Abstract
During the course of HIV-1 disease, virus neuroinvasion occurs as an early event, within weeks following infection. Intriguingly, subsequent central nervous system (CNS) complications manifest only decades after the initial virus exposure. Although CNS is commonly regarded as an immune-privileged site, emerging evidence indicates that innate immunity elicited by the CNS glial cells is a critical determinant for the establishment of protective immunity. Sustained expression of these protective immune responses, however, can be a double-edged sword. As protective immune mediators, cytokines have the ability to function in networks and co-operate with other host/viral mediators to tip the balance from a protective to toxic state in the CNS. Herein, we present an overview of some of the essential elements of the cerebral innate immunity in HIV neuropathogenesis including the key immune cell types of the CNS with their respective soluble immune mediators: (1) cooperative interaction of IFN-γ with the host/virus factor (platelet-derived host factor (PDGF)/viral Tat) in the induction of neurotoxic chemokine CXCL10 by macrophages, (2) response of astrocytes to viral infection, and (3) protective role of PDGF and MCP-1 in neuronal survival against HIV Tat toxicity. These components of the cerebral innate immunity do not act separately from each other but form a functional immunity network. The ultimate outcome of HIV infection in the CNS will thus be dependent on the regulation of the net balance of cell-specific protective versus detrimental responses.
Keywords: HIV neuropathogenesis, MCP-1, CXCL10
Introduction
It is estimated that almost 25% of untreated HIV-1-infected individuals and ~7% of HIV-1-infected patients treated with combined antiretroviral therapy develop HIV-associated dementia (HAD) (Budka 1991; Spencer and Price 1992; McArthur et al. 1993; Sacktor et al. 2001), a neurodegenerative syndrome that is clinically characterized by progressive cognitive, motor, and behavioral abnormalities (Gendelman et al. 1994; Lipton and Gendelman 1995). The pathological manifestation of the syndrome, HIV encephalitis (HIVE), is accompanied by prominent microglial activation, formation of microglial nodules, perivascular accumulations of mononuclear cells, presence of multinucleated giant cells, and neuronal damage and loss (Gendelman et al. 1994; Bell 1998; Nath 1999). The primary cells infected by HIV-1 in the brain are macrophages/microglia and, to a lesser extent, astrocytes, but not neurons (Kaul et al. 2001). One broad explanation frequently advocated to explain the loss of neurons in this disease is that cellular and/or viral proteins released from the infected cells have a direct toxic effect on the neurons (Brenneman et al. 1988; Dreyer et al. 1990; Adamson et al. 1996; Hof et al. 1998; Kruman et al. 1998; Chen et al. 2000; Patel et al. 2000).
HIV-1 neuroinvasion occurs during the early stages of disease; however, pathological phenotypes, like microglial activation, astrogliosis, and neuronal loss, are not manifested until late in the disease and, again, not in all infected individuals. This leads to the speculation that the ultimate disease outcome is a result of the balance of neuroprotective versus neurotoxic responses. Additionally, the same pathways that mediate a protective response have also been shown to play critical roles in mediating toxicity. For example, antiviral cytokine such as IFN-γ can, under certain conditions, synergize with other inflammatory mediators such as TNF-α, resulting in induction of inflammatory mediators, such as the chemokine CXCL10 (Bokhari et al. 2009; Williams et al. 2009), thereby tipping the balance from a protective physiological response to a pathological one. Such cooperation and interplay of host and viral factors often culminates in end-organ HIV pathology observed in later stages of the disease. It is this dysregulation of host responses and the dual roles of these mediators in neuroAIDS that are the focus of this review. Herein, we will present an overview of some of the essential elements of the cerebral innate immunity in HIV-1 neuropathogenesis, specifically in the key immune cell types of the central nervous system (CNS).
