Abstract
Purpose
To demonstrate a high-level expression of transferrin (Tf)-based fusion proteins by inserting a helical linker between two protein domains.
Methods
Tf-based fusion proteins were designed to contain oligonucleotides encoding a helical linker inserted between the protein domains. Plasmid constructs were transfected into HEK293 cells and the secreted fusion proteins were purified from conditioned serum free media. Expression was assessed using both SDS-PAGE and Western Blot using anti-hGH, G-CSF, or Tf antibodies; protein bands were analyzed using Quantity One software. The function of fusion proteins consisting of human growth hormone (hGH) and Tf was evaluated in Nb2 cell proliferation assays.
Results
The fusion proteins containing a helical linker, hGH-(H4)2-Tf and Tf-(H4)2-hGH, were expressed 1.7- and 2.4-fold higher, respectively, with a 2-fold lower ED50 than the hGH-Tf fusion protein without a helical linker. The Tf-(H4)2-G-CSF fusion protein exhibited a greater expression with an 11.2-fold increase compared with Tf-G-CSF fusion protein.
Conclusions
The helical linker introduced in Tf-fusion proteins resulted in a high-level of expression with improved in vitro bioactivity. This approach provides a simple method to increase poor expression of other fusion proteins.
Keywords: Helical linker, increased-expression, transferrin, fusion protein, domain switch, G-CSF, hGH
1. Introduction
High quantities of recombinant proteins ranging from hundreds of milligrams to grams must be produced in order to carry out preclinical evaluations and clinical trials (1–3). Unfortunately, the potential therapeutic proteins with poor expression face an obstacle to make it through clinical trials to final approval by the FDA. Protein therapeutics developed from recombinant hormones, growth factors and cytokines express at relatively low levels, not only increasing the manufacturing cost but also delaying further product evaluation. Some successful protein therapeutics are recombinant fusion proteins consisting of cytokines or growth factors fused with the Fc portion of IgG1 or immunotoxin and are expressed as single polypeptides with dual biological activities (4, 5). These therapeutic fusion proteins, including Enbrel® (TNF-R/Fc-IgG1), Ontak® (IL-2/diphtheria toxin), Orencia® (CTLA-4/Fc-IgG1) and Amevive® (LFA-3/Fc-IgG1) (6), may experience poor expression as the fusion partners interfere with each other for optimal translation, especially in mammalian cells. Since mammalian cells are the preferred choice for producing some therapeutic proteins, as posttranslational modifications in these cells may be associated with reduced immunogenecity compared to other systems (3), a simple strategy that enhances the expression of therapeutic fusion proteins in mammalian cells would be desirable.
Typically, the problem of low expression is improved by incorporating carbohydrate-binding module (CBM) and maltose-binding protein (MBP) as fusion partners to the target protein (7, 8). However, these fusion partners are generally removed during or after purification by introducing peptide linkers with cleavage sites for endopeptidases such as thrombin and factor Xa (8). Conceivably, this approach is not feasible for large-scale production of target proteins because it requires numerous steps of column purification and enzymatic processing, limiting the production capacity and possibly causing non-specific cleavage.
The selection of a peptide linker with the ability to maintain domain function of the fusion protein is becoming important (9–12). Recently, we designed a helical linker with 50 amino acids using an EAAAK helix-forming motif based on a previous study (10), and inserted the linker between granulocyte colony stimulating factor (G-CSF) and Tf moieties, leading to increased biological activity (13). Most recently, we found that the insertion of the same helical linker in Tf-fusion proteins resulted in a high-level expression in HEK293 cells as compared to the same fusion proteins without the helical linker. Here we report the helical linker-dependent increase of expression in two Tf-based fusion proteins, G-CSF and human growth hormone (hGH), and provide evidence of a high-level of expression for both proteins regardless the level of original expression without the linker. Conceivably, this approach can be introduced and applied to other fusion proteins with limited to no expression, greatly improving the production yield for downstream applications.
2. Materials and Methods
2.1. Preparing gene fusion constructs in pcDNA3.1(+)
Fusion constructs for Tf-based fusion proteins containing either hGH or human G-CSF were designed and established in mammalian expression vector pcDNA3.1(+) (Invitrogen) based on a previous report (14). Briefly, the DNA sequences encoding for hGH or G-CSF were subcloned and fused in frame to the sequences encoding for Tf. The DNA sequences encoding for the signal peptide from the N-terminus domain was incorporated in the polypeptide; however, the stop codon from the N-terminus domain was deleted for uninterrupted translation. The final constructs were verified by DNA sequence analysis.
