Abstract
Purpose
In animal models, the small intestine responds to massive small bowel resection (SBR) through a compensatory process termed adaptation, characterized by increases in both villus height and crypt depth. This study seeks to determine whether similar morphologic alterations occur in humans following SBR.
Methods
Clinical data and pathologic specimens of infants who had both a SBR for necrotizing enterocolitis (NEC) and an ostomy takedown from 1999–2009 were reviewed. Small intestine mucosal morphology was compared in the same patients at the time of SBR and the time of ostomy takedown.
Results
For all samples, there was greater villus height (453.6±20.4 vs. 341.2±12.4 μm, p<0.0001) and crypt depth (178.6±7.2 vs. 152.6±6 μm, p<0.01) in the ostomy specimens compared to the SBR specimens. In infants with paired specimens, there was an increase of 31.7±8.3% and 22.1±10.0% in villus height and crypt depth, respectively. There was a significant correlation between the amount of intestine resected and the percent change in villus height (r=0.36, p<0.05).
Conclusion
Mucosal adaptation after SBR in human infants is similar to what is observed in animal models. These findings validate the use of animal models of SBR utilized to understand the molecular mechanisms of this important response.
Keywords: small intestine, adaptation, small bowel resection, adaptation in humans
Introduction
In animals models of massive small bowel resection (SBR) resulting in the short bowel syndrome (SBS), intestinal adaptation is characterized by a variety of physiologic, cellular, and molecular responses that serve to enhance absorptive function to compensate for surgically diminished length. These interactions are triggered by a mitogenic response within the intestinal mucosa, resulting in significant morphologic alterations. These are manifested primarily as increases in villus height, crypt depth, bowel caliber, and to some extent, length [1]. Along with increased expression of a variety of transport proteins and alterations in secretory profiles of gut and pancreatobiliary hormones, these structural augmentations in the mucosa are critical for adaptation to occur [2–4]. Understanding the exact mechanisms of adaptation is a critical prerequisite toward the design of targeted clinical interventions designed to amplify adaptation. Greater adaptation may result in improved tolerance of enteral feeding, thus, attenuating requirements for parenteral nutrition and its allied morbidity and cost.
While many studies have sought to elucidate the mechanisms of the processes involved in intestinal adaptation using various animal models, the direct translation of these processes to the intestinal response aftermassive SBR in humans is less clear. Clinically, adaptation is demonstrated as patients are able to tolerate increasing amounts of enteral nutrition over time. A thorough adaptation response culminates in complete autonomy from parenteral nutrition. Despite this important clinical progression, the scientific literature has failed to consistently reveal significant increases in villus height and/or crypt depth in the remnant bowel after massive SBR in human patients [5–9]. Unfortunately, these prior studies are comprised of small numbers of patients and generally do not compare adaptive structural alterations in the same patient and/or region of intestine. Specifically, we are unaware of any prior studies evaluating resection-induced adaptation in the neonatal population. In order to more explicitly delineate the human neonatal response to SBR, we sought to characterize morphologic changes in the intestinal mucosa in a uniform population of infants with neonatal necrotizing enterocolitis (NEC) in response to intestinal resection.
1. Methods
1.1. Setting
St. Louis Children’s Hospital is a 235-bed free-standing pediatric hospital on the campus of Washington University School of Medicine in St. Louis.
1.2. Patients
Institutional Review Board approval was obtained from the Washington University School of Medicine Institutional Review Board (HRPO 09-0761). We sought to compare intestinal morphology at the time of SBR in neonates treated for NEC with the same infants at the time of subsequent ostomy closure. Patients were included in our study if they had a diagnosis of NEC based on ICD-9 CM disease codes (ICD-9 code 777.5) and underwent a procedure on the digestive system during the time period from January 1, 1999 to June 30, 2009. Patients were excluded from our study if they did not have a clinical diagnosis of NEC, available pathologic specimens, or evaluable tissue upon review of pathologic slides.
