Abstract
Purpose:
To assess our institution's ability to minimize local and distant recurrence with a preference for sphincter preserving surgery in the management of rectal cancer.
Methods:
A retrospective analysis of all patients treated between 1982 and 1998. Patients with Stage 0 (AJCC) disease and those treated for palliation were not included. Clinical and pathologic stage, operation type, adjuvant therapy, recurrence, and survival were compared. Kaplan-Meier analysis was also performed.
Results:
Rectal cancer was identified in 332 patients (mean follow-up: 5.5 years). One hundred and seventy-three patients (52.1%) underwent low anterior resection, while 107 patients (32.2%) required abdominoperineal resection, 6 patients (1.8%) required exenteration to control disease, and 46 (13.9%) patients were treated with local excision. Of the 332 patients, 63 (19.0%) received adjuvant radiotherapy alone, 85 (25.6%) received combination chemoradiotherapy, and 4 (1.2%) received chemotherapy. Sphincter preserving procedures were used more frequently in the later half of the experience. Local/regional recurrences occurred in 5 patients (3.3%) treated with adjuvant therapy, and in 16 patients (8.9% of total) who did not receive adjuvant therapy (p=0.02, Chi-square test) although the total risk of recurrence (local and/or distant) was not different (30.2% vs. 27.7%, p=0.54). The actuarial rate of local recurrence (regardless of adjuvant therapy) for all stages was 7% at 5 years, and the risk of any recurrence (local or distant) was 21.1% at 5 years. Cancer specific 5-year survival was 77% overall.
Conclusions:
In rectal cancer, the therapeutic objectives are to control disease, limit recurrence, and preserve sphincter function; these goals were met for many patients at this institution. These data compare favorably with the current literature. Careful surgical technique and adjuvant therapy can allow successful treatment, even of advanced rectal cancers.
Keywords: Rectal cancer, local recurrence, survival, adjuvant therapy
INTRODUCTION
The management of rectal cancer has received significant attention in the recent literature (1,2). In the past, the primary therapy for rectal cancer was an abdominoperineal resection. This operation removes the rectum and anus and leaves patients with a permanent colostomy. Recent surgical alternatives to this operation include low anterior resection with a colorectal anastomosis or a coloanal pull-through or local excision (Figures 1, 2 and 3). An anterior resection allows removal of the rectum (containing the tumor) with preservation of the anal muscles and maintenance of the normal evacuation route. Local excision involves a transanal removal of very early cancers. These operations are often referred to as sphincter preserving procedures. The increasingly widespread use of preoperative chemoradiotherapy, delineation of anatomic perirectal dissection, and advanced techniques for reconstruction allowing continence have all altered the traditional concepts of therapy for rectal cancer (3,4). To assess the effectiveness of current surgery and of adjuvant therapy on the specific issues of local control, recurrence, and survival, the experience of the last 20 years at our institution was reviewed.
Figure 1.
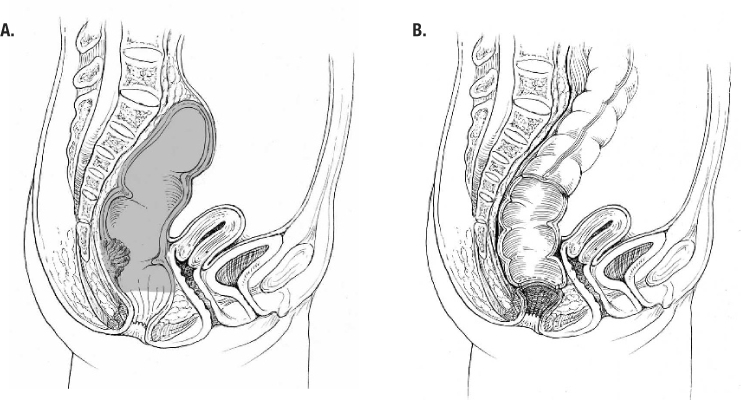
Low anterior resection. A, sagittal view with low rectal lesion (shaded area is distal portion of resection); B, completed low stapled colorectal anastomosis.
Figure 2.