2.2. Helical linker insertion
Two domains between Tf and hGH or G-CSF in the fusion protein were fused by leucine (L) and glutamic acid (E), a product of Xho I restriction site. The helical linker, H4 and (H4)2, LEA(EAAAK)4ALE and LEA(EAAAK)4ALEA(EAAAK)4ALE, respectively, were prepared and inserted according to the previous study (13). The orientation, sequences and copy numbers of the helical linker were confirmed by DNA sequence analysis.
2.3. Production of fusion protein
The human embryonic kidney 293 cells (HEK293 or HEK293T) (ATCC) were cultured in DMEM media (Mediatech) containing 10% FBS, 50 units penicillin/50 μg streptomycin in a humidified incubator at 37 °C with 5% CO2. HEK293 cells were seeded at near confluence in 6-well plates (Costar) and transiently transfected with 2 μg expression constructs and 5.5 μl Lipofectamine 2000 (Invitrogen). The transfected cells were allowed to express fusion proteins in serum free CD293 media (Invitrogen) for five days. The conditioned media containing the fusion protein was then harvested, clarified by centrifugation, and concentrated using Amicon Ultra-4 or Ultra-15 filtering devices (Millipore).
2.4. SDS-PAGE and Western blots
The fusion proteins were fractionated on a 10% SDS-PAGE or 4–20% pre-cast gel (Thermo Scientific) and visualized by staining with Coomassie blue. For Western blot analysis, the fusion proteins were transferred to a PVDF membrane (GE healthcare) and blocked with 5% non-fat milk for 1 hr at room temperature, after separating on SDS-PAGE. hGH and Tf were identified by using goat anti-hGH monoclonal antibody (1:1000) (R&D Systems) and goat anti-human Tf antibody (1:5000) as primary antibodies and rabbit anti-goat antibody conjugated to HRP as secondary antibody. Likewise, the G-CSF was detected by using rabbit anti-human G-CSF (1:10000) as primary antibody and donkey anti-rabbit antibody conjugated to HRP (1:10000) as secondary antibody. All antibodies were obtained from Sigma, unless mentioned otherwise. ECL plus reagents (GE Healthcare) and ChemiDoc XBR (Bio-Rad) were used for developing and capturing the hGH-fusion proteins. X-ray film was used to develop G-CSF-fusion proteins. The expression of both hGH- and G-CSF-fusion proteins were analyzed using Quantity One software (Bio-Rad), and results from either anti-hGH or anti-G-CSF Western blots are comparable to that of anti-Tf Western blots.
2.5. In vitro cell proliferation
Nb2 cells (Sigma) derived from rat T lymphoma cells were cultured as suspension in RPMI 1640 media (Mediatech) supplemented with 2 mM glutamine, 10% FBS, 10% horse serum (HS) (Invitrogen), 50 units of penicillin/50 μg streptomycin, and 50 μM 2-mercaptoethanol (15) in a humidified incubator at 37 °C with 5% CO2. For proliferation assays, Nb2 cells were washed extensively in a serum free RPMI 1640 media, re-suspended in assay media that included 10% HS but not FBS, and counted with a Z1 Coulter particle counter (Beckman Coulter). About 5000 Nb2 cells per well were seeded into 96-well plates in 200 μl assay media, starved for 24 h, treated with hGH or fusion protein whose dose was normalized to that of hGH, and incubated for 4 days. Next, the cells were added with 20 μl of Alamar Blue dye (Biosource) and incubated overnight for color development. The UV absorbance was measured at 570 nm using a Genios spectrophotometer (Tecan) and corrected by subtracting the control without treatment. The ED50 was defined as the dose of fusion protein that led to half of maximum proliferation.
3. Results
3.1. Establishing gene fusion constructs
To investigate whether the insertion of a helical linker between the protein domains in the Tf-based fusion protein improves the expression, we constructed three pairs of gene fusion plasmids with or without the inserted helical linker, and confirmed that the insertion, orientation and number of copies were correct. Subsequently, the plasmid constructs were transfected to HEK293 or HEK293T cells to produce fusion proteins.
