Abstract
There is increasing evidence that opiates accelerate the pathogenesis and progression of acquired immunodeficiency syndrome (AIDS), as well as the incidence of human immunodeficiency virus (HIV) encephalitis (HIVE), a condition characterized by inflammation, leukocyte infiltration, and microglial activation. The mechanisms, by which the HIV-1 transactivating protein Tat and opioids exacerbate microglial activation, however, are not fully understood. In the current study, we explored the effects of morphine and HIV-1 Tat1–72 on the activation of mouse BV-2 microglial cells and primary mouse microglia. Both morphine and Tat exposure caused up-regulation of the chemokine receptor CCR5, an effect blocked by the opioid receptor antagonist naltrexone. Morphine in combination with Tat also induced morphological changes in the BV-2 microglia from a quiescent to an activated morphology, with a dramatic increase in the expression of the microglial activation marker CD11b, as compared with cells exposed to either agent alone. In addition, the mRNA expression of inducible nitric oxide synthase (iNOS), CD40 ligand, Interferon-gamma-inducible protein 10 (IP-10), and the proinflammatory cytokines tumor necrosis factor alpha (TNFα), interleukin (IL)-1fβ, and IL-6, which were elevated with Tat alone, were dramatically enhanced with Tat in the presence of morphine. In summary, these findings shed light on the cooperative effects of morphine and HIV-1 Tat on both microglial activation and HIV coreceptor up-regulation, effects that could result in exacerbated neuropathogenesis.
Keywords: microglia, activation, morphine, Tat, cytokines
Introduction
AIDS or acquired immunodeficiency syndrome is a multisystem disorder involving the central nervous system (CNS). Neurological impairment affects about 60% of human immunodeficiency virus (HIV)-1 patients (Fischer-Smith and Rappaport, 2005). HIV-1–associated dementia (HAD), characterized by macrophage infiltration in the brain with the formation of microglial nodules and multinucleated giant cells, develops in the later stages of AIDS (Budka, 1991). Other characteristic features of HAD include microglial activation, astrocytosis, and neuronal damage/death (Ghafouri et al, 2006).
Microglial activation is one of the hallmark features of HAD. Microglia are the resident patrol of the CNS that keeps the brain environment under constant surveillance. They constitute 10% of the total glial population in the adult CNS (Pessac et al, 2001) and are distributed throughout the brain and the spinal cord. Microglia in the adult mouse brain are derived from the monocyte/macrophage precursor cells that migrate from the yolk sac into the developing CNS where they actively proliferate during development, thus giving rise to the resident microglial pool (Alliot et al, 1999; Pessac et al, 2001). In the normal mature brain, microglia typically exist in a resting state characterized by ramified morphology, and function to monitor the brain environment (Davalos et al, 2005; Nimmerjahn et al, 2005). In response to certain external insults such as brain injury or infection, however, microglia become rapidly activated (Davalos et al, 2005; Fetler and Amigorena, 2005; Nimmerjahn et al, 2005). These activated microglia undergo a dramatic alteration from their resting ramified state into an ameboid phenotype accompanied by up-regulated expression of cell surface markers, such as CD11b, CD14, major histocompatibility complex (MHC) molecules, chemokine receptors, and several other markers (Rock et al, 2004). Microglial activation in HIV infection is a result of interaction with the viral proteins, such as HIV Tat (transactivator of transcription) (D'Aversa et al, 2004) or gp120 (Bonwetsch et al, 1999; Garden et al, 2004; Kong et al, 1996; Kruman et al, 1998).
HIV-infected individuals who are also opiate drug users account for a third of the total HIV patients (UNAIDS, 2006). They not only demonstrate a higher incidence of HAD but also have a faster progression of AIDS (Chen et al, 2002; Donahoe and Vlahov, 1998) and more severe neurocognitive and pathological abnormalities (Bell et al, 1996). Although the mechanism by which opiates exacerbate the disease is still largely obscure, it is known that these individuals have increased numbers of macrophages and microglia in the CNS (Chen et al, 2002). The biological effects of opioids are mediated through the opioid receptors, which are also present on microglia (Chao et al, 1997; Ruzicka et al, 1995; Stiene-Martin et al, 1998). It is of key importance to understand the underlying mechanisms by which opiates accelerate and enhance HAD.