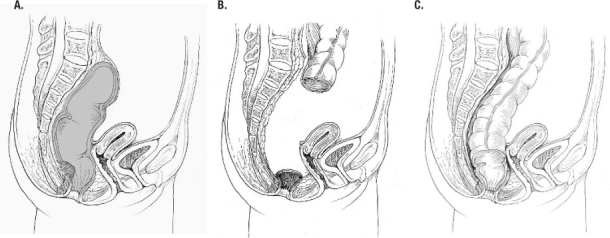
Coloanal pullthrough. A, sagittal view with very low rectal lesion (shaded area is distal portion of resection); B, sigmoid colon, and rectum removed, cuff of anal muscles remains; C, completed coloanal pullthrough with handsewn anastomosis at dentate line.
Figure 3.
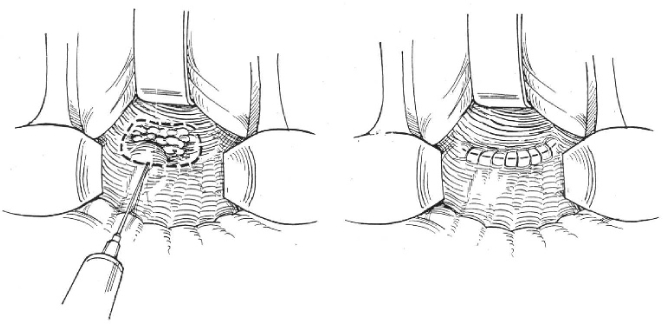
Local excision.
METHODS
Following Ochsner Institutional Review Board approval, a prospectively maintained database of all patients with rectal cancer treated at the Ochsner Clinic Foundation from 1982 to 1998 was analyzed. Analysis was limited to 1998 to allow at least 3 years of follow-up. Patients' records were reviewed and pertinent clinical data were analyzed and compared. The American Joint Committee on Cancer (AJCC) Tumor Nodal Metastasis (TNM) staging criteria were used to classify each patient's pathologic stage (5).
Patients excluded were those with carcinoma-insitu (Stage 0 AJCC) rectal cancer and those who were felt to be candidates only for palliative surgery. The remaining patients' clinical stage, pathologic stage, adjuvant therapy, surgical therapy, and outcome were analyzed. All operations were performed by the colorectal staff or by surgical trainees under the direct supervision of the colorectal staff. These staff surgeons were all experienced, board certified in general surgery and colon and rectal surgery, and adhered to accepted colorectal oncologic principles of rectal cancer surgery (proximal ligation of colonic vessels, adequate mesenteric resection, obtaining appropriate proximal, distal, and radial margins, and sharp dissection in anatomic tissue planes).
Continuous variables were compared with a two-tailed Students t-test, and discrete variables were compared with Chi-square test.
RESULTS
The database identified 332 patients for analysis. All patients were managed with an intention of cure, although 6 patients required pelvic exenteration (posterior or total) as the surgical intervention. Patients ranged in age from 25.2 to 89.8 years (mean = 63.2 years) and 63.6 % were female. A summary of treatment by Stage is included in Table 1. Table 2 shows the operative choice for each patient. Treatment for 152 patients (45.8%) included adjuvant therapy. Of the total 332 patients, 63 patients (19%) received adjuvant radiotherapy, 85 (25.6%) received combination chemoradiotherapy, and 4 (1.2 %) received chemotherapy. Sphincter preserving operations were used more frequently in the later half of the study period (Figure 4) without any significant change on survival. During the entire study period, 6 staff colon and rectal surgeons were involved in the management of these patients.
Table 1: Therapy by Stage
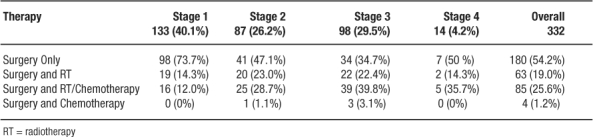
Table 2: Surgical Therapy in each Stage (operation may be after adjuvant therapy)
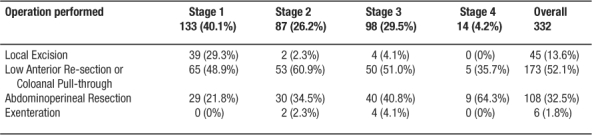
Figure 4.