1.3. Data Collection
The paraffin blocks from patients with evaluable tissues were sectioned by the Morphology Core of the Washington University Digestive Diseases Research Center and stained using hematoxylin and eosin (H & E). When possible, the most proximal sample from a segment of resected small bowel was evaluated, as this region would most closely approximate the same region of small bowel excised at the time of ostomy closure. The scoring of all slides was done in a blinded manner and morphologic characteristics of the small bowel were measured using a microscopic, video-assisted software package(Metamorph, UIC, Dowington, PA). Only samples with structurally intact mucosa were measured; many of the samples had patchy areas of necrosis that obliterated mucosal architecture. For each sample, at least 10 well-oriented crypts and villi were measured to calculate mean villus height and mean crypt depth. Crypts were considered well-oriented if they extended from the crypt-villus junction to the basement membrane and had Paneth cells present in their base. Villi were considered well-oriented if the central lacteal was evident from base to tip and the epithelium was contiguous with an adjacent, well-oriented crypt. Both intra- and inter-observer variability in the measurement of morphologic indexes were <10%.
To obtain clinical data for correlation with resection-induced architectural alterations in the intestinal mucosa, the medical records, including charts, daily flow sheets, laboratory reports, and radiographic reports were reviewed retrospectively. Data on patient demographics, prenatal course, delivery history, comorbidities, date of NEC diagnosis, various risk factors for NEC, Bell’s stage, intubation history, laboratory values, feeds prior to operations, location of resection, amount of small and large bowel resected, retention of ileocecal valve, feeds at discharge, dependence on TPN, cholestasis, and survival were recorded [10].
1.4. Statistics
Data analysis was performed using SAS 9.2 (Cary, NC). Categorical variables were compared using χ2analyses. Continuous variables were compared using the Wilcoxon rank-sum test for non-normally distributed variables. Continuous results are presented as a mean ±standard error (SE) of measure. Correlations between variables were calculated using Pearson’s correlation coefficient (Pearson’s r). All p-values less than 0.05 were considered statistically significant. All tests of significance were two-tailed.
2. Results
2.1. Sample Characteristics
Sixty-four patients met the inclusion criteria. Of these, 4 had missing or unavailable tissue from the pathology archives and an additional 11 patients were excluded because of poor slide quality due to extensive necrosis and poor fixation and/or orientation. The remaining forty-nine patients (77%) had at least one evaluable tissue sample with 33 (52%) patients having both SBR and ostomy specimens available.
2.2. Clinical Data
The clinical characteristics of the 33 paired patients in the study are shown in Table 1. The majority of patients were male, and most patients (79%) had no significant comorbidities other than prematurity. The mean time between SBR and ostomy takedown was 74 days (±13). The mean amount of small bowel resected was 15.7 cm (range 1.5 - 65). The overall survival rate for this cohort of patients was 86%. 92% of the surviving patients had weaned completely from TPN at the time of discharge.
Table 1.
Characteristics of patients with paired tissue samples: demographics, disease severity, hospital course, and underlying disease. In parentheses is the standard error of the mean or percentages, when applicable.
