behavioral syndrome that resembled achromatopsia in humans (Heywood et al, 1995). Other, independent, lesion experiments confirmed the powerful effects on color vision of lesions to inferotemporal cortex (Huxlin et al, 2000). Thus there is support from the lesion work for the idea that in inferotemporal cortex there may be a color area important for perception--but that color center is not in V4.
Support from physiological experiments for the importance of inferotemporal cortex in color vision has been accumulating for decades and there also was a burst of activity recently on this topic (Komatsu et al, 1992; Takechi et al, 1997; Komatsu, 1998; Koida and Komatsu, 2007; Conway and Tsao, 2006; Conway et al, 2007; Yasuda et al, 2010). The posterior inferotemporal (PIT) cortex in the macaque monkey contains many highly color selective neurons that also are somewhat shape selective (Conway et al, 2007; Yasuda et al 2010). Earlier, Komatsu and colleagues explored the color selectivity in anterior inferotemporal cortex where they found many cells that were highly selective for hue. An fMRI study of macaque brain revealed that there were multiple, distinct color-selective regions in IT cortex consistent with the electrophysiology both anterior and posterior in IT (Harada et al, 2009). The fact that the color "centers" are located in inferotemporal cortex in monkeys may be very significant in interpreting the functional role of these areas in visual perception. We will take that up next when we consider color responses in the human brain.
Is there a color center in human cortex?
Imaging activity in the human brain, first with PET and then with fMRI, has had a tremendous impact on neuroscience. The impact on the study of cortical mechanisms of color perception was as great as in any other area of brain research during the period under review, 1985–2010. What is now obvious, that the response to color in V1 cortex in humans and monkeys is strong compared to its response to achromatic stimuli, only became obvious after the results of fMRI experiments on human cortex made it clear (Kleinschmidt et al, 1996; Engel et al, 1997; McKeefry and Zeki, 1997; Hadjikhani et al 1998; Beauchamp et al 1999; Brewer et al., 2005; Mullen et al, 2007; Wade et al., 2008). Subsequently, a similar picture of the distributed nature of color signal processing in macaque visual cortex also became clearer through studies with fMRI imaging (Conway and Tsao, 2006).
The search for the color center in human cortex postulated by Zeki (1993) has been pursued with fMRI as the searching tool. Motivated by Zeki’s prior work on macaque monkeys, Zeki and colleagues sought a human analogue to V4 cortex as the color center of the human brain, using brain imaging (Lueck et al, 1989; McKeefry and Zeki, 1997; Zeki and Bartels, 1999; Bartels and Zeki, 2000). Lueck et al (1989) and Zeki and Bartels (1999) found an area excited by color more than by black/white in the ventral occipital cortex and called it human V4, hV4. They also found another more anterior color-preferring area that they called V4α. These areas identified in normal human observers were located near or at the cortical sites that had been found to be damaged in human achromatopsia patients (Zeki, 1993). One curious fact was that the human color activations were all very ventral while in macaque monkey there are dorsal and ventral subdivisions of V4 as there are for V2 cortex for instance. The location of area V4 seems to be quite different in human and monkey cortex (Wade et al, 2008).
The assignment of color-preferring cortex as hV4 was not universally accepted and there has been controversy about this topic from 1989 until now. In monkey, V4 is a visuotopically mappable area in a sequence of such areas from V1—V4. The point of contention is whether the human visuotopically mapped V4 is the same area that Lueck et al (1989) called hV4 and that Bartels and Zeki continued to call V4 in humans. One problem is methodological. Color areas have been labeled as those areas that activate more to color patterns than to achromatic, and have been identified with a subtraction paradigm. Engel et al (1997) improved on this method by stimulating in many directions of color space and inferring color vs luminance responsiveness from the orientation of equal response contours in color space (as in Fig.3). This is clearly a better approach than the subtraction paradigm because subtraction hides the contribution of color-luminance cells that are so numerous in macaque V1 and V4 (Johnson et al, 2001; Friedman et al, 2003; Schluppeck and Engel, 2002). Another problem is determining whether the color-activated area is part of the visuotopically mapped V4 or whether it is yet another distinct mappable area anterior to V4 (Hadjikhani et al, 1998). What seems to be an emerging consensus is that there are mappable areas in the ventral occipital cortex that are anterior to hV4 that are highly activated by color. Brewer et al (2005) call these two regions VO-1 and VO-2. Mullin et al (2007) found that when cone contrasts for chromatic and achromatic stimuli were equated, only a cortical region anterior to hV4, possibly VO-1 revealed a clear color preference (Figure 8). There are more anterior color areas such as area V4α that may also qualify as a color center (Beauchamp et al, 1999; Murphey et al, 2008). In analogy with the work on macaque inferotemporal cortex, there may be posterior and anterior color centers, all in cortex that is analogous to macaque inferotemporal cortex that is object-related. In fact, the crucial concept of a separate, independent color center has not been validated by all the investigations of color centers in ventral occipital cortex because the regions where the putative color center has been found are surrounded by cortical areas that respond to faces, houses, and scenes.