3.2.a. Comparing hGH-Tf and hGH-(H4)2-Tf fusion proteins for expression
The hGH-Tf fusion protein consists of human growth hormone and Tf linked by a short di-peptide linker; whereas the hGH-(H4)2-Tf fusion protein is linked by a helical linker with 50 amino acids. To assess the level of expression, the fusion proteins with or without the helical linker were analyzed by Western blot as well as SDS-PAGE with Coomassie stain. Both anti-hGH and anti-Tf Western blots detected a band corresponding to approximately 100 kDa, which is the sum of molecular weights from Tf (79 kDa) and hGH (22 kDa), and confirmed that the fusion protein was composed of two moieties including Tf and hGH (Fig. 2 lanes 3 and 4). To evaluate and compare the level of expression, band-densities for each fusion protein were quantified and analyzed. The density data revealed that the fusion protein with the helical linker expressed at a level 1.7 fold higher than the original fusion protein without the helical linker (Table 1, Fig. 1 lanes 3 and 4).
Fig. 2.
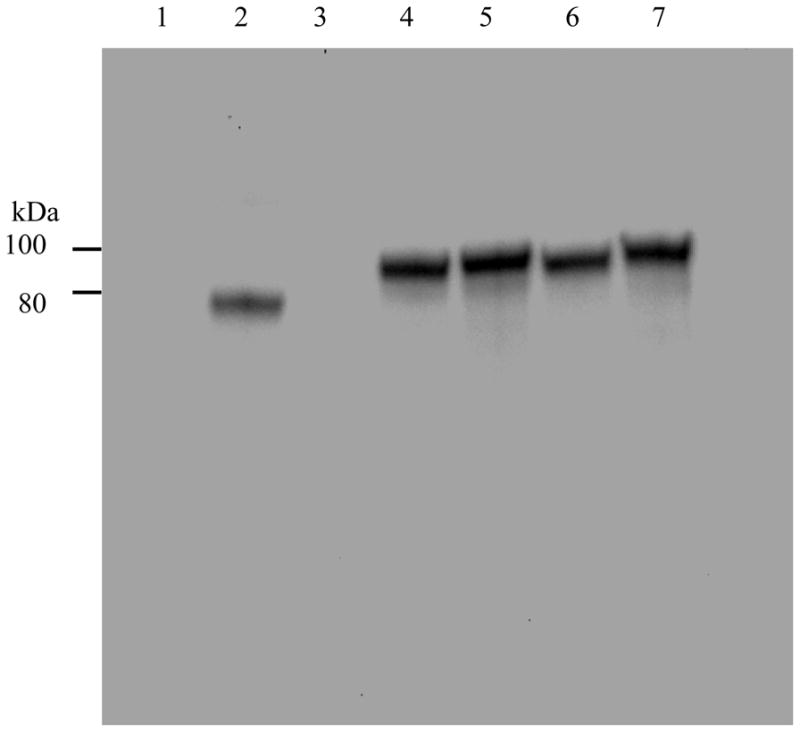
High-level expression by the insertion of helical linker in both hGH-Tf and Tf-hGH fusion proteins as analyzed by Anti-Tf Western blot. Four fusion proteins with or without the inserted helical linker, expressed in serum free media, were analyzed by Western blot using goat anti-Tf antibody (1:5000). The signal was detected using rabbit anti-goat secondary antibody conjugated to HRP (1:1000) and ECL reagents. The image was recorded and analyzed by ChemiDoc XBR. Lane 1: Tf (50 ng); lane 2: hGH (negative control); lane 3: hGH-Tf; lane 4: hGH-(H4)2-Tf; lane 5: Tf-hGH; lane 6: Tf-(H4)2-hGH.
Table 1.
The ratio of expression of Tf-fusion proteins with or without helical linker
| Linker (x) | G-CSF Fusion Proteins | hGH Fusion Proteins | ||
|---|---|---|---|---|
| Tf-x-G-CSF | G-CSF-x-Tf | Tf-x-hGH | hGH-x-Tf | |
| No Linker | 1 | 1 | 1 | 1 |
| H4 | 7.8 | ND | ND | ND |
| (H4)2 | 11.2 | 1.44 | 2.39 | 1.66 |
x: linker inserted between Tf and hGH or G-CSF.
