Abstract
Background
Glomerular podocyte number declines and urinary excretion of podocytes increases as kidney disease progresses in persons with type 2 diabetes mellitus (T2DM).
Methods
Using high-power electron microscopy, we quantified podocyte detachment in T2DM.
Results
We evaluated 106 glomeruli (range 1–6 per subject) from 40 Pima Indian subjects with T2DM enrolled in a clinical trial. On high-power electron micrographs, 35% of the subjects had no evidence of podocyte detachment. Among the remaining subjects, the median percentage of basement membrane with podocyte detachment was 0.62% (interquartile range = 0.32–1.52%).
Conclusion
Podocyte detachment from the glomerular basement membrane has been described and measured in type 1 diabetes mellitus using a different method. We now document podocyte detachment microscopically and quantify it morphometrically in humans with T2DM. The findings offer quantitative histologic support to a potential mechanism for the functional impairment, and possibly the sclerosis of glomeruli, in diabetic glomerular injury.
Key Words: Diabetic kidney disease, Electron microscopy, Histology, Morphometry, Podocyte
Introduction
The role of the glomerular podocyte in the evolution of diabetic kidney disease is of increasing interest [1]. Using stereologic methods to quantify the total number of podocytes per glomerulus, we have earlier reported a decrease in podocyte number in diabetic nephropathy [2]. Podocyte number predicts progression of nephropathy better than mesangial expansion [3] and, when assessed by serial kidney biopsy, correlates with the transition from microalbuminuria to more advanced nephropathy [4]. The number of glomerular podocytes may decline because podocytes detach from the glomerular basement membrane (GBM) and are lost in the urine of patients with type 2 diabetes mellitus (T2DM) [5]. Although replenishment of podocytes from parietal epithelial precursors may help to mitigate these losses [6], it is not known whether such replacement can keep up with ongoing losses. Another process that restores coverage of basement membrane is local foot process broadening (effacement) adjacent to areas of detachment [7], although, when this restorative function reaches its limits, synechiae and segmental sclerosis may occur [8]. We developed a morphometric method to quantify podocyte detachment from the basement membrane in a study of glomerular structural characteristics in 40 Pima Indians with T2DM.
Methods
Participants in this study were enrollees in a randomized, placebo-controlled, clinical trial to evaluate the renoprotective efficacy of losartan in T2DM (ClinicalTrials.gov No. NCT00340678). This study was approved by the Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant gave informed consent. Subjects were at least 18 years old at enrollment in the trial and underwent percutaneous kidney biopsy at the end of the randomized treatment period (∼5.5 years). Kidney biopsies were performed under ultrasound guidance with a 15-gauge Boston Scientific Easy Core® biopsy needle. Specimens were fixed in buffered 4% glutaraldehyde and shipped to the Beckman Center for Electron Microscopy at Stanford University, where the biopsy cores were passed through a graded series of ethanols, and the fixed tissue was embedded in Epon 812® in preparation for light and electron microscopy.
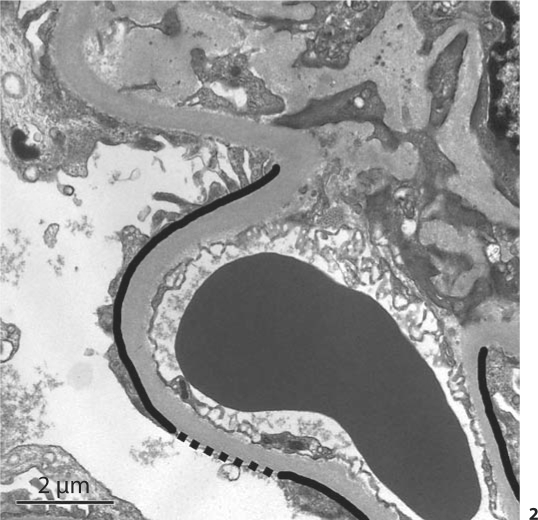
For transmission electron microscopy (TEM), 0.5-μm sections stained with toluidine blue were surveyed to identify blocks containing at least two open glomerular profiles. Further sections, 70 nm thick, were collected on 3-mm copper grids, stained with lead citrate and uranyl acetate, and examined in a Hitachi H-600 TEM. Twenty high-power photomicrographs (×11,280) were obtained from each of the glomerular profiles. These images were used to determine the percentage of GBM that was denuded of podocytes. Podocyte detachment was defined as complete absence of foot processes along the interface of the GBM and the podocyte layer, and was limited to the filtration surface. The majority of these denuded areas were free of any podocyte membrane or cytoplasm; Bowman's space was in direct contact with the denuded GBM. If podocyte cell membrane was observed along the GBM but the cytoplasm was electrolucent, that area was not considered denuded. Similarly, if only alternating foot processes from a single podocyte were missing, this was not considered denuded (fig. 1). The denuded length of GBM in each image was divided by the total GBM length measured (along the filtration surface) to calculate the percentage of denuded GBM (fig. 2). The values from each image were summed across all images from each glomerulus. The mean percentage of podocyte detachment was then calculated from all glomeruli in each individual.