Macrophages
Cooperative interaction of IFN-γ with the host/virus factor (PDGF/viral Tat) in the induction of neurotoxic chemokine CXCL10
Chemokines in the brain have been recognized as essential elements in neurodegenerative disease and related neuro-inflammation through their regulation of inflammatory responses (Luster and Ravetch 1987; Conant et al. 1998; Kelder et al. 1998), thereby contributing to injury and eventual loss of neurons (Asensio et al. 2001). Cerebral expression of various chemokines, including CXCL10 (interferon γ-inducible peptide or IP-10), and its receptor is increased in HIVE (McArthur et al. 1993; Kolson and Pomerantz 1996; Sanders et al. 1998; Kolb et al. 1999). Increased levels of CXCL10 have been detected in the cerebrospinal fluid and plasma of individuals with HIV-1 infection (Kolb et al. 1999) and in the brains of individuals with HAD (McArthur et al. 1993; Kolson and Pomerantz 1996; Sanders et al. 1998). Importantly, the CXCL10 levels in the CNS of HIV-1-infected individuals correlated positively with disease progression. There is also evidence that CXCL10 participates in the neuropathogenesis in simian HIV (SHIV)-infected macaques (Sasseville et al. 1996; Westmoreland et al. 1998) by contributing to the degeneration of neurons, possibly through the activation of a calcium-dependent apoptotic pathway (Potula et al. 2004; Sui et al. 2006). Increased CXCL10 levels are critical for the increased migration of inflammatory cells into the CNS, a hallmark feature of HAD (Navia et al. 1986; Nath 1999).
In the brain, CXCL10 can be induced by a variety of factors, including viral gp120, Tat, and Nef, and cellular host factors such as IFN-γ (Kutsch et al. 2000; Asensio et al. 2001; van Marle et al. 2004). Although both the cellular (IFN-γ and TNF-α) (Ohmori and Hamilton 1993; Majumder et al. 1998b) and viral factors (Tat and gp120) (Kutsch et al. 2000; Asensio et al. 2001) are known to cooperatively induce CXCL10, it remains unclear how the interplay of host factors and virus modulates chemokine expression. In fact, it has been shown that interactions of soluble host factors can synergistically induce the expression of CXCL10 (Ohmori and Hamilton 1993, 1995; Majumder et al. 1998a, b). In addition to the known inflammatory mediators, over-expression of the growth factor such as the platelet-derived growth factor (PDGF)-B chain has been demonstrated in the brains of macaques with SHIV-E (Sui et al. 2003; Potula et al. 2004). PDGF has been implicated in the pathogenesis of various inflammatory diseases (Ross et al. 1984, 1985, 1986; Ross and Raines 1988; Antoniades et al. 1990). In addition to enhanced expression of IFN-γ, there is also an increased expression of PDGF-B chain in the brains of macaques with SHIV-E (Lane et al. 1996; Orandle et al. 2002; Shapshak et al. 2004).
There is an increasing cumulative evidence that activated mononuclear phagocytes (macrophages/microglia) releasing inflammatory mediators in the CNS are a better correlate of HIV-associated neurocognitive disorders (HAND) than the actual viral load in the brain. The complex interplay of inflammatory mediators released by macrophages often leads to the induction of neurotoxins in HAD. Dissection of these complex interplays has led to the finding that, while PDGF alone had no effect on the induction of CXCL10 in human macrophages, in conjunction with IFN-γ, it significantly augmented the expression of CXCL10 RNA and protein through transcriptional and posttranscriptional mechanisms. Signaling molecules, such as JAK and STATs, PI3K, mitogen-activated protein kinase (MAPK), and NF-κB, were found to play a role in the synergistic induction of CXCL10 (Dhillon et al. 2007). Furthermore, PDGF via its activation of p38 MAPK was also able to increase the stability of IFN-γ-induced CXCL10 mRNA (Dhillon et al. 2007).
Another mechanism of increased CXCL10 expression in the CNS occurs via the interplay of HIV-1 protein Tat and IFN-γ in macrophages (Dhillon et al. 2008). Synergistic induction of CXCL10 by both Tat and IFN-γ was susceptible to inhibition by the MEK1/2 inhibitor U0126 and the p38 MAPK inhibitor SB203580. In addition, JAK/STAT pathway was shown to play a major role in Tat/IFN-γ-mediated CXCL10 induction in macrophages (Dhillon et al. 2008). Thus, the cooperative interaction of Tat and IFN-γ resulted in enhanced chemokine expression, which in turn can manifest as an amplified inflammatory immune response within the CNS of patients with HAD, by a mechanism involving an increased recruitment of lymphocytes/monocytes in the brain.