(H4): helical linker.
(H4)2: two copies of helical linker.
no linker: two domains were fused by LE peptide
ND: not determined.
Fig. 1.
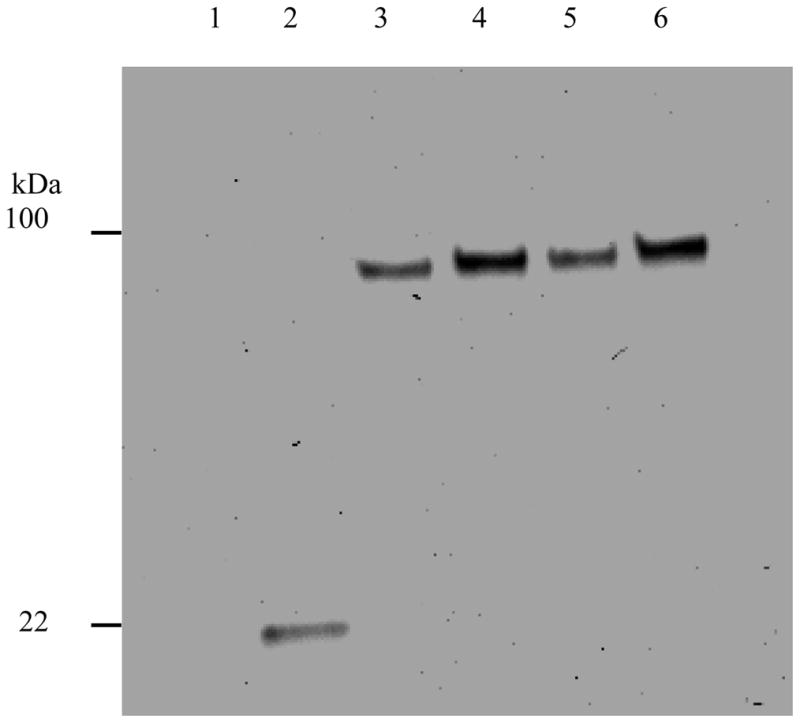
High-level expression by the insertion of helical linker in both hGH-Tf and Tf-hGH fusion proteins as analyzed by Anti-hGH Western blot. Four fusion proteins with or without the inserted helical linker, expressed in serum free media, were analyzed by Western blot using goat anti-hGH monoclonal antibody (1:1000). The signal was detected using HRP-conjugated rabbit anti-goat secondary antibody (1:1000) and ECL reagents. The image was recorded and analyzed by ChemiDoc XBR (Bio-Rad). Lane 1: Tf (negative control); lane 2: hGH (10 ng); lane 3: hGH-Tf; lane 4: hGH-(H4)2-Tf; lane 5: Tf-hGH; lane 6: Tf-(H4)2-hGH.
In addition, fusion proteins were analyzed by SDS-PAGE stained with Coomassie blue to confirm results from the band-density analysis from Western blots, and to evaluate relative purity and abundance. The results from SDS-PAGE demonstrated that the fusion proteins were expressed with high purity (~ 90%) and high abundance (~ 95%) at a molecular weight of 100 kDa (Fig. 3 lane 5). Furthermore, band-density analysis from Coomassie blue-stained SDS-PAGE showed that the expression of fusion protein with the helical linker was about 1.7 fold higher than the original fusion protein without the helical linker (Fig. 3 lane 4), confirming results from Western blot analysis.
Fig. 3.
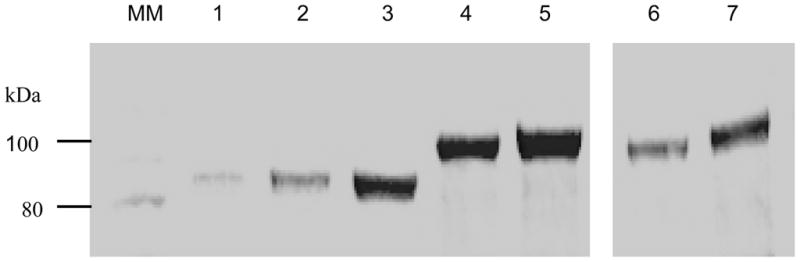
Helical linker insertion led to high-level expression in both hGH-Tf and Tf-hGH fusion protein as analyzed by SDS-PAGE. Same volume of conditioned media (5 μl) from the transfected HEK-293 cells were fractionated using SDS-PAGE, stained with Coomassie blue, and analyzed with ChemiDoc XBR. MM: molecular weight marker; lanes 1–3: Tf control; lane 4: hGH-Tf; lane 5: hGH-(H4)2-Tf ; lane 6: Tf-hGH; lane 7: Tf-(H4)2-hGH.