In the present study, we investigated the combined effects of the opiate morphine and the HIV-1 protein Tat on the activation of murine microglia, a phenomenon that has implications in the progression of HAD. Study into the multiple effects of cytokines and chemokines on the CNS will enhance our understanding of the initiation, maintenance, and modification of microglial activation. Because proinflammatory cytokines produced as a result of microglial activation have been shown to be protective as well as deleterious for the host, attempts at developing potential therapeutics strategies aimed at controlling overactivation of microglia rather than inhibiting activation are warranted (Hanisch, 2002).
Results
Effect of morphine and Tat on CCR5 expression
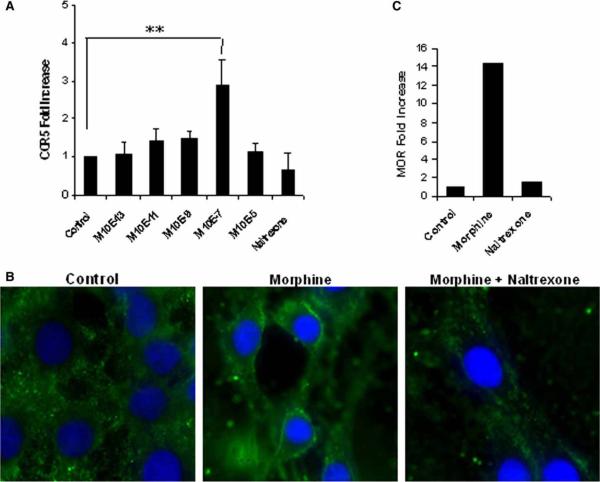
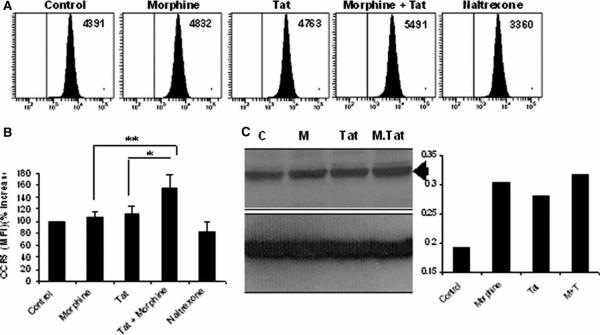
In order to determine the optimal dose of morphine, BV-2 mouse microglia were treated with morphine doses (10−13 to 10−5 M) for 6 h, followed by isolation of RNA, which was subjected to reverse transcription and real-time polymerase chain reaction (PCR) analysis using primers specific for CCR5 or hypoxanthine phosphoribosyl-transferase (HPRT) as an internal control. Data were plotted as fold increase compared to the untreated control. Morphine, when used at a concentration of 10−7 M, resulted in an average 2.8-fold increase in the mRNA expression of CCR5 (Figure 1A). Morphine (10−7 M) up-regulated the expression of CCR5 on the membranes of microglial cells (Figure 1B) and increased mRNA expression of μ-opioid receptor by more than 1.5-fold (Figure 1C). Pretreatment with naltrexone abolished these effects (Figure 1B and C). Fluorescence-activated cell sorting (FACS) analysis of morphine- and/or Tat-treated BV-2 cells, using a CCR5 antibody conjugated to phycoerythrin (PE), resulted in an up-regulation of the CCR5 receptor, as evidenced by an increase in the MFI (mean fluorescence intensity) values (Figure 2A and B). The results were further confirmed by Western blot analysis of proteins using total cell lysates from treated cells (Figure 2C). Morphine or Tat treatment alone resulted in a modest increase in CCR5 expression, and this was further increased with the combined treatment of morphine and Tat.
Figure 1.
Morphine up-regulates CCR5 through the μ-opioid receptor in BV-2 mouse microglia. Cells were treated with 10−7 M morphine (10−13 to 10−5 M for dose response) for 6 h (24-h treatment for immunocytochemistry). Cells were pretreated with 10−6 M naltrexone for 1 h to block opioid receptor. RNA was isolated and subjected to reverse transcription and real-time PCR analysis using primers specific for CCR5 or MOR (μ-opioid receptor) and HPRT as an internal control. Data were plotted as fold increase compared to the untreated control. (A) Dose response effect of morphine on the mRNA expression of CCR5 chemokine receptor. **P<.01 (B) Immunocytochemical analysis using antibody specific to the CCR5 chemokine receptor. (C) Fold increase in the MOR mRNA.