Figure 8.
Human color centers – fMRI from Mullen et al (2007). Average t-statistical map (n = 8) comparing Achromatic and Chromatic conditions, displayed on average unfolded cortical surfaces (from Mullen et al 2007). The oblique medial views of the left and right hemispheres are shown on the left and right, respectively. On the averaged surfaces, locations of the parietal–occipital sulcus (POS) and corpus callosum (CC) are indicated to facilitate orientation on the surfaces. Black-and-white dashed lines indicate the average visual area and border locations for V1, V2, V3, VP, V3A, hV4 and hMT+. The stars indicate the cortical representations of the fovea in each hemisphere based on the average of eight subjects. Significantly stronger responses to Achromatic than to the average of the two Chromatic stimuli are indicated by positive t-values (red–yellow scale) and significantly stronger responses to the average of the two Chromatic than to Achromatic stimuli are indicated by negative t-values (purple–blue scale).
This brings us back to human achromatopsia. The syndrome of achromatopsia was always one of the conceptual foundations for the idea of separate, independent analysis of color. A meta-analysis of a large number of achromatopsia cases (92 cases) cast doubt on the purity of the color deficit and therefore of the concept of a separate color center (Bouvier and Engel, 2006). For instance, the overlap of the lesions that produce achromatopsia was very close to the region of overlap of lesions that cause face-blindness, prosopagnosia. Behavioral deficits in most cases of achromatopsia are not limited only to deficits in color discrimination or identification but also involved spatial deficits including prosopagnosia. Therefore, even the data on achromatopsia are not such strong support for the segregated-color or modular hypothesis as once was thought.
One last consideration is about the function of the color areas in ventral occipital cortex in humans, or the inferotemporal cortical color areas in monkeys. The function of anterior ventral occipital cortex, and inferotemporal cortex, is not certain but what is certain is that understanding their functions will be a challenge for neuroscience going forward. These areas of occipital cortex are getting close to temporal cortex, to cortical areas that are considered to be important for visual learning and memory. Rather than being near the end of a parallel hierarchical cascade, perhaps VO-1 is a memory bank of colors that is compared with incoming color signals from V1 where color computations are in fact carried out. Color may serve as a model system for understanding the visual system generally as a feedback system between sensory areas and memory areas that produce recognition of objects, and of colors, by association of current input from the world with prior experience. There are experiments that support such speculations (Hansen et al 2006).
Theoretical Note
We conclude not with a summary but with a review of some theoretical work that illustrates the complexity and controversy of studying color vision. This is work in the spirit of the times circa 2010, about the statistical nature of the world and how the visual system is matched to the statistics of natural scenes. Two major studies have been done, one by Tailor et al (2000) and one by Caywood et al (2004). The earlier Tailor study concluded that the optimal independent components that match color and pattern in natural scenes would be a group of separate and independent color and spatial channels, rather like the ideas of the modularists. The later work by Caywood et al (2004) offered technical criticism of Tailor et al, and concluded that optimal filters in V1 would resemble the population of single- and (orientation-selective) double-opponent cells that we have reported (Johnson et al 2004, 2008). Stay tuned.