| Total (N=29) | |
|---|---|
| DEMOGRAPHICS | |
| Birthweight (g) | 1254 (870–2177) |
| Estimated Gestational Age (wks) | 30 (26–34) |
| Sex | |
| Male | 17 (59%) |
| Female | 12 (41%) |
| Race | |
| African American | 15 (52%) |
| Caucasian | 13 (45%) |
| Other | 1 (3%) |
| Comorbidities | |
| None | 23 (79%) |
| Cardiac | 4 (14%) |
| CNS | 1 (3%) |
| Other | 1 (3%) |
| Post-Conceptional Age at Dx (wks) | 31.6 (29.4–35.5) |
|
| |
| DIAGNOSIS | |
| Radiographs | |
| Pneumatosis Intestinalis | 20 (69%) |
| Portal Venous Gas | 8 (28%) |
| Free Air Under Diaphragm | 13 (45%) |
| Bells Stage | |
| I | 0 |
| II | 12 (41%) |
| III | 17 (59%) |
|
| |
| MANAGEMENT | |
| Post Conceptional Age at SBR (wks) | 32.3 (29.9–34.9) |
| Section of Intestine Resected | |
| Jejunum | 5 (17%) |
| Jejunum/Illeum | 6 (21%) |
| Illeum | 9 (31%) |
| Illeum/Colon | 9 (31%) |
| Ileocecal Valve Resected (cm) | 11 (38%) |
| Resection Length (cm) | 15.7 (8.0–24.5) |
| Post Conceptional Age at Ostomy Takedown (wks) | 42.7 (40–49.1) |
| Time between SBR and Ostomy Takedown (days) | 74 (61–90) |
|
| |
| OUTCOMES | |
| Survive to Discharge | 25 (86%) |
| Enteral Feeds Only at time of Discharge | 27 (92%) |
2.3. Small Intestine Morphology: SBR vs. ostomy
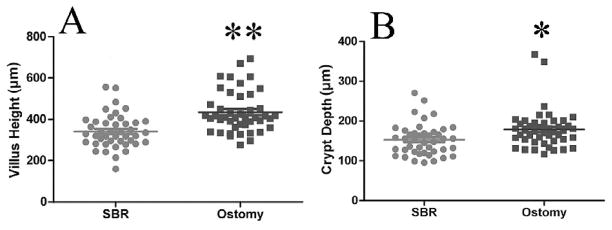
For all samples, villus height in the ostomy samples was greater when compared with the SBR samples (453.6±20.4 vs. 341.2±12.4 μm, p<0.0001; Fig. 1A). Similarly, crypt depth was significantly deeper in the ostomy samples compared with samples taken at the time of SBR (178.6±7.2 μm vs. 152.6±6.1 μm, p<0.01 Fig. 1B). For patients with paired samples only (n=33), an increase in both villus height and crypt depth (Fig. 2), corresponding to 31.7±8.3% and 22.1±10.0% growth, respectively (p<0.01, Fig. 3), was observed.
Figure 1.
A) Comparison of villus height and B) crypt depth for all samples combined. The mean (±SEM) villus height was 453.6±20.4 vs. 341.2±12.4 μm in the ostomy samples and SBR samples, respectively. **= p<0.0001. The average (±SEM) crypt depth was 178.6±7.2 vs. 152.6±6.1 μm in the ostomy samples and SBR samples, respectively. *= p<0.01.
Figure 2.
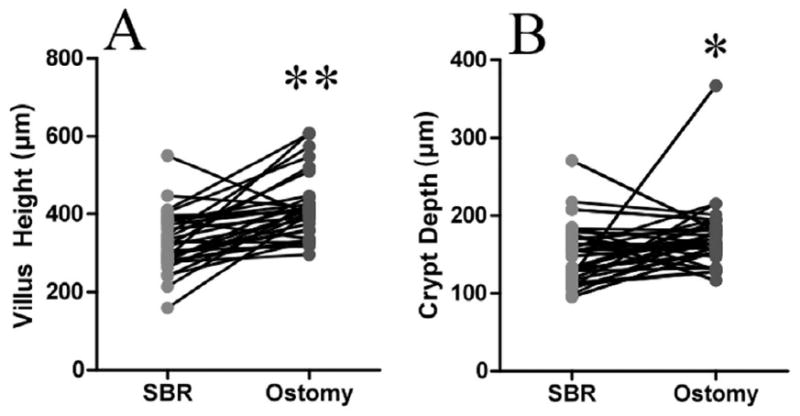
Comparison of A) villi and B) crypts in paired samples (patients that had evaluable specimens at both times of resection and ostomy closure). There was a significant increase in villus height in the ostomy samples compared to the resection samples. **= p<0.0001. There was a significant increase in crypt depth in the ostomy samples compared to the resection samples. *= p<0.05.
Figure 3.