Figure 2.
Morphine plus Tat enhance the expression of CCR5 in BV-2 microglia. Cells were pretreated with morphine (10−7 M) for 1 h and treated with 20 nM of Tat1–72 for 24 h and stained for the CCR5 receptor. (A and B) FACS analysis of mouse microglial cells using antibody specific to the CCR5 chemokine receptor. Samples were run on the BD LSRII cytometer. Data were expressed as averages of the mean fluorescence intensities (MFIs), whereas the graphical data were obtained by converting the MFIs into percentage increase/decrease. **P<.01; *P<.05. (C) Western blot analysis of mouse microglial cells using antibody specific to the CCR5 chemokine receptor. Total proteins were isolated, denatured, separated by SDS-PAGE, and analyzed by Western blotting.
Effect of morphine and Tat on microglial activation
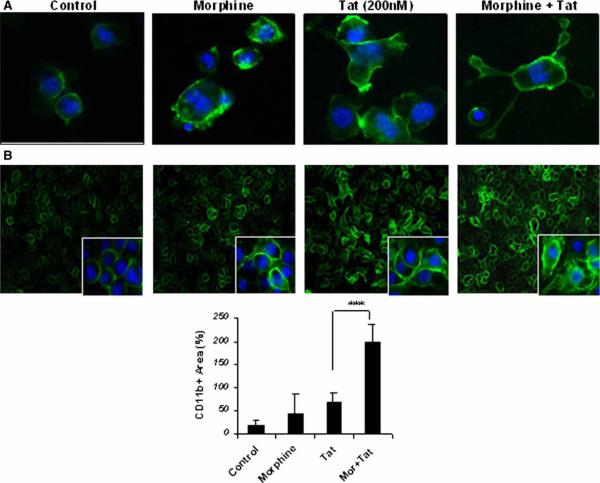
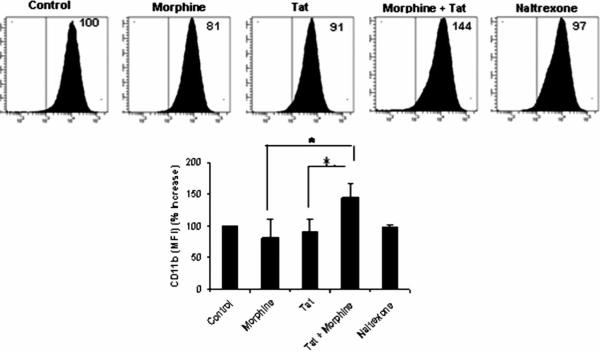
In order to determine the activation of BV-2 microglia following treatment with morphine or Tat, we examined the expression of surface marker CD11b on these cells. BV-2 microglia treated with either morphine or Tat demonstrated a change in cell morphology from quiescent to an activated macrophage-like phenotype (Figure 3A). Morphine or Tat treatment of the microglial cells showed an up-regulation of CD11b on the membranes (Figure 3A and B). This effect was further enhanced with combined treatment of morphine and Tat. FACS analysis of BV-2 cells treated with morphine and Tat in combination demonstrated an increase in CD11b expression by approximately 144% as compared to morphine or Tat treatment alone (Figure 4).
Figure 3.
(A) Morphine and Tat effect changes in microglial morphology from quiescent to an activated macrophage-like. Cells were treated with morphine (10−7 M) for 6 h and treated with 20 nM of Tat1–72 for 24 h and stained for the CCR5 receptor. Morphine enhances the effect of Tat on microglial activation. (B) Immunocytochemical analysis of mouse microglial cells pretreated with morphine (10−7 M) for 1 h and treated with 20 nM of Tat1–72 for 24 h. Cells were stained for the activation marker CD11b. Data were expressed as averages of the mean fluorescence intensities (MFIs). ***P<.001.
Figure 4.
Morphine enhances the effect of Tat on microglial activation. FACS analysis of mouse microglial cells using antibodies specific to CD11b microglial activation marker. Cells were pretreated with morphine (10−7 M) for 6 h, followed by treatment with 20 nM of Tat1–72 for 24 h. Samples were run on the BD LSRII cytometer. Data were expressed as average of mean fluorescence intensities (MFIs) expressed as percentages. *P<.05.