3.2.b. Comparing Tf-hGH and Tf-(H4)2-hGH fusion proteins for expression
Thus far, the fusion proteins evaluated were oriented to have a N-terminal hGH domain and C-terminal Tf domain. To investigate the effect of the helical linker on the expression of fusion proteins with different orientation, the Tf-and hGH-domains were switched to produce two new fusion proteins: Tf-hGH and Tf-(H4)2-hGH. The two fusion proteins were expressed and the yield of expression was compared. The Western blot data with fusion protein specific antibodies including anti-hGH and anti-Tf confirmed the identity and molecular weight (100 kDa) of the fusion protein, as well as the insertion of helical linker as shown by slightly increased molecular weight (Fig. 1 lanes 5 and 6, Fig. 2 lanes 5 and 6). The band-density analysis from Western blot data showed the fusion protein with the inserted helical linker expressed at a higher level with a ~ 2.4 fold increase as compared to the original fusion protein without the helical linker (Table 1). The SDS-PAGE with Coomassie stain revealed that both fusion proteins were expressed with high purity (~ 90%) and high abundance (90%) but the fusion protein with the helical linker expressed at high levels with a ~ 2.4 fold increase compared to the original fusion protein without the helical linker (Fig. 3 lane 7 and 6, respectively), further confirming the Western blot data.
3.2.c. Comparing Tf-G-CSF and Tf-(H4)2-G-CSF fusion proteins for expression
To study the broader implication of the helical linker insertion on the increased expression of Tf-based fusion proteins, Tf-G-CSF fusion protein consisting of transferrin and granulocyte colony-stimulating factor was constructed, and subsequently, the helical linker was inserted. These two fusion proteins were expressed to compare the level of expression. The Western blot using anti-G-CSF antibody detected a 100-kDa band equivalent to the molecular weight of Tf-G-CSF fusion protein (~ 80 + 19 kDa), confirming the identity and molecular weight (Fig. 4). Moreover, the Western blot data revealed that the expression from the Tf-G-CSF fusion protein was too low to detect convincingly (Fig. 4 lane 3). In contrast, the Tf-(H4)-G-CSF and Tf-(H4)2-G-CSF fusion proteins expressed at elevated levels with a 7.8 and an 11.2 fold increase as compared to original fusion protein without the linker, respectively, providing notable evidence for the improved expression of Tf-based fusion proteins by the insertion of a helical linker (Fig. 4 lanes 4 and 5, Table 1). The fusion protein expressed with a reversed coding sequence for the helical linker (Tf-r(H4)2-G-CSF) failed to express (Fig. 4 lane 6). Taken together, the helical linker insertion between Tf and G-CSF domains led to a significant increase in expression, comparing with the original fusion protein without the helical linker.
Fig. 4.