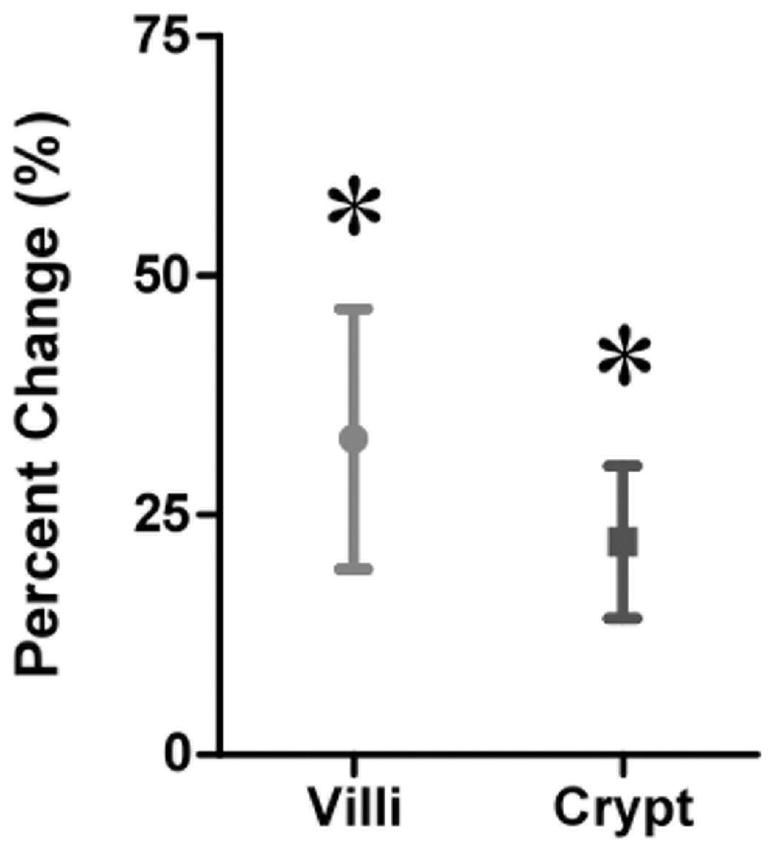
Percent change for paired samples. There was an increase in mean villus height and crypt depth of 31.7±8.3% and 22.1±10.0%, respectively, when comparing the ostomy samples to the SBR samples. *= p<0.01.
2.4. Clinical Correlations with Adaptation
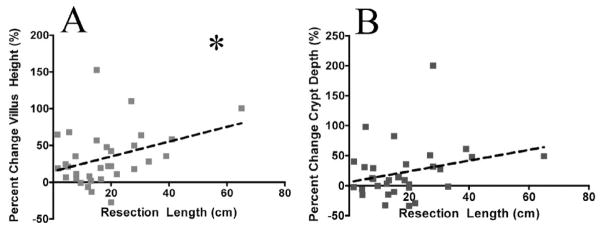
No clinical characteristics were significantly correlated with the magnitude of adaptation, except for the length of small intestine resected. There was a statistically significant positive correlation between the amount of small intestine that was removed and the percent increase in villus height in the ostomy samples compared to the SBR samples (r=0.36, p<0.05; Fig. 4A). There was a statistically insignificant trend towards a positive correlation between the amount of small intestine resected and the percent increase in crypt depth (r=0.27, p=0.14; Fig. 4B).
Figure 4.
Correlation between amount of intestine resected and A) villus and B) crypt changes. There was a significant positive correlation between the amount of intestine resected and the percent increase in villus height for paired samples (r=0.36). *= p<0.05. There was a trend towards a positive correlation between the amount of intestine resected and the percent increase in crypt depth for paired samples, but this difference was not significant (r=0.27, p=0.14).
3. Discussion
In the present study, we have demonstrated significant increases in both villus height and crypt depth following intestinal resection in human neonates with NEC. Further, we noted a significant correlation between the magnitude of SBR and the degree of villus growth. To our knowledge, this is the first study examining small intestinal adaptation responses in infants following SBR.