Morphine potentiates the mRNA expression of inflammatory cytokines in BV-2 and in primary mouse microglia
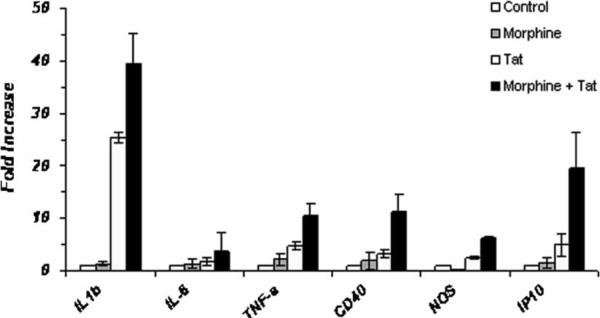
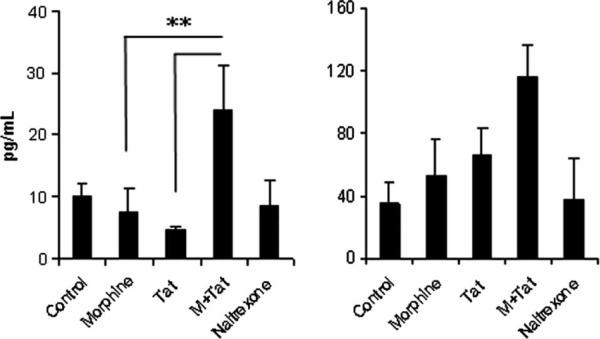
Next we asked whether morphine- and Tat-mediated microglial activation was accompanied by an inflammatory response by these cells. For this purpose, we investigated mRNA expression of the key proinflammatory cytokines, interleukin (IL)-1β, tumor necrosis factor alpha (TNFα), and IL-6. BV-2 cells or primary mouse microglia were pretreated with morphine (10−7 M) for 1 h, followed by treatment with Tat1−72 for 6 h (Figure 5). RNA from treated cells was isolated and subjected to reverse transcription and real-time PCR analysis using primers specific for the selected cytokines and HPRT as an internal control. Data were plotted as fold increase compared to the untreated control. Morphine treatment induced a modest increase in mRNA expression of the inflammatory cytokines as well as the ligand CD40 and the chemokine IP-10. On the other hand, morphine treatment of microglia demonstrated a decrease in expression of inducible nitric oxide synthase (iNOS). Interestingly, treatment with Tat resulted in increased induction of cytokines, with about 20- and 25-fold increase in expression levels of IL-6 and IL-1β, respectively, and about 2.5- to 4.9-fold increase in TNFα, CD40, iNOS, and IP-10 expression. In combination, morphine and Tat produced a dramatic up-regulation of almost 40-fold in expression of IL-1β and IL-6 RNA and about 6- to 19-fold increase in TNFα, CD40, iNOS, and IP-10 expression. Primary mouse microglial cultures exhibited similar results, except that in presence of morphine alone, there was an overall decrease in cytokine production. In combination with morphine and Tat, however, the expression of TNFα, IP-10, iNOS, and CD40 RNA were elevated (Figure 6). We next examined the protein expression of cytokines released from the microglial cells using the Milliplex mouse cytokine panel. IL-6 and TNFα were modestly increased with morphine or Tat treatment alone; however, the combined treatment resulted in enhanced production of these proinflammatory cytokines (Figure 7).
Figure 5.
Morphine potentiates the mRNA expression of inflammatory cytokines in BV-2 mouse microglia. Cells were pretreated with morphine (10−7 M) for 1 h and treated with Tat1–72 for 6 h. RNA was isolated and subjected to reverse transcription and real-time PCR analysis using primers specific for the selected cytokines and HPRT as internal control. Data were plotted as fold increase compared to the untreated control.
Figure 6.
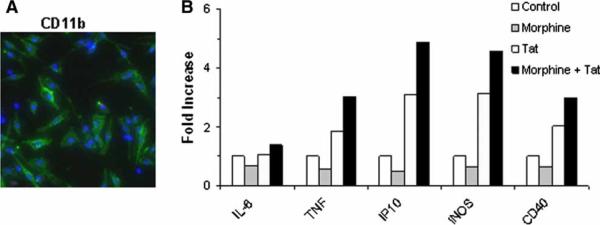
Morphine enhances the mRNA expression of inflammatory cytokines in primary mouse microglia. (A) Immunofluorescence staining for CD11b (green) showing the purity of primary mouse microglial cultures. (B) Quantitative PCR of cDNA from primary mouse microglia. Cells were pretreated with morphine (10−7 M) for 1 h and treated with Tat1–72 for 6 h. RNA was isolated and subjected to reverse transcription and real-time PCR analysis using primers specific for the selected cytokines and HPRT as internal control. Data were plotted as fold increase compared to the untreated control.