Astrocytes
Response of astrocytes to viral infection
While abundant studies support the role of macrophages and microglia in HIV-1-induced CNS damage, other glial cells such as astrocytes are also important contributors to neuronal pathology and inflammation. In addition to macrophages, astrocytes, the most numerous cell type within the brain, provide a very important reservoir for the generation CXCL10 (Dong and Benveniste 2001; Thompson et al. 2001; Minagar et al. 2002). In fact, it is becoming increasingly appreciated that the severity of HAD/HIVE correlates more closely with the presence of activated glial cells rather than with the presence and amount of HIV-infected cells in the brain. It is known that proinflammatory cytokines, IFN-γ and TNF-α, are markedly increased in CNS tissues during HIV-1 infection in the brain and are implicated in the pathophysiology of HAD (Wesselingh et al. 1997; Shapshak et al. 2004). One mechanism for the induced expression of CXCL10 in astrocytes has been via the synergistic induction of both IFN-γ and TNF-α (Majumder et al. 1998b). Additionally, HIV-1/HIV-1 proteins in the CNS can further interact with these proinflammatory cytokines to further dramatically induce the expression of CXCL10 in the brain (Kutsch et al. 2000; Asensio et al. 2001). In fact, recent findings have demonstrated a synergistic induction of CXCL10 mRNA and protein in human astrocytes exposed to HIV-1/HIV proteins Tat and the proinflammatory cytokines. Signaling molecules, including JAK, STATs, MAPK (via activation of ERK1/ 2 and p38), and NF-κB, were identified as key players in the synergistic induction of CXCL10.
Furthermore, dissection of mechanisms involved in the synergistic induction of CXCL10 clearly points to the role of oxidative stress as a major player in this process. HIV-1-induced oxidative stress in astrocytes has been shown to be a critical determinant of the NF-κB-targeted genes, specifically CXCL10 (Song et al. 2007). One mechanism by which oxidative stress is able to impact signaling pathways and their corresponding transcription factors is through a respiratory burst orchestrated by the activation of NADPH oxidase (Sundaresan et al. 1995; Wang et al. 1998; Adler et al. 1999; Park et al. 2004; Turchan-Cholewo et al. 2009). NADPH oxidase, a multi-subunit membrane associated enzyme, is capable of producing superoxide (Chanock et al. 1994; Babior 1999; El-Benna et al. 2005; Raad et al. 2008). It has recently been demonstrated that treatment of astrocytes with a mixture of Tat and the inflammatory cytokines resulted in a respiratory burst, an effect that was abrogated by apocynin, an NADPH oxidase inhibitor (Williams et al. 2010). Treatment with apocynin also decreased CXCL10 expression in Tat-, IFN-γ-, and TNF-α-stimulated astrocytes. This synergistic induction involved the activation of the ERK1/2, JNK, and Akt pathways with downstream translocation of NF-κB.
Neurons
Paradoxical roles of chemokines in HAND
In the brains of individuals with HAD, upregulation of chemokines in the CNS is often considered a correlate of neuroinflammation. However, recent increasing evidence raises the possibility that, in addition to their role as chemoattractants, chemokines might also act as neurotransmitters or neuromodulators (Rostene et al. 2007). A classic example of this is the chemokine fractalkine, which is not only a neuroimmune modulator recruiting peripheral macrophages into the brain but can also function as a neuroprotective factor (Tong et al. 2000). Similar to fractalkine, CCL2 has also been demonstrated to protect cardiac myocytes from hypoxia-induced apoptosis (Tarzami et al. 2005). CCL2 is a constitutively expressed chemokine in the normal brain that is produced by a variety of CNS cells. It has been demonstrated that CCL2 can function not only to recruit monocytes into the CNS of HIV-1 patients but also to play a critical role in the protection of fetal neurons against HIV-1 Tat toxicity (Eugenin et al. 2003). A recent study also identified an additional role for CCL2, that of neuroprotection against HIV-1 Tat toxicity in rat primary midbrain neurons involving transient receptor potential canonical (TRPC) (Yao et al. 2009b).
Similarly, newer pleiotrophic roles of the chemokine fractalkine are also becoming increasingly appreciated in the literature. Specifically, although fractalkine is a known chemoattractant, it can also function to regulate neuronal survival via its antiapoptotic effects (Meucci et al., 1998; Meucci et al., 2000).