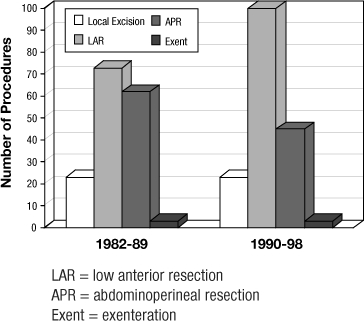
Operative trends over time
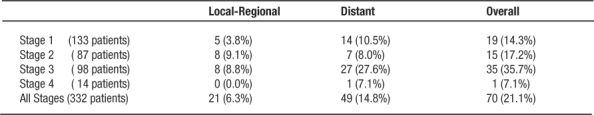
Follow-up ranged from 0.1 to 17.7 years (mean 5.5 years). Table 3 lists the local-regional, distant and overall recurrence by stage. Simultaneous local and distant recurrences were counted together as only 1 patient was found to have true simultaneous recurrence. Local/regional recurrences occurred in 5 patients (3.3%) treated with adjuvant therapy, and in 16 patients (8.9%) who did not receive adjuvant therapy (p=0.02, Chi-square test) although the total risk of recurrence (local and/or distant) was not different (30.2% vs. 27.7%, p=0.54). The actuarial rate of local recurrence (regardless of adjuvant therapy) for all stages was 6.3% at 5 years; the risk of any recurrence (local or distant) is 21.1% at 5 years. Five-year survival was 77.8% for Stage 1, 69.1% for Stage 2, 52% for Stage 3, and 15.4% for Stage 4 patients. Overall cancer specific survival was 77%.
Table 3: Recurrences by Stage
DISCUSSION
This summary of almost 20 years of a single institution's management of rectal cancer by seven colorectal surgeons can provide some insights into the complexities of managing rectal cancer. The distribution of pathologic stages reflects the referral patterns of a large institution, managed care population, and the consequences of adjuvant therapy. Forty percent were Stage 1, 26% were Stage 2, and 30% were Stage 3. Adjuvant therapy was used more frequently in Stage 2–4 patients.
These data suggest that local control of disease is very achievable with low rates of local recurrence. Our management strategy has been that of attempts at sphincter preservation, adequate margins, and careful dissection. Thus abdominoperineal resection was performed less frequently in the later years of this study and there has been a trend to more frequent preoperative chemoradiotherapy in more recent years. Certainly there have been some changes in the specific management strategies (dose and type of chemotherapy, number of fields used for radiotherapy.)
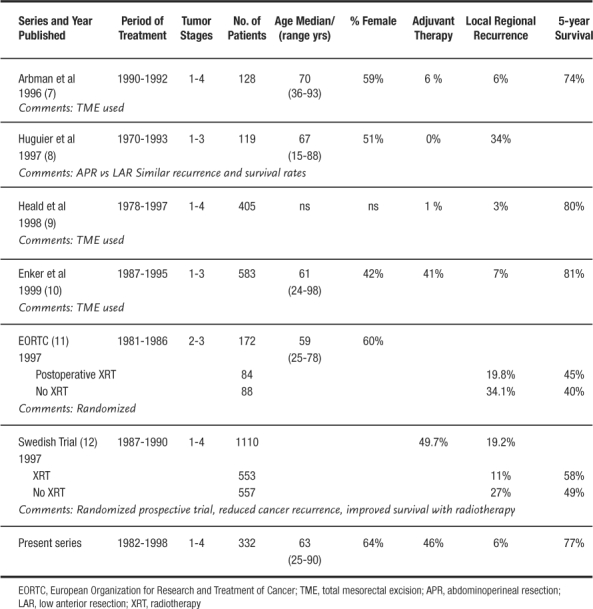
Selecting the correct surgical procedure must take into account tumor biology, patient factors, and surgical skill. Correct choices allow sphincter preservation with equivalent local control and survival (6). The survival of patients in this series is very comparable to published literature on the subject (Table 4). The only spurious finding is the number of patients with stage 3 disease who developed metastatic distant disease. Several recent series have demonstrated that high volume centers, such as Ochsner, have lower operative mortality, increased sphincter preservation, and improved survival in rectal cancer surgery (13–15).