Figure 7.
Morphine increases the protein expression of IL-6 and TNFα in mouse microglia. Cells were pretreated with morphine (10−7 M) for 6 h and treated with Tat1–72 for 24 h. Cell culture supernatants were analyzed for the expression of various cytokines using the Milliplex mouse cytokine panel.
Discussion
Although activated microglia are associated with neuroprotection through the elimination of cellular debris and the release of neurotrophic and anti-inflammatory factors, their overactivation or dysregulation can be highly detrimental and is recognized as one of the key factors in the neuropathogenesis of HAD and the associated neuropathology. Microglial activation is accompanied by an increase in their numbers, a condition termed as microgliosis; by a change in their morphology—from a quiescent, ramified form to an activated, macrophage-like phenotype (Adler et al, 1994); and by up-regulation of an array of cell surface antigens.
In this study, we have demonstrated the effect of morphine on the activation of murine microglia treated with the HIV transactivating protein Tat. Because Tat has known proinflammatory effects and has been localized in microglia in the brains of AIDS patients, it is a likely mediator of the proinflammatory responses in the brains of HIV-infected individuals. Morphine, a prototypical opioid and the principal active agent of opium, is a highly potent analgesic. It has been shown to up-regulate the expression of the HIV coreceptor CCR5 in CD3+ lymphoblasts and CD14+ monocytes (Steele et al, 2003). In this study, we demonstrate up-regulation of CCR5 mRNA in murine microglia (almost 3-fold) with 6-h treatment of morphine, an effect that was blocked by pretreatment with naltrexone, a general opioid-receptor antagonist. Furthermore, immunocytochemical analysis of cells treated with morphine demonstrated the up-regulation in CCR5 expression localized on the cell membranes, an effect that was blocked by pretreatment with naltrexone (Figure 1B). Analysis of the opioid receptor expression showed a 14-fold up-regulation in the mRNA levels of the μ-opioid receptor (Figure 1C), whereas those for κ- and δ-opioid receptors remained unchanged (data not shown), supporting previous studies that morphine mediates its analgesic effects specifically through modulation of the μ-receptor (Matthes et al, 1996).
We next wanted to explore the effect of morphine on the expression of CCR5 in the presence of HIV-1 Tat. Using FACS analysis, it was demonstrated that combined treatment of morphine and Tat resulted in a 154% increase in CCR5 expression as compared to a modest 104% or 115% increase in microglia treated with morphine or Tat alone, respectively (Figure 2A). Further analysis of CCR5 expression by Western blot analysis did reflect the up-regulation of CCR5 expression with combined morphine and Tat treatment, albeit not as pronounced as that seen with FACS. This discrepancy could be due to the fact that Western blot analysis detects total cellular CCR5 protein whereas FACS analysis only detects the surface bound CCR5.
Having established the effect of morphine and Tat on CCR5 up-regulation in BV-2 murine microglia, we next asked if morphine or HIV-1 Tat could induce activation of these cells. Microglial activation is associated with intense ramification and cytoskeletal rearrangement, resulting in change in shape and motility of cells. Classically microglia in the brain have a ramified appearance in the quiescent state. Transformation of BV-2 cell morphology following treatment with phorbol myrisitate (PMA) has been reported (Pottler et al, 2006). BV-2 cells appear small and elongated under normal culture conditions; however, when treated with morphine or Tat alone or in combination, the cells dramatically changed in size and morphology. Treated cells increased in size and became more rounded to resemble macrophages, some displaying short processes and/or multiple nuclei (Figure 3). This change in phenotype upon activation has been associated with an increase in the CD11b expression (Gonzalez-Scarano and Baltuch, 1999; Ling and Wong, 1993; Rock et al, 2004). CD11b has immense biological significance among various markers for microglial activation. It binds ICAM-1 (intracellular cell adhesion molecule-1) and the complement C3bi (Schwarz et al, 2002). We looked at the effect of morphine and Tat on the morphology of BV-2 cells because change in morphology is associated with a state of activation. Tat or morphine treatment of BV-2 cells resulted in change in cell shape; normally small and elongated or rounded, the cells appeared enlarged and macrophage-like. This change in cell shape was accompanied by an increase in the cell membrane expression of the microglial activation marker CD11b. Interestingly, CD11b expression was significantly increased with combinatorial treatment of morphine and Tat. With both treatments, cells became distinctly large in size, with some displaying podia-like processes. Further confirmation of CD11b expression by FACS analysis also demonstrated a potentiation of CD11b expression and microglial activation with combined morphine and Tat treatment (Figure 4).