In addition to the ability of the chemokines to exert diverse functions in varying cell types, there are other intrinsic mechanisms within the host that can manifest the pleiotropic nature of these chemokines. For example, it is well recognized that the activity of many neuroactive peptides is modulated by bioactive fragments, which are formed by the action of a variety of peptidases. This phenomenon appears to represent an important regulatory mechanism that modulates many neuropeptide systems. Similar processing of chemokines resulting in the alteration of their functions has recently been reported for the chemokine SDF-1α. These studies showed that, in addition to its direct neurotoxic effect, cleaved chemokine SDF (5–67) could actually amplify the HIV-induced “bystander” neurotoxicity. Herein, the authors elegantly demonstrated that the proteolytic cleavage of SDF resulted in a peptide that was highly immunogenic and that contributed to HIV neurodegeneration through engagement of a chemokine receptor, CXCR3 (Vergote et al. 2006). Furthermore, this indirect effect occurred at a concentration range that was 100 times lower than the concentration required to induce a direct neuronal death. This is a classic example demonstrating yet another mechanism by which proteins can acquire neuropathogenic properties following proteolytic processing.
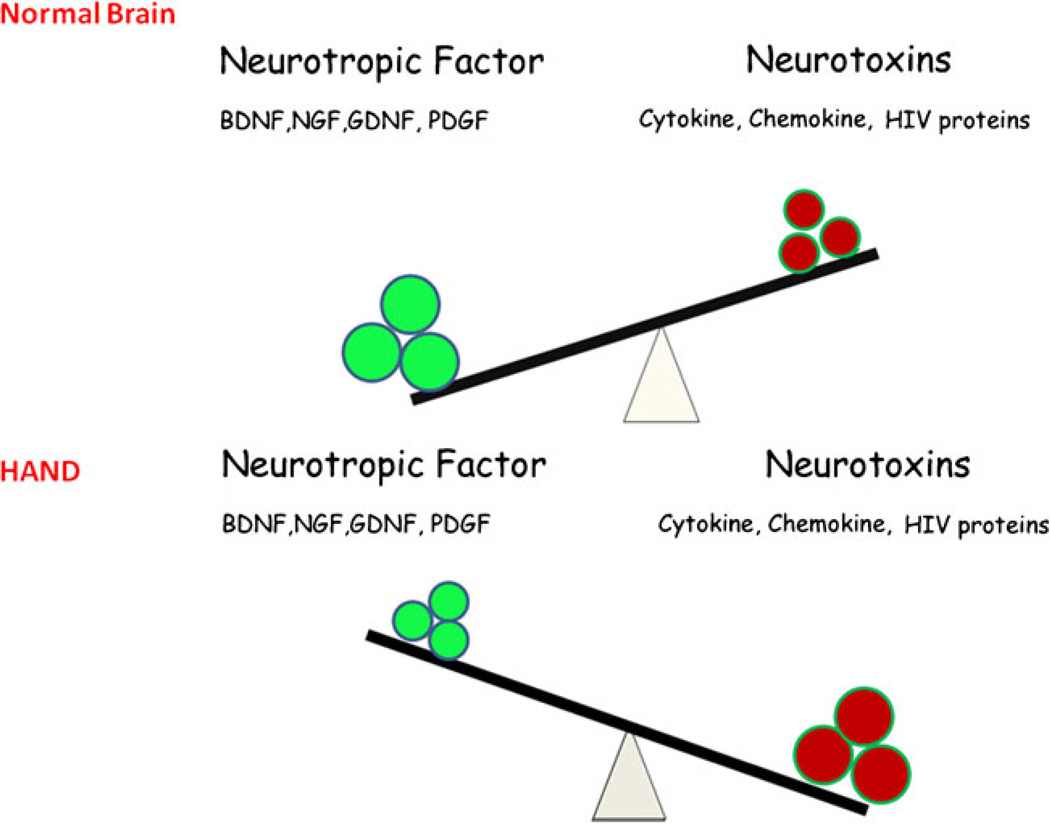
In response to cellular damage, the host is also capable of producing trophic growth factors (PDGF, fibroblast growth factor—FGF, or nerve growth factor—NGF) that may protect the neuronal, glial, and other resident cells of the brain. These neurotropic growth factors also play somewhat paradoxically diverse roles during the progression of CNS infection by promoting increased viral replication or cooperating with viral and/or host factors that promote neuronal degeneration. Many of these trophic factors also provide protection of neurons against the neurotoxic factors (cytokines, chemokines, and viral products). We previously demonstrated that, while PDGF expression in activated mononuclear phagocytes was closely associated in macaques with SHIV-E (Potula et al. 2004), its expression in the neurons correlated with neuronal fitness (Peng et al. 2008b). Thus, depending on the cell type within the tissue, the same host factor can manifest diverse activation responses. Figure 1 illustrates the complex interactions that exist among trophic growth factors and cytokines/chemokines/viral proteins in the brains of HIV-infected patients. The ultimate outcome of infection in the CNS (neuronal survival versus damage) is a result of the ensuing shift in balance between the neurotrophic versus neurotoxic products manifested over time following infection. Recent findings demonstrated that PDGF-BB protected the neurons against gp120-mediated apoptosis as measured by lactate dehydrogenase and deoxynucleotidyltransferasemediated dUTP nick end labeling assay (Peng et al. 2008b). Antiapoptotic effects of PDGF-BB were also confirmed by regulation of caspase-3 activation and by examining levels of anti- and proapoptotic genes, Bcl-xL and Bax, respectively (Peng et al. 2008b). Further studies indicated the roles for both PI3K/Akt and Bcl family pathways in PDGF-mediated neuroprotection in SH-SY5Y cells (Peng et al. 2008a). In addition, it was also demonstrated that PDGF was able to rescue dopaminergic neurons from HIV Tat neurotoxicity in vivo (Yao et al. 2009a). The confirmation of the neuroprotective role of PDGF against Tat toxicity was also corroborated in vitro in primary cultures of rat midbrain neurons. H. Yao and colleagues demonstrated a novel role of the Ca2+-permeable channel TRPC in PDGF-mediated neuroprotection in rat neurons that indicated an exogenous PDGF-activated TRPC results in the amplification of downstream ERK signaling via the Pyk2 pathway, followed by nuclear translocation of CREB and ultimately culminating in neuronal survival (Yao et al. 2009a). These studies implicate the robustness of PDGF neuroprotective ability against a range of diverse HIV-1 proteins.
Fig. 1.