Table 4.
Rectal Cancer Results
Attempts to analyze subsets of patients are severely limited by group size and lack sufficient power to make any statistically supported statements. Confounding issues such as the timing of therapy may also play a role. Neoadjuvant therapy (chemotherapy administered before surgical therapy) may result in clinical down-staging, but patients receiving neoadjuvant therapy may have a more insidious course than some Stage 1 patients. The number of staff surgeons and surgical trainees involved in the management of these patients may produce some biases. Rectal cancer surgery is one of the procedures in which the quality and quantity of surgery has been shown to have an impact on outcome (13–15).
In rectal cancer, the therapeutic objectives are to control disease, limit recurrence, and preserve sphincter function; these goals were met for many patients at this institution. Our data compare favorably with the current literature. Careful surgical technique and adjuvant therapy can allow successful treatment, even of advanced rectal cancers.
REFERENCES
- Moore H. G., Guillem J. G. Total mesorectal excision in rectal cancer resection. Clin Colon Rect Surg. 2002;15:27–34. [Google Scholar]
- Ko C., Stamos M. J., Zimmerman A., Yang H. C. Adjuvant therapy for rectal cancer. Clin Colon Rect Surg. 2002;15:55–62. [Google Scholar]
- Beck D. E. Surgical management of colon and rectal cancer. Ochsner Journal. 2002;4:156–162. [PMC free article] [PubMed] [Google Scholar]
- Beck D. E. Malignancy of the colon, rectum and anus. 2003:447–482. In Beck DE (ed). Handbook of Colorectal Surgery. 2nd ed. New York: Marcel Dekker. [Google Scholar]
- American Joint Committee on Cancer AJCC Cancer Staging Manual. 1997 5th ed. Chicago:AJCC. [Google Scholar]
- Cawthorne S. J., Parums D. V., Gibbs N. M. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer. Lancet. 1990;335:1055–1059. doi: 10.1016/0140-6736(90)92631-q. [DOI] [PubMed] [Google Scholar]
- Arbman G., Nilsson E., Hallbook O., Sjodahl R. Local recurrence following total mesorectal excision for rectal cancer [comments] Br J Surg. 1996;83:375–379. doi: 10.1002/bjs.1800830326. [DOI] [PubMed] [Google Scholar]
- Huguier M., Chastang C., Houry S. Sphincter-saving resection, or not, for cancer of the midrectum. Am J Surg. 1997;174:11–15. doi: 10.1016/S0002-9610(97)00023-8. [DOI] [PubMed] [Google Scholar]
- Heald R. J., Moran B. J., Ryall R. D. Rectal cancer: the Basing-stoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- Enker W. E., Merchant N., Cohen A. M. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg. 1999;230:544–552. doi: 10.1097/00000658-199910000-00010. discussion 552–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud J. P., Nordlinger B., Bosset J. F. Radical surgery and postoperative radiotherapy as combined treatment in rectal cancer. Final results of a phase III study of the European Organization for Research and Treatment of Cancer. Br J Surg. 1997;84:352–357. [PubMed] [Google Scholar]
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial [comments][published erratum appears in N Engl J Med 1997;May 22;336 (21):1539] N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- Ho V., Heslin M. J., Yun H., Howard L. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13:851–858. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Wibe A., Eriksen M. T., Syse A., Tratli S., Myrvold H. E., Soreide O. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg. 2005;92:217–224. doi: 10.1002/bjs.4821. Norwegian Rectal Cancer Group. [DOI] [PubMed] [Google Scholar]
- McGrath D. R., Leong D. C., Gibberd R., Armstrong B., Spigelman A. D. Surgeon and hospital volume and the management of colorectal cancer patients in Australia. ANZ J Surg. 2005;75:901–910. doi: 10.1111/j.1445-2197.2005.03543.x. [DOI] [PubMed] [Google Scholar]