Acknowledgement
This preparation of this article was supported in part by grants from the US National Eye Institute, EY01472, EY08300, EY17945, and from the National Science Foundation, 0745253. Thanks for helpful comments to Elizabeth Johnson, Jim Gordon, Karl Gegenfurtner, and Paul Martin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: new results and a review. Eur. J. Neurosci. 2000;12:172–193. doi: 10.1046/j.1460-9568.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Summation and inhibition in the frog's retina. J Physiol. 1953;119:69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Haxby JV, Jennings JE, DeYoe EA. An fMRI version of the Farnsworth-Munsell 100-Hue test reveals multi- ple color-selective areas in human ventral occipitotemporal cortex. Cereb. Cortex. 1999;9:257–263. doi: 10.1093/cercor/9.3.257. [DOI] [PubMed] [Google Scholar]
- Beaudot WH, Mullen KT. Orientation selectivity in luminance and color vision assessed using 2-d band-pass filtered spatial noise. Vision Res. 2005;45:687–696. doi: 10.1016/j.visres.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E. Dynamics of primate P retinal ganglion cells: responses to chromatic and achromatic stimuli. J Physiol. 1999;519:775–790. doi: 10.1111/j.1469-7793.1999.0775n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Zucker SW. Hue geometry and horizontal connections. Neural Netw. 2004;17:753–771. doi: 10.1016/j.neunet.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Zucker SW. Good Continuation in Layers: Shading flows, color flows, surfaces and shadows. In: Albertazzi Liliana, van Tonder GertJ, Vishwanath Dhanraj., editors. Perception beyond Inference: The Information Content of Visual Processes. Chapter 8. The MIT Press; 2010. [Google Scholar]
- Berliner A. Lectures on Visual Psychology. Chicago: Professional Press; 1949. [Google Scholar]
- Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb. Cortex. 2006;16:183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- Bradley A, Switkes E, De Valois K. Orientation and spatial frequency selectivity of adaptation to color and luminance gratings. Vision Res. 1988;28(7):841–856. doi: 10.1016/0042-6989(88)90031-4. [DOI] [PubMed] [Google Scholar]
- Brainard D. Color constancy. In: Chalupa L, Werner J, editors. Visual neuroscience. Cambridge: MIT Press; 2004. pp. 948–961. [Google Scholar]
- Brewer AA, Liu J, Wade AR, Wandell BA. Visual field maps and stimulus selectivity in human ventral occipital cortex. Nat. Neurosci. 2005;8:1102–1109. doi: 10.1038/nn1507. [DOI] [PubMed] [Google Scholar]
- Buzas P, Szmajda BA, Hashemi-Nezhad M, Dreher B, Martin PR. Color signals in the primary visual cortex of marmosets. J Vis. 2008;8:7.1–7.16. doi: 10.1167/8.10.7. [DOI] [PubMed] [Google Scholar]
- Casagrande VA. A third parallel visual pathway to primate area V1. Trends Neurosci. 1994;17:305–310. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Yazar F, Jones KD, Ding Y. The morphology of the koniocellular axon pathway in the macaque monkey. Cereb Cortex. 2007;17:2334–2345. doi: 10.1093/cercor/bhl142. [DOI] [PubMed] [Google Scholar]
- Caywood M, Willmore B, Tolhurst D. Independent components of color natural scenes resemble V1 neurons in their spatial and color tuning. Journal of Neurophysiology. 2004;91:2859–2873. doi: 10.1152/jn.00775.2003. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature. 2003;426 doi: 10.1038/nature02167. 668-67. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Spehar B, Solomon SG, Martin PR, Zaidi Q. Interactions between color and luminance in the perception of orientation. J. Vis. 2003;3:106–115. doi: 10.1167/3.2.1. [DOI] [PubMed] [Google Scholar]
- Conway BR. Spatial structure of cone inputs to color cells in alert macaque primary visual cortex (V1) J Neurosci. 2001;21:2768–2783. doi: 10.1523/JNEUROSCI.21-08-02768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Hubel DH, Livingstone MS. Color contrast in macaque V1. Cereb Cortex. 2002;12:915–925. doi: 10.1093/cercor/12.9.915. [DOI] [PubMed] [Google Scholar]
- Conway BR, Livingstone MS. Spatial and temporal properties of cone signals in alert macaque primary visual cortex. J. Neurosci. 2006;26:10826–10846. doi: 10.1523/JNEUROSCI.2091-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Tsao DY. Color architecture in alert macaque cortex revealed by FMRI. Cereb Cortex. 2006;16(11):1604–1613. doi: 10.1093/cercor/bhj099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Moeller S, Tsao DY. Specialized color modules in macaque extrastriate cortex. Neuron. 2007;56:560–573. doi: 10.1016/j.neuron.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Heywood CA. There's more to colour than meets the eye. Behav Brain Res. 1995;71:89–100. doi: 10.1016/0166-4328(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Cropper SJ, Wuerger SM. The perception of motion in chromatic stimuli. Behav Cogn Neurosci Rev. 2005;4(3):192–217. doi: 10.1177/1534582305285120. [DOI] [PubMed] [Google Scholar]