Microglia when activated produce proinflamma-tory cytokines, which could be detrimental to the neurons. Because HIV-infected individuals who use opiates show higher pathological abnormalities in their brains, we hypothesized that morphine can exacerbate the effect of HIV Tat by potentiating release of proinflammatory cytokines. Treatment of microglia with morphine alone did not produce any significant effects on expression of proinflammatory cytokines, or iNOS and CD40. Treatment of microglia with Tat, however, induced a robust inflammatory response. Interestingly, when BV-2 cells were treated with morphine and Tat in combination, they demonstrated a potentiation in the mRNA expression of the proinflammatory cytokines IL-6, IL-1β, TNFα, as well as IP-10, iNOS, and CD40 ligand (Figure 5). The protein expression of proinflammatory cytokines (Figure 7), although enhanced by the combined treatment with morphine and Tat, was not as robust as the level of mRNA expression (Figure 6), thus underscoring the role of post-transcriptional and translational mechanisms in this process.
TNFα levels, which are elevated in the serum, cerebrospinal fluid (CSF), and brains of HIV-infected individuals, have been implicated in the pathogenesis of HAD, with levels of the cytokine corresponding to the severity of HAD (Glass et al, 1993; Grimaldi et al, 1991; D'Aversa et al, 2002). CD40 protein, which is expressed on microglia and is up-regulated by activating signals such as interferon (IFN)-γ from CD4+T cells, monocytes, or B cells, is engaged by the CD40 ligand (CD40L) (D'Aversa et al, 2002; de Goer de Herve et al, 2001). This interaction has been shown to play an important role in microglial activation and in the inflammatory response in HAD (D'Aversa et al, 2002). In summary, the observed increase in the expression of proinflammatory cytokines such as TNFα, IL-6, and also of CD40 by microglia exposed to Tat and morphine suggests a possible mechanism by which opiates could exacerbate HIV pathogenesis. The enhanced release of proinflammatory molecules could lead to enhanced neurotoxicity, thus activating a self-perpetuating mechanism in which the injured neurons, in turn, could trigger microgliosis.
Materials and methods
Cells, antibodies, and reagents
Morphine sulphate and Naltrexone HCl were obtained from NIDA (National Institutes on Drug Abuse). HIV-1 Tat1–72 was either obtained from the AIDS Research and Reference Reagent Program of National Institutes of Health (Bethseda, MD) or purchased from Ray D. Phillip (UK College of Medicine, Lexington, KY). BV-2 mouse microglial cells were a kind gift from Dr. Sanjay Maggirwar (University of Rochester, Medical Center, Rochester, NY). PE (phycoerythrin)-conjugated rat anti-mouse CCR5 antibodies were obtained from BD Biosciences (San Jose, CA). Unconjugated rat anti-mouse CD11b antibody was obtained from eBioscience (San Diego, CA), unconjugated monoclonal anti-human CCR5 antibodies were obtained from R&D Systems (Minneapolis, MN); anti-CD16/CD32 antibodies were used for blocking Fc-γIII receptors. GolgiPlug containing Brefeldin A for blocking protein transport, CytoFix/CytoPerm, and Perm/Wash buffer were purchased from BD Biosciences. Ethidium monoazide bromide (EMA) (Invitrogen Life Technologies, Carlsbad, CA) was used to stain dead cells prior to flow cytometry.