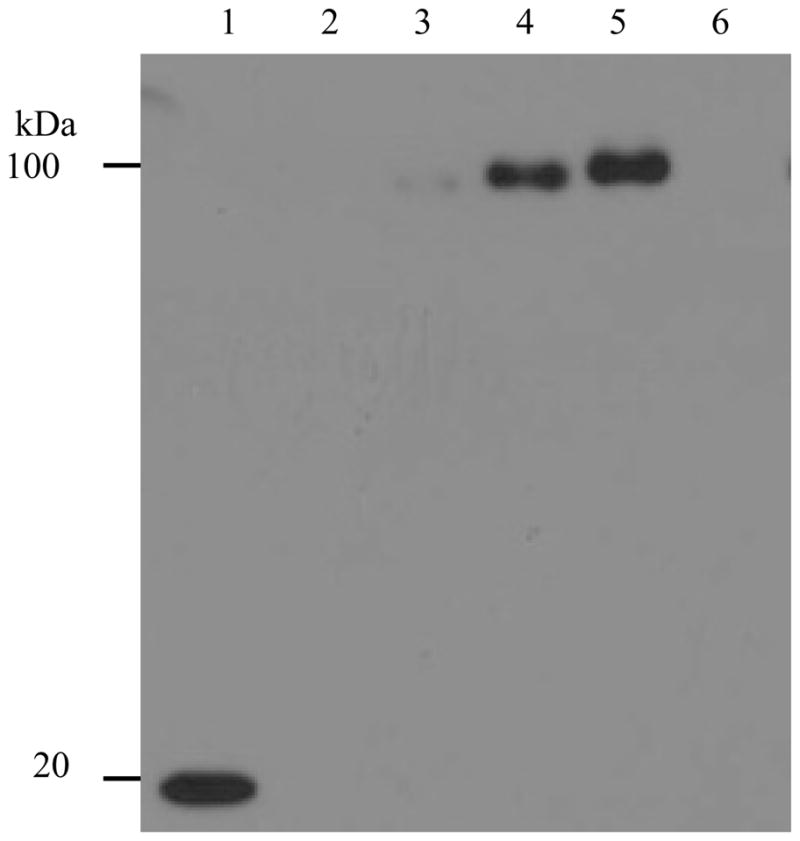
Helical linker insertion led to high-level expression in both G-CSF-Tf and Tf-G-CSF fusion protein as analyzed by anti-G-CSF Western blot. 15 μl-conditioned media from the transfected HEK-293 cells were analyzed using anti-GCSF Western blot. Lane 1: G-CSF control; lane 2: pcDNA3.1(+) without the insert; lane 3: Tf-G-CSF; lane 4: Tf-(H4)-G-CSF; lane 5: Tf-(H4)2-G-CSF; lane 6: Tf-r(H4)2-G-CSF (fusion protein with a reversed (H4)2 DNA sequence inserted, thus a non-helical linker in the product).
3.3. Comparing in vitro cell proliferation
The hGH-(H4)2-Tf and Tf-(H4)2-hGH fusion proteins were tested in vitro to determine whether they induce Nb2 cell proliferation. The Nb2 cells treated with either hGH-(H4)2-Tf or Tf-(H4)2-hGH fusion protein demonstrated higher proliferation activity as compared to Nb2 cells treated with the hGH-Tf fusion protein without helical linker (Table 2).
Table 2.
Comparing Nb2 cell proliferation activity of hGH-Tf fusion protein, with or without helical linker.
| Linker (x) | ED50 (ng/mL)
|
||
|---|---|---|---|
| hGH | hGH-x-Tf | Tf-x-hGH | |
| No linker | 0.25 | 1.85 | NA* |
| (H4)2 | -- | 0.85 | 0.80 |
Was not determined due to low yield of the fusion protein.
4. Discussion
The success of constructing biologically active recombinant G-CSF-Tf fusion protein with the helical linker (13, 14) led us to pursue the feasibility of producing other Tf fusion proteins. To achieve this goal, we constructed recombinant fusion protein consisting of Tf and hGH, and introduced two copies of a helical linker (H4)2 as a spacer between two protein domains. As previously reported in G-CSF fusion protein (13), the insertion of the helical linker also increases the in vitro biological function of hGH fusion protein as shown in the Nb2 cell proliferation assay (Table 2).
Besides the increase of in vitro biological activity, we found that the expression level of both fusion proteins with the helical linker, i.e., G-CSF-(H4)2-Tf and hGH-(H4)2-Tf, was significantly higher than that of fusion proteins without the linker. We further investigated the effect of helical linker insertion on the expression of fusion proteins with a Tf-domain switch, i.e., Tf-(H4)2-G-CSF and Tf-(H4)2-hGH, which created a fusion protein with a different orientation. As shown in Fig. 3 and 4, the expression of fusion protein with Tf at the amino-terminus is generally very poor. However, the insertion of the helical linker significantly increased the expression of both fusion proteins with Tf at the amino-terminus (Table 1).