- Daw NW. Goldfish retina: organization for simultaneous color contrast. Science. 1967;158:942–944. doi: 10.1126/science.158.3803.942. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Ohzawa I, Freeman RD. Receptive-field dynamics in the central visual pathways. Trends Neurosci. 1995;18:451–458. doi: 10.1016/0166-2236(95)94496-r. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois RL. Analysis and coding of color vision in the primate visual system. Cold Spring Harb Symp Quant Bio. 1965;38:567–580. doi: 10.1101/sqb.1965.030.01.055. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Pease PL. Contours and contrast: Responses of monkey lateral geniculate nucleus cells to luminance and colour figures. Science. 1971;171:694–696. doi: 10.1126/science.171.3972.694. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. In: Spatial Vision. Broadbent DE, editor. New York: Oxford University Press; 1988. [Google Scholar]
- De Valois RL, Cottaris NP, Elfar SD, Mahon LE, Wilson JA. Some transformations of color information from lateral geniculate nucleus to striate cortex. Proc Natl Acad Sci U S A. 2000;97:4997–5002. doi: 10.1073/pnas.97.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe A, Van Essen DC. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Dow BM, Gouras P. Color and spatial specificity of single units in Rhesus monkey foveal striate cortex. J Neurophysiol. 1973;36:79–100. doi: 10.1152/jn.1973.36.1.79. [DOI] [PubMed] [Google Scholar]
- Edwards DP, Kaplan E. How sharp is the orientation tuning of single cells in the cytochrome oxidase blobs of primate visual cortex? Invest.Ophthalmol.Visual Sci. 1992;3(4) suppl:1255(#2812). [Google Scholar]
- Engel SA, Furmanski CS. Selective adaptation to color contrast in human primary visual cortex. J Neurosci. 2001;21:3949–3954. doi: 10.1523/JNEUROSCI.21-11-03949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Zhang X, Wandell B. Colour tuning in human visual cortex measured with functional magnetic resonance imaging. Nature. 1997;388:68–71. doi: 10.1038/40398. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson J. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol (Lond) 1966;187:517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federer F, Ichida JM, Jeffs J, Schiessl I, McLoughlin N, Angelucci A. Four projection streams from primate V1 to the cytochrome oxidase stripes of V2. J Neurosci. 2009;29:15455–15471. doi: 10.1523/JNEUROSCI.1648-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS, Zhou H, von der Heydt R. The coding of uniform colour figures in monkey visual cortex. J. Physiol. 2003;548:593–613. doi: 10.1113/jphysiol.2002.033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR. Cortical mechanisms of colour vision. Nat Rev Neurosci. 2003;4:563–572. doi: 10.1038/nrn1138. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC. Color vision. Ann Rev Neurosci. 2003;26:181–206. doi: 10.1146/annurev.neuro.26.041002.131116. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC, Fenstemaker SB. Processing of color, form and motion in macaque V2. Vis. Neurosci. 1996;13:161–172. doi: 10.1017/s0952523800007203. [DOI] [PubMed] [Google Scholar]
- Girard P, Morrone MC. Spatial structure of chromatically opponent receptive fields in the human visual system. Vis. Neurosci. 1995;12:103–116. doi: 10.1017/s0952523800007355. [DOI] [PubMed] [Google Scholar]
- Gordon J, Shapley R. Brightness contrast inhibits color induction: evidence for a new kind of color theory. Spat Vis. 2006;19:133–146. doi: 10.1163/156856806776923498. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Mingolla E. Neural dynamics of form perception: boundary completion, illusory figures, and neon color spreading. Psychol. Rev. 1985;92:173–211. [PubMed] [Google Scholar]
- Hadjikhani N, Liu AK, Dale AM, Cavanagh P, Tootell RB. Retinotopy and color sensitivity in visual cortical area V8. Nat Neurosci. 1998;1:235–241. doi: 10.1038/681. [DOI] [PubMed] [Google Scholar]
- Hamburger K, Hansen T, Gegenfurtner KR. Geometric-optical illusions at isoluminance. Vision Res. 2007;47:3276–3285. doi: 10.1016/j.visres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Handa T, Inoue M, Mikami A. Neuronal activity during discrimination of shapes defined by motion in area V4. Neuroreport. 2010;21:532–536. doi: 10.1097/WNR.0b013e3283393a5f. [DOI] [PubMed] [Google Scholar]