Primary murine microglial–enriched cultures
Murine microglial–enriched cultures were isolated from 1- to 2-day-old C57BL/6 mice according to a previously described protocol (Liu et al, 2000). Briefly, cerebral cortices from 2-day-old mouse pups were isolated under sterile conditions and were kept at 4°C prior to mechanical dissociation. Cells were plated onto 75-cm2 flasks coated with poly-D-lysine, in complete medium (consisting of Dulbecco's modified Eagle's medium [DMEM], 10% fetal bovine serum [FBS], and penicillin/streptomycin), and grown at 37°C in 5% CO2. Microglia were dislodged from the astrocyte layer by forceful shaking several times. Following centrifugation, microglia were plated at a density of 105 cells per well of a 24-well culture plate. The enriched microglia were >99% pure as determined by CD11b (Mac-1) staining (see Figure 7A).
Culture conditions
BV-2 immortalized cell line and primary murine microglia were grown and routinely maintained in DMEM (4.5 g/L glucose; 2% fetal bovine serum; 50 μg/ml gentamycin) and incubated at 37°C and 5% CO2. BV-2 cells were used up to passage 20.
Cell treatments
Morphine was used at 10−7 M concentration for all experiments except the dose-response study, for which a concentration range of 10−13 to 10−5 M morphine was used. The opioid receptor antagonist naltrexone was used at a concentration of 10−6 M. Tat was used at a 20 nM concentration. Cells were generally pretreated with morphine for 1 h followed by Tat treatment. Naltrexone was added 1 h prior to morphine. A 6-h combined incubation with morphine and Tat was adequate for a peak mRNA response whereas the treatment was extended to 24 h for protein analysis.
Flow cytometry
Cells were stained for flow cytometry according to previously published protocol, with some modifications (Bokhari et al, 2008). Following scraping from plates, BV-2 cells were washed once and resuspended in 1 ml of staining buffer (phosphate buffered saline (DPBS) with 2% fetal bovine serum). After counting, the cells were incubated with anti-CD16/CD32 (1 μg/106 cells) to block FcγII/III receptors. Fluorochrome-labeled antibodies to CCR5 or unconjugated CD11b antibodies were added to cells along with 0.5 μg/ml of EMA, and the mixtures were incubated for 10 min on ice in dark. Cell suspensions were then exposed to direct fluorescent light for 15 min at room temperature. Following two washes with staining buffer, cells were fixed and permeabilized with CytoFix/CytoPerm, washed, and restained with anti-CCR5 antibodies to detect internalized receptor. The cells were analyzed on an LSR II flow cytometer (BD Biosciences) using FACSDiva software after gating to exclude EMA-positive cells.
Immunocytochemistry
Cells were grown on coverslips in 24-well plates and treated with either morphine or Tat alone, or morphine and Tat in combination. Before fixation, cells were washed once with phosphate-buffered saline (PBS) and fixed in zinc-formalin solution for 20 min at room temperature, followed by blocking in 10% normal serum for 30 min and subsequently by incubation in primary antibody. The cells were washed 3 times with PBS (0.05% Tween-20) and incubated in fluorochrome-conjugated secondary antibody for 45 min. Cells were washed 3 times in buffer and mounted with Vectashield onto slides (Vector Laboratories, Burlingame, CA). Slides were viewed on the Nikon Eclipse 80i scope with a Nikon DSFi1 camera. Images from five random areas of each slide were captured and saved as grey-scale images in Adode Photoshop. Grey-scale images were thresholded and subjected to particle analysis. Data were compared to the control images.
Reverse transcription and real-time PCR
The quantitative polymerase chain reaction (qPCR) primers for mouse CCR5, HPRT, IL-1β, TNFα, iNOS, CD40, and IP-10 were obtained from SABiosciences (Frederick, MD). The sequences of primers used for IL-6 qPCR are as follows: sense: ATCCAGTTGCCTTCTTGGGACTGA; antisense: TAAGCCTCCGACTTGTGAAGTGGT. Total RNA was extracted with Trizol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. RNA was resuspended in RNase-free water and treated with DNase using the Turbo Free DNase kit from Ambion (Austin, TX) to remove any contaminating DNA. The RNA was quantified and reverse transcribed using the Taqman reverse transcription reagents from Applied Biosystems (Austin, TX). The cDNA was then subjected to quantitative real-time PCR using the SYBR green PCR reagents from Applied Biosystems on the AB7500 Thermal Cycler, Applied Biosystems. Data were analyzed using the 7500 Fast System software (version 1.4) and calculated as fold increase or decrease relative to the control.