The present literature regarding structural adaptation in humans is both limited and conflicting. For example, in a 1965 report, the mucosal morphology of 4 adult SBS patients was compared with age-matched controls; no significant differences in villus height or crypt depth were identified [5]. Along these lines, Ziegler, et al. compared 13 adult SBS patients with controls, and failed to demonstrate any significant differences in the mucosal morphology of either the small intestine or the colon [8]. In another study of nine adult SBS patients with jejunostomies, villus heights were actually found to be lower than a similarly aged control group[7]. Collectively, these studies were comprised of adult patients with SBS that had tissue collected at a singular time point, which was compared with age-matched controls. The results of the current study may be more valid because our study design allowed for direct comparison of tissue from two time points within the same patients. While the previous studies were mostly comprised of patients who had undergone massive SBR in the distant past, the time frame for tissue analysis in our study was within an expected period of adaptation (1st year after SBR). Thus, the earlier reports might have missed significant earlier adaptive changes. Finally, the earlier studies are limited by their small sample sizes, which we were able to improve on in the present study.
Several caveats must be considered in the interpretation of our findings. First, the length of intestinal resection in our NEC patients was relatively short (average of 15.7 cm). Our patients, therefore, did not truly mimic animal models of adaptation in the context of massive intestinal loss. On the other hand, our finding that villus growth significantly correlated with the magnitude of intestinal resection would support the concept that more extensive small bowel resections may lead to greater adaptive responses [2,4,11]. On the other hand, we have previously reported that removing more than 50% of the small bowel in a murine model does not lead to greater intestinal adaptation [12]. Perhaps the capacity for adaptation correlates with the degree of intestinal loss up to a specific point, beyond which greater resections have no further effect.
Another explanation for the observed villus and crypt growth could be that baseline levels of these parameters were lower at the time of SBR as a consequence of NEC. Villus height measurements were found to be reduced in several animal models of NEC [13,14]. To address this consideration, we attempted to compare villus and crypt measurements in our NEC group at the time of resection with age-matched autopsy-derived samples of small intestine taken from infants without NEC. While we found no differences in crypt and villus lengths between infants with NEC and infants without NEC (data not shown), we acknowledge that drawing any conclusions from these data would be premature due to the small sample size for each age, as well as the unknown site of intestine (jejunum versus ileum) from which the samples were taken at the time of autopsy. Therefore, it is possible that the villus and crypt measurements were attenuated in infants with NEC and became greater in the context of intestinal recovery (rather than resection-induced adaptation).
An alternative reason for the increase in crypt and villus growth between the time of bowel resection and the ostomy reversal could be due to our patient population being largely comprised of premature infants. Thus, the augmented morphologic changes observed may represent normal postnatal growth rather than an adaptive response to SBR. From our review of intestine sampled at the time of autopsy, we noted that villus height and crypt depth measurements were similar across a wide spectrum of post-conceptual ages (data not shown). In addition, the correlation found between the amount of intestine resected and the degree of morphologic adaptation would be consistent with adaptation, rather than normal postnatal growth..
The present study was limited by its retrospective design. Therefore, protocols for intestinal resection, pathologic tissue processing, and preservation could not be standardized. Our ability to find evaluable tissue specimens was further compromised by the obliteration of mucosal architecture in the most diseased sections of bowel in patients with NEC. As a result, we were forced to exclude many patients. Despite this, our sample size was adequate to achieve statistical significance over a variety of parameters, and represents a larger sample size than any other study addressing human adaptation. The present study is also limited by its evaluation of the adaptive response over only a single, relatively short, time course. Here, ostomy takedown was performed 2–3 months after SBR. While adaptation has been shown to occur days after SBR in animal models, adaptation responses may require several years to fully mature in humans. Thus, our present measurements may underestimate the true magnitude of adaptive changes that might take place over longer periods of time.
Overall, the results of this study offer evidence that morphologic intestinal adaptation occurs in infants with NEC following SBR. These changes are characterized by increases in both villus height and crypt depth, the magnitude of which correlate with the amount of intestine resected. These findings mirror what has been described in animal models of adaptation, thereby validating their use as a means to gain mechanistic insight into the cellular and molecular mechanisms involved in the important resection-induced adaptation process. Future prospective studies are needed to more definitively characterize resection-induced intestinal adaptation responses in humans.