- Hansen T, Olkkonen M, Walter S, Gegenfurtner KR. Memory modulates color appearance. Nat Neurosci. 2006;9(11):1367–1368. doi: 10.1038/nn1794. [DOI] [PubMed] [Google Scholar]
- Harada T, Goda N, Ogawa T, Ito M, Toyoda H, Sadato N, Komatsu H. Distribution of colour-selective activity in the monkey inferior temporal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 2009;30:1960–1970. doi: 10.1111/j.1460-9568.2009.06995.x. [DOI] [PubMed] [Google Scholar]
- Hartline HK. The Receptive Field of the Optic Nerve Fibers. Am. J. Physiol. 1940;130:690. [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994;264:575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Heywood CA, Gadotti A, Cowey A. Cortical area V4 and its role in the perception of color. J. Neurosci. 1992;12:4056–4065. doi: 10.1523/JNEUROSCI.12-10-04056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood CA, Gaffan D, Cowey A. Cerebral achromatopsia in monkeys. Eur J Neurosci. 1995;7:1064–1073. doi: 10.1111/j.1460-9568.1995.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292:762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Chichilinsky EJ, Albright TD. Cone inputs to simple and complex cells in V1 of awake macaque. J. Neurophysiol. 2007;97:3070–3081. doi: 10.1152/jn.00965.2006. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Livingstone MS. Segregation of form, color, and stereopsis in primate area 18. J Neurosci. 1987;7:3378–3415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert A, Wolf K. Color contrast: a contributory mechanism to color constancy. Prog. Brain Res. 2004;144:147–160. doi: 10.1016/s0079-6123(03)14410-x. [DOI] [PubMed] [Google Scholar]
- Hurvich LM, Jameson D. An opponent- process theory of color vision. Psych Rev. 1957;64:384–404. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- Huxlin KR, Saunders RC, Marchionini D, Pham HA, Merigan WH. Perceptual deficits after lesions of inferotemporal cortex in macaques. Cer Cortex. 2000;10:671–683. doi: 10.1093/cercor/10.7.671. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Primate color vision: a comparative perspective. Vis Neurosci. 2008;25:619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- Jameson D, Hurvich LM. From contrast to assimilation; in art and in the eye. Leonardo. 1975;8:125–131. [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nature Neurosci. 2001;4:409–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. Cone inputs in macaque primary visual cortex. J.Neurophysiol. 2004;91:2501–2514. doi: 10.1152/jn.01043.2003. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The orientation selectivity of color-responsive neurons in macaque V1. J Neurosci. 2008;28:8096–8106. doi: 10.1523/JNEUROSCI.1404-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EN, Van Hooser SD, Fitzpatrick D. The representation of S-cone signals in primary visual cortex. J Neurosci. 2010;30:10337–10350. doi: 10.1523/JNEUROSCI.1428-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanizsa G. Organization in Perception. New York: Praeger; 1979. [Google Scholar]
- Kaplan E, Shapley R. X and Y cells in the lateral geniculate nucleus of the macaque monkey. J.Physiol. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley R. Two types of ganglion cell in the monkey retina with different contrast sensitivity. Proc.Nat.Acad.Sci. US. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. In: The world of colour. MacLeod RB, Fox CW, editors. London: Kegan, Paul, Trench, Truebner & Co. Ltd.; 1935. transl. [Google Scholar]
- Kirschmann A. Ueber die quantitativen Verhaeltnisse des simultanen Helligkeitsund Farben-contrastes. Philosophische Studien. 1891;6:417–491. [Google Scholar]
- Kleinschmidt A, Lee BB, Requardt M, Frahm J. Functional mapping of color processing by magnetic resonance imaging of responses to selective P- and M-pathway stimulation. Experimental Brain Research. 1996;110:27–88. doi: 10.1007/BF00228558. [DOI] [PubMed] [Google Scholar]
- Koida K, Komatsu H. Effects of task demands on the responses of color selective neurons in the inferior temporal cortex. Nat Neurosci. 2007;10:108–116. doi: 10.1038/nn1823. [DOI] [PubMed] [Google Scholar]