We have reported previously that the expression of G-CSF-Tf fusion protein failed when a flexible and non-helical linker, consisting of glycine and serine (GSSSS)3, was inserted between Tf and G-CSF domains (13). Others have also found that the insertion of IgG hinge region as a flexible linker between Tf and nerve growth factor (NGF) domains was ineffective for the expression of this fusion protein (12). To further demonstrate the requirement of the helical structure of the linker peptide for the increase of the fusion protein expression, we reversed the DNA sequence coding for the helical peptide to produce a linker with an identical peptide length, but a non-helical structure. Our results showed that there was no expression of the fusion protein, Tf-r(H4)2-G-CSF (Fig. 4 lane 6). These findings led us to the conclusion that the increase of the expression of Tf-based fusion protein is due to the non-flexible and helical nature of the linker that is inserted between the two protein domains.
The exact mechanism responsible for this increased expression is unclear. However, the rigid, extended nature, as well as the composition, of the helical linker may help increase the rate of hGH- and Tf- domain folding. A proper folding will enhance the stability of the newly translated polypeptide and, consequently, will increase the expression of Tf fusion proteins in the conditioned media. By using fluorescent resonance energy transfer (FRET) technique, Arai et al. found that the insertion of a helical linker between the enhanced green fluorescent protein (EGFP) domain and enhanced blue fluorescent protein (EBFP) domain in a chimeric protein increased the distance and kept two domains apart (10). Furthermore, Robinson et al. reported that the composition and length of linker between two domains were important in controlling the rate of folding, unfolding, and stability of chimeric protein (16). It is likely that a large molecule, such as Tf-G-CSF or Tf-hGH fusion protein with a molecular weight of 100 kDa, requires large conformational space to fold correctly. The stable and efficient folding may drive the equilibrium towards an increased expression and accumulation of the fusion protein. Conceivably, the linker with a helical structure can hold the domains at a distance, providing a larger space for correct folding.
Another possible reason for the increased expression is that a helical linker with its secondary structure may be resistant to enzymatic cleavage by protecting the target amino acids from protease recognition, thereby increasing overall stability of the fusion protein (17). Further studies that evaluate the stability of the helical linker against common proteolytic enzymes including trypsin and chymotrypsin, would aid partially in our understanding of the mechanisms responsible for the increased expression.
In general, a high level expression of fusion proteins may be achieved: 1) by designing expression vectors with strong promoters and episomal origins; 2) by transfecting host cells such as HEK293T and HEK293 EBNA1 with expression of episomal antigens including SV40 large-T antigen and EBV nuclear antigen, respectively; 3) by selecting transfection reagents with highest gene transfer efficiency; and 4) by developing or using culture media with enhanced cell proliferation and survival but with minimum cell death and apoptosis (1–3). Furthermore, the addition of sodium butyrate and peptone into the culture media containing the transfected cells can have positive effect on the expression of target protein. However, long-term consequences of using such components in culture media remain controversial and unknown (18, 19). Results in this report demonstrate that a high level expression of Tf-fusion proteins can be achieved by the insertion of a helical peptide linker between the two protein domains. If the increase of expression can be demonstrated in fusion proteins other than Tf, it will provide a simple and practical technique to improve the production of recombinant fusion proteins for therapeutic and diagnostic uses.
5. Conclusions
We found that the insertion of a helical peptide as the linker in Tf-based fusion proteins led to a high level of expression, with superior in vitro bioactivity. Given the straightforward approach and ease of both designing and introducing the helical linker in the fusion protein, this qualifies as a feasible strategy for the production of therapeutic fusion proteins at high levels in mammalian cells.
Abbreviations
- hGH
human growth hormone
- G-CSF
granulocyte colony stimulating factor
- Tf
human serum transferrin
- (H4)2
2 copies of helical linker [A(EAAAK)4A]2
- H4
1 copy of helical linker A(EAAAK)4A
- hGH-Tf
human growth hormone-transferrin recombinant fusion protein
- hGH-(H4)2-Tf
hGH-Tf fusion protein with two copies of helical linker
- Tf-hGH
transferrin-human growth hormone fusion protein with Tf at N-terminus
- Tf-(H4)2-hGH
Tf-hGH fusion protein with 2 copies of helical linker
- G-CSF-Tf
granulocyte colony stimulating factor-transferrin recombinant fusion protein
- Tf-G-CSF
transferrin-granulocyte colony stimulating factor recombinant fusion protein with Tf at N-terminus
- Tf-(H4)2-G-CSF
Tf-G-CSF fusion protein with 2 copies of helical linker
- Tf-r(H4)2-G-CSF
Tf-G-CSF fusion protein with a reversed (H4)2 DNA sequence inserted.