Imbalance of neurotrophic versus neurotoxic factors in the CNS leads to development of HAND. In the normal brain, neuronal homeostasis is maintained by an increased expression of neurotrophic factors compared with the neurotoxins. High levels of neurotrophic factors are neuroprotective. On the other hand, following injury or virus infection, an increased expression of neurotoxins in the CNS shifts the balance to a diseased state that is exemplified as neuronal loss
Conclusion
During HIV-1 infection, cytokines/chemokines, elaborated initially as protective immune mediators, have the potential to function in networks and can co-operate with other host/viral mediators to tip the balance from a protective to a toxic response in the CNS. A better understanding of the basic mechanisms of HAND is thus critical for the development of treatments for neurological compliant of AIDS.
Acknowledgement
This work was supported by grants MH-068212, DA020392, DA023397, DA024442, and DA 027729 from the National Institutes of Health (SB).
Contributor Information
Honghong Yao, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880, USA.
Crystal Bethel-Brown, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880, USA.
Cicy Zidong Li, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880, USA.
Shilpa J. Buch, Email: sbuch@unmc.edu, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880, USA.
References
- Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Antoniades HN, Bravo MA, Avila RE, Galanopoulos T, Neville-Golden J, Maxwell M, Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS, Campbell IL. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75:7067–7077. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Bell JE. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;22:1–10. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- Chen Z, Huang Y, Zhao X, Skulsky E, Lin D, Ip J, Gettie A, Ho DD. Enhanced infectivity of an R5-tropic simian/human immunodeficiency virus carrying human immunodeficiency virus type 1 subtype C envelope after serial passages in pig-tailed macaques (Macaca nemestrina) J Virol. 2000;74:6501–6510. doi: 10.1128/jvi.74.14.6501-6510.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemo-attractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Peng F, Ransohoff RM, Buch S. PDGF synergistically enhances IFN-gamma-induced expression of CXCL10 in blood-derived macrophages: implications for HIV dementia. J Immunol. 2007;179:2722–2730. doi: 10.4049/jimmunol.179.5.2722. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Zhu X, Peng F, Yao H, Williams R, Qiu J, Callen S, Ladner AO, Buch S. Molecular mechanism(s) involved in the synergistic induction of CXCL10 by human immunodeficiency virus type 1 Tat and interferon-gamma in macrophages. J Neurovirol. 2008;14:196–204. doi: 10.1080/13550280801993648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53:199–206. [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Hof PR, Lee PY, Yeung G, Wang RF, Podos SM, Morrison JH. Glutamate receptor subunit GluR2 and NMDAR1 immunoreactivity in the retina of macaque monkeys with experimental glaucoma does not identify vulnerable neurons. Exp Neurol. 1998;153:234–241. doi: 10.1006/exnr.1998.6881. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Pomerantz RJ. AIDS dementia and HIV-1-induced neurotoxicity: possible pathogenic associations and mechanisms. J Biomed Sci. 1996;3:389–414. doi: 10.1007/BF02258044. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Buchmeier MJ, Watry DD, Fox HS. Expression of inflammatory cytokines and inducible nitric oxide synthase in brains of SIV-infected rhesus monkeys: applications to HIV-induced central nervous system disease. Mol Med. 1996;2:27–37. [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J Neurosci Res. 1998a;54:169–180. doi: 10.1002/(SICI)1097-4547(19981015)54:2<169::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol. 1998b;161:4736–4744. [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268:6677–6688. [PubMed] [Google Scholar]
- Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76:5797–5802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Choi K, Jeong E, Kwon D, Benveniste EN, Choi C. Reactive oxygen species mediate chloroquine-induced expression of chemokines by human astroglial cells. Glia. 2004;47:9–20. doi: 10.1002/glia.20017. [DOI] [PubMed] [Google Scholar]
- Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuro-protection—implications in HIV dementia. Eur J NeuroSci. 2008a;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon N, Callen S, Yao H, Bokhari S, Zhu X, Baydoun HH, Buch S. Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol. 2008b;14:62–72. doi: 10.1080/13550280701809084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R, Dhillion N, Sui Y, Zien CA, Funa K, Pinson D, Mayo MS, Singh DK, Narayan O, Buch S. Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;165:815–824. doi: 10.1016/S0002-9440(10)63344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. Faseb J. 2008 doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Raines EW. Platelet-derived growth factor—its role in health and disease. Adv Exp Med Biol. 1988;234:9–21. doi: 10.1007/978-1-4757-1980-2_2. [DOI] [PubMed] [Google Scholar]
- Ross R, Faggiotto A, Bowen-Pope D, Raines E. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70:III77–III82. [PubMed] [Google Scholar]
- Ross R, Bowen-Pope DF, Raines EW. Platelet-derived growth factor: its potential roles in wound healing, atherosclerosis, neoplasia, and growth and development. Ciba Found Symp. 1985;116:98–112. doi: 10.1002/9780470720974.ch7. [DOI] [PubMed] [Google Scholar]
- Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes: multicenter AIDS cohort study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- Shapshak P, Duncan R, Minagar A, Rodriguez de la Vega P, Stewart RV, Goodkin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Front Biosci. 2004;9:1073–1081. doi: 10.2741/1271. [DOI] [PubMed] [Google Scholar]
- Song HY, Ryu J, Ju SM, Park LJ, Lee JA, Choi SY, Park J. Extracellular HIV-1 Tat enhances monocyte adhesion by up-regulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-kappaB activation in astrocytes. Exp Mol Med. 2007;39:27–37. doi: 10.1038/emm.2007.4. [DOI] [PubMed] [Google Scholar]
- Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–693. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Sui Y, Potula R, Pinson D, Adany I, Li Z, Day J, Buch E, Segebrecht J, Villinger F, Liu Z, Huang M, Narayan O, Buch S. Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIV-encephalitis. J Med Primatol. 2003;32:229–239. doi: 10.1034/j.1600-0684.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J NeuroSci. 2006;23:957–964. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Tarzami ST, Calderon TM, Deguzman A, Lopez L, Kitsis RN, Berman JW. MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a G(alphai)-independent pathway. Biochem Biophys Res Commun. 2005;335:1008–1016. doi: 10.1016/j.bbrc.2005.07.168. [DOI] [PubMed] [Google Scholar]
- Thompson KA, McArthur JC, Wesselingh SL. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann Neurol. 2001;49:745–752. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164:1333–1339. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga VM, Gupta S, Gorospe RM, Keller JN, Bruce-Keller AJ. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid Redox Signal. 2009;11:193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Rottman JB, Williams KC, Lackner AA, Sasseville VG. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am J Pathol. 1998;152:659–665. [PMC free article] [PubMed] [Google Scholar]
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Yao H, Peng F, Yang Y, Bethel-Brown C, Buch S. Cooperative induction of CXCL10 involves NADPH oxidase: implications for HIV dementia. Glia. 2010;58:611–621. doi: 10.1002/glia.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Fan Y, Zhu X, Hu G, Buch SJ. TRPC channel-mediated neuroprotection by PDGF involves Pyk2/ERK/CREB pathway. Cell Death Differ. 2009a;16:1681–1693. doi: 10.1038/cdd.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009b;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]