- Komatsu H. Mechanisms of central color vision. Curr Opin Neurobiol. 1998;8:503–508. doi: 10.1016/s0959-4388(98)80038-x. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Ideura Y, Kaji S, Yamane S. Color selectivity of neurons in the inferior temporal cortex of the awake macaque monkey. J. Neurosci. 1992;12:408–424. doi: 10.1523/JNEUROSCI.12-02-00408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauskopf J. Effect of retinal image stabilization on the appearance of heterochromatic targets. J. Opt. Soc. Amer. 1963;53:741–744. doi: 10.1364/josa.53.000741. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Williams DR, Heeley DW. Cardinal directions of color space. Vision Res. 1982;22:1123–1131. doi: 10.1016/0042-6989(82)90077-3. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Ts'o DY. Color processing in macaque striate cortex: relationships to ocular dominance, cytochrome oxidase, and orientation. J Neurophysiol. 2002a;87:3126–3137. doi: 10.1152/jn.2002.87.6.3126. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Ts'o DY. Color processing in macaque striate cortex: electrophysiological properties. J Neurophysiol. 2002b;87:3138–3151. doi: 10.1152/jn.00957.1999. [DOI] [PubMed] [Google Scholar]
- Lehky SR, Sejnowski TJ. Seeing white: Qualia in the context of decoding population codes. Neural Comput. 1999;11(6):1261–1280. doi: 10.1162/089976699300016232. [DOI] [PubMed] [Google Scholar]
- Lennie P. Color coding in the cortex. In: Gegenfurtner K, Sharpe L, editors. Color: from genes to perception. Chapter 12. Cambridge: Cambridge U.P.; 1999. pp. 235–248. [Google Scholar]
- Lennie P, D'Zmura M. Mechanisms of color vision. Crit Rev Neurobiol. 1988;3:333–400. [PubMed] [Google Scholar]
- Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J. Neurosci. 1990;10:649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P, Movshon JA. Coding of color and form in the geniculostriate visual pathway. J Opt Soc Am A Opt Image Sci Vis. 2005;22(10):2013–2033. doi: 10.1364/josaa.22.002013. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Thompson KG, Liu D, Zhou Y, Ault SJ. Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. J. Neurosci. 1995;15:1808–1818. doi: 10.1523/JNEUROSCI.15-03-01808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Kiper DC, Movshon JA. Receptive fields and functional architecture of macaque V2. J. Neurophysiol. 1994;71:2517–2542. doi: 10.1152/jn.1994.71.6.2517. [DOI] [PubMed] [Google Scholar]
- Liu J, Wandell BA. Specializations for chromatic and temporal signals in human visual cortex. J Neurosci. 2005;25(13):3459–3468. doi: 10.1523/JNEUROSCI.4206-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J. Neurosci. 1987;7:3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Losada MA, Mullen KT. The spatial tuning of chromatic mechanisms identified by simultaneous masking. Vision Res. 1994;34:331–341. doi: 10.1016/0042-6989(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Lu HD, Roe AW. Functional organization of color domains in V1 and V2 of macaque monkey revealed by optical imaging. Cereb Cortex. 2008;18:516–533. doi: 10.1093/cercor/bhm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueck CJ, Zeki S, Friston KJ, Deiber MP, Cope P, Cunningham VJ, Lammertsma AA, Kennard C, Frackowiak RS. The colour centre in the cerebral cortex of man. Nature. 1989;340:386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- McKeefry DJ, Zeki S. The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain. 1997;120:2229–2242. doi: 10.1093/brain/120.12.2229. [DOI] [PubMed] [Google Scholar]
- Martin PR, White AJ, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. Eur J Neurosci. 1997;9(7):1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Mollon JD. Monge: The Verriest Lecture. Visual Neuroscience. 2006;23:297–309. doi: 10.1017/S0952523806233479. [DOI] [PubMed] [Google Scholar]
- Mullen KT. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J. Physiol. 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KT, Dumoulin SO, McMahon KL, de Zubicaray GI, Hess RF. Selectivity of human retinotopic visual cortex to S-cone-opponent, L/M-cone-opponent and achromatic stimulation. Eur. J. Neurosci. 2007;25:491–502. doi: 10.1111/j.1460-9568.2007.05302.x. [DOI] [PubMed] [Google Scholar]
- Murphey DK, Yoshor D, Beauchamp MS. Perception matches selectivity in the human anterior color center. Curr Biol. 2008;18:216–220. doi: 10.1016/j.cub.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Orban GA. Higher order visual processing in macaque extrastriate cortex. Physiol Rev. 2008;88:59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Population coding of shape in area V4. Nature Neuroscience. 2002;5:1332–1338. doi: 10.1038/nn972. [DOI] [PubMed] [Google Scholar]
- Peterhans E, von der Heydt R. Functional organization of area V2 in the alert macaque. Eur. J. Neurosci. 1993;5:509–524. doi: 10.1111/j.1460-9568.1993.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Poirson AB, Wandell BA, Varner DC, Brainard DH. Surface characterizations of color thresholds. J Opt Soc Am A. 1990;7(4):783–789. doi: 10.1364/josaa.7.000783. [DOI] [PubMed] [Google Scholar]