Mouse cytokine assay
The Cytokine assay was performed on 24-h cell culture supernatants using Millipore's Milliplex Mouse Cytokine/Chemokine 9-plex panel according to the manufacturer's instructions. Twenty-five microliters of undiluted cell culture supernatants were captured by antibody-coated beads for various cytokines. Following incubation with a biotinylated antibody, the reaction mixture was incubated with streptavidin-PE–conjugated reporter molecule and run on the LUMINEX 100 system, which excites the internal dyes on microspheres representing various cytokines by a laser, leading to identification of each molecule and quantification based on fluorescent reporter signals. Data were analyzed on the Upstate Beadview Multiplex data analysis software (version 1.0)
Statistics
Each experiment was repeated at least 3 times and data shown are representative. Results are expressed as mean±SD. Data were analyzed by applying the Student's t test using the Microsoft Office Excel 2003 software.
Acknowledgments
This work was supported by grants MH62969, RR016443, MH068212, DA020392, and DA024442 from the National Institutes of Health (to S.M.B.). The authors thank Dr. Sanjay Maggirwar at the University of Rochester Medical Center for his kind gift of the BV-2 mouse microglial cell line and for his valuable advice. The authors also thank Shannon Callen for data analysis and Xuhui Zhu for technical assistance.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adler H, Peterhans E, Jungi TW. Generation and functional characterization of bovine bone marrow-derived macrophages. Vet Immunol Immunopathol. 1994;41:211–227. doi: 10.1016/0165-2427(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10:493–499. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Kim KJ, Pinson DM, Slusser J, Yeh HW, Parmely MJ. NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect Immun. 2008;76:1379–1389. doi: 10.1128/IAI.00745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonwetsch R, Croul S, Richardson MW, Lorenzana C, Del Valle L, Sverstiuk AE, Amini S, Morgello S, Khalili K, Rappaport J. Role of HIV-1 Tat and CC chemokine MIP-1alpha in the pathogenesis of HIV associated central nervous system disorders. J Neuro-Virol. 1999;5:685–694. doi: 10.3109/13550289909021297. [DOI] [PubMed] [Google Scholar]
- Budka H. The definition of HIV-specific neuro-pathology. Acta Pathol Jpn. 1991;41:182–191. doi: 10.1111/j.1440-1827.1991.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther. 1997;281:998–1004. [PubMed] [Google Scholar]
- Chen AC, LaForge KS, Ho A, McHugh PF, Kellogg S, Bell K, Schluger RP, Leal SM, Kreek MJ. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. Am J Med Genet. 2002;114:429–435. doi: 10.1002/ajmg.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am J Pathol. 2002;160:559–567. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aversa TG, Yu KO, Berman JW. Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein Tat. J NeuroVirol. 2004;10:86–97. doi: 10.1080/13550280490279807. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- de Goer de Herve MG, Delfraissy JF, Taoufik Y. Following direct CD40 activation, human primary microglial cells produce IL-12 p40 but not bioactive IL-12 p70. Cytokine. 2001;14:88–96. doi: 10.1006/cyto.2000.0855. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Fetler L, Amigorena S. Neuroscience. Brain under surveillance: the microglia patrol. Science. 2005;309:392–393. doi: 10.1126/science.1114852. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7:1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Grimaldi LM, Martino GV, Franciotta DM, Brustia R, Castagna A, Pristera R, Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol. 1991;29:21–25. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Kong LY, Wilson BC, McMillian MK, Bing G, Hudson PM, Hong JS. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell Immunol. 1996;172:77–83. doi: 10.1006/cimm.1996.0217. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS. Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience. 2000;97:749–756. doi: 10.1016/s0306-4522(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Pessac B, Godin I, Alliot F. Microglia: origin and development. Bull Acad Natl Med. 2001;185:337–346. discussion 346–347. [PubMed] [Google Scholar]
- Pottler M, Zierler S, Kerschbaum HH. An artificial three-dimensional matrix promotes ramification in the microglial cell-line, BV-2. Neurosci Lett. 2006;410:137–140. doi: 10.1016/j.neulet.2006.09.082. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Nordt T, Bode C, Peter K. The GP IIb/IIIa inhibitor abciximab (c7E3) inhibits the binding of various ligands to the leukocyte integrin Mac-1 (CD11b/CD18, alphaMbeta2) Thromb Res. 2002;107:121–128. doi: 10.1016/s0049-3848(02)00207-4. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- UNAIDS . report on the global aids epidemic. UNAIDS; Geneva: 2006. 2006. [Google Scholar]