Acknowledgments
This work was supported by The Doris Duke Charitable Foundation Clinical Research Fellowship for Medical Students (LAM) and The National Institutes of Health grants R01 DK059288 (BWW), T32 CA009621 (DW), T32 GM00879509 (BTB), The St. Louis Children’s Hospital Foundation - Children’s Surgical Sciences Research Institute, and P30 DK52574 - Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longshore SW, Wakeman D, McMellen M, Warner BW. Bowel resection induced intestinal adaptation: progress from bench to bedside. Minerva Pediatr. 2009;61(3):239–51. [PubMed] [Google Scholar]
- 2.Drozdowski L, Thompson AB. Intestinal mucosal adaptation. World J Gastroenterol. 2006;12(29):4614–27. doi: 10.3748/wjg.v12.i29.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cisler JJ, Buchman AL. Intestinal adaptation in short bowel syndrome. J Investig Med. 2005;53(8):402–13. doi: 10.2310/6650.2005.53804. [DOI] [PubMed] [Google Scholar]; Thiesen A, Drozdowski L, Iordache C, Neo CC, Woudstra TD, Xenodemetropoulos T, et al. Adaptation following intestinal resection: mechanisms and signals. Best Pract Res Clin Gastroenterol. 2003;17(6):981–95. doi: 10.1016/s1521-6918(03)00097-0. [DOI] [PubMed] [Google Scholar]
- 4.Porus RL. Epithelial hyperplasia following massive small bowel resection in man. Gastroenterol. 1965;48:753–7. [PubMed] [Google Scholar]
- 5.Weinstein LD, Shoemaker CP, Hersh T, Wright HK. Enhanced intestinal absorption after small bowel resection in man. Arch Surg. 1969;99(5):560–2. doi: 10.1001/archsurg.1969.01340170012003. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe SJ, Haymond MW, Bennet WM, Oswald B, Nelson DK, Shorter RG. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterol. 1994;107(2):379–88. doi: 10.1016/0016-5085(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler TR, Fernández-Estívariz C, Gu LH, Bazargan N, Umeakunne K, Wallace TM, et al. Distribution of the H+/peptide transporter PepT1 in human intestine: up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am J Clin Nutr. 2002;75(5):922–30. doi: 10.1093/ajcn/75.5.922. [DOI] [PubMed] [Google Scholar]
- 8.Pironi L, Paganelli GM, Miglioli M, Biasco G, Santucci R, Ruggeri E, et al. Morphologic and Cytoproliferative Patterns of Duodenal Mucosa in Two Patients After Long-term Total Parenteral Nutrition: Changes With Oral Refeeding and Relation to Intestinal Resection. J Parenter Enteral Nutr. 1994;18:351–4. doi: 10.1177/014860719401800413. [DOI] [PubMed] [Google Scholar]
- 9.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978;298(25):1393–402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- 11.Wakeman D, Longshore SW, McMellen ME, Santos JA, Guo J, Erwin CR, Warner BW. Extent of small bowel resection does not influence the magnitude of intestinal adaptation in the mouse. J Pediatr Surg. 2010 Jun;45(6):1274–9. doi: 10.1016/j.jpedsurg.2010.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siggers RH, Siggers J, Boye M, Thymann T, Molbak L, Leser T, et al. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J Nutr. 2008;138(8):1437–44. doi: 10.1093/jn/138.8.1437. [DOI] [PubMed] [Google Scholar]
- 13.Siggers RH, Thymann T, Jensen BB, Molbak L, Heegaard PM, Schmidt M, et al. Elective cesarean delivery affects gut maturation and delays microbial colonization but does not increase necrotizing enterocolitis in preterm pigs. Am J Physiol Regul Integr Comp Physiol. 2008;294:R929–R938. doi: 10.1152/ajpregu.00705.2007. [DOI] [PubMed] [Google Scholar]