- Rabin J, Switkes E, Crognale M, Schneck ME, Adams AJ. Visual evoked potentials in three-dimensional color space: correlates of spatio-chromatic processing. Vis Res. 1994;34:2657–2671. doi: 10.1016/0042-6989(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Reid RC, Shapley R. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992;356:716–718. doi: 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Reid RC, Shapley RM. Space and time maps of cone photoreceptor signals in macaque lateral geniculate nucleus. J. Neurosci. 2002;22:6158–6175. doi: 10.1523/JNEUROSCI.22-14-06158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Victor J, Shapley R. The use of m-sequences in the analysis of visual neurons. Visual Neurosci. 1997;14:1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- Ringach DL. Spatial structure and symmetry of simple-cell receptive fields in macaque primary visual cortex. J. Neurophysiol. 2002;88:455–463. doi: 10.1152/jn.2002.88.1.455. [DOI] [PubMed] [Google Scholar]
- Ringach D, Carandini M, Sapiro G, Shapley R. A subspace reverse correlation method for the study of visual neurons. Vis Res. 1997;37:2455–2464. doi: 10.1016/s0042-6989(96)00247-7. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965;28:832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Roe AW, Ts'o DY. Visual topography in primate V2: multiple representation across functional stripes. J Neurosci. 1995;15:3689–3715. doi: 10.1523/JNEUROSCI.15-05-03689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW, Ts'o DY. Specificity of color connectivity between primate V1 and V2. J Neurophysiol. 1999;82(5):2719–2730. doi: 10.1152/jn.1999.82.5.2719. [DOI] [PubMed] [Google Scholar]
- Roy S, Jayakumar J, Martin PR, Dreher B, Saalmann YB, Hu D, Vidyasagar TR. Segregation of short-wavelength-sensitive (S) cone signals in the macaque dorsal lateral geniculate nucleus. Eur J Neurosci. 2009;30(8):1517–1526. doi: 10.1111/j.1460-9568.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein SJ, Desimone R. Spectral properties of V4 neurons in the macaque. J. Neurosci. 1990;10:3369–3389. doi: 10.1523/JNEUROSCI.10-10-03369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Engel SA. Color opponent neurons in V1: a review and model reconciling results from imaging and single-unit recording. J. Vis. 2002;2:480–492. doi: 10.1167/2.6.5. [DOI] [PubMed] [Google Scholar]
- Shapley R, Hawken M. Neural mechanisms for color perception in the primary visual cortex. Current Opinion in Neurobiology. 2002;12:426–432. doi: 10.1016/s0959-4388(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Shevell SK, Kingdom FA. Color in complex scenes. Annu. Rev. Psychol. 2008;59:143–166. doi: 10.1146/annurev.psych.59.103006.093619. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. The functional organization of area V2, I: specialization across stripes and layers. Vis Neurosci. 2002;19:187–210. doi: 10.1017/s0952523802191164. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. annu Rev Neurosci. 2005;28:303–326. doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lennie P. Chromatic gain controls in visual cortical neurons. J Neurosci. 2005;25:4779–4792. doi: 10.1523/JNEUROSCI.5316-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Lennie P. The machinery of colour vision. Nat. Rev. Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Peirce JW, Lennie P. The impact of suppressive surrounds on chromatic properties of cortical neurons. J Neurosci. 2004;24:148–160. doi: 10.1523/JNEUROSCI.3036-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromeyer CF, 3rd, Cole GR, Kronauer RE. Second-site adaptation in the red-green chromatic pathways. Vision Res. 1985;25(2):219–237. doi: 10.1016/0042-6989(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Switkes E, Bradley A, De Valois KK. Contrast dependence and mechanisms of masking interactions among chromatic and luminance gratings. J. Opt. Soc. Am. A. 1988;5:1149–1162. doi: 10.1364/josaa.5.001149. [DOI] [PubMed] [Google Scholar]
- Tailor D, Finkel L, Buchsbaum G. Color opponent receptive fields derived from independent component analysis of natural images. Vision Research. 2000;40:2671–2676. doi: 10.1016/s0042-6989(00)00105-x. [DOI] [PubMed] [Google Scholar]
- Takechi H, Onoe H, Shizuno H, Yoshikawa E, Sadato N, Tsukada H, Watanabe Y. Mapping of cortical areas involved in color vision in non-human primates. Neuroscience Letters. 1997;230:17–20. doi: 10.1016/s0304-3940(97)00461-8. [DOI] [PubMed] [Google Scholar]
- Thorell LG, De Valois RL, Albrecht DG. Spatial mapping of monkey V1 cells with pure color and luminance stimuli. Vision Res. 1984;24:751–769. doi: 10.1016/0042-6989(84)90216-5. [DOI] [PubMed] [Google Scholar]
