Abstract
Background
The podocyte serves the important function of maintaining the glomerular filtration barrier, and many studies report a decrease in podocyte number relative to the development of proteinuric states. However, there is significant inconsistency in the number of podocytes counted, possibly due to different counting methods. We previously counted the three glomerular cell types in the mouse kidney and showed that the fractionator/disector method is a close approximation of the exhaustive count or the gold standard method. In this study, we compared the commonly used model-based approach with the design-based approach to count podocytes in the db/m and db/db mouse and illustrate that the design-based approach, which uses the fractionator/disector method, provides an accurate determination of podocyte number.
Methods
In the design-based approach, toluidine blue-stained 1-μm-thick sections from glutaraldehyde perfusion-fixed kidneys were used (n = 15) with the fractionator/disector method. In the model-based approach, WT-1-immunolabeled podocyte nuclei in 3- to 4-μm-thick formalin-fixed paraffin-embedded sections of the same kidneys were counted with the Weibel-Gomez method. Glomerular volume was determined for each method.
Results
We discovered that the fractionator/disector method counted 89 ± 10 podocytes compared to the Weibel-Gomez method, which counted 137 ± 38 podocytes and overestimated podocyte number by 54% (p < 0.05). In addition, glomerular volume (231 ± 52 × 103 vs. 192 ± 64 × 103 μm3) was significantly underestimated by 17% (p < 0.0002). Moreover, the model-based approach was more time consuming.
Conclusion
We conclude that the fractionator/disector method offers an unbiased and efficient determination of podocyte counts.
Key Words: db/db mouse, Design-based approach, Fractionator/disector method, Glomerular volume, Podocyte number, Stereology, Weibel-Gomez method
Introduction
The concept of making a paradigm shift has long been in existence and has contributed to the evolution of our craft. The discovery of ‘the germ theory’ in the 17th and 18th centuries with the identification of microorganisms [1] created a significant shift in the way infectious diseases were medically viewed and eventually successfully treated. When Crick, Watson and Wilkins received the Nobel Prize for the identification of the DNA double helix [2], their discovery revolutionized our understanding of the genetic foundation of numerous diseases. Fast forward to the information age and the advent of Facebook, we can certainly attest to the transformation of the word ‘friend’ and its impact on social networking as well as its potential to permeate academia and the practice of medicine [3,4,5]. Yet, paradigm shifts and ‘thinking outside the box’ do not occur overnight and are neither easy nor straightforward, but are often revered in hindsight. In most cases, paradigm shifts are stimulated by a desire to acquire new knowledge. The epidemic of diabetes and its complications, particularly diabetic kidney disease (DKD), may require a paradigm shift in how anatomical structures, such as podocytes, and podocyte numbers are commonly investigated as we expand our knowledge of disease mechanisms in order to develop new and innovative targeted therapies for both early and late disease progression.
The scientific approach to uncover novel interventions that will ultimately improve health outcomes typically require designing suitable in vitro and in vivo experiments, utilizing appropriate animal disease models, andespecially making accurate measurements that lead to the acquisition of true information, which may then direct feasible interventional large-scale clinical studies. This ‘bench-to-bedside’ notion is not new, but the application of basic stereologic principles to acquire accurate measurements of renal morphology may be a new concept to some. In this supplement of the American Journal of Nephrology, we provide several original papers from the Proceedings from the Inaugural Stereology and Its Application in Kidney Disease Symposium: ‘Using Stereology to Quantify Renal Structural Pathology’, May 19–21, 2010, that demonstrate the utility of stereologic methods to investigate key features of chronic kidney diseases in both humans and animal models. Teiken et al. (this issue) describe the salient aspects of the OVE26 transgenic diabetic mouse that attest to its suitability as an appropriate model of human DKD; Weil et al. (this issue) demonstrate the potential impact of quantified podocyte loss on the development of sclerosed glomeruli in type 2 diabetic patients, and Najafian and Mauer (this issue) provide new data on the quantification of glomerular endothelial fenestrae in patients with Fabry's disease. In addition, in-depth reviews by Hoy et al. (this issue) compare the variability in individual glomerular volumes in several ethnic groups; Sutherland et al. (this issue) provide a stereologic assessment of renal development during preterm birth, and Pedersen et al. (this issue) describe the potential of non-invasive imaging to assess intrarenal volume. This supplement is designed to highlight the many aspects of renal investigation in which stereology principles have been and may be applied to advance our thinking and acquisition of correct appraisals of renal structure.
We have previously compared stereologic methods to count the three cell types of the glomerulus in a mouse model and demonstrated that the fractionator/disector method is an unbiased substitute for the exhaustive count or gold standard method when compared to the Weibel-Gomez method, which overestimates the number of cells [6]. While all three cell types of the glomerulus contribute greatly to the overall structure and function of the kidney, the importance of podocytes in the development and progression of proteinuric diseases, particularly DKD, has been well established [7,8,9], and the potential to target podocytes is becoming more evident [10]. Therefore, the assessment of podocyte number has emerged as a marker of disease activity. Indeed, the podocyte is an important component of the glomerular filtration barrier [11]. In 1997, Pagtalunan et al. [12] first reported a decrease in podocyte number associated with type 2 diabetes. Since that report, numerous studies have corroborated a decrease in podocyte number in clinical renal diseases [12,13] as well as in rat and mouse models [14]. However, there has been inconsistency in the number of podocytes reported, possibly due to the use of different counting methods.
A common approach is to assess podocyte number using immunolabeled materials and image analysis programs. This approach may be inaccurate and assumes that (1) the antibody is highly specific; (2) the glomerulus is perfectly round; (3) the distribution of podocytes is homogeneous throughout the glomerulus, and (4) podocyte shape, size, and distribution remain unchanged in health and disease. This is therefore a model-based approach as all podocyte nuclei are presumed to have a homogeneous shape. Unfortunately, this technique may lead to significant overestimation of podocyte number. On the other hand, a design-based approach to counting podocytes makes no assumptions on the size, shape, or orientation of the cells. In this study, we compared the design-based approach using the fractionator/disector method and the model-based approach using the Weibel-Gomez method of WT-1-stained podocytes, and show that knowledge and use of a design-based method provides a more accurate and less time-consuming determination of podocyte number.
Methods
Kidney Harvest and Tissue Preparation
The left kidneys of male non-diabetic db/m (BKS.Cg-m+/+Lepdb/J)and type 2 diabetic db/db mice (BKS.Cg-Dock7m+/+Leprdb/J; Jackson Laboratories, Bar Harbor, Me., USA; n = 15) were perfusion-fixed with 1% glutaraldehyde in Millonig's buffer as previously described [6]. The right kidneys were used for immmunolabeling studies. Animal surgery protocols were approved by the Institutional Animal Care and Use Committee of the David Geffen School of Medicine at UCLA.
After glutaraldehyde fixation, 1-mm3 blocks were arbitrarily selected from the kidney cortex, dehydrated through a series of graded ethanol and embedded in Poly/Bed 812® resin (Polysciences, Inc., Warrington, Pa., USA). Using an ultramicrotome and a Histo Jumbo diamond knife (Diatome US, Warrington, Pa., USA), serial 1-μm-thick sections were cut, collected as ribbons on standard microscope slides and stained with 1% aqueous toluidine blue. The first section from the ribbon to be imaged was selected using a random number between 1 and 10, followed by every 10th and its adjacent section. Glomeruli in the first selected section were not used because an unknown number of podocytes in those glomeruli were lost. Pairs of sections from throughout the first 10 glomeruli were imaged with a ×100 objective and a digital camera (fig. 1).
Fig. 1.
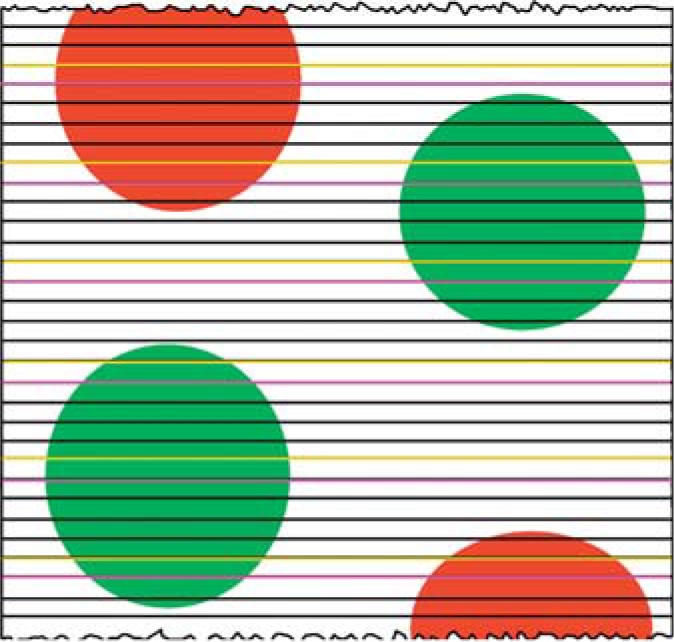
Identification of disector pairs. The figure depicts a series of sections through 4 particles, such as glomeruli. Partial glomeruli (red) are not used, while whole glomeruli (green) are used for the analysis. A fraction of the sections, one fifth in this example, is selected as the reference or sampling section (yellow line). Here, a random number from 1 to 5 was used to select the first section without bias. The adjacent section is used as the look-up section (purple line).
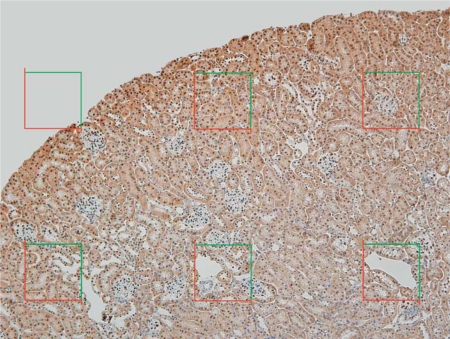
Tissue preparation for immunostaining was performed as previously described [15]. Formalin-fixed kidney slices (2 mm) were dehydrated in ethanol and xylene, embedded in paraffin, and 3- to 4-μm-thick sections obtained. Sections were deparaffinized and rehydrated, and endogeneous peroxidase activity was blocked (3% H2O2 in PBS for 5 min) and boiled for 5 min in high-pH antigen unmasking solution (Vector Laboratories, Burlingame, Calif., USA). Sections were incubated with non-immune goat serum (Sigma-Aldrich, 10% in PBS) for 1 h. Podocyte nuclei were stained using antibody to WT-1 (Abcam, San Francisco, Calif., USA; 1:200 dilution) at 4°C overnight. The slides were washed and incubated with secondary anti-rabbit antibody (1:200 dilution) and avidin-biotin peroxidase complex, and immunoreactivity was visualized with a substrate chromagen mixture (Vector Laboratories), counterstained with hematoxylin, dehydrated and cover slipped. A motorized stage attached to the microscope was used to obtain a systematic sample with random start of the first 10 glomeruli (fig. 2), and images of entire glomerular profiles were obtained with a ×100 objective lens and a digital camera.
Fig. 2.
Systematic sampling of immunolabeled kidney sections. This is a representative formalin-fixed, paraffin-embedded immunolabeled kidney cortex section. Unbiased sampling frames were used to systematically select the first 10 glomeruli to be analyzed using the Weibel-Gomez method. Glomerular profiles entirely within the sampling frame, or intersecting the green line but not the red line, were imaged using the ×100 objective lens and a digital camera.
Imaging
All Images were observed at the 100% window magnification on a 24′′ Apple iMac® monitor using Adobe Photoshop® CS3 software. Magnification was validated by imaging a micrometer slide and the Adobe Photoshop® measuring tool to measure the appropriate distance.
Design-Based Approach for Podocyte Counting
Since the boundaries of podocytes are not completely resolved by light microscopy, podocyte nuclei were used as surrogates assuming 1 nucleus per podocyte.
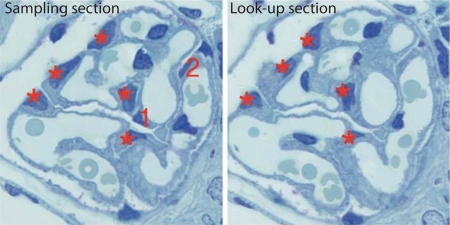
In the fractionator/disector method, podocytes were identified by location. In the fractionator portion of the method, a known fraction of sections through an object is typically selected to count particles within the object [16] (fig. 1). The disector portion of the method provides a mechanism to count the number of 3D particles in 3D space using 2D images [17]. Thus, the profiles of podocyte nuclei were directly visualized, and pairs of sections consisting of the ‘sampling’ section and the ‘look-up’ section were identified. Figure 3 shows a disector pair and two profiles from podocyte nuclei which appeared in the sampling section, but not in the look-up section. These were counted as a Q–. By combining the fractionator and disector principles, the number of podocytes in a glomerulus was calculated using the equation, Npodo = ΣQ– × 10, where ΣQ– was the number of profiles from podocyte nuclei seen in the sampling sections but not in the look-up sections, summed over all the disector pairs, and 10 was the reciprocal of the sampling fraction. The design-based Cavalieri principle was used to measure glomerular volume as previously described [6].
Fig. 3.
Disector pair from a glomerulus. A disector pair, consisting of a sampling section and the adjacent look-up section, was used to count podocyte nuclei. Profiles from nuclei seen in both sections are not counted (*). Profiles from nuclei seen in the sampling section but not the look-up sections (1, 2) are counted as Q– and used to calculate the number of podocytes in the glomerulus.
Model-Based Approach for Counting Podocytes
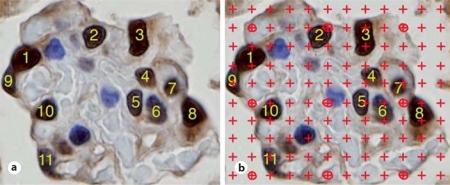
In contrast to the fractionator/disector method which directly visualizes and counts cells, the Weibel-Gomez method ascertains cell number from determination of cell density [18]. The Weibel-Gomez method was performed on a single immunolabeled section in which WT-1-stained podocyte nuclear profiles were identified and counted (fig. 4a). A counting grid was superimposed over each glomerular image using the Photoshop Layers function. The number of grid points ‘hitting’ podocyte nuclear profiles and the number of grid points ‘hitting’ the glomerular profile were counted (fig. 4b). The density of podocytes per glomerulus was calculated using the equation:
Fig. 4.
WT-1-stained podocyte nuclei used with the Weibel-Gomez counting method. a The WT-1-stained nuclei were first counted. b A grid was superimposed on the image and the number of points ‘hitting’ podocyte nuclei and the number of points ‘hitting’ glomeruli were counted. Counts were used to calculate podocyte density (see text for details).
where n is the number of podocyte nuclear profiles, Pglom is the number of grid points ‘hitting’ the glomerular tuft profiles, d is the distance between the grid points, mag is the magnification, Pnuclei is the number of grid points ‘hitting’ the podocyte nuclear profiles, and 0.645 is the shape factor for the nuclei. The number of podocytes per glomerulus was then calculated using the equation,
Glomerular volume (Vglom) was determined using the Weibel-Gomez equation[16],
where ΣPglom is the number of points hitting a glomerular tuft, 10 is the number of glomeruli analyzed per animal, d is the distance between grid points, mag is the magnification, 1.38 is the shape factor, and 1.01 is the variability among glomerular factors.
Statistics
Results are reported as means ± SD. Data were analyzed using GraphPad InStat 3. Paired t test determined the statistical difference between the two methods. Bland-Altman analysis assessed the average difference between the methods.
Results
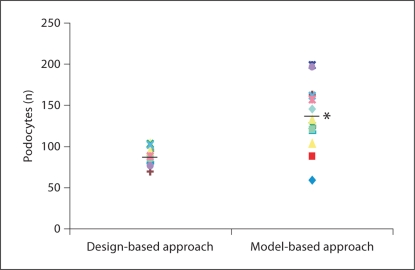
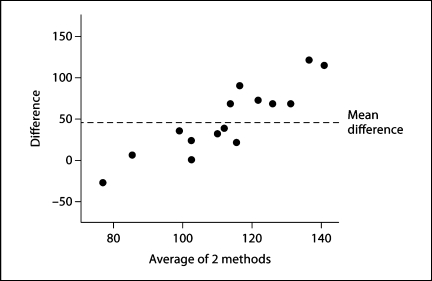
The number of podocytes per mouse glomerulus counted by the design-based approach using the fractionator/disector method was 89 ± 10. The model-based approach using the Weibel-Gomez method counted 137 ± 38 podocytes (p < 0.05; fig. 5). Our earlier studies demonstrated that the fractionator/disector method closely approximates the exhaustive count method for counting glomerular cells [6]. The Bland-Altman analysis determined that the Weibel-Gomez method overestimated the number of podocytes by an average of 48 cells (54%) compared to the fractionator/disector method (fig. 6).
Fig. 5.
Podocyte number obtained from the design-based and model-based approaches. Tissue from the same animals (n = 15) was used to count podocytes with the design-based and the model-based approach. * p < 0.05.
Fig. 6.
Bland-Altman plot of the two counting methods. The Bland-Altman plot shows the average differences between the means of the podocyte number ascertained by the two counting methods.
The glomerular volume measured in the design-based approach was 231 ± 65 × 103 compared to 192 ± 64 × 103 μm3 in the model-based approach. The latter approach significantly underestimated the glomerular volume by 17% (p < 0.0002) compared to the design-based approach.
Discussion
As the number of effective interventions to treat DKD remains relatively stagnant, it becomes prudent to identify new potential therapeutic targets. The podocyte is an important player in both the development and progression of DKD and other proteinuric diseases and may provide prognostic significance during disease management. In recent studies, investigation of the repair and regeneration of podocytes has further emphasized these cells as possible targets for therapeutic intervention. It therefore becomes intuitive to utilize every available resource to optimize the evaluation of podocyte pathology, and since reporting podocyte counts has become a focus of numerous studies, we opted to compare two commonly used counting methods. Here, we illustrated the salient features of the two counting methods using tissues from the db/m and db/db mouse, which is a well-characterized and commonly used model of human DKD [19]. In the design-based approach, toluidine blue-stained, glutaraldehyde-fixed 1-μm-thick kidney sections were used with the unbiased fractionator/disector method. In the model-based approach, formalin-fixed, paraffin-embedded 3- to 4-μm-thick kidney sections were counted with the Weibel-Gomez method. We showed that the model-based approach significantly overestimated podocyte number by 54% and underestimated the glomerular volume by 17% on thick sections when compared to the design-based approach. Further, the Bland-Altman plot indicated that the number of podocytes counted by the model-based approach was increased by an average of 48. In addition, the plot shows the bias increases when more cells are counted. Therefore, the model-based approach, which assumes no change in podocyte size, shape, or orientation within the glomerulus, is biased. In an earlier study [6], we demonstrated that the Weibel-Gomez method may overestimate glomerular cell number by 10%. Some of the factors that may contribute to the 54% overestimation in podocyte number by the model-based approach may be attributed to the greater thickness of paraffin-embedded sections as well as the specificity of the WT-1 antibody.
Indeed, all podocyte nuclei are not of the same shape and size throughout the glomerulus [20]. Quantification of podocyte number from light microscopy images of immunolabeled kidneys using image analysis programs introduces significant bias, even when single sections are presumed to be obtained at the same location within the glomerulus. The consistency of sectioning can only be ascertained from serial sections. Based on stereologic principles, larger nuclei on these single sections will have a greater chance to be counted compared to smaller nuclei [16]. The Weibel-Gomez method may improve this commonly used approach by assessing several sections throughout the glomerulus. However, this method remains biased as no change in cell morphology is assumed. As a consequence, it significantly overestimates podocyte number, which is calculated indirectly from cell density.
The fractionator/disector method has been used to estimate podocyte number by direct visualization of cells on images of 1-μm-thick sections in both humans and animals [6,21,22,23,24] and may require additional expertise not typically available to the investigator. This could explain the common use of the model-based approach that utilizes image analysis programs of immunolabeled kidney sections. Indeed, a 54% error in podocyte number obtained from application of the Weibel-Gomez method in the model-based approach may not be relevant to the goals of a particular study. However, it is unknown whether this degree of difference in podocyte number might explain critical differences in disease mechanisms that otherwise could be the foundation for innovative therapies. As recent studies suggest a potential for podocytes to regenerate from the parietal glomerular epithelium of Bowman's capsule [25] which may be reflected in changes in renal physiology and pathology, the use of antibody-staining techniques to identify the origin of ‘new’ podocytes may become necessary. Hence, it becomes a practical endeavor to optimize laboratory techniques for more efficient immunolabeling of podocyte-specific proteins on 1-μm-thick plastic-embedded sections in order to broaden application of the fractionator/disector method for accurate quantification of podocyte number. In preliminary studies, our laboratory has demonstrated that this type of innovative technique is certainly possible [pers. commun.].
Therefore, it makes sense to adopt an efficient, unbiased method for podocyte counting. Importantly, we observed that the fractionator/disector method was actually less time consuming and required only ∼5 h per animal compared with the Weibel-Gomez method, which required 1–2 days per animal, primarily due to performing appropriate immmunolabeling. The acquisition of new knowledge and skills, possibly through broader collaborations with skilled investigators and laboratories that are equipped to perform detailed morphometry, are important factors to consider in order to effectively shift the paradigm of our current culture. Are you connected?
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgements
The work was supported by NIH/NIDDK grant DK08-057303 and NIH/NCRR grant 1U54RR026138.
References
- 1.Barnard FA. The germ theory of disease and its relations to hygiene. Public Health Pap Rep. 1873;1:70–87. [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Shampo MA. Wilkins, Crick, and Watson: Nobel Prize for work on the structure of DNA. Mayo Clin Proc. 1998;73:362. doi: 10.1016/S0025-6196(11)63703-8. [DOI] [PubMed] [Google Scholar]
- 3.Cain J. Online social networking issues within academia and pharmacy education. Am J Pharm Educ. 2008;72:10. doi: 10.5688/aj720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene JA, Choudhry NK, Kilabuk E, Shrank WH. Online social networking by patients with diabetes: a qualitative evaluation of communication with facebook. J Gen Intern Med. 2011;26:287–292. doi: 10.1007/s11606-010-1526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moubarak G, Guiot A, Benhamou Y, Benhamou A, Hariri S. Facebook activity of residents and fellows and its impact on the doctor-patient relationship. J Med Ethics. 2011;37:101–104. doi: 10.1136/jme.2010.036293. [DOI] [PubMed] [Google Scholar]
- 6.Basgen JM, Nicholas SB, Mauer M, Rozen S, Nyengaard JR. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp Nephrol. 2006;103:e139–e148. doi: 10.1159/000092905. [DOI] [PubMed] [Google Scholar]
- 7.Mathieson PW. Update on the podocyte. Curr Opin Nephrol Hypertens. 2009;18:206–211. doi: 10.1097/mnh.0b013e328326f3ca. [DOI] [PubMed] [Google Scholar]
- 8.Ruotsalainen V, Ljungberg P, Wartiovaara J, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stitt-Cavanagh E, MacLeod L, Kennedy C. The podocyte in diabetic kidney disease. Sci World J. 2009;9:1127–1139. doi: 10.1100/tsw.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev. 2010;62:1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kriz W, Elger M, Nagata M, et al. The role of podocytes in the development of glomerular sclerosis. Kidney Int Suppl. 1994;45:S64–S72. [PubMed] [Google Scholar]
- 12.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 14.Teiken JM, Audettey JL, Laturnus DI, Zheng S, Epstein PN, Carlson EC. Podocyte loss in aging OVE26 diabetic mice. Anat Rec (Hoboken) 2008;291:114–121. doi: 10.1002/ar.20625. [DOI] [PubMed] [Google Scholar]
- 15.Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int. 2009;76:32–43. doi: 10.1038/ki.2009.90. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- 17.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 18.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol. 1962;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- 19.Breyer MD, Bottinger E, Brosius FC, 3rd, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 20.Olivetti G, Anversa P, Melissari M, Loud AV. Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int. 1980;17:438–454. doi: 10.1038/ki.1980.52. [DOI] [PubMed] [Google Scholar]
- 21.Bai XY, Basgen JM. Podocyte number in the maturing rat kidney. Am J Nephrol. 2011;33:91–96. doi: 10.1159/000322701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res. 1992;270:37–45. doi: 10.1007/BF00381877. [DOI] [PubMed] [Google Scholar]
- 23.White KE, Bilous RW. Estimation of podocyte number: a comparison of methods. Kidney Int. 2004;66:663–667. doi: 10.1111/j.1523-1755.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- 24.White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 25.Appel D, Kershaw DB, Smeets B, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]