Fig. 1.
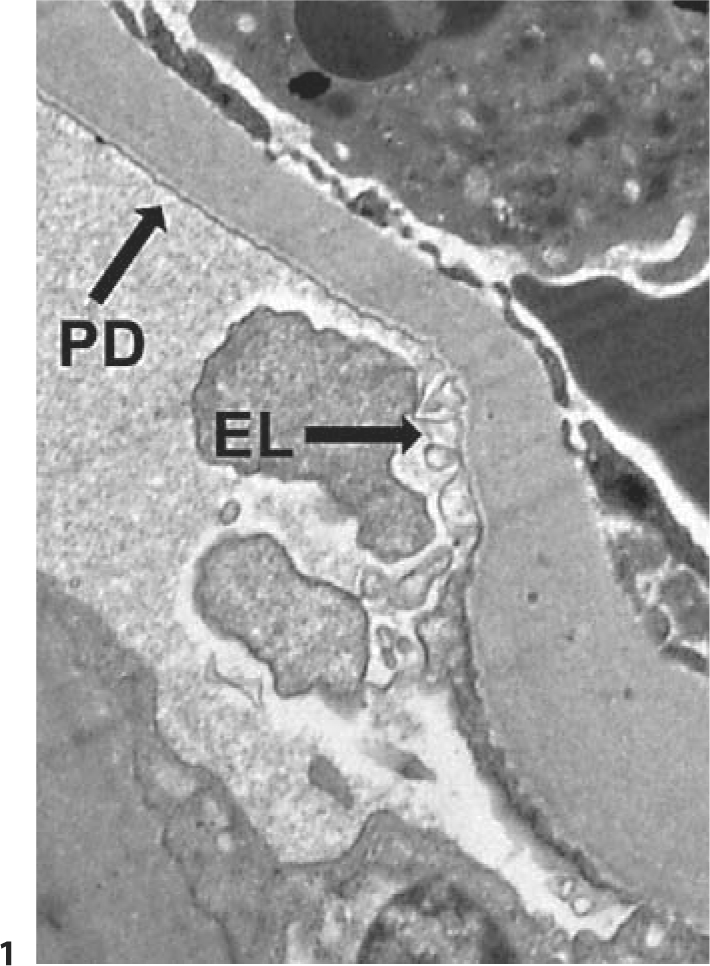
Electrolucent foot processes (EL) adjacent to an area of podocyte foot process detachment (PD). Only areas of complete PD were considered in the computation of detachment.
Fig. 2.
Method for calculating the percentage of podocyte detachment. The dashed black line represents the length of GBM with podocyte detachment and the solid black line + dashed black line lengths represent the entire visible length of filtering basement membrane in a glomerular capillary. Detachment (%) = [length of dashed line/(length of dashed line + length of solid line)] × 100. The values from each image were summed across all images from each glomerulus. The mean percentage of podocyte detachment was then calculated from all glomeruli in each individual.
Statistical Methods
Results are expressed as medians and interquartile ranges. The analyses were carried out using SAS® software (version 9.1; SAS, Cary, N.C., USA).
Results
Kidney biopsies were examined from 12 men and 28 women. The mean age of these participants was 47.5 years (range 32.5–64.8); the mean duration of diabetes was 16.8 years (range 8.6–36.6). From these subjects, 106 glomeruli (range 1–6 per subject) were evaluated. No evidence of podocyte detachment was noted in 35% of the subjects on high-power electron micrographs. Among the remaining subjects (65%), the median percentage of basement membrane with podocyte detachment was 0.62% (interquartile range = 0.32–1.52%). Images of normal and detached podocytes are shown in figure 3.