- Tobimatsu S, Tomoda H, Kato M. Parvocellular and magnocellular contributions to visual evoked potentials in humans: stimulation with chromatic and achromatic gratings and apparent motion. J Neurol Sci. 1995;134:73–82. doi: 10.1016/0022-510x(95)00222-x. [DOI] [PubMed] [Google Scholar]
- Ts'o DY, Roe AW, Gilbert CD. A hierarchy of the functional organization for color, form and disparity in primate visual area V2. Vision Res. 2001;41:1333–1349. doi: 10.1016/s0042-6989(01)00076-1. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18(3):477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- Vautin RG, Dow BM. Color cell groups in foveal striate cortex of the behaving macaque. J Neurophysiol. 1985;54:273–292. doi: 10.1152/jn.1985.54.2.273. [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura K, Katz E, Mao B. Population encoding of spatial frequency, orientation, and color in macaque V1. J Neurophysiol. 1994;72:2151–2166. doi: 10.1152/jn.1994.72.5.2151. [DOI] [PubMed] [Google Scholar]
- Wachtler T, Sejnowski TJ, Albright TD. Representation of color stimuli in awake macaque primary visual cortex. Neuron. 2003;37:681–691. doi: 10.1016/s0896-6273(03)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade AR, Brewer AA, Rieger JW, Wandell BA. Functional measurements of human ventral occipital cortex: retino- topy and colour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:963–973. doi: 10.1098/rstb.2002.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade A, Augath M, Logothetis N, Wandell B. fMRI measurements of color in macaque and human. J Vis. 2008;8:6.1–6.19. doi: 10.1167/8.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach H. Ueber visuell wahrgenommene Bewegungsrichtung. Psychologische Forschung. 1935;20:325–380. [Google Scholar]
- Walsh V, Butler SR, Carden D, Kulikowski JJ. The effects of V4 lesions on the visual abilities of macaques: Shape discrimination. Behavioural Brain Research. 1992;50:115–126. doi: 10.1016/s0166-4328(05)80293-1. [DOI] [PubMed] [Google Scholar]
- Webster MA, De Valois KK, Switkes E. Orientation and spatial-frequency discrimination for luminance and chromatic gratings. J. Opt. Soc. Am. A. 1990;7:1034–1049. doi: 10.1364/josaa.7.001034. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wuerger S, Shapley R, Rubin N. "On the visually perceived direction of motion" by Hans Wallach, 1935: translation. Perception. 1996;25:1317–1368. [Google Scholar]
- Xiao Y, Wang Y, Felleman DJ. A spatially organized representation of colour in macaque cortical area V2. Nature. 2003;421:535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Casti A, Xiao J, Kaplan E. Hue maps in primate striate cortex. Neuroimage. 2007;35:771–786. doi: 10.1016/j.neuroimage.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbus AL. Eye Movements and Vision. New York: Plenum; 1967. [Google Scholar]
- Yasuda M, Banno T, Komatsu H. Color Selectivity of Neurons in the Posterior Inferior Temporal Cortex of the Macaque Monkey. Cer Cortex. 2010;20:1630–1646. doi: 10.1093/cercor/bhp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CI, Xing D, Williams PE, Shapley RM. Stimulus ensemble and cortical layer determine V1 spatial receptive fields. Proc Natl Acad Sci U S A. 2009;106:14652–14657. doi: 10.1073/pnas.0907406106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Colour coding in rhesus monkey prestriate cortex. Brain Res. 1973;53:422–427. doi: 10.1016/0006-8993(73)90227-8. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J Physiol. 1978a;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Functional specialisation in the visual cortex of the rhesus monkey. Nature. 1978b;274:423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]
- Zeki S. Color coding in the cerebral-cortex—the reaction of cells in monkey visual-cortex to wavelengths and colors. Neuroscience. 1983a;9:741–765. doi: 10.1016/0306-4522(83)90265-8. [DOI] [PubMed] [Google Scholar]
- Zeki S. Colour coding in the cerebral cortex: the responses of wavelength-selective and colour-coded cells in monkey visual cortex to changes in wavelength composition. Neuroscience. 1983b;9:767–781. doi: 10.1016/0306-4522(83)90266-x. [DOI] [PubMed] [Google Scholar]
- Zeki S. A century of cerebral achromatopsia. Brain. 1990;113:1721–1777. doi: 10.1093/brain/113.6.1721. [DOI] [PubMed] [Google Scholar]
- Zeki S. a vision of the brain. Cambridge (Ma): Blackwell Scientific; 1993. [Google Scholar]
- Zeki S, Bartels A. The clinical and functional measurement of cortical (in)activity in the visual brain, with special reference to the two subdivisions (V4 and V4 alpha) of the human colour centre. Philos Trans R Soc Lond B Biol Sci. 1999;354:1371–1382. doi: 10.1098/rstb.1999.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]