- Nb2 cells
rat T lymphoma cells that proliferate upon stimulation by the bioactive hGH
References
- 1.Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett. 2007;29:677–684. doi: 10.1007/s10529-006-9297-y. [DOI] [PubMed] [Google Scholar]
- 2.Wurm F, Bernard A. Large-scale transient expression in mammalian cells for recombinant protein production. Current Opinion in Biotechnology. 1999;10:156–159. doi: 10.1016/s0958-1669(99)80027-5. [DOI] [PubMed] [Google Scholar]
- 3.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian GM, Fiscella M, Lamousé-Smith A, Zeuzem S, McHutchison JG. Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nature Biotechnology. 2007;25:1411–1419. doi: 10.1038/nbt1364. [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nature Biotechnology. 2007;25:1290–1297. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 6.Leader B, Baca QJ, Golan DE. Opinion: Protein therapeutics: a summary and pharmacological classification. Nature Reviews Drug Discovery. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 7.Kavoosi M, Creagh AL, Kilburn DG, Haynes CA. Strategy for selecting and characterizing linker peptides for CBM9-tagged fusion proteins expressed in Escherichia coli. Biotechnology and Bioengineering. 2007;98:599–610. doi: 10.1002/bit.21396. [DOI] [PubMed] [Google Scholar]
- 8.Pryor KD, Leiting B. High-Level Expression of Soluble Protein inEscherichia coliUsing a His6-Tag and Maltose-Binding-Protein Double-Affinity Fusion System. Protein Expression and Purification. 1997;10:309–319. doi: 10.1006/prep.1997.0759. [DOI] [PubMed] [Google Scholar]
- 9.Maeda Y, Ueda H, Kazami J, Kawano G, Suzuki E, Nagamune T. Engineering of Functional Chimeric Protein G–VargulaLuciferase. Analytical Biochemistry. 1997;249:147–152. doi: 10.1006/abio.1997.2181. [DOI] [PubMed] [Google Scholar]
- 10.Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Engineering. 2001;14:529–532. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- 11.Arai R, Wriggers W, Nishikawa Y, Nagamune T, Fujisawa T. Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins Structure Function and Bioinformatics. 2004;57:829–838. doi: 10.1002/prot.20244. [DOI] [PubMed] [Google Scholar]
- 12.Park E, Starzyk RM, McGrath JP, Lee T, George J, Schutz AJ, Lynch P, Putney SD. Production and characterization of fusion proteins containing transferrin and nerve growth factor. J Drug Target. 1998;6:53–64. doi: 10.3109/10611869808997881. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharmaceutical Research. 2006;23:2116–2121. doi: 10.1007/s11095-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 14.Bai Y, Ann DK, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proceedings of the National Academy of Sciences. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa M, Nimura A, Horikawa R, Katsumata N, Arisaka O, Wada M, Honjo M, Tanaka T. A Novel Specific Bioassay for Serum Human Growth Hormone. Endocrine Soc. 2000;85:4274–4279. doi: 10.1210/jcem.85.11.6983. [DOI] [PubMed] [Google Scholar]
- 16.Robinson CR, Sauer RT. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proceedings of the National Academy of Sciences. 1998;95:5929–5934. doi: 10.1073/pnas.95.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marqusee S, Baldwin RL. Helix Stabilization by Glu−\ cdots Lys+ Salt Bridges in Short Peptides of De novo Design. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham PL, Perret S, Cass B, Carpentier E, St-Laurent G, Bisson L, Kamen A, Durocher Y. Transient gene expression in HEK293 cells: Peptone addition posttransfection improves recombinant protein synthesis. Biotechnology and Bioengineering. 2005;90:332–344. doi: 10.1002/bit.20428. [DOI] [PubMed] [Google Scholar]
- 19.Crowell CK, Qin Q, Grampp GE, Radcliffe RA, Rogers GN, Scheinman RI. Sodium butyrate alters erythropoietin glycosylation via multiple mechanisms. Biotechnol Bioeng. 2008;99:201–213. doi: 10.1002/bit.21539. [DOI] [PubMed] [Google Scholar]