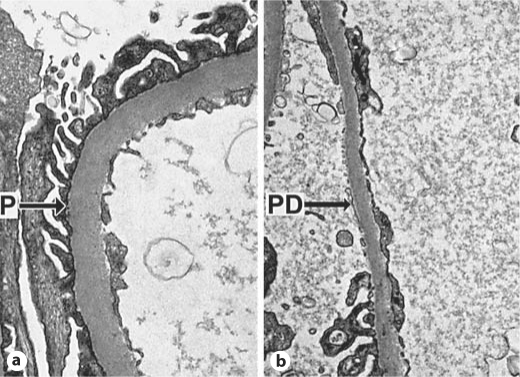
Fig. 3.
Peripheral glomerular capillaries from Pima Indians with T2DM (TEM, ×11,280). a Intact podocyte foot processes (P). b Local podocyte detachment (PD).
Discussion
Podocyte detachment occurs in persons with type 2 diabetic nephropathy. High-power images of areas of podocyte detachment illustrate that replacement of detached glomerular podocytes and/or broadening of adjacent foot processes is neither immediate nor complete enough to cover all areas of denuded basement membrane. If the detachment is persistent, it could lead to segmental sclerosis and hyalinosis [9].
We defined podocyte detachment as complete absence of podocyte foot processes along the peripheral capillary GBM. We wanted to avoid characterizing electrolucent foot processes as areas of detachment, since filtration slits and diaphragms between foot processes are potentially intact, and the functional characteristics of the filtration barrier may remain unchanged. We chose this very conservative definition so as not to overstate structural abnormalities in studies of structural-functional correlations.
Areas of GBM devoid of podocytes were described previously in patients with type 1 diabetes mellitus [10]. Toyoda et al. [10] used a point-counting method on high-power electron micrographs to quantify the extent of GBM from which podocytes were detached. In contrast to our definition, they included electrolucent foot processes and areas where normal foot processes were mixed with absent or electrolucent foot processes in their definition of detachment. The fact that they reported 22% denudation, whereas we report 1% detachment, is probably, in large part, a result of our different definitions of podocyte detachment. Despite these differences, the two studies demonstrate a process common to both type 1 and type 2 diabetic glomerulopathy [11].
Integrity of podocytes on the urinary side of the glomerular filtration barrier is essential for normal glomerular capillary permeability, and areas of podocyte detachment are undoubtedly associated with alterations in local permselectivity. The current study did not focus on the functional significance of podocyte detachment, but earlier dextran-sieving experiments conducted in Pima Indians with T2DM found that subjects with macroalbuminuria had increased filtration of high-molecular-weight dextrans through an excess of large pores acting as a macromolecular shunt. This shunt was not present in subjects with either normo- or microalbuminuria [12]. Further studies are needed to determine if areas of podocyte detachment correlate with these large pores.
In conclusion, this study describes a quantitative method to document podocyte detachment in humans with T2DM. The detachment we observed is consistent with that reported previously in type 1 diabetes mellitus [10], and offers quantitative histologic support to a potential mechanism for the functional impairment, and possibly the sclerosis of glomeruli, in diabetic glomerular injury.
Disclosure Statement
No potential conflicts of interest relevant to this article were reported.
Acknowledgements
The authors are indebted to the participants in this investigation, and to the doctors, nurses and support staff involved in collecting and processing the data. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and by the American Diabetes Association (Clinical Science Award 1-08-CR-42).
References
- 1.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 2.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 4.Lemley KV, Abdullah I, Myers BD, Meyer TW, Blouch K, Smith WE, Bennett PH, Nelson RG. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney Int. 2000;58:1228–1237. doi: 10.1046/j.1523-1755.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 6.Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ. The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney Int. 2009;76:1225–1238. doi: 10.1038/ki.2009.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 8.Kriz W, Elger M, Nagata M, Kretzler M, Uiker S, Koeppen-Hageman I, Tenschert S, Lemley KV. The role of podocytes in the development of glomerular sclerosis. Kidney Int Suppl. 1994;45:S64–S72. [PubMed] [Google Scholar]
- 9.Rennke HG, Klein PS. Pathogenesis and significance of nonprimary focal and segmental glomerulosclerosis. Am J Kidney Dis. 1989;13:443–456. doi: 10.1016/s0272-6386(89)80001-0. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AH, Mampaso F, Zamboni L. Glomerular podocyte degeneration in human renal disease: an ultrastructural study. Lab Invest. 1977;37:30–42. [PubMed] [Google Scholar]
- 12.Lemley KV, Blouch K, Abdullah I, Boothroyd DB, Bennett PH, Myers BD, Nelson RG. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11:2095–2105. doi: 10.1681/ASN.V11112095. [DOI] [PubMed] [Google